The Incidence of Skin Cancer in Relation to Climate Change in South Africa
Abstract
:1. Introduction
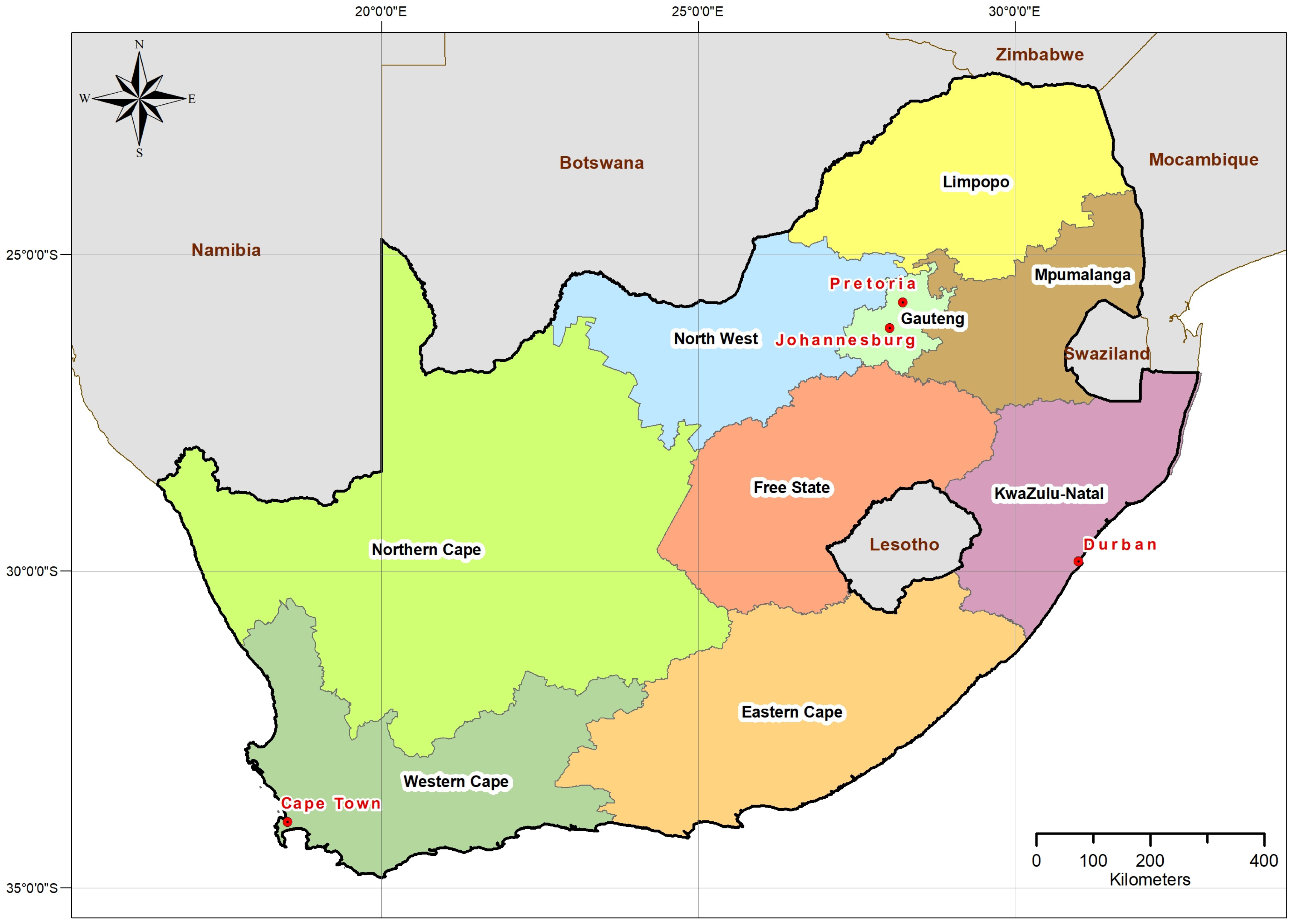
2. South African Geography and Population
3. Current Incidence of Skin Cancer and Mortality Data
4. Costs Due to Skin Cancer in South Africa
5. Air Pollution and Skin Cancer
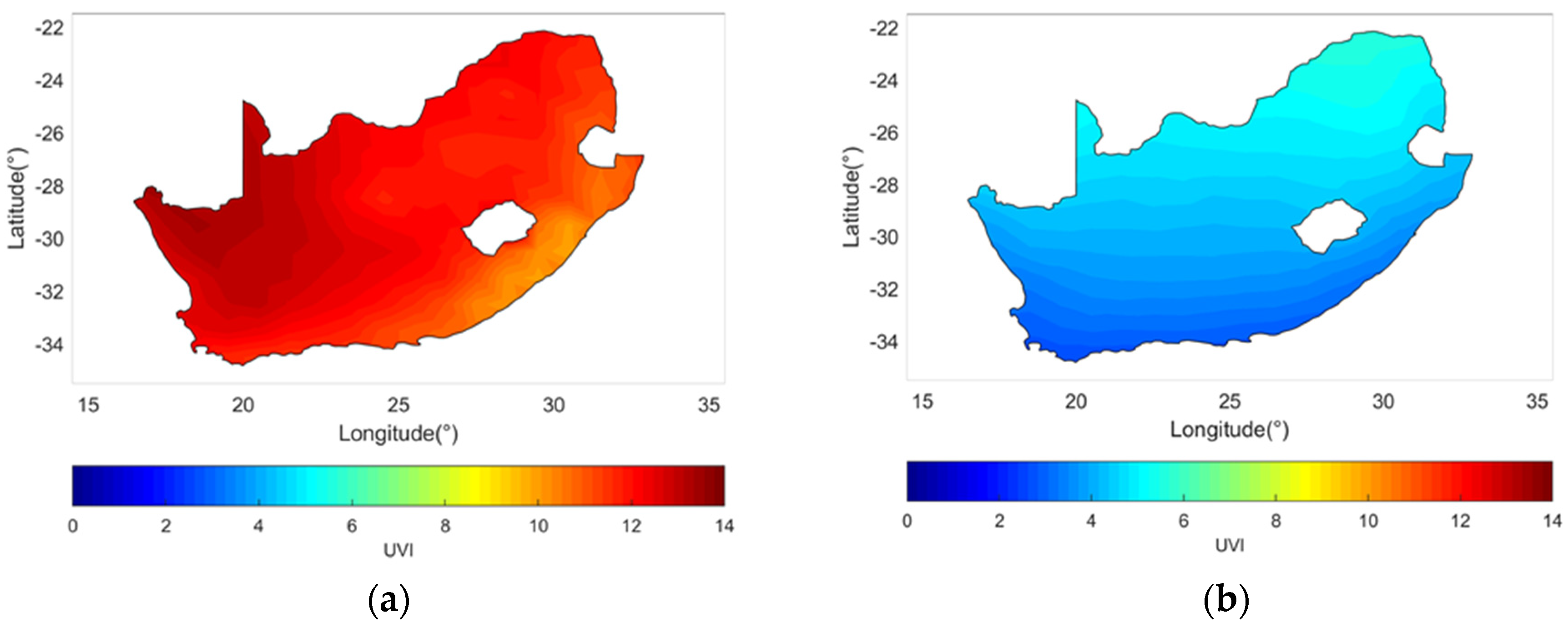
6. Solar Ultraviolet Radiation Exposure, Temperature and Skin Cancer
7. Rainfall, Cloud Cover and Skin Cancer
8. Adaptation Strategies
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Watts, N.; Amann, M.; Arnell, N.; Ayeb-Karlsson, S.; Beleson, K.; Berry, K.; Bouley, T.; Boykoff, M.; Byass, P.; Cai, W.; et al. The 2018 report of the Lancet Countdown on health and climate change: Shaping the health of nations for centuries to come. Lancet 2018, 392, 2479–2514. [Google Scholar] [CrossRef]
- Stammes, P.; Noordhoek, R. OMI Algorithm Theoretical Basis Document Volume III: Clouds, Aerosols, and Surface UV Irradiance; Technology Report ATBD-OMI-03; KNMI: De Bilt, The Netherlands, 2002. [Google Scholar]
- OMI Team. Ozone Monitoring Instrument (OMI) Data user’s Guide; NASA: Washington DC, USA, 2012.
- Hovila, J.; Arola, A.; Tamminen, J. OMI/Aura Surface UVB Irradiance and Erythemal Dose Daily L3 Global Gridded 1.0 degree x 1.0 degree V3, NASA Goddard Space Flight Center, Goddard Earth Sciences Data and Information Services Center (GES DISC). Available online: 10.5067/Aura/OMI/DATA3009 (accessed on 14 March 2019).
- World Health Organization. UV Index. Available online: https://www.who.int/uv/intersunprogramme/activities/uv_index/en/ (accessed on 30 September 2019).
- Statistics SA. Mortality and Causes of Death in South Africa, 2016—Findings from Death Notification. Statistical release P0309.3. Available online: http://www.statssa.gov.za/publications/P03093/P030932016.pdf (accessed on 14 March 2019).
- Cameron, M.C.; Lee, E.; Hibler, B.P.; Barker, C.B.; Mori, S.; Cordova, M.; Nehal, K.S.; Rossi, A.M. Basal Cell Carcinoma: Epidemiology; Pathophysiology; Clinical and Histological Subtypes; and Disease Associations. J. Am. Acad Dermatol. 2018, 80, 303–317. [Google Scholar] [CrossRef]
- Lucas, R.M.; Tazar, S.; Young, A.R.; Norval, M.; de Gruijl, F.R.; Rhodes, L.E.; Sinclair, C.A.; Neale, R.E. Human health in relation to exposure to solar ultraviolet radiation under changing stratospheric ozone and climate. Photochem. Photobiol. Sci. 2019, 18, 641–680. [Google Scholar] [CrossRef] [PubMed]
- Norval, M.; Kellett, P.; Wright, C.Y. The incidence and body site of skin cancers in the population groups of South Africa. Photodermatol. Photoimmunol. Photomed. 2014, 30, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.Y.; Kapwata, T.; Singh, E.; Green, A.; Baade, P.; Kellett, P.; Norval, M. Trends in melanoma mortality in population groups of South Africa. Dermatology 2019, 235, 396–399. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Registry (of SA). Cancer in SA: 2014 Full Report. Available online: http://www.nicd.ac.za/wp-content/uploads/2017/03/2014-NCR-tables-1.pdf (accessed on 22 March 2019).
- Gordon, L.G.; Elliott, T.M.; Wright, C.Y.; Deghaye, N.; Visser, W. Modelling the healthcare costs of skin cancer in South Africa. BMC Health Serv. Res. 2016, 16, 113. [Google Scholar] [CrossRef]
- Tod, B.; Kellett, P.; Singh, E.; Visser, W.; Lombard, C.; Wright, C.Y. The incidence of melanoma in South Africa: An exploratory analysis of National Cancer Registry data from 2005 to 2013 with a specific focus on melanoma in Black Africans. S. Afr. Med. J. 2019, 109, 246–253. [Google Scholar] [CrossRef]
- Discovery Health. Healthcare Claims Tracker: A Focus on Oncology Claims. Available online: https://www.discovery.co.za/assets/discoverycoza/medical-aid/find-a-document/guides/healthcare-claims-tracker-oncology-claims.pdf (accessed on 18 March 2019).
- Piacentini, R.D.; Della Ceca, L.S.; Ipina, A. Climate change and its relationship with non-melanoma skin cancers. Photochem. Photobiol. Sci. 2018, 17, 1913–1917. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef]
- Krutmann, J.; Liu, W.; Li, L.; Pan, X.; Crawford, M.; Sore, G. Pollution and skin: From epidemiological and mechanistic studies to clinical implications. J. Dermatol. Sci. 2014, 76, 163–168. [Google Scholar] [CrossRef]
- Mancebo, S.E.; Wand, S.Q. Recognizing the impact of ambient air pollution on skin health. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2326–2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zegarska, B.; Pietkun, K.; Giemza-Kucharska, P.; Zegarski, T.; Nowacki, M.S.; Romanska-Gocka, K. Changes in Langerhans cells during skin ageing. Postepy. Dermatol. Alergol. 2017, 34, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Burke, K.E.; Wei, H. Synergistic damage by UVA radiation and pollutants. Toxicol. Indus. Health 2009, 25, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Glavan, U.; Dahmane, R. Skin Cancer, Free radicals and antioxidants. Int. J. Res. Prev. 2011, 4, 193–217. [Google Scholar]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilberte, Y.; Gonzalez, S.; Juarranz, A. Environmental Stressors on Skin Aging. Mechanistic Insights. Front. Pharmacol. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Republic of South Africa. Government Gazette National Environment Management: Air Quality Act. 2005; Volume 476, p. 27318. Available online: https://www.environment.gov.za/sites/default/files/legislations/nema_amendment_act39.pdf (accessed on 27 August 2019).
- Josipovic, M.; Annegarn, H.J.; Keen, M.A.; Pienaar, J.J.; Piketh, S.J. Concentrations, distributions and critical level exceedance assessment of SO2, NO2 and O3 in South Africa. Environ. Monit. Assess. 2010, 171, 181–196. [Google Scholar] [CrossRef]
- Garland, R.M.; Naidoo, M.; Sibiya, B.; Oosthuizen, M.A. Air quality indicators from the Environmental Performance Index: Potential use and limitations in South Africa. Clean Air J. 2017, 27, 1–9. Available online: https://www.cleanairjournal.org.za/journals/volume27_no1_2017/volume27_no1_2017_p33.php (accessed on 27 August 2019). [CrossRef]
- Morakinyo, O.M.; Adebowale, A.S.; Mokgobu, M.I.; Mukhola, M.S. Health risk of inhalation exposure to sub-10 µm particulate matter and gaseous pollutants in an urban-industrial area in South Africa: An ecological study. BMJ 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Pollet, B.G.; Staffell, I.; Adamson, K. Current energy landscape in the Republic of South Africa. Int. J. Hydrogen Energ. 2015, 40, 16685–16701. [Google Scholar] [CrossRef]
- Arndt, C.; Davies, R.; Thurlow, J. Urbanisation, Structural Transformation and Rural-Urban Linkages in South Africa (2018). Available online: https://csp.treasury.gov.za/Resource%20_Centre/Conferences/Documents/Urbanization%20Review%20Papers/Paper%203%20-%20RSA%20Urbanization.pdf (accessed on 27 August 2019).
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef]
- Armstrong, B.K.; Cust, A.E. Sun exposure and skin cancer, and the puzzle of cutaneous melanoma: A perspective on Fears et al. Mathematical models of age and ultraviolet effects on the incidence of skin cancer among whites in the United States. Cancer Epidemiol. 2011, 48, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Kricker, A.; Weber, M.; Sitas, F.; Banks, E.; Rahman, B.; Goumas, C.; Kabir, A.; Hodgkinson, V.S.; van Lemenade, C.H.; Waterboer, T.; et al. Early life UV and risk of basal and squamous cell carcinoma in New South Wales, Australia. Photochem. Photobiol. 2017, 93, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Weather2travel. Available online: www.weather2travel.com (accessed on 27 August 2019).
- Holiday-Weather. Available online: www.holiday-weather.com (accessed on 27 August 2019).
- World Population Review. Available online: http://worldpopulationreview.com/countries/south-africa-population (accessed on 27 August 2019).
- Newman, P.A.; McKenzie, R. UV impacts avoided by the Montreal Protocol. Photochem. Photobiol. Sci. 2011, 10, 1152–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bais, A.F.; Bernhard, G.; McKenzie, R.L.; Aucamp, P.J.; Young, P.J.; Ilyes, M.; Jockel, P.; Deushi, M. Ozone-climate interactions and effects on solar ultraviolet radiation. Photochem. Photobiol. Sci. 2019, 18, 602–640. [Google Scholar] [CrossRef]
- van der Leun, J.C.; de Gruijl, F.R. Climate change and skin cancer. Photochem. Photobiol. Sci. 2002, 1, 324–326. [Google Scholar] [CrossRef]
- Kuttippurath, J.; Nair, P.J. The signs of Antarctic ozone hole recovery. Sci. Rep. 2017, 7, 585. [Google Scholar] [CrossRef]
- Bain, J.A.; Rusch, H.P.; Kline, B.E. The effect of temperature upon ultraviolet carcinogenesis with wavelengths 2800–3400 Å. Cancer Res. 1943, 3, 610–612. Available online: https://cancerres.aacrjournals.org/content/3/9/610.short (accessed on 3 October 2019).
- van der Leun, J.C.; Piacentini, R.D.; de Gruijl, F.R. Climate change and human skin cancer. Photochem. Photobiol. Sci. 2008, 7, 730–733. [Google Scholar] [CrossRef]
- Kaffenberger, B.H.; Shetlar, D.; Norton, S.A.; Rosenbach, M. The effect of climate change on skin disease in North America. J. Am. Acad. Dermatol. 2017, 76, 140–147. [Google Scholar] [CrossRef]
- Calapre, L.; Gray, E.S.; Kurdykowski, S.; David, A.; Hart, P.; Descargues, P.; Ziman, M. Heat-mediated reduction of apoptosis in UVB-damaged keratinocytes in vitro and in human skin ex vivo. BMC Dermatol. 2016, 16, 6. [Google Scholar] [CrossRef]
- Kapwata, T.; Gebreslasie, M.T.; Mathee, A.; Wright, C.Y. Current and potential future seasonal trends on indoor dwelling temperature and likely health risks in rural Southern Africa. Int. J. Environ. Res. Public Health 2018, 15, 952. [Google Scholar] [CrossRef] [PubMed]
- Garland, R.M.; Matooane, M.; Engelbrecht, F.A.; Bopape, M.-J.M.; Landman, W.A.; Naidoo, M.; van der Merwe, J.; Wright, C.Y. Regional projections of extreme apparent temperature days in Africa and the related potential risk to human health. Int. J. Environ. Res. Public Health 2015, 12, 12577–12604. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Hoshino, T.; Yamakawa, N.; Tahara, K.; Adachi, H.; Sobue, G.; Maji, D.; Ihn, H.; Mizushima, T. Suppression of UV-induced wrinkle formation by induction of HSP70 expression in mice. J. Invest. Derm. 2013, 135, 919. [Google Scholar] [CrossRef] [PubMed]
- Schmalwieser, A.W.; Schmalwieser, V.T.; Schmalwieser, S.S. Influence of air temperature on the UV exposure of different body sites due to clothing of young women during daily errands. Photochem. Photobiol. 2019, 95, 1068–1075. [Google Scholar] [CrossRef]
- Slaper, H.; Velders, G.M.; Daniel, J.S.; de Gruijl, F.R.; van der Leun, J.C. Estimates of ozone depletion and skin cancer incidence to examine the Vienna Convention achievements. Nature 1996, 384, 256–258. [Google Scholar] [CrossRef]
- Diffey, B.L. Climate change, ozone depletion and the impact on ultraviolet exposure of human skin. Phys. Med. Biol. 2003, 49, R1–R11. [Google Scholar] [CrossRef]
- Bharath, A.K.; Turner, R.J. Impact of climate change on skin. J. R. Soc. Med. 2009, 102, 215–218. [Google Scholar] [CrossRef]
- Cook, K.H.; Vizy, E.K. Impact of climate change on mid-twenty-first century growing seasons in Africa. Clim. Dynam. 2012, 39, 2937–2955. [Google Scholar] [CrossRef] [Green Version]
- Lohmann, U.; Feichter, J. Global indirect aerosol effects: A review. Atmos. Chem. Phys. 2005, 5, 715–737. [Google Scholar] [CrossRef]
- American Meteorological Society. Glossary of Meteorology. Available online: http://glossary.ametsoc.org/wiki/Solar_zenith_angle (accessed on 26 February 2019).
- Jury, M.R. Climate trends in southern Africa. S. Afr. J. Sci. 2012, 109, 1–11. [Google Scholar] [CrossRef]
- Ruppel, I.O.C.; Abdrado, M.A.; Essel, A.; Lennard, C.; Padghamp, J.; Urqhaurt, P. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Available online: https://www.ipcc.ch/report/ar5/wg2/ (accessed on 27 August 2019).
- World Health Organization. Sun Protection. Available online: https://www.who.int/uv/sun_protection/en/ (accessed on 27 August 2019).
- Wu, Y.P.; Parsons, B.G.; Mooney, R.; Aspinwall, L.G.; Cloyes, K.; Hay, J.L.; Kohlmann, W.; Grossman, D.; Leachman, S.A. Barriers and Facilitators to melanoma prevention and control behaviours among at-risk children. J. Community Health 2018, 43, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Religi, A.; Backes, C.; Moccozet, L.; Vuilleumier, L.; Vernez, D.; Bulliard, J.-L. Body anatomical UV protection predicted by shade structures: A modelling study. Photochem. Photobiol. 2018, 94, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Mathee, A.; Oba, J.; Rose, A. Climate change impacts on working people (the HOTHAPS initiative): Findings of the South African pilot study. Global Health Action 2010, 3, 1–9. Available online: https://www.tandfonline.com/doi/abs/10.3402/gha.v3i0.5612. (accessed on 3 October 2019). [CrossRef] [PubMed]
| Age Standardised Incidence | Rate per 100,000 | |
|---|---|---|
| Cutaneous Melanoma | All | 2.7 |
| Black African | 0.5 | |
| White | 23.2 | |
| Basal Cell Carcinoma | Females | 28.6 |
| Males | 55.6 | |
| Basal Cell Carcinoma | White Females | 138.0 |
| White Males | 223.2 | |
| Black African Females | 2.2 | |
| Black African Males | 3.5 | |
| Cutaneous Squamous Cell Carcinoma | Females | 10.6 |
| Males | 25.6 | |
| Cutaneous Squamous Cell Carcinoma | White Females | 38.8 |
| White Males | 84.5 | |
| Black African Females | 2.0 | |
| Black African Males | 3.5 | |
| Characteristic | Cape Town | Durban | Johannesburg Including Soweto | Pretoria | |
|---|---|---|---|---|---|
| 2018 population size estimate (millions) | 3.4 | 3.1 | 3.7 | 1.6 | |
| Latitude (°S) | 34 | 29 | 26 | 26 | |
| Altitude (m) | 0–300 | 0–21 | 1750 | 1340 | |
| Ultraviolet Index (average values at noon) | Summer | 9–10 | 10–11 | 11+ | 11+ |
| Winter | 2–3 | 3–4 | 4–6 | 4–6 | |
| Average day-time temperatures (°C) | Summer | 26 | 29 | 26 | 30 |
| Winter | 19 | 24 | 19 | 21 | |
| Average night-time temperatures (°C) | Summer | 15 | 21 | 15 | 18 |
| Winter | 8 | 11 | 4 | 5 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wright, C.Y.; Norval, M.; Kapwata, T.; du Preez, D.J.; Wernecke, B.; Tod, B.M.; Visser, W.I. The Incidence of Skin Cancer in Relation to Climate Change in South Africa. Atmosphere 2019, 10, 634. https://doi.org/10.3390/atmos10100634
Wright CY, Norval M, Kapwata T, du Preez DJ, Wernecke B, Tod BM, Visser WI. The Incidence of Skin Cancer in Relation to Climate Change in South Africa. Atmosphere. 2019; 10(10):634. https://doi.org/10.3390/atmos10100634
Chicago/Turabian StyleWright, Caradee Y., Mary Norval, Thandi Kapwata, David Jean du Preez, Bianca Wernecke, Bianca M. Tod, and Willem I. Visser. 2019. "The Incidence of Skin Cancer in Relation to Climate Change in South Africa" Atmosphere 10, no. 10: 634. https://doi.org/10.3390/atmos10100634
APA StyleWright, C. Y., Norval, M., Kapwata, T., du Preez, D. J., Wernecke, B., Tod, B. M., & Visser, W. I. (2019). The Incidence of Skin Cancer in Relation to Climate Change in South Africa. Atmosphere, 10(10), 634. https://doi.org/10.3390/atmos10100634






