Dry-Heat Cooking of Meats as a Source of Airborne N-Nitrosodimethylamine (NDMA)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
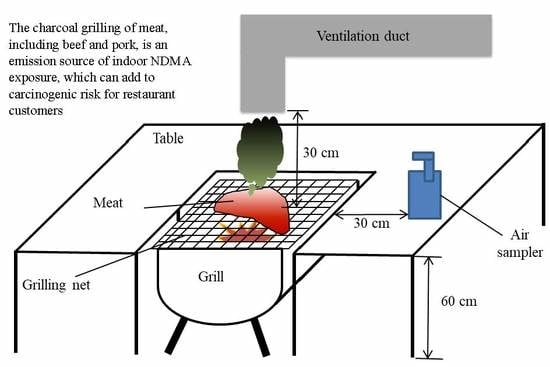
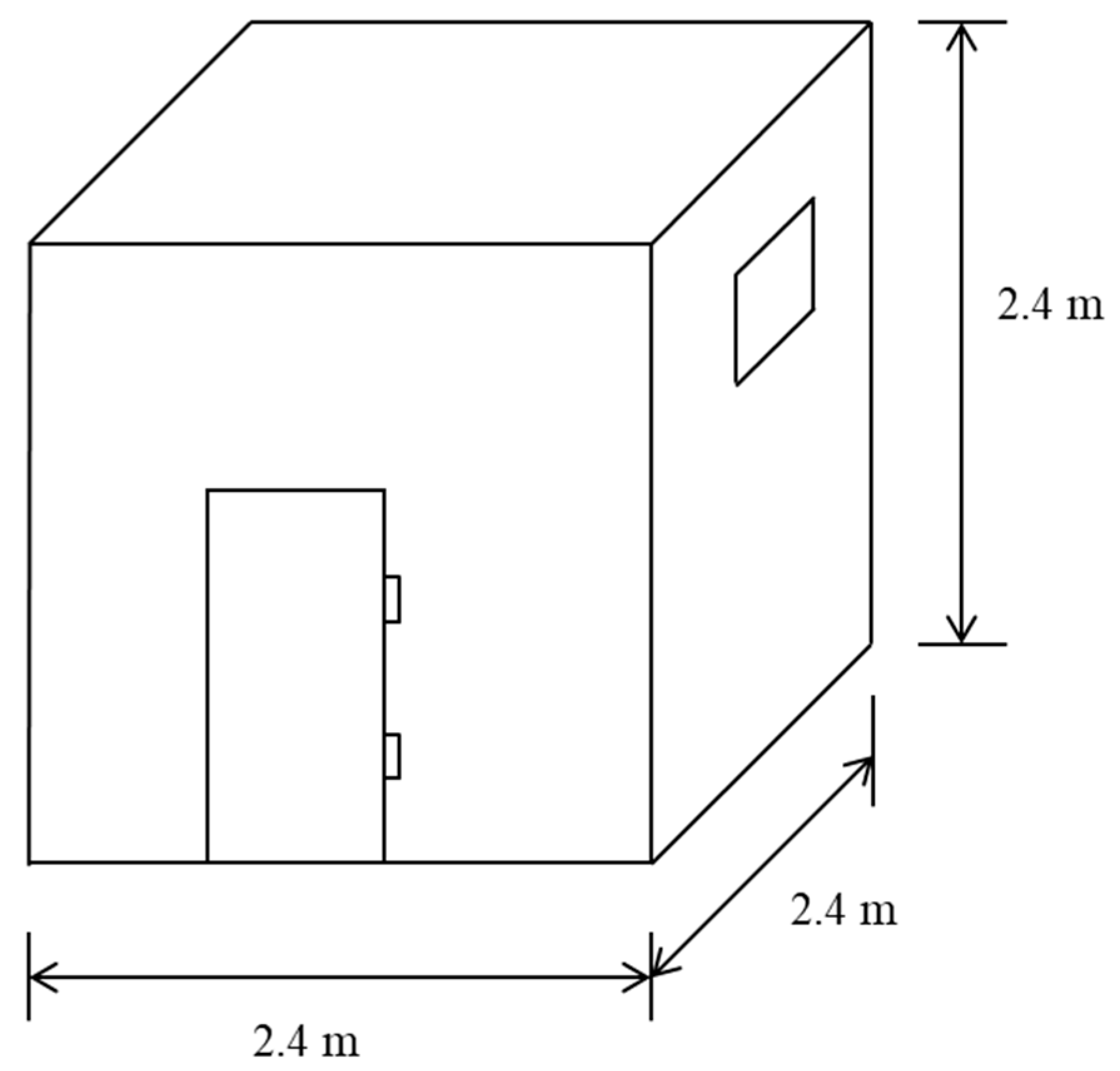
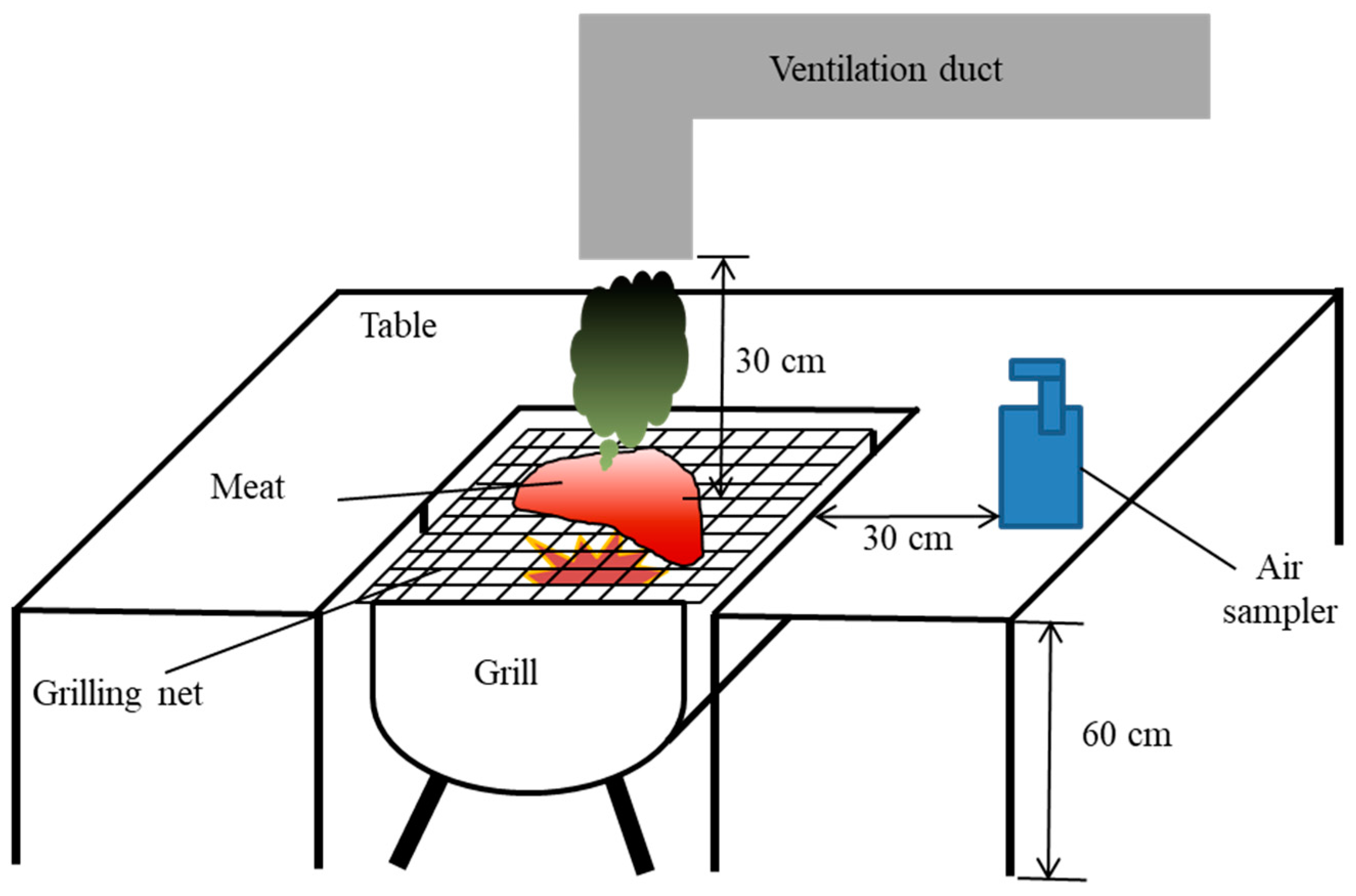
2.2. Experimental Setting for the Dry-Heat Cooking of Meat
2.3. Ambient Air Sampling
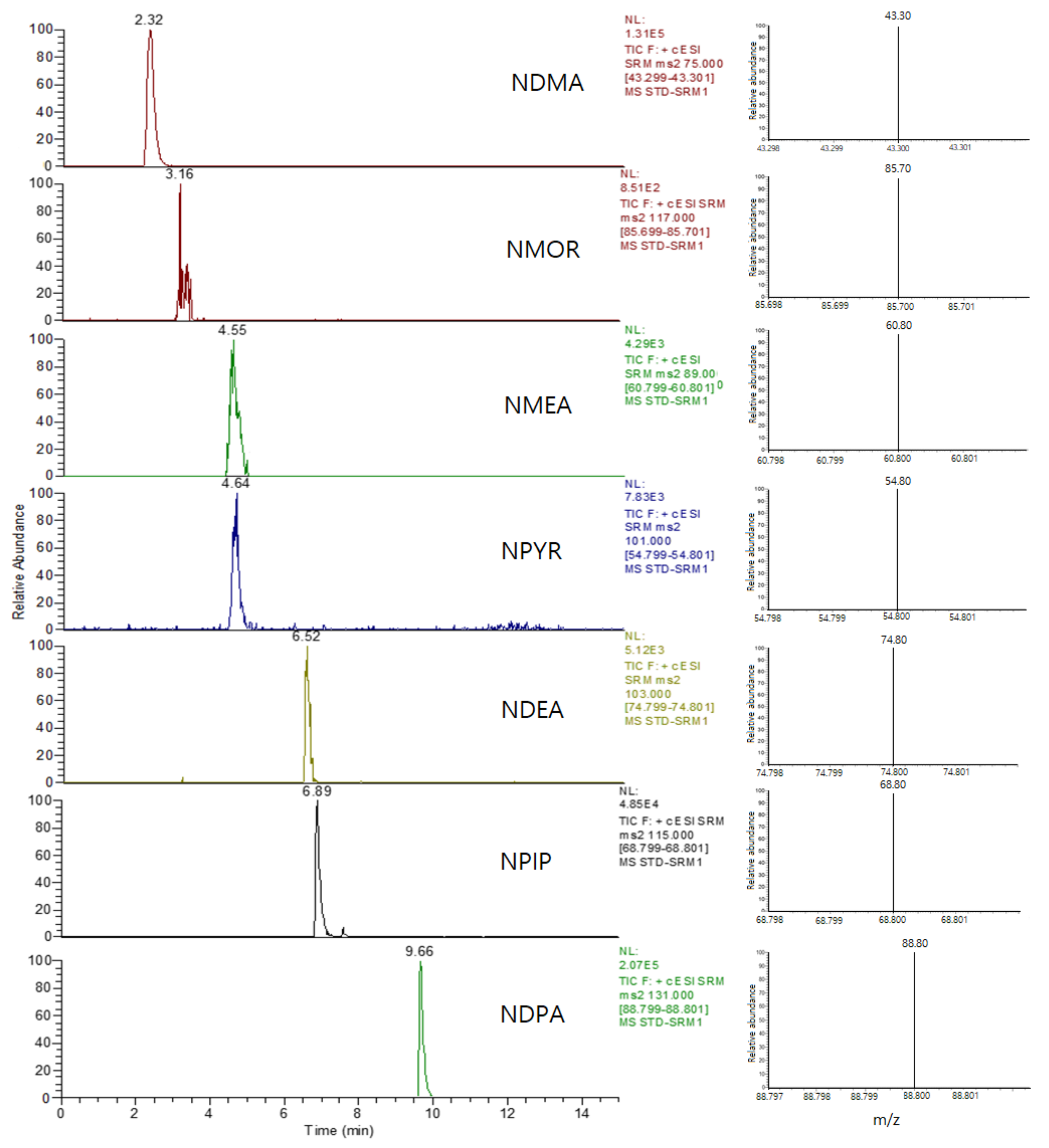
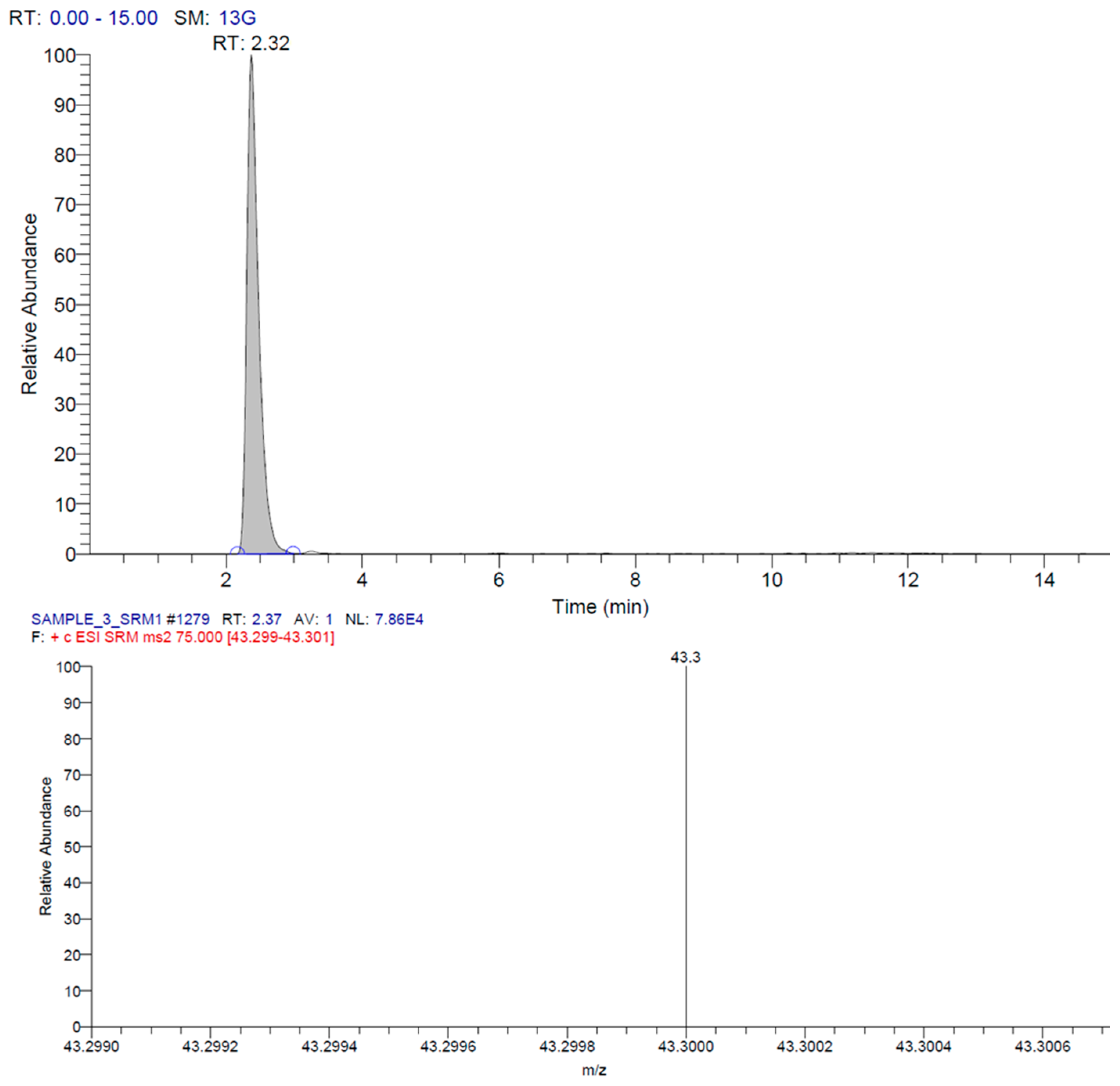
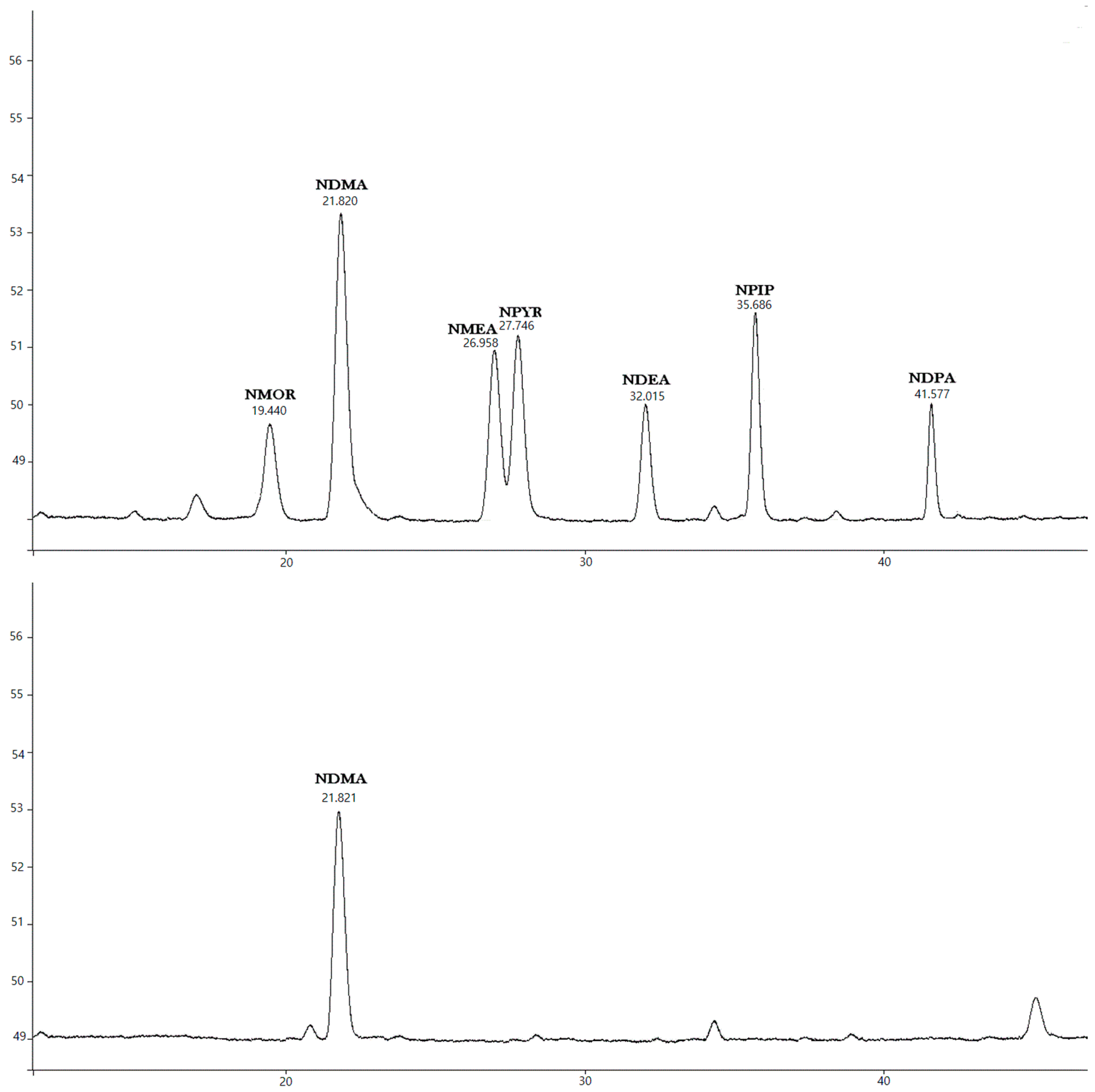
2.4. Qualitative and Quantitative Analyses for N-Nitrosamines
2.5. Method Validation and Data Analysis
2.6. Inhalation Cancer Risk Assessment
- LADDinh: Lifetime average daily dose via inhalation (mg/kg-day)
- Cair: NDMA concentration in the air (mg/m3)
- IR: Inhalation rate (17.65 m3/day)
- ET: Exposure time (1.5 h/day)
- EF: Exposure frequency (72, 145, and 55 day/yr for beef, pork, and duck meats, respectively)
- ED: Exposure duration (79 yr)
- BW: Body weight (62.8 kg)
- LT: Lifetime (82 yr)
- ELCRinh: Excess lifetime cancer risk via inhalation
- CSF: cancer slope factor for NDMA [49 (mg/kg/day)−1].
3. Results
3.1. Surface Temperature
3.2. Qualitative and Quantitative Analyses
3.3. NDMA Concentrations by Cooking Method and Type of Meat
3.4. Cancer Risk Assessment for Charcoal Grilling
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bansal, V.; Kim, K.-H. Review of PAH contamination in food products and their health hazards. Environ. Int. 2015, 84, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Aygün, S.F.; Kabadayi, F. Determination of benzo[a]pyrene in charcoal grilled meat samples by HPLC with fluorescence detection. Int. J. Food Sci. Nutr. 2005, 56, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-J.; Jin, S.-H.; Lee, K.-H.; Choi, D.-M. Determination of polycyclic aromatic hydrocarbons in processed foods. Anal. Sci. Technol. 2010, 23, 196–204. [Google Scholar] [CrossRef]
- Lee, H.M.; Yoon, E.K.; Park, K.A.; Kim, Y.H.; Jung, S.Y.; Kwon, K.S.; Kim, M.C.; Song, I.S.; Lee, C.H.; Yang, J.S.; et al. Dietary risk assessment for polycyclic aromatic hydrocarbons in foods. J. Food Hyg. Safety 2004, 19, 1–8. [Google Scholar]
- Lee, J.-G.; Kim, S.-Y.; Moon, J.-S.; Kim, S.-H.; Kang, D.-H. Effects of grilling procedures on levels of polycyclic aromatic hydrocarbons in grilled meats. Food Chem. 2016, 199, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Cao, Y. Optimization of pretreatment procedures for analysis of polycyclic aromatic hydrocarbons in charcoal-grilled pork. Anal. Lett. 2010, 43, 97–109. [Google Scholar] [CrossRef]
- Bartle, K.D. Analysis and occurrence of PAHs in food. In Food Contaminants: Sources and Surveillance; Creaser, C., Purchase, R., Eds.; Royal Society of Chemistry: Cambridge, UK, 1991; pp. 41–60. [Google Scholar]
- Lijinsky, W. The formation and occurrence of polynuclear aromatic hydrocarbons associated with food. Mutat. Res. 1991, 259, 251–261. [Google Scholar] [CrossRef]
- Phillips, D.H. Polycyclic aromatic hydrocarbons in the diet. Mutat. Res. 1999, 443, 139–147. [Google Scholar] [CrossRef]
- Wu, J.; Wong, M.K.; Lee, H.K.; Shi, C.Y.; Ong, C.N. Determination of PAHs in Rougan, a traditional Chinese barbecued food, by capillary gas chromatography. Environ. Monit. Assess. 1997, 44, 577–585. [Google Scholar] [CrossRef]
- Magee, P.N.; Barnes, J.M. The production of malignant primary hepatic tumours in the rat by feeding dimethylnitrosamine. Brit. J. Cancer 1956, 10, 114–122. [Google Scholar] [CrossRef]
- Gushgari, A.J.; Halden, R.U. Critical review of major sources of human exposure to N-nitrosamines. Chemosphere 2018, 210, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Integrated Risk Information System (IRIS) Chemical Assessment Summary of N-Nitrosodimethylamine (CASRN 62-75-9). Available online: https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=45 (accessed on 7 November 2018).
- Integrated Risk Information System (IRIS) Chemical Assessment Summary of Benzo[a]pyrene (CASRN 50-32-8). Available online: https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=136 (accessed on 7 November 2018).
- Scanlan, R.A. Formation and occurrence of nitrosamines in food. Cancer Res. 1983, 43, 2435s–2440s. [Google Scholar] [PubMed]
- De Mey, E.; De Maere, H.; Paelinck, H.; Fraeye, I. Volatile nitrosamines in meat products: Potential precursors, influence of processing, and mitigation strategies. Crit. Rev. Food Sci. Nutr. 2017, 57, 2909–2923. [Google Scholar] [CrossRef] [PubMed]
- Kocak, D.; Ozel, M.Z.; Gogus, F.; Hamilton, J.F.; Lewis, A.C. Determination of volatile nitrosamines in grilled lamb and vegetables using comprehensive gas chromatography–nitrogen chemiluminescence detection. Food Chem. 2012, 135, 2215–2220. [Google Scholar] [CrossRef] [PubMed]
- Sen, N.P.; Seaman, S.; Miles, W.F. Dimethylnitrosamine and nitrosopyrrolidine in fumes produced during the frying of bacon. Food Cosmet. Toxicol. 1976, 14, 167–170. [Google Scholar] [CrossRef]
- Kataoka, H.; Kurisu, M.; Shindoh, S. Determination of volatile N-nitrosamines in combustion smoke samples. Bull. Environ. Contam. Toxicol. 1997, 59, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Aragón, M.; Marcé, R.M.; Borrull, F. Determination of N-nitrosamines and nicotine in air particulate matter samples by pressurized liquid extraction and gas chromatography-ion trap tandem mass spectrometry. Talanta 2013, 115, 896–901. [Google Scholar] [CrossRef]
- Farren, N.J.; Ramírez, N.; Lee, J.D.; Finessi, E.; Lewis, A.C.; Hamilton, J.F. Estimated exposure risks from carcinogenic nitrosamines in urban airborne particulate matter. Environ. Sci. Technol. 2015, 49, 9648–9656. [Google Scholar] [CrossRef]
- Hong, Y.; Kim, K.H.; Sang, B.-I.; Kim, H. Simple quantification method for N-nitrosamines in atmospheric particulates based on facile pretreatment and GC-MS/MS. Environ. Pollut. 2017, 226, 324–334. [Google Scholar] [CrossRef]
- Rounbehler, D.P.; Reisch, J.; Fine, D.H. Nitrosamines in new motor-cars. Food Cosmet. Toxicol. 1980, 18, 147–151. [Google Scholar] [CrossRef]
- U.S. EPA Method T0-7. Method for the Determination of N-Nitrosodimethylamine in Ambient Air Using Gas Chromatography—Revision 1.0; U.S. Environmental Protection Agency: Washington, DC, USA, 1986.
- Fadlallah, S.; Cooper, S.F.; Perrault, G.; Truchon, G.; Lesage, J. N-Nitroso compounds in the ambient air of metal factories using metal-working fluids. Bull. Environ. Contam. Toxicol. 1996, 57, 867–874. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Definition and Procedure for the Determination of the Method Detection Limit—Revision 1.11; U.S. Environmental Protection Agency: Washington, DC, USA, 2011.
- Hornung, R.W.; Reed, L.D. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- U.S. EPA. Guidelines for Carcinogen Risk Assessment; U.S. Environmental Protection Agency: Washington, DC, USA, 2005.
- Jang, J.Y.; Jo, S.N.; Kim, S.; Kim, S.; Jung, H. Korean Exposure Factors Handbook; Ministry of Environment: Sejong, Korea, 2007.
- Korean Statistical Information Service (KOSIS), National Statistics: Daejeon, Korea. Available online: http://kosis.kr/statisticsList/statisticsListIndex.do?menuId=M_01_01&vwcd=MT_ZTITLE&parmTabId=M_01_01 (accessed on 7 November 2018).
- Korean Rural Economic Institute (KREI). Supply, Demand, and Price of Beef, Pork, Chicken, Duck, and Chicken Eggs: Trend (2010–2014) and Outlook (2015–2025); KREI: Naju, Jeollanam-do, Korea, 2016. [Google Scholar]
- Asante-Duah, K. Public Health Risk Assessment for Human Exposure to Chemicals, 2nd ed.; Springer: New York, NY, USA, 2002; p. 27. [Google Scholar]
- Hotchkiss, J.H. Sources of N-Nitrosamine Contamination in Foods. In Nutritional and Toxicological Aspects of Food Safety—Advances in Experimental Medicine and Biology; Friedman, M., Ed.; Springer: Boston, MA, USA, 1984; Volume 177, pp. 287–298. ISBN 978-1-4684-4790-3. [Google Scholar]
- Lijinsky, W. Environmental Cancer Risks. In Handbook of Hazardous Material, 1st ed.; Corn, M., Ed.; Academic Press: Cambridge, MA, USA, 1993; p. 247. ISBN 9780323139557. [Google Scholar]
- Ibrahim, M.; Gilbert, K. Management of gastric cancer in Indian population. Transl. Gastroenterol. Hepatol. 2017, 2, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, D.J.; Nawar, W.W. Thermal decomposition of lysine. J. Agr. Food Chem. 1979, 27, 511–514. [Google Scholar] [CrossRef]
- Nerín, C.; Aznar, M.; Carrizo, D. Food contamination during food process. Trends Food Sci. Technol. 2016, 48, 63–68. [Google Scholar] [CrossRef]
- USDA. USDA Food Composition Databases. Available online: https://ndb.nal.usda.gov/ndb/search/list (accessed on 7 November 2018).
- U.S. EPA. Technical Fact Sheet—N-Nitrosodimethyalamine (NDMA). Available online: https://www.epa.gov/sites/production/files/2014-03/documents/ffrrofactsheet_contaminant_ndma_january2014_final.pdf (accessed on 7 November 2018).
| Cooking Method | Type of Meat | Number of Experiments |
|---|---|---|
| Charcoal Grilling | Beef sirloin | 12 |
| Pork belly | 12 | |
| Duck | 7 | |
| Pan-Broiling | Beef sirloin | 4 |
| Pork belly | 4 | |
| Duck | 4 |
| Descriptive Statistics | Charcoal (n = 7) | Grill (n = 7) | Frying Pan (n = 4) | |||
|---|---|---|---|---|---|---|
| 0 min | 25 min | 0 min | 25 min | 0 min | 25 min | |
| Mean ± SD | 484 ± 42 | 351 ± 65 | 186 ± 36 | 184 ± 35 | 181 ± 34 | 175 ± 22 |
| Median | 474 | 376 | 182 | 186 | 168 | 173 |
| Range | 431–561 | 224–420 | 130–237 | 122–225 | 156–230 | 151–202 |
| Type of Meat | Descriptive Statistics | Charcoal Grilling | Pan-Broiling |
|---|---|---|---|
| Beef Sirloin | Mean ± SD | 410 ± 195 | 58.2 |
| Median | 326 * | 65.6 | |
| Range | 179–906 | NQ 1)–81.0 | |
| Pork Belly | Mean ± SD | 202 ± 103 | 29.2 |
| Median | 192 | 20.5 | |
| Range | 69.4–413 | NQ–55.5 | |
| Duck | Mean ± SD | 109 ± 27 | 27.1 |
| Median | 109 | 20.4 | |
| Range | 69.4–154 | ND 2)–63.1 |
| Category | Beef Sirloin | Pork Belly | Duck | |||
|---|---|---|---|---|---|---|
| Average | 95th Percentile | Average | 95th Percentile | Average | 95th Percentile | |
| LADD | 1.42 × 10−6 | 2.64 × 10−6 | 1.41 × 10−6 | 2.71 × 10−6 | 2.89 × 10−7 | 3.86 × 10−7 |
| ELCR | 6.96 × 10−5 | 1.29 × 10−4 | 6.91 × 10−5 | 1.33 × 10−4 | 1.41 × 10−5 | 1.89 × 10−5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Tcha, J.; Shim, M.-y.; Jung, S. Dry-Heat Cooking of Meats as a Source of Airborne N-Nitrosodimethylamine (NDMA). Atmosphere 2019, 10, 91. https://doi.org/10.3390/atmos10020091
Kim H, Tcha J, Shim M-y, Jung S. Dry-Heat Cooking of Meats as a Source of Airborne N-Nitrosodimethylamine (NDMA). Atmosphere. 2019; 10(2):91. https://doi.org/10.3390/atmos10020091
Chicago/Turabian StyleKim, Hekap, Jiyeon Tcha, Man-yong Shim, and Sungjin Jung. 2019. "Dry-Heat Cooking of Meats as a Source of Airborne N-Nitrosodimethylamine (NDMA)" Atmosphere 10, no. 2: 91. https://doi.org/10.3390/atmos10020091
APA StyleKim, H., Tcha, J., Shim, M.-y., & Jung, S. (2019). Dry-Heat Cooking of Meats as a Source of Airborne N-Nitrosodimethylamine (NDMA). Atmosphere, 10(2), 91. https://doi.org/10.3390/atmos10020091