Air Quality and Potential Health Risk Impacts of Exposure to Bacterial Aerosol in a Waste Sorting Plant Located in the Mountain Region of Southern Poland, Around Which There Are Numerous Rural Areas
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Sampling and Analysis Methods
2.3. Identification of Selected Bacteria
2.4. Antibiotic Susceptibility Test
- <15 mm—resistant to antibiotics (R),
- 16 to 25 mm—the average level of resistance to antibiotics (I),
- >25 mm—bacteria were sensitive to antibiotics (S).
3. Results and Discussion
3.1. The Concentration of Culturable Bacterial Aerosol (CCBA)
3.2. The Size Distribution of the Bacterial Aerosol
3.3. Identification of Bacterial Aerosol
3.4. Antibiotic Susceptibility Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalwasińska, A.; Burkowska, A. Municipal landfill sites as sources of microorganisms potentially pathogenic to humans. Environ. Sci. Process. Impacts 2013, 15, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Ivens, U.I.; Ebbehøj, N.; Poulsen, O.M.; Skov, T. Gastrointestinal symptoms among waste recycling workers. Ann. Agric. Environ. Med. 1997, 4, 153–158. [Google Scholar]
- Poulsen, O.M.; Breum, N.O.; Ebbehøj, N.; Hansen, Å.M.; Ivens, U.I.; van Lelieveld, D.; Malmros, P.; Matthiasen, L.; Nielsen, B.H.; Nielsen, E.M.; et al. Sorting and recycling of domestic waste. Review of occupational health problems and their possible causes. Sci. Total Environ. 1995, 168, 33–56. [Google Scholar] [CrossRef]
- Solans, X.; Alonso, R.M.; Constans, A.; Mansilla, A. Occupational exposure to airborne fungi and bacteria in a household recycled container sorting plant. Rev. Iberoam. Micol. 2007, 24, 131–135. [Google Scholar] [CrossRef]
- Estillore, A.D.; Trueblood, J.V.; Grassian, V.H. Atmospheric chemistry of bioaerosols: Heterogeneous and multiphase reactions with atmospheric oxidants and other trace gases. Chem. Sci. 2016, 7, 6604–6616. [Google Scholar] [CrossRef] [PubMed]
- Malta-Vacas, J.; Viegas, S.; Sabino, R.; Viegas, C. Fungal and microbial volatile organic compounds exposure assessment in a waste sorting plant. J. Toxicol. Environ. Health-Part A Curr. Issues 2012, 75, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Reponen, T.; Willeke, K.; Grinshpun, S.; Nevalainen, A. Biological Particle Sampling. In Aerosol Measurement: Principles, Techniques, and Applications, 3rd ed.; John Wiley and Sons: New York, NY, USA, 2011; pp. 549–570. [Google Scholar]
- Marta, S.; Canha, N.; Silva, A.; Freitas, C.; Pegas, P.; Alves, C.; Evtyugina, M.; Adrião, C. Children exposure to atmospheric particles in indoor of Lisbon primary schools. Atmos. Environ. 2011, 45, 7594–7599. [Google Scholar]
- Aydogdu, H.; Asan, A.; Tatman Otkun, M. Indoor and outdoor airborne bacteria in child day-care centers in Edirne City (Turkey), seasonal distribution and influence of meteorological factors. Environ. Monit. Assess. 2010, 164, 53–66. [Google Scholar] [CrossRef]
- Brandl, H. Bioaerosols in Indoor Environment-A Review with Special Reference to Residential and Occupational Locations. Open Environ. Biol. Monit. J. 2011, 4, 83–96. [Google Scholar] [CrossRef]
- Brągoszewska, E.; Biedroń, I.; Kozielska, B.; Pastuszka, J.S. Microbiological indoor air quality in an office building in Gliwice, Poland: Analysis of the case study. Air Qual. Atmos. Health 2018, 11, 729–740. [Google Scholar] [CrossRef]
- Brągoszewska, E.; Biedroń, I. Indoor Air Quality and Potential Health Risk Impacts of Exposure to Antibiotic Resistant Bacteria in an Office Rooms in Southern Poland. Int. J. Environ. Res. Public Health 2018, 15, 2604. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Nam, I.S.; Yun, H.; Kim, J.; Yang, J.; Sohn, J.R. Characterization of indoor air quality and efficiency of air purifier in childcare centers, Korea. Build. Environ. 2014, 82, 203–214. [Google Scholar] [CrossRef]
- Nasir, Z.A.; Colbeck, I.; Sultan, S.; Ahmed, S. Bioaerosols in residential micro-environments in low income countries: A case study from Pakistan. Environ. Pollut. 2012, 168, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Breza-Boruta, B. The assessment of airborne bacterial and fungal contamination emitted by a municipal landfill site in Northern Poland. Atmos. Pollut. Res. 2016, 7, 1043–1052. [Google Scholar] [CrossRef]
- Canha, N.; Almeida, S.M.; Freitas, M.D.C.; Wolterbeek, H.T. Assessment of bioaerosols in urban and rural primary schools using passive and active sampling methodologies. Arch. Environ. Prot. 2015, 41, 11–22. [Google Scholar] [CrossRef]
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, D. Bioaerosol health effects and exposure assessment: Progress and prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar] [PubMed]
- Dz.U. 2005 In Regulation of the Minister of Health on harmful biological factors for health in the work environment and health protection of workers professionally exposed to these factors. Dz.U.2005.81.716. Available online: http://prawo.sejm.gov.pl/isap.nsf/download.xsp/WDU20050810716/O/D20050716.pdf (accessed on 28 June 2019).
- Brągoszewska, E.; Pastuszka, J.S. Influence of meteorological factors on the level and characteristics of culturable bacteria in the air in Gliwice, Upper Silesia (Poland). Aerobiologia 2018, 34, 241–255. [Google Scholar] [CrossRef]
- Bragoszewska, E.; Mainka, A.; Pastuszka, J.S. Concentration and size distribution of culturable bacteria in ambient air during spring and winter in Gliwice: A typical urban area. Atmosphere 2017, 8, 239. [Google Scholar] [CrossRef]
- Kallawicha, K.; Lung, S.C.C.; Chuang, Y.C.; Wu, C.D.; Chen, T.H.; Tsai, Y.J.; Chao, H.J. Spatiotemporal distributions and land-use regression models of ambient bacteria and endotoxins in the greater Taipei area. Aerosol Air Qual. Res. 2015, 15, 1448–1459. [Google Scholar] [CrossRef]
- Nevalainen, A.; Willeke, K.; Liebhaber, F.; Pastuszka, J.S.; Burge, H.; Henningson, E. Bioaerosol sampling. In Aerosol Measurement: Principles, Techniques and Applications; Willeke, K., Baron, P., Eds.; Van Nostrand Reinhold: New York, NY, USA, 1993; pp. 471–492. [Google Scholar]
- Andersen, A.A. New sampler for the collection, sizing, and enumeration of viable airborne particles. J. Bacteriol. 1958, 76, 471. [Google Scholar]
- PN-EN 12322 In Vitro Diagnostic Medical Devices. Culture Media for Microbiology. Performance Criteria for Culture Media. 2005. Available online: https://ec.europa.eu/growth/single-market/european-standards/harmonised-standards/iv-diagnostic-medical-devices_en (accessed on 28 June 2019).
- ISO 11133 Microbiology of Food, Animal Feed and Water—Preparation, Production, Storage and Performance Testing of Culture Media. 2014. Available online: https://www.iso.org/standard/53610.html (accessed on 28 June 2019).
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. 2016. Available online: http://www.asmscience.org/content/education/protocol/protocol.3189 (accessed on 28 June 2019).
- Faridi, S.; Hassanvand, M.S.; Naddafi, K.; Yunesian, M.; Nabizadeh, R.; Sowlat, M.H.; Kashani, H.; Gholampour, A.; Niazi, S.; Zare, A.; et al. Indoor/outdoor relationships of bioaerosol concentrations in a retirement home and a school dormitory. Environ. Sci. Pollut. Res. 2015, 22, 8190–8200. [Google Scholar] [CrossRef] [PubMed]
- Menteşe, S.; Arisoy, M.; Rad, A.Y.; Güllü, G. Bacteria and fungi levels in various indoor and outdoor environments in Ankara, Turkey. Clean-Soilairwater 2009, 37, 487–493. [Google Scholar]
- Law, A.K.Y.; Chau, C.K.; Chan, G.Y.S. Characteristics of bioaerosol profile in office buildings in Hong Kong. Build. Environ. 2001, 36, 527–541. [Google Scholar] [CrossRef]
- Gołofit-Szymczak, M.; Górny, R.L. Bacterial and fungal aerosols in air-conditioned office buildings in Warsaw, Poland-the winter season. Int. J. Occup. Saf. Ergon. 2010, 16, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, J.; Tolvanen, O.; Nivukoski, U.; Veijanen, A.; Hänninen, K. Occupational hygiene in terms of volatile organic compounds (VOCs) and bioaerosols at two solid waste management plants in Finland. Waste Manag. 2013, 33, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Park, D.U.; Ryu, S.H.; Kim, S.B.; Yoon, C.S. An Assessment of Dust, Endotoxin, and Microorganism Exposure during Waste Collection and Sorting. J. Air Waste Manag. Assoc. 2011, 61, 461–468. [Google Scholar] [CrossRef]
- Fang, Z.; Ouyang, Z.; Zheng, H.; Wang, X.; Hu, L. Culturable airborne bacteria in outdoor environments in Beijing, China. Microb. Ecol. 2007, 54, 487–496. [Google Scholar] [CrossRef]
- Di Giorgio, C.; Krempff, A.; Guiraud, H.; Binder, P.; Tiret, C.; Dumenil, G. Atmospheric pollution by airborne microorganisms in the city of Marseilles. Atmos. Environ. 1996, 30, 155–160. [Google Scholar] [CrossRef]
- Mouli, P.C.; Mohan, S.V.; Reddy, S.J. Assessment of microbial (bacteria) concentrations of ambient air at semi-arid urban region: Influence of meteorological factors. Appl. Ecol. Environ. Res. 2005, 3, 139–149. [Google Scholar] [CrossRef]
- Kummer, V.; Thiel, W.R. Bioaerosols-Sources and control measures. Int. J. Hyg. Environ. Health 2008, 211, 299–307. [Google Scholar] [CrossRef]
- Lacey, J.; Dutkiewicz, J. Bioaerosols and occupational lung disease. J. Aerosol Sci. 1994, 25, 1371–1404. [Google Scholar] [CrossRef]
- Owen, M.K.; Ensor, D.S.; Sparks, L.E. Airborne particle sizes and sources found in indoor air. Atmos. Environ. Part A Gen. Top. 1992, 26, 2149–2162. [Google Scholar] [CrossRef]
- Wolfgang, W.J.; Carpenter, A.N.; Cole, J.A.; Gronow, S.; Habura, A.; Jose, S.; Nazarian, E.J.; Kohlerschmidt, D.J.; Limberger, R.; Schoonmaker-Bopp, D.; et al. Neisseria wadsworthii sp. nov. and Neisseria shayeganii sp. nov., isolated from clinical specimens. Int. J. Syst. Evol. Microbiol. 2011, 61, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Holcombe, L.J.; Patel, N.; Colyer, A.; Deusch, O.; O’Flynn, C.; Harris, S. Early canine plaque biofilms: Characterization of key bacterial interactions involved in initial colonization of enamel. PLoS ONE 2014, 9, e113744. [Google Scholar] [CrossRef] [PubMed]
- Paściak, M.; Pawlik, K.; Gamian, A.; Szponar, B.; Skóra, J.; Gutarowska, B. An airborne actinobacteria Nocardiopsis alba isolated from bioaerosol of a mushroom compost facility. Aerobiologia 2014, 30, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Arévalo, A.P.; Camarena-Pozos, D.A.; Castellanos-Arévalo, D.C.; Rangel-Córdova, A.A.; Peña-Cabriales, J.J.; Arévalo-Rivas, B.; De Peña, D.G.; Maldonado-Vega, M. Microbial contamination in the indoor environment of tanneries in Leon, Mexico. Indoor Built Environ. 2016, 25, 524–540. [Google Scholar] [CrossRef]
- Morris, D.L.; Wilson, S.R.; Pain, J.; Edwardson, K.F.; Jones, J.; Strachan, C.; Slack, R. A comparison of aztreonam/metronidazole and cefotaxime/metronidazole in elective colorectal surgery: Antimicrobial prophylaxis must include Gram-positive cover. J. Antimicrob. Chemother. 1990, 25, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.D.; Klutman, N.E.; Lamp, K.C. Metronidazole. A therapeutic review and update. Drugs 1997, 54, 679–708. [Google Scholar] [CrossRef]
- Bennett, J.S.; Jolley, K.A.; Maiden, M.C.J. Genome sequence analyses show that Neisseria oralis is the same species as ‘Neisseria mucosa var. Heidelbergensis’. Int. J. Syst. Evol. Microbiol. 2013, 63 Pt 10, 3920. [Google Scholar] [CrossRef]
- Liu, G.; Tang, C.M.; Exley, R.M. Non-pathogenic neisseria: Members of an abundant, multi-habitat, diverse genus. Microbiology 2015, 161, 1297–1312. [Google Scholar] [CrossRef]
- Choi, S.M.; Park, M.H.; Jung, T.S.; Moon, K.H.; Kim, K.M.; Kang, J.S. Characterization of Bacillus mojavensis KJS-3 for industrial applications. Arch. Pharm. Res. 2011, 34, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Takaku, H.; Kodaira, S.; Kimoto, A.; Nashimoto, M.; Takagi, M. Microbial communities in the garbage composting with rice hull as an amendment revealed by culture-dependent and -independent approaches. J. Biosci. Bioeng. 2006, 101, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Heyrman, J.; Rodríguez-Díaz, M.; Devos, J.; Felske, A.; Logan, N.A.; De Vos, P. Bacillus arenosi sp. nov., Bacillus arvi sp. nov. and Bacillus humi sp. nov., isolated from soil. Int. J. Syst. Evol. Microbiol. 2005, 55, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Karadag, D.; Özkaya, B.; Ölmez, E.; Nissilä, M.E.; Çakmakçi, M.; Yildiz, Ş.; Puhakka, J.A. Profiling of bacterial community in a full-scale aerobic composting plant. Int. Biodeterior. Biodegrad. 2013, 77, 85–90. [Google Scholar] [CrossRef]
- Horiike, T.; Dotsuta, Y.; Nakano, Y.; Ochiai, A.; Utsunomiya, S.; Ohnuki, T.; Yamashita, M. Removal of soluble strontium via incorporation into biogenic carbonate minerals by halophilic bacterium Bacillus sp. strain TK2d in a highly saline solution. Appl. Environ. Microbiol. 2017, 83, e00855–e00917. [Google Scholar] [CrossRef] [PubMed]
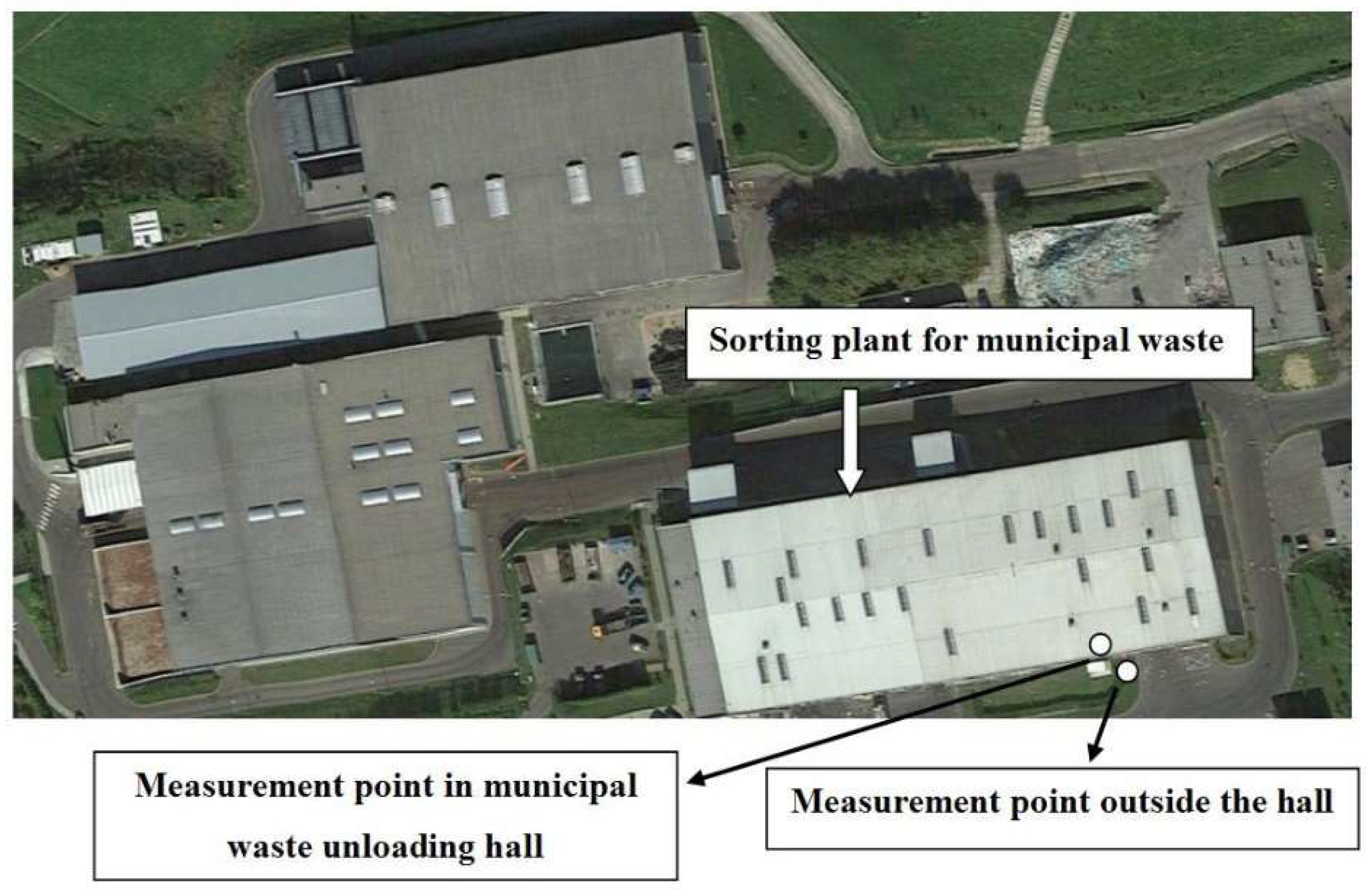


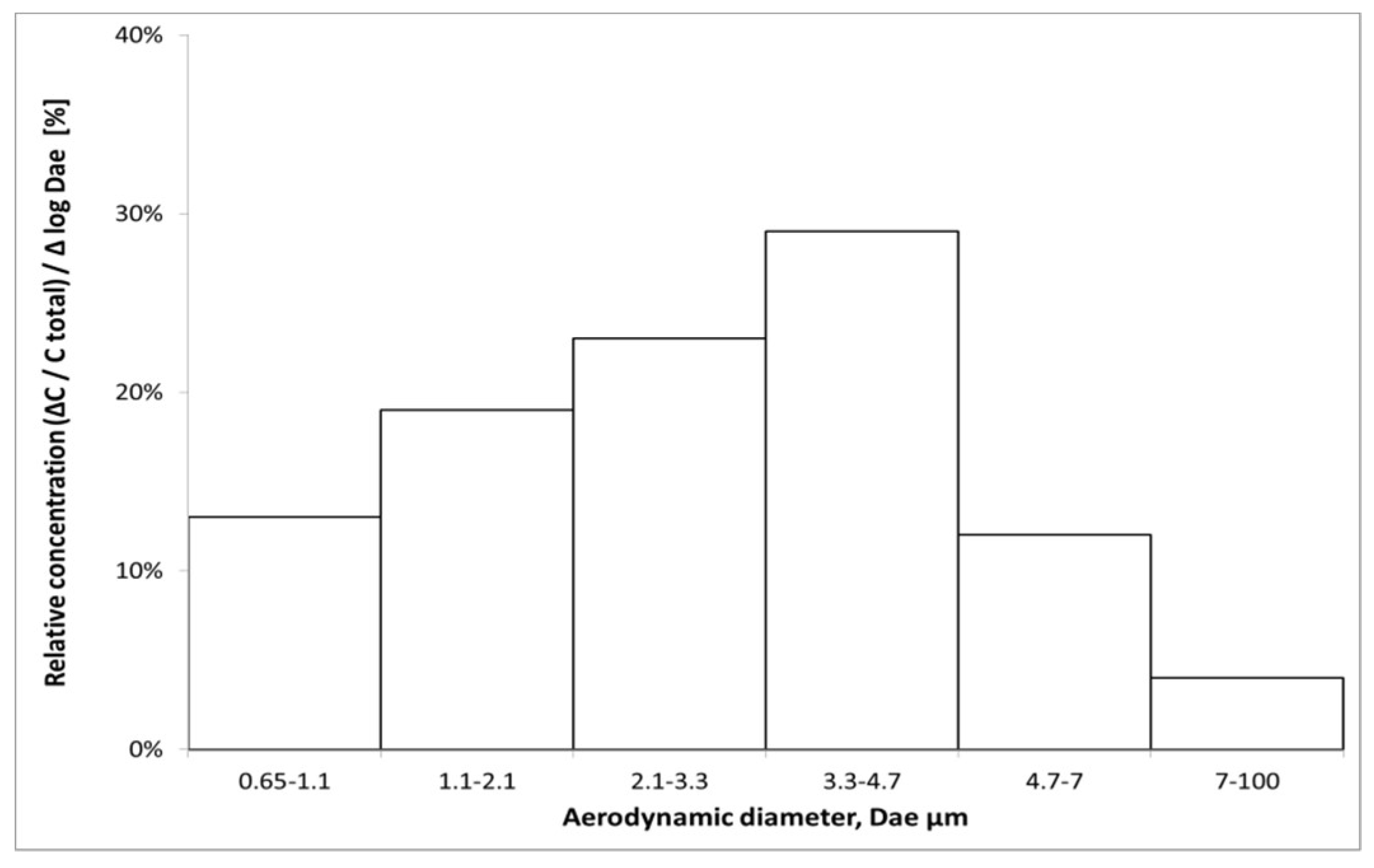
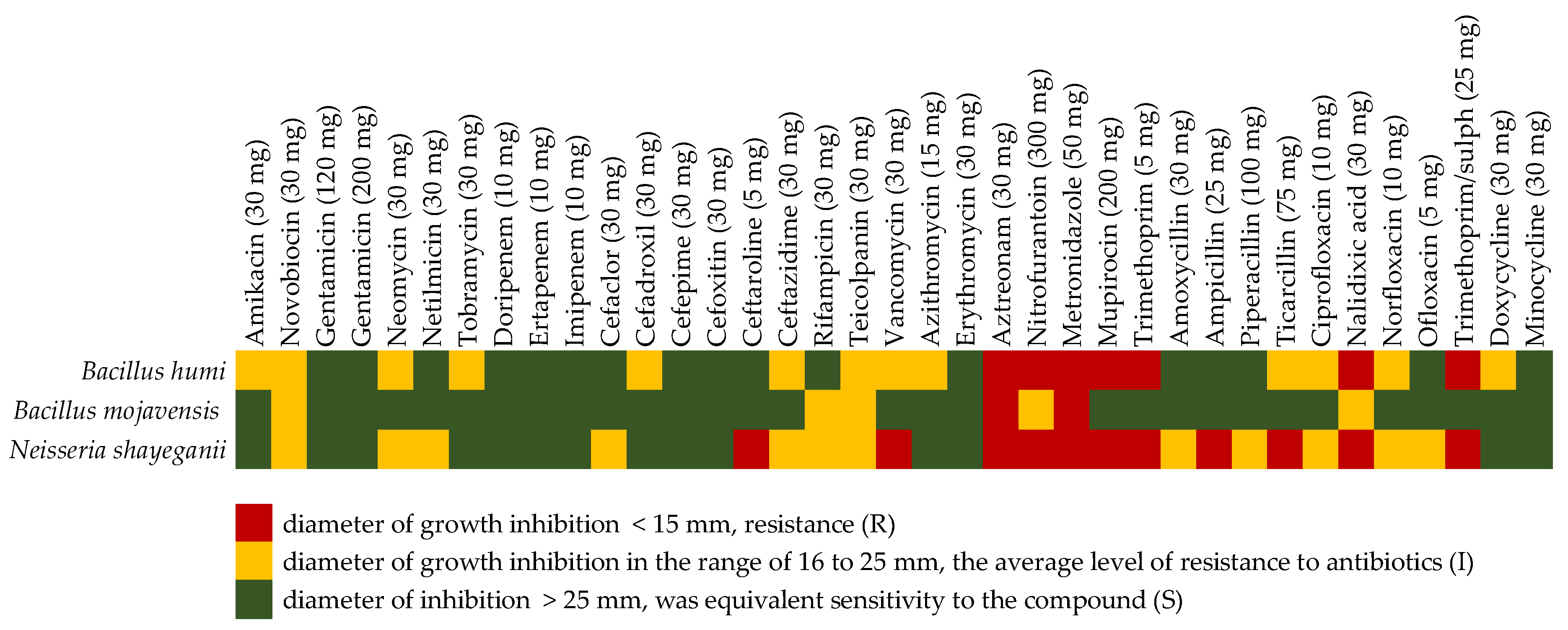
| Environmental parameters | Measurement Point Outside the Hall | Measurement Point in Municipal Waste Unloading Hall |
|---|---|---|
| Temperature, °C | 10.2 | 12–14.2 |
| Relative Humidity (RH), % | 28 | 47–53 |
| Air Pressure, hPa | 961 | 961 |
| Wind Velocity, m/s | 3.6 | - |
| Wind Direction | Variable, with south-west dominant | - |
| Location | Average Concentration CFU/m3 | SD | Min. | Max. | I/O Ratio |
|---|---|---|---|---|---|
| UHSP | 2687 | 912 | 2124 | 3121 | 2.36 |
| OSP | 1138 | 401 | 692 | 1714 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brągoszewska, E.; Biedroń, I.; Hryb, W. Air Quality and Potential Health Risk Impacts of Exposure to Bacterial Aerosol in a Waste Sorting Plant Located in the Mountain Region of Southern Poland, Around Which There Are Numerous Rural Areas. Atmosphere 2019, 10, 360. https://doi.org/10.3390/atmos10070360
Brągoszewska E, Biedroń I, Hryb W. Air Quality and Potential Health Risk Impacts of Exposure to Bacterial Aerosol in a Waste Sorting Plant Located in the Mountain Region of Southern Poland, Around Which There Are Numerous Rural Areas. Atmosphere. 2019; 10(7):360. https://doi.org/10.3390/atmos10070360
Chicago/Turabian StyleBrągoszewska, Ewa, Izabela Biedroń, and Wojciech Hryb. 2019. "Air Quality and Potential Health Risk Impacts of Exposure to Bacterial Aerosol in a Waste Sorting Plant Located in the Mountain Region of Southern Poland, Around Which There Are Numerous Rural Areas" Atmosphere 10, no. 7: 360. https://doi.org/10.3390/atmos10070360
APA StyleBrągoszewska, E., Biedroń, I., & Hryb, W. (2019). Air Quality and Potential Health Risk Impacts of Exposure to Bacterial Aerosol in a Waste Sorting Plant Located in the Mountain Region of Southern Poland, Around Which There Are Numerous Rural Areas. Atmosphere, 10(7), 360. https://doi.org/10.3390/atmos10070360






