The Characteristics of Ambient Non-Methane Hydrocarbons (NMHCs) in Lanzhou, China
Abstract
1. Introduction
2. Experiment and Methods
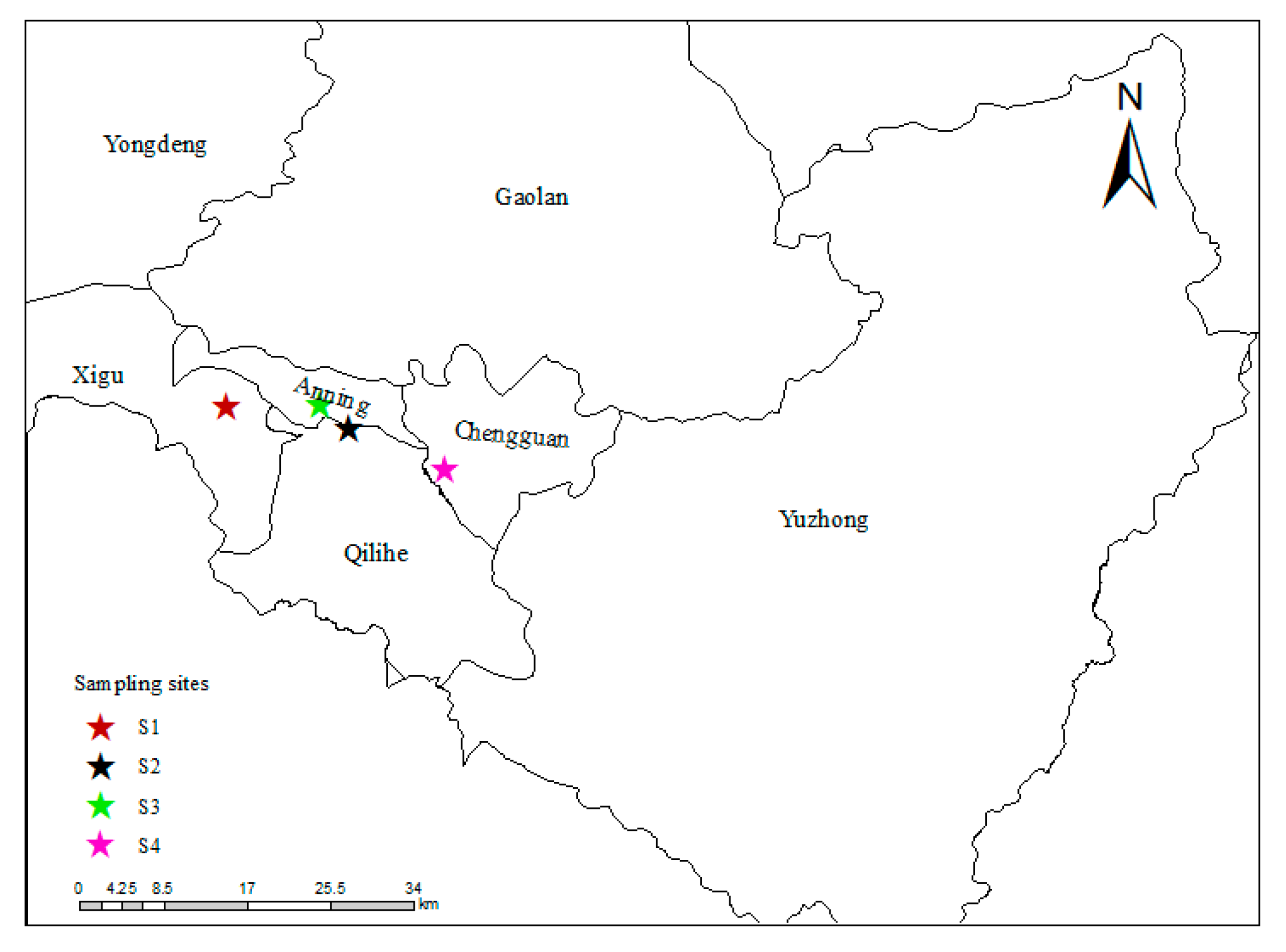
2.1. Sampling Sites Description
2.2. Sample Collection and Analysis
2.3. Quality Control and Quality Assurance
2.4. Meteorological Parameters
3. Results and Discussion
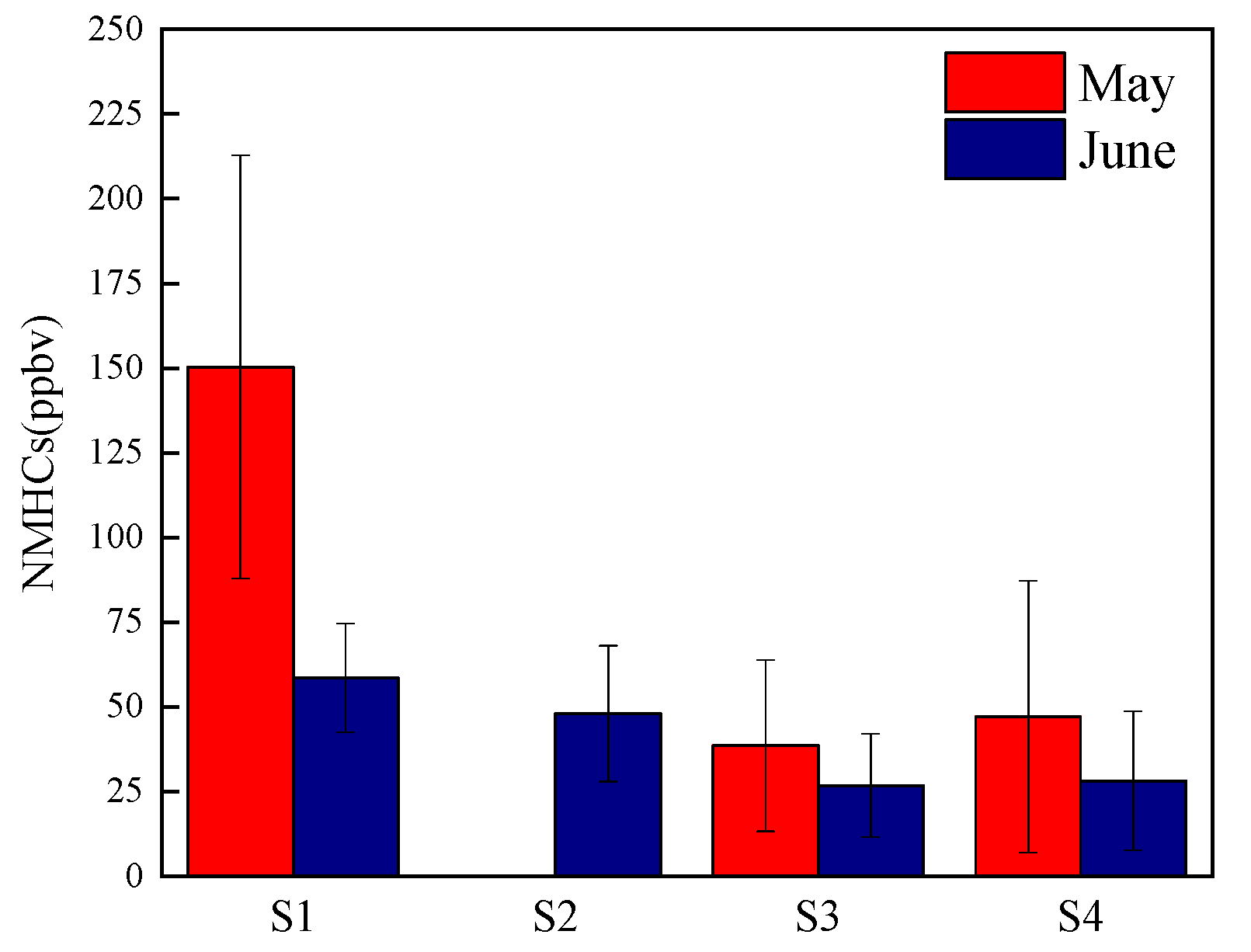
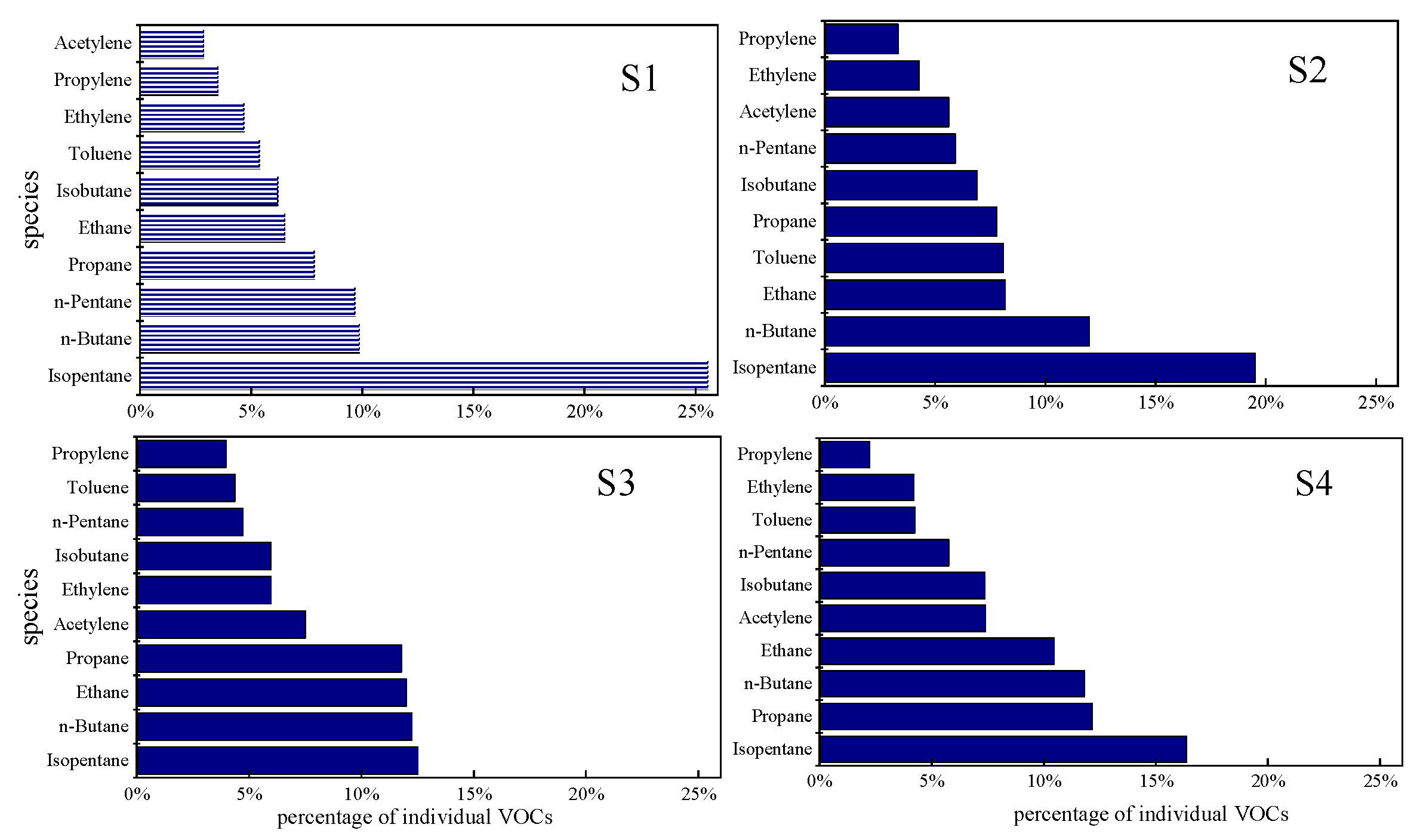
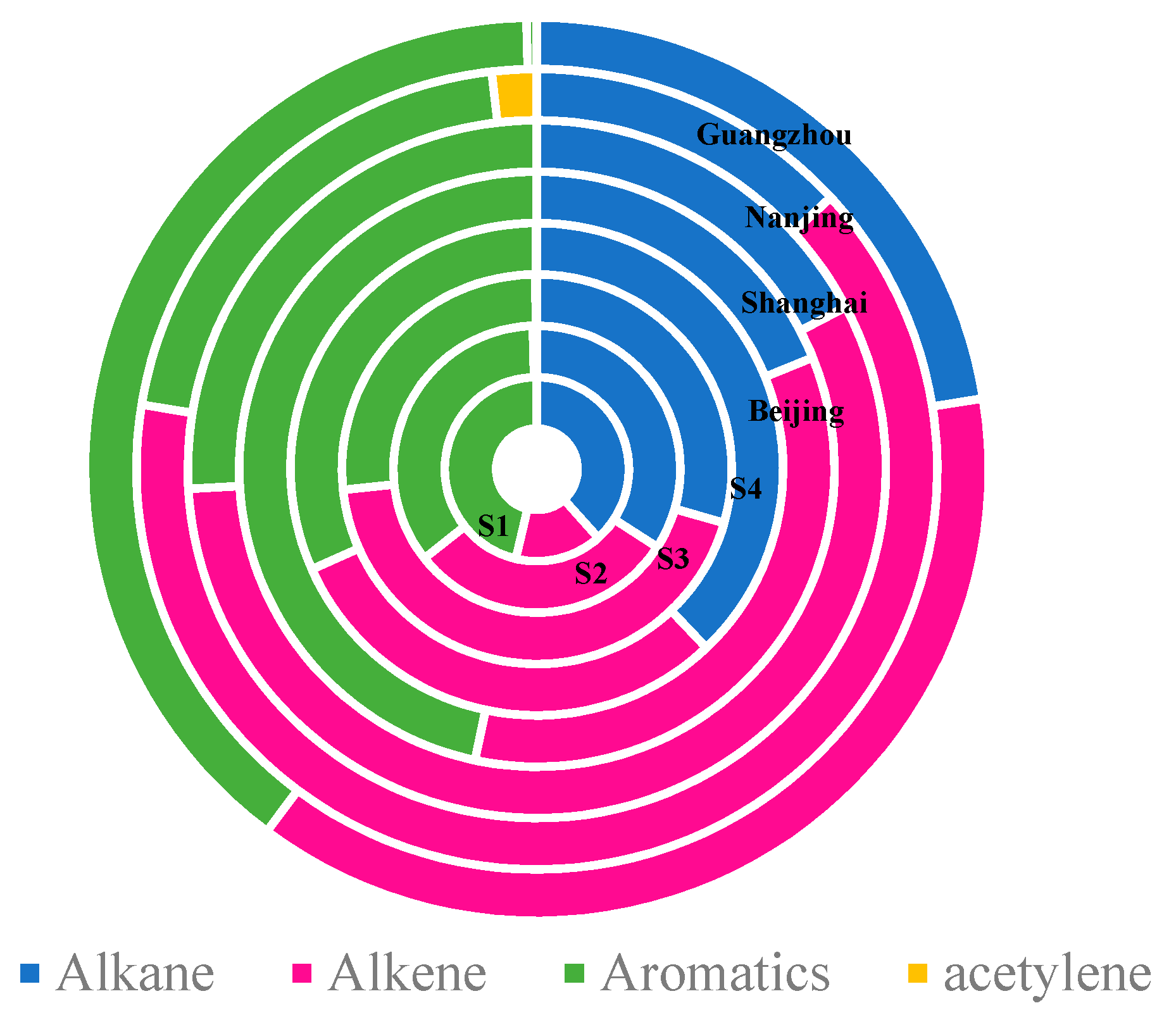
3.1. General Characteristics of NMHCs
3.2. Ozone and SOA Formation Potential for NMHCs
3.2.1. Ozone Formation Potential for NMHCs
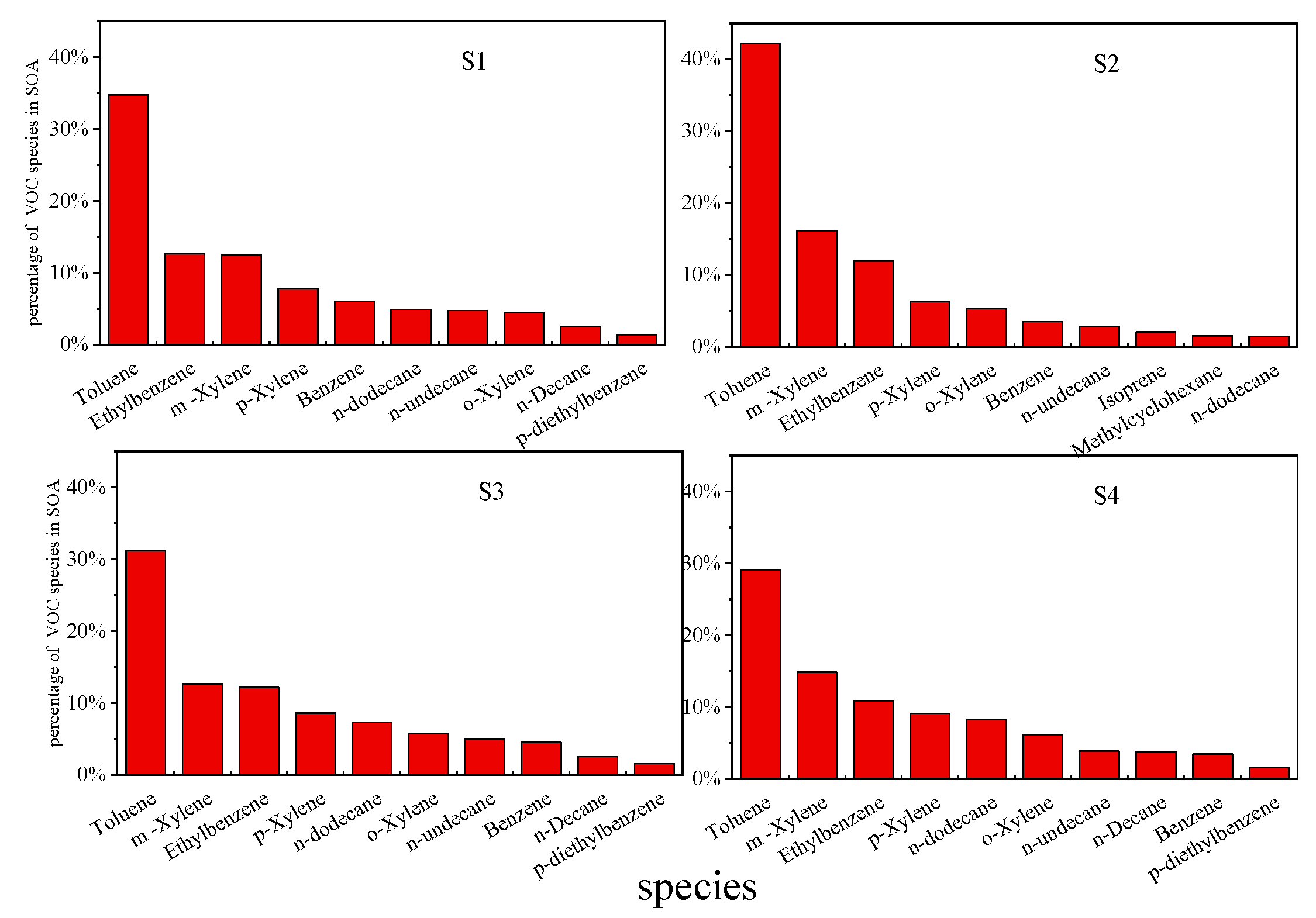
3.2.2. Secondary Organic Aerosol Formation Potential
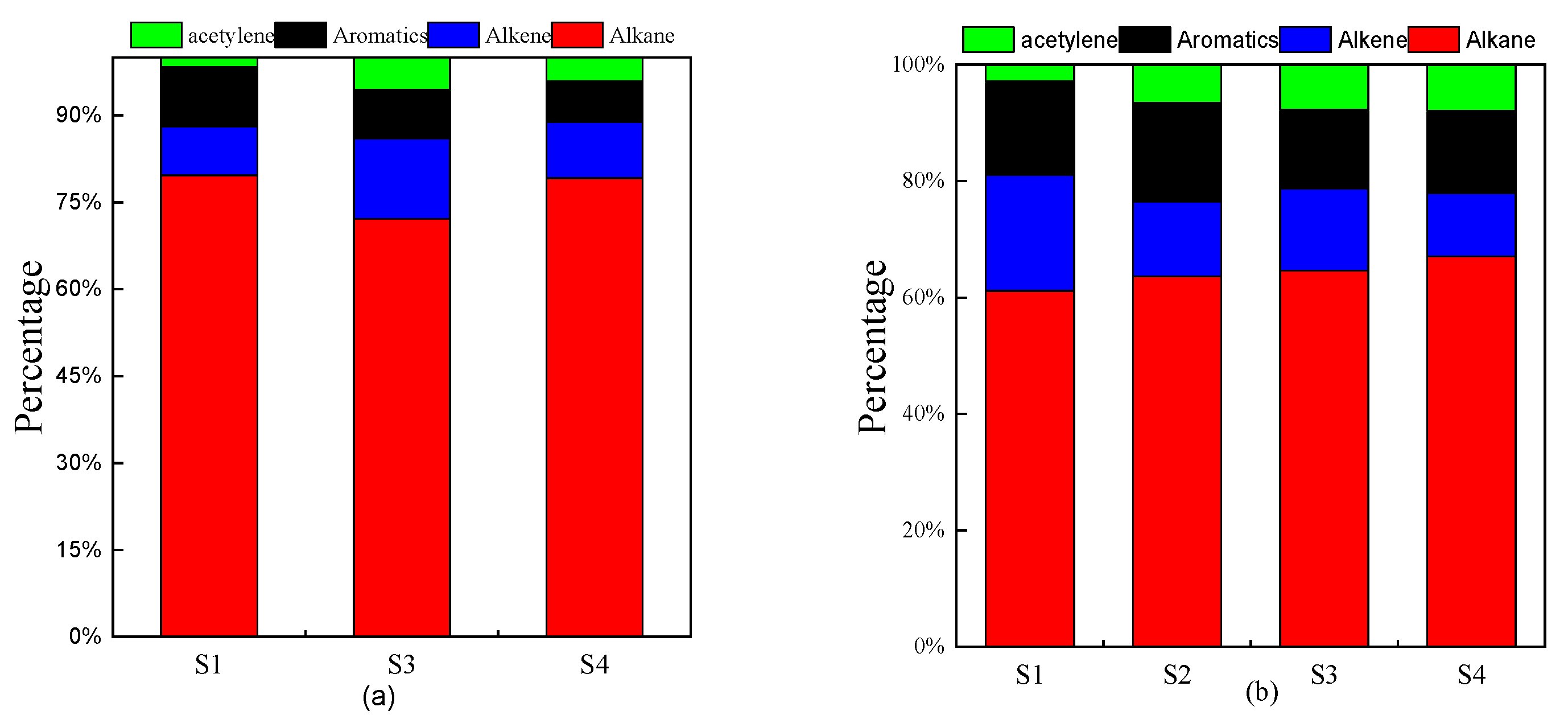
3.3. Source Analysis of NMHCs
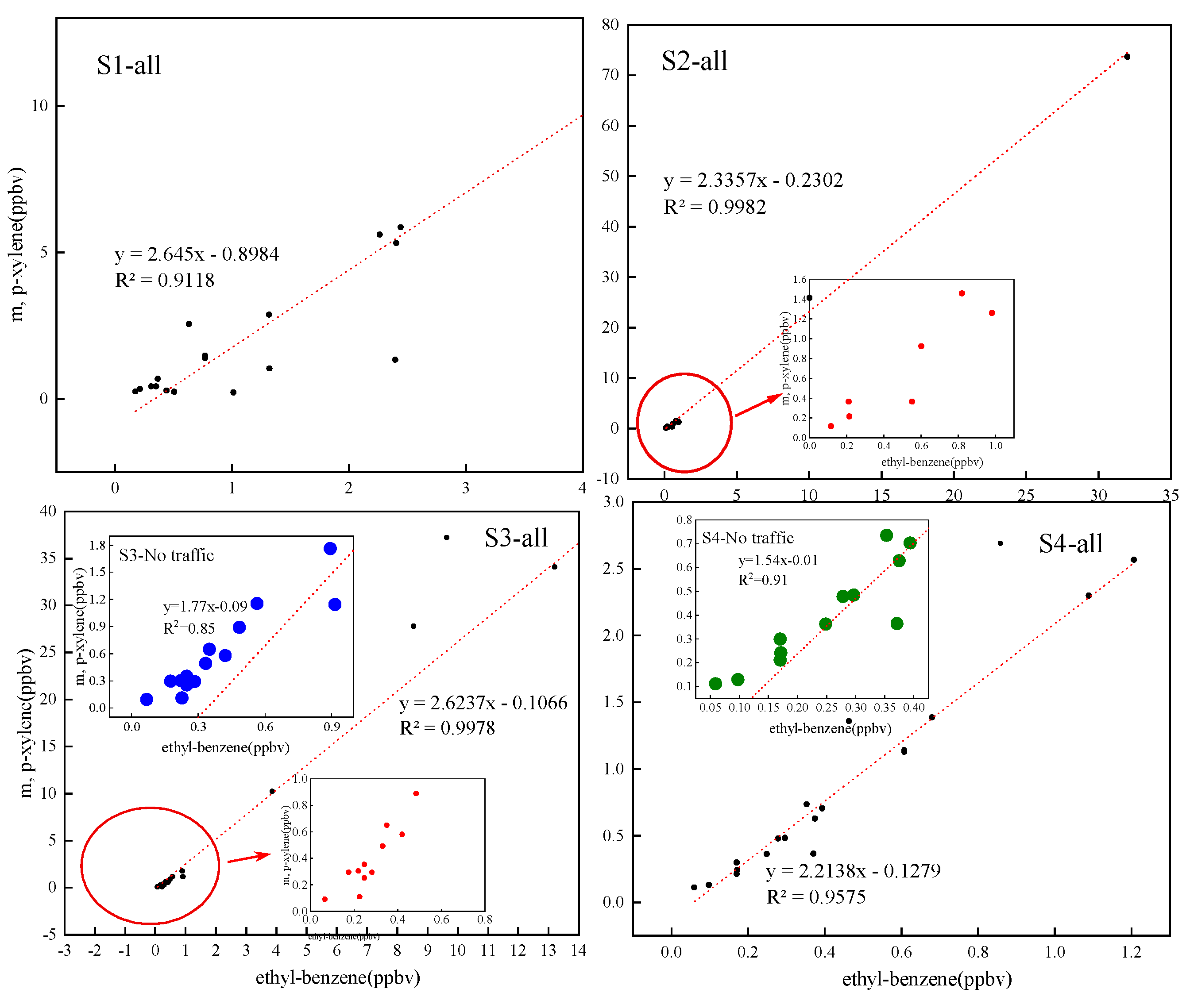
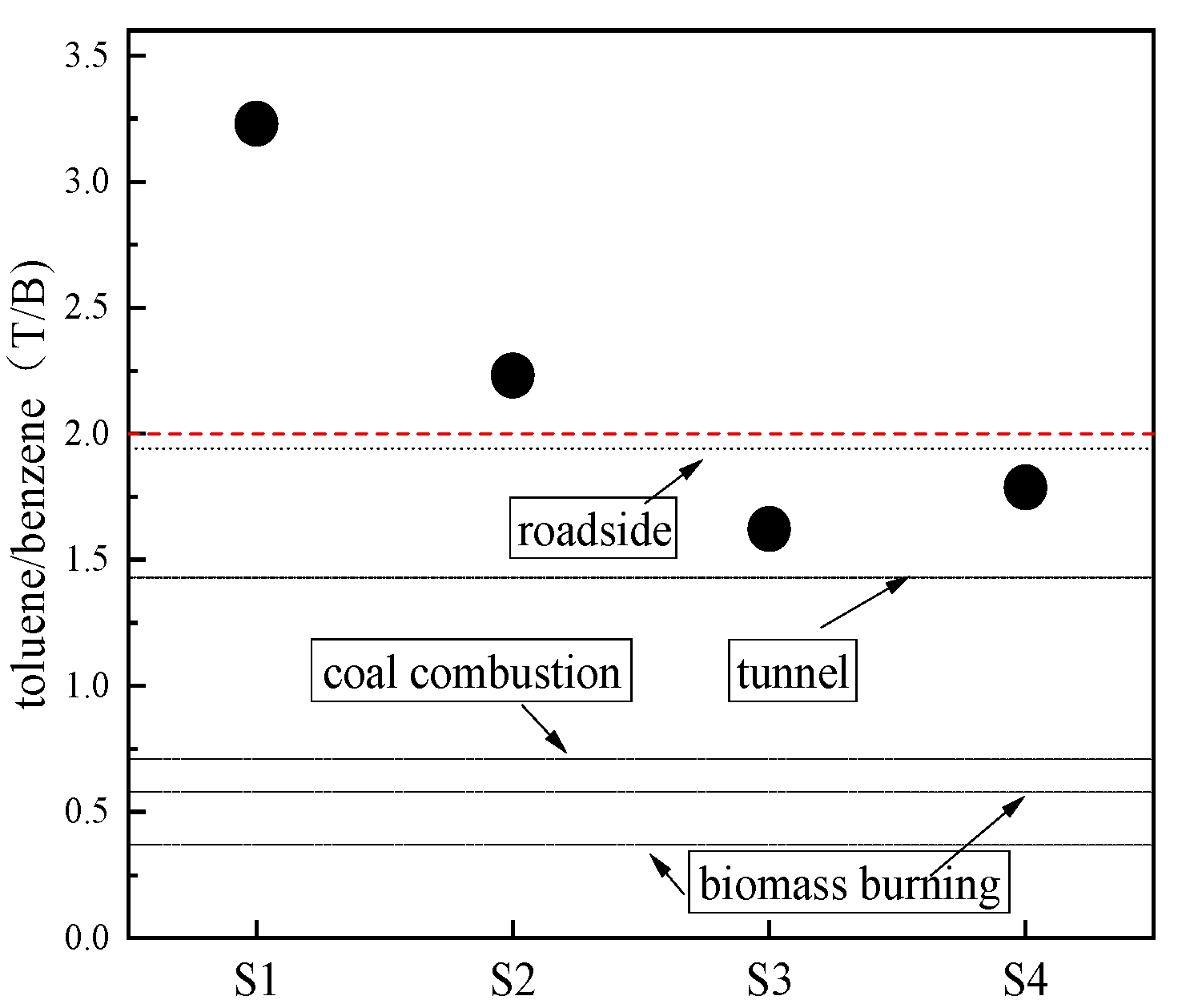
3.3.1. Ratios of the Specific NMHCs
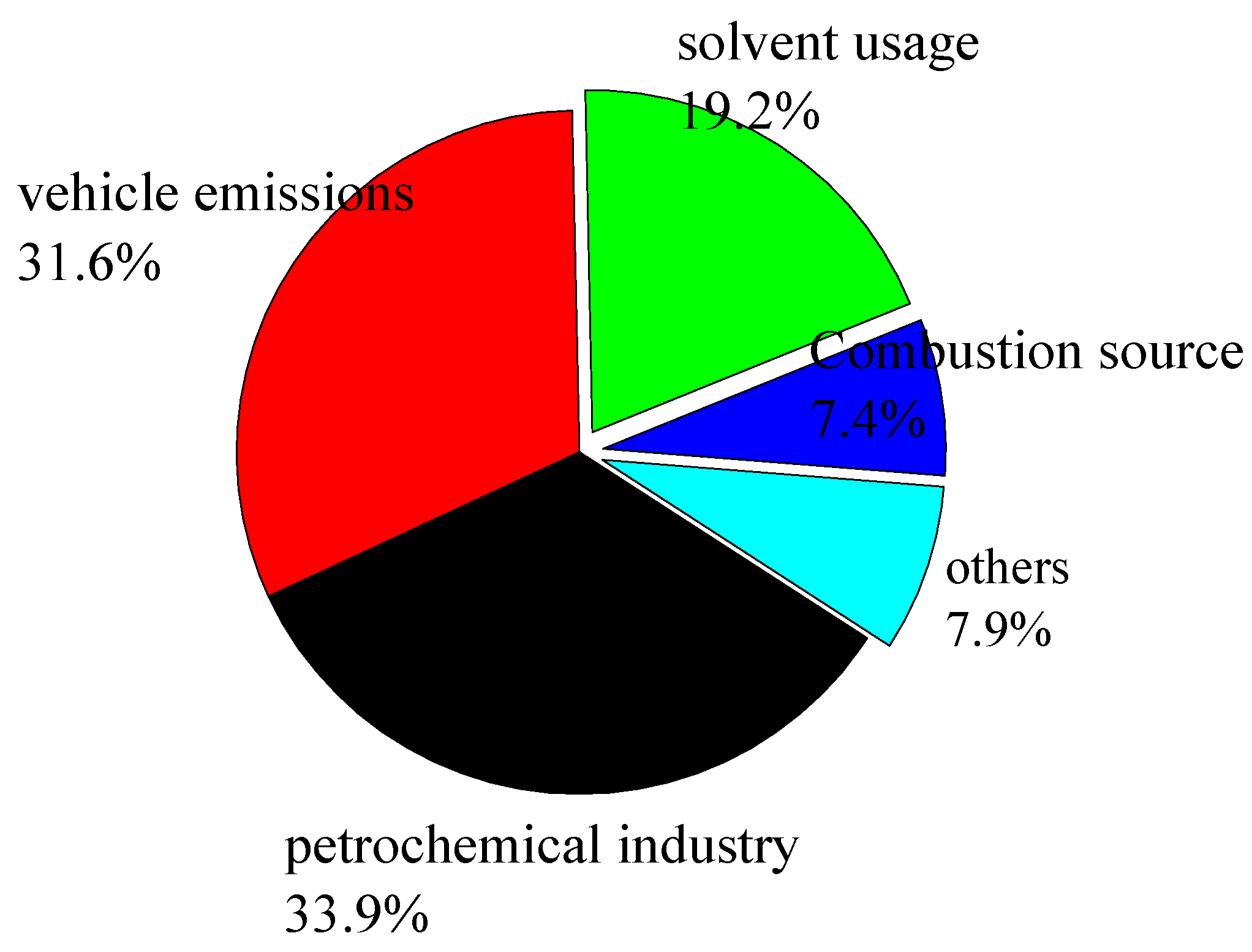
3.3.2. Source attribution of NMHCs by PCA
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Allen, S.K.; Plattner, G.K.; Nauels, A.; Xia, Y.; Stocker, T.F. Climate Change 2013: The Physical Science Basis. An overview of the Working Group 1 contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC). Comput. Geom. 2007, 18, 95–123. [Google Scholar]
- Duan, J.; Tan, J.; Yang, L.; Wu, S.; Hao, J. Concentration, sources and ozone formation potential of volatile organic compounds (VOCs) during ozone episode in Beijing. Atmos. Res. 2008, 88, 25–35. [Google Scholar] [CrossRef]
- Jie, S.; Wu, F.; Hu, B.; Tang, G.; Wang, Y. VOC characteristics, emissions and contributions to SOA formation during hazy episodes. Atmos. Environ. 2016, 141, 560–570. [Google Scholar]
- Mills, G.; Hayes, F.; Simpson, D.; Emberson, L.; Norris, D.; Harmens, H. Evidence of widespread effects of ozone on crops and (semi-)natural vegetation in Europe (1990–2006) in relation to AOT40- and flux-based risk maps. Glob. Chang. Biol. 2015, 17, 592–613. [Google Scholar] [CrossRef]
- Nie, T.; Li, X.; Wang, X.; Shao, M.; Zhang, Y. Characteristics of the Spatial Distributions of Ozone-Precursor Sensitivity Regimes in summer over Beijing. Acta Sci. Nat. Univ. Pekin. 2014, 50, 557–564. [Google Scholar]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Kroll, J.H.; Seinfeld, J.H. Chemistry of secondary organic aerosol: Formation and evolution of low-volatility organics in the atmosphere. Atmos. Environ. 2008, 42, 3593–3624. [Google Scholar] [CrossRef]
- Huang, R.-J.; Zhang, Y.; Bozzetti, C.; Ho, K.F.; Cao, J.J.; Han, Y.; Daellenbach, K.R.; Slowik, J.G.; Platt, S.M.; Canonaco, F.; et al. High secondary aerosol contribution to particulate pollution during haze events in China. Nature 2014, 514, 218–222. [Google Scholar]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Bale, A.S.; Meacham, C.A.; Benignus, V.A.; Bushnell, P.J.; Shafer, T.J. Volatile organic compounds inhibit human and rat neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. Toxicol. Appl. Pharmacol. 2005, 205, 77–88. [Google Scholar] [CrossRef][Green Version]
- Ying, L.; Shao, M.; Lu, S.; Chang, C.C.; Wang, J.L.; Fu, L. Source apportionment of ambient volatile organic compounds in the Pearl River Delta, China: Part II. Atmos. Environ. 2008, 42, 6261–6274. [Google Scholar]
- Baker, A.; Beyersdorf, A.J.; Doezema, L.A.; Katzenstein, A.; Meinardi, S.; Simpson, I.J. Measurements of nonmethane hydrocarbons in 28 United States cities. Atmos. Environ. 2008, 42, 170–182. [Google Scholar] [CrossRef]
- Lu, S.; Ying, L.; Min, S.; Shan, H. Chemical speciation and anthropogenic sources of ambient volatile organic compounds (VOCs) during summer in Beijing, 2004. Front. Environ. Sci. Eng. China 2007, 1, 147–152. [Google Scholar] [CrossRef]
- Han, M.; Lu, X.; Zhao, C.; Ran, L.; Han, S. Characterization and Source Apportionment of Volatile Organic Compounds in Urban and Suburban Tianjin, China. Adv. Atmos. Sci. 2015, 32, 439–444. [Google Scholar] [CrossRef]
- Cai, C.; Geng, F.; Tie, X.; Yu, Q.; An, J. Characteristics and source apportionment of VOCs measured in Shanghai, China. Atmos. Environ. 2010, 44, 5005–5014. [Google Scholar] [CrossRef]
- Geng, F.; Zhao, C.; Tang, X.; Lu, G.; Tie, X. Analysis of ozone and VOCs measured in Shanghai: A case study. Atmos. Environ. 2007, 41, 989–1001. [Google Scholar] [CrossRef]
- Xue, L.; Wang, T.; Gao, J.; Ding, A.; Zhou, X.; Blake, D. Ground-level ozone in four Chinese cities: Precursors, regional transport and heterogeneous processes. Atmos. Chem. Phys. 2014, 14, 13175–13188. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, C.H.; Li, S.X.; Zhang, F. Air Pollution and Potential Control Schemes in Lanzhou. Res. Environ. 2000, 13, 18–21. [Google Scholar]
- Tang, X.Y.; Li, J.L.; Dong, Z.X. Photochemical pollution in Lanzhou, China—A case study. J. Environ. Sci. 1989, 1, 31–38. [Google Scholar]
- Cheng, C.; Huang, J.; Ren, Z.; Peng, X. Meteorological conditions of photochemical smog in Xigu industrial area, lanzhou. Acta Sci. Circumstantiae 1986, 6, 334–342. [Google Scholar]
- Jia, C.; Mao, X.; Huang, T.; Liang, X.; Wang, Y.; Shen, Y.; Jiang, W.; Wang, H.; Bai, Z.; Ma, M.; et al. Non-methane hydrocarbons (NMHCs) and their contribution to ozone formation potential in a petrochemical industrialized city, Northwest China. Atmos. Res. 2016, 169, 225–236. [Google Scholar] [CrossRef]
- Yan, Y.; Peng, L.; Li, R.; Li, Y.; Li, L.; Bai, H. Concentration, ozone formation potential and source analysis of volatile organic compounds (VOCs) in a thermal power station centralized area: A study in Shuozhou, China. Environ. Pollut. 2017, 223, 295–304. [Google Scholar] [CrossRef]
- An, J.; Zhu, B.; Wang, H.; Li, Y.; Lin, X.; Yang, H. Characteristics and source apportionment of VOCs measured in an industrial area of Nanjing, Yangtze River Delta, China. Atmos. Environ. 2014, 97, 206–214. [Google Scholar] [CrossRef]
- Ho, K.F.; Lee, S.C.; Guo, H.; Tsai, W.Y. Seasonal and diurnal variations of volatile organic compounds (VOCs) in the atmosphere of Hong Kong. Sci. Total Environ. 2004, 322, 155–166. [Google Scholar] [CrossRef]
- Li, L.; Xie, S.; Zeng, L.; Wu, R.; Li, J. Characteristics of volatile organic compounds and their role in ground-level ozone formation in the Beijing-Tianjin-Hebei region, China. Atmos. Environ. 2015, 113, 247–254. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zhu, B.; An, J.L.; Gao, J.H.; Tang, L.L. Characteristics of VOCs and Their Photochemical Reactivity in Autumn in Nanjing Northern Suburb. Environ. Sci. 2013, 34, 2933–2942. [Google Scholar]
- Lyu, X.P.; Chen, N.; Guo, H.; Zhang, W.H.; Liu, M. Ambient volatile organic compounds and their effect on ozone production in Wuhan, central China. Sci. Total Environ. 2016, 548, 483. [Google Scholar] [CrossRef]
- Cai, C.J.; Geng, F.H.; Tie, X.X.; Yu, Q.; Peng, L.; Zhou, G.Q. Characteristics of ambient volatile organic compounds (VOCs) measured in Shanghai, China. Sensors 2010, 10, 7843–7862. [Google Scholar] [CrossRef]
- Alghamdi, M.A.; Khoder, M.; Harrison, R.M.; Hyvärinen, A.P.; Hussein, T.; Al-Jeelani, H.; Abdelmaksoud, A.S.; Goknil, M.H.; Shabbaj, I.I.; Almehmadi, F.M.; et al. Temporal variations of O3 and NOx in the urban background atmosphere of the coastal city Jeddah, Saudi Arabia. Atmos. Environ. 2014, 94, 205–214. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, C.; Mu, Y.; Liu, C.; Xue, C.; Ye, C. The levels, variation characteristics and sources of atmospheric non-methane hydrocarbon compounds during wintertime in Beijing, China. Atmos. Chem. Phys. Discuss. 2017, 17, 10633–10649. [Google Scholar] [CrossRef]
- Wu, Y.J.; Hu, J.; Zhang, H.F.; Zhang, J.Q.; Zhang, M.; Chai, F.H.; Wang, S.L. Characteristics and Chemical Reactivity of Fugitive Volatile Organic Compounds from Typical Industries in Lanzhou City. Res. Environ. Sci. 2019, 32, 802–812. [Google Scholar]
- Watson, J.G.; Chow, J.C.; Fujita, E.M. Review of volatile organic compound source apportionment by chemical mass balance. Atmos. Environ. 2001, 35, 1567–1584. [Google Scholar] [CrossRef]
- Carter, W.P.L. Development of Ozone Reactivity Scales for Volatile Organic Compounds. Air Waste 1994, 44, 881–899. [Google Scholar] [CrossRef]
- Wang, H. Chemical Loss of Volatile Organic Compounds and Its Impact on the Formation of Ozone in Shanghai. Environ. Sci. 2015, 36, 3159–3167. [Google Scholar]
- Lin, X.; Zhu, B.; An, J.L.; Yang, H. Potential contribution of secondary organic aerosols and ozone of VOCs in the Northern Suburb of Nanjing. China Environ. Sci. 2015, 35, 976–986. [Google Scholar]
- Yu, X.; Cheng, P.; Gu, Y.; Li, M.; Tian, Z. Formation potential of ozone and secondary organic aerosol from VOCs oxidation in summer in Guangzhou, China. China Environ. Sci. 2018, 38, 830–837. [Google Scholar]
- Ying, L.; Shao, M.; Fu, L.; Lu, S.; Zeng, L.; Tang, D. Source profiles of volatile organic compounds (VOCs) measured in China: Part I. Atmos. Environ. 2008, 42, 6247–6260. [Google Scholar]
- Grosjean, D. In situ organic aerosol formation during a smog episode: Estimated production and chemical functionality. Atmos. Environ. 1992, 26, 953–963. [Google Scholar] [CrossRef]
- Lv, Z.; Hao, J.M.; Duan, J.C.; Li, J.H. Estimate of the Formation Potential of Secondary Organic Aerosol in Beijing Summertime. Environ. Sci. 2009, 30, 969–975. [Google Scholar]
- Wu, W.; Zhao, B.; Wang, S.; Hao, J. Ozone and secondary organic aerosol formation potential from anthropogenic volatile organic compounds emissions in China. J. Environ. Sci. 2017, 53, 224–237. [Google Scholar] [CrossRef]
- Dechapanya, W.; Russell, M.; Allen, D.T. Estimates of Anthropogenic Secondary Organic Aerosol Formation in Houston, Texas Special Issue of Aerosol Science and Technology on Findings from the Fine Particulate Matter Supersites Program. Aerosol Sci. Technol. 2004, 38, 156–166. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Barletta, B.; Simpson, I.J.; Blake, D.R.; Fu, X. Source attributions of hazardous aromatic hydrocarbons in urban, suburban and rural areas in the Pearl River Delta (PRD) region. J. Hazard. Mater. 2013, 250, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Barletta, B.; Meinardi, S.; Rowland, F.S.; Chan, C.Y.; Wang, X.; Zou, S. Volatile organic compounds in 43 Chinese cities. Atmos. Environ. 2005, 39, 5979–5990. [Google Scholar] [CrossRef]
- Garzón, J.P.; Huertas, J.I.; Magaña, M.; Huertas, M.E.; Cárdenas, B.; Watanabe, T.; Blanco, S. Volatile organic compounds in the atmosphere of Mexico City. Atmos. Environ. 2015, 119, 415–429. [Google Scholar] [CrossRef]
- Mehta, D.; Nguyen, A.; Montenegro, A.; Li, Z. A Kinetic Study of the Reaction of OH with Xylenes Using the Relative Rate/Discharge Flow/Mass Spectrometry Technique. J. Phys. Chem. A 2009, 113, 12942–12951. [Google Scholar] [CrossRef]
- Baldasano, J.M.; Delgado, R.; Calbó, J. Applying Receptor Models to Analyze Urban/Suburban VOCs Air Quality in Martorell (Spain). Environ. Sci. Technol. 2001, 32, 405–412. [Google Scholar] [CrossRef]
- Wang, M.; Shao, M.; Lu, S.H.; Yang, Y.D.; Chen, W.T. Evidence of coal combustion contribution to ambient VOCs during winter in Beijing. Chin. Chem. Lett. 2013, 24, 829–832. [Google Scholar] [CrossRef]
- Mo, Z.; Shao, M.; Lu, S.; Qu, H.; Zhou, M.; Sun, J. Process-specific emission characteristics of volatile organic compounds (VOCs) from petrochemical facilities in the Yangtze River Delta, China. Sci. Total Environ. 2015, 533, 422–431. [Google Scholar] [CrossRef]
- Shi, J.; Deng, H.; Bai, Z.; Kong, S.; Wang, X.; Hao, J.; Han, X. Emission and profile characteristic of volatile organic compounds emitted from coke production, iron smelt, heating station and power plant in Liaoning Province, China. Sci. Total Environ. 2015, 515, 101–108. [Google Scholar] [CrossRef]
- Na, K. Determination of VOC source signature of vehicle exhaust in a traffic tunnel. J. Environ. Manag. 2006, 81, 392–398. [Google Scholar] [CrossRef]
- Thurston, G.D.; Spengler, J.D. A quantitative assessment of source contributions to inhalable particulate matter pollution in metropolitan Boston. Atmos. Environ. 1987, 19, 9–25. [Google Scholar] [CrossRef]
- Cetin, E.; Odabasi, M.; Seyfioglu, R. Ambient volatile organic compound (VOC) concentrations around a petrochemical complex and a petroleum refinery. Sci. Total Environ. 2003, 312, 103–112. [Google Scholar] [CrossRef]
- Chang, C.C.; Wang, J.L.; Lung, S.C.C.; Liu, S.C.; Shiu, C.J. Source characterization of ozone precursors by complementary approaches of vehicular indicator and principal component analysis. Atmos. Environ. 2009, 43, 1771–1778. [Google Scholar] [CrossRef]
- Scheff, P.A.; Wadden, R.A. Receptor modeling of volatile organic compounds. 1. Emission inventory and validation. Environ. Sci. Technol. 1993, 27, 617–625. [Google Scholar] [CrossRef]
- Barletta, B.; Meinardi, S.; Simpson, I.J.; Khwaja, H.A.; Blake, D.R.; Rowland, F.S. Mixing ratios of volatile organic compounds (VOCs) in the atmosphere of Karachi, Pakistan. Atmos. Environ. 2002, 36, 3429–3443. [Google Scholar] [CrossRef]
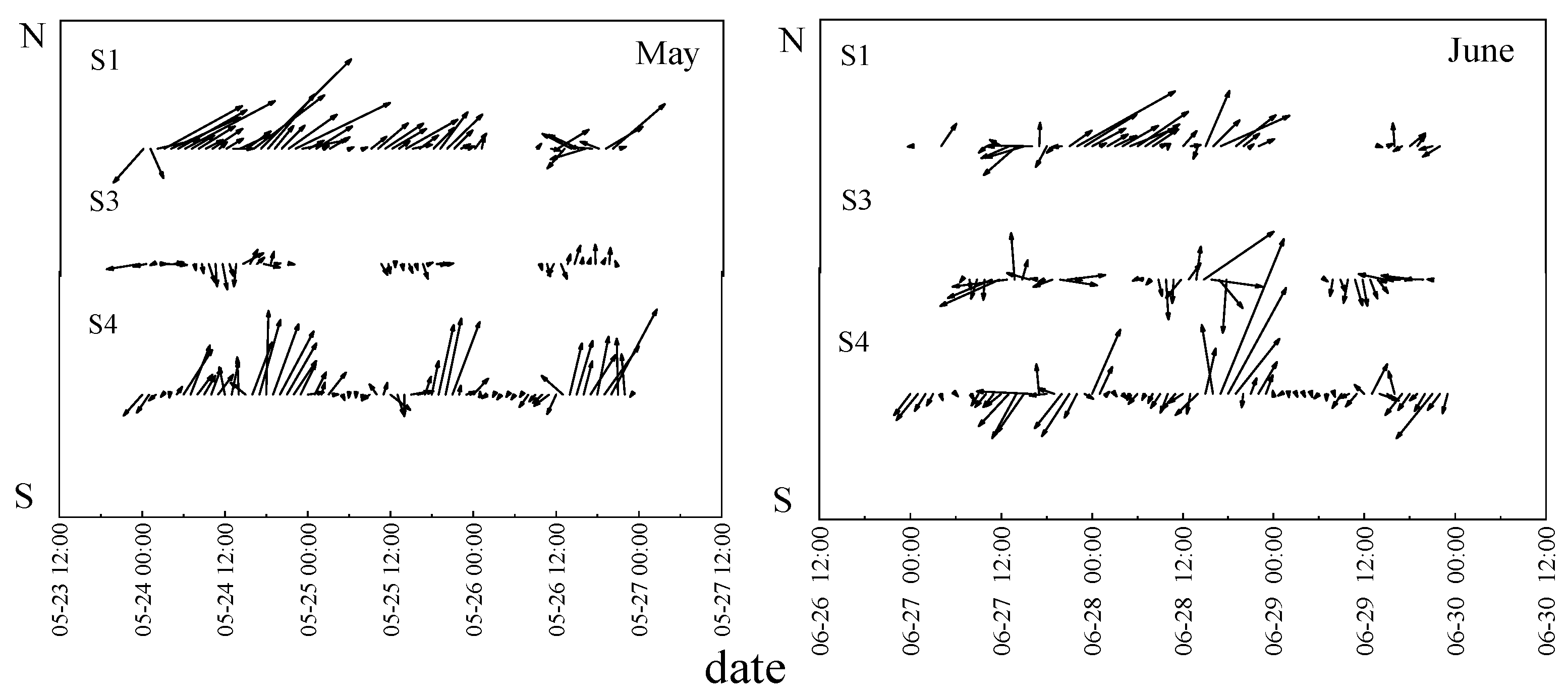

| Alkanes | Alkenes and Alkyne | Aromatics | |
|---|---|---|---|
| Ethane | 2,3-dimethylpentane | Ethylene | Benzene |
| Propane | 3-methylhexane | Propylene | Toluene |
| Isobutane | 2,2,4-trimethylpentane | Trans-2-butene | Ethylbenzene |
| N-butane | N-heptane | 1-butene | m, p-xylene |
| Cyclopentane | Methylcyclohexane | Cis-2-butene | O-xylene |
| Isopentane | 2,3,4-trimethylpentane | 1-pentene | Styrene |
| N-pentane | 2-methylheptane | Trans-2-pentene | Iso-propylbenzene |
| 2,2-dimethylbutane | 3-methylheptane | Isoprene | N-propylbenzene |
| 2,3-dimethylbutane | N-octane | Cis-2-pentene | M-ethyltoluene |
| 2-methylpentane | N-nonane | 1-hexene | P-ethyltoluene |
| 3-methylpentane | N-decane | Acetylene | 1,3,5-trimethylbenzene |
| N-hexane | N-undecane | O-ethyltoluene | |
| 2,4-dimethylpentane | N-dodecane | 1,2,4-trimethylbenzene | |
| Methylcyclopentane | 1,2,3-trimethylbenzene | ||
| 2-methylhexane | M-diethylbenzene | ||
| Cyclohexane | P-diethylbenzene | ||
| S1 | OFP | S2 | OFP | S3 | OFP | S4 | OFP |
|---|---|---|---|---|---|---|---|
| ethylbenzene | 14.13% | propene | 15.38% | propene | 15.85% | propene | 12.39% |
| propene | 12.97% | toluene | 14.94% | n-butene | 13.01% | ethylbenzene | 11.47% |
| styrene | 12.48% | isopentane | 11.19% | ethylbenzene | 9.09% | n-Pentane | 10.50% |
| n-pentane | 9.18% | m/p-xylene | 10.49% | styrene | 8.92% | styrene | 10.29% |
| m/p-xylene | 9.16% | ethylene | 7.85% | m/p-xylene | 7.58% | Trans-2-butene | 10.25% |
| n-butene | 7.76% | styrene | 4.60% | 1-Pentene | 5.59% | m/p-xylene | 6.41% |
| toluene | 5.28% | cis-2-butene | 4.00% | isopentane | 5.27% | 1-Butene | 5.87% |
| Trans-2-butene | 4.73% | n-pentane | 3.89% | n-pentane | 4.33% | isopentane | 5.17% |
| 2,2-Dimethylbutane | 2.72% | n-butane | 3.80% | toluene | 4.14% | 1-Pentene | 4.04% |
| 1-Pentene | 2.32% | n-butene | 3.35% | isobutane | 3.26% | Benzene | 2.71% |
| Species | FAC | Fvocr | SOAp (μg/m3) | ||||
|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | ||||
| alkane | Methylcyclopentane | 0.002 | 0.100 | 0.001 | 0.001 | 0.001 | 0.001 |
| Cyclohexane | 0.002 | 0.140 | 0.001 | 0.000 | 0.000 | 0.001 | |
| n-Heptane | 0.001 | 0.140 | 0.000 | 0.001 | 0.000 | 0.000 | |
| Methylcyclohexane | 0.027 | 0.200 | 0.012 | 0.015 | 0.008 | 0.007 | |
| 2-Methylheptane | 0.005 | 0.100 | 0.001 | 0.001 | 0.001 | 0.001 | |
| 3-Methylheptane | 0.005 | 0.100 | 0.001 | 0.001 | 0.001 | 0.001 | |
| n-octane | 0.001 | 0.170 | 0.001 | 0.001 | 0.000 | 0.000 | |
| n-Nonane | 0.015 | 0.200 | 0.011 | 0.006 | 0.006 | 0.005 | |
| n-Decane | 0.020 | 0.220 | 0.035 | 0.011 | 0.020 | 0.032 | |
| n-undecane | 0.025 | 0.250 | 0.065 | 0.028 | 0.039 | 0.032 | |
| n-dodecane | 0.030 | 0.260 | 0.068 | 0.014 | 0.058 | 0.070 | |
| alkene | Isoprene | 0.026 | 0.230 | 0.016 | 0.020 | 0.010 | 0.008 |
| aromatic | Benzene | 0.020 | 0.100 | 0.084 | 0.035 | 0.035 | 0.029 |
| Toluene | 0.054 | 0.120 | 0.480 | 0.418 | 0.246 | 0.244 | |
| Ethylbenzene | 0.054 | 0.150 | 0.175 | 0.118 | 0.096 | 0.091 | |
| m -Xylene | 0.047 | 0.340 | 0.173 | 0.160 | 0.100 | 0.124 | |
| p-Xylene | 0.047 | 0.340 | 0.107 | 0.062 | 0.068 | 0.076 | |
| o-Xylene | 0.050 | 0.260 | 0.062 | 0.052 | 0.046 | 0.051 | |
| iso-Propylbenzene | 0.040 | 0.130 | 0.008 | 0.003 | 0.004 | 0.004 | |
| n-Propylbenzene | 0.016 | 0.120 | 0.003 | 0.002 | 0.002 | 0.002 | |
| m-ethyltoluene | 0.063 | 0.310 | 0.011 | 0.006 | 0.008 | 0.011 | |
| p-ethyltoluene | 0.025 | 0.210 | 0.004 | 0.002 | 0.003 | 0.004 | |
| 1,3,5-Trimethylbenzene | 0.029 | 0.740 | 0.005 | 0.002 | 0.003 | 0.003 | |
| o-ethyltoluene | 0.056 | 0.230 | 0.005 | 0.003 | 0.004 | 0.004 | |
| 1,2,4-Trimethylbenzene | 0.020 | 0.580 | 0.009 | 0.005 | 0.005 | 0.006 | |
| 1,2,3-Trimethylbenzene | 0.036 | 0.510 | 0.009 | 0.006 | 0.006 | 0.010 | |
| m-diethylbenzene | 0.063 | 0.470 | 0.017 | 0.007 | 0.007 | 0.009 | |
| p-diethylbenzene | 0.063 | 0.470 | 0.019 | 0.011 | 0.012 | 0.013 | |
| NMHCs | SUM | - | - | 1.38 | 0.99 | 0.79 | 0.84 |
| VOCs | Principal Component | |||
|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | |
| Ethane | - | 0.83 | 0.5 | 0.1 |
| Propane | 0.13 | 0.11 | 0.97 | 0.04 |
| n-hexane | 0.2 | 0.85 | - | 0.35 |
| Isopentane | 0.83 | 0.02 | 0.48 | 0.07 |
| Dodecane | 0.12 | 0.06 | 0.95 | - |
| Ethylene | - | 0.92 | - | - |
| Propylene | - | 0.85 | 0.47 | 0.03 |
| Isoprene | 0.12 | 0.87 | - | - |
| Acetylene | - | 0.13 | 0.05 | 0.98 |
| Benzene | - | 0.98 | 0.63 | 0.01 |
| ethyl benzene | 0.98 | 0.05 | 0.75 | - |
| p-xylene | 0.08 | 0.02 | 0.95 | - |
| Styrene | 0.95 | 0.03 | - | - |
| variance contribution | 33% | 32% | 19% | 7% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Hu, J.; Wang, H.; Li, H.; Zhang, H.; Chai, F.; Wang, S. The Characteristics of Ambient Non-Methane Hydrocarbons (NMHCs) in Lanzhou, China. Atmosphere 2019, 10, 745. https://doi.org/10.3390/atmos10120745
Wu Y, Hu J, Wang H, Li H, Zhang H, Chai F, Wang S. The Characteristics of Ambient Non-Methane Hydrocarbons (NMHCs) in Lanzhou, China. Atmosphere. 2019; 10(12):745. https://doi.org/10.3390/atmos10120745
Chicago/Turabian StyleWu, Yajun, Jun Hu, Han Wang, Hui Li, Hefeng Zhang, Fahe Chai, and Shulan Wang. 2019. "The Characteristics of Ambient Non-Methane Hydrocarbons (NMHCs) in Lanzhou, China" Atmosphere 10, no. 12: 745. https://doi.org/10.3390/atmos10120745
APA StyleWu, Y., Hu, J., Wang, H., Li, H., Zhang, H., Chai, F., & Wang, S. (2019). The Characteristics of Ambient Non-Methane Hydrocarbons (NMHCs) in Lanzhou, China. Atmosphere, 10(12), 745. https://doi.org/10.3390/atmos10120745





