Effects of Grazing Pattern on Ecosystem Respiration and Methane Flux in a Sown Pasture in Inner Mongolia, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Gas Flux Measurements
2.4. Auxiliary Measurements
2.5. Statistical Analysis
3. Results
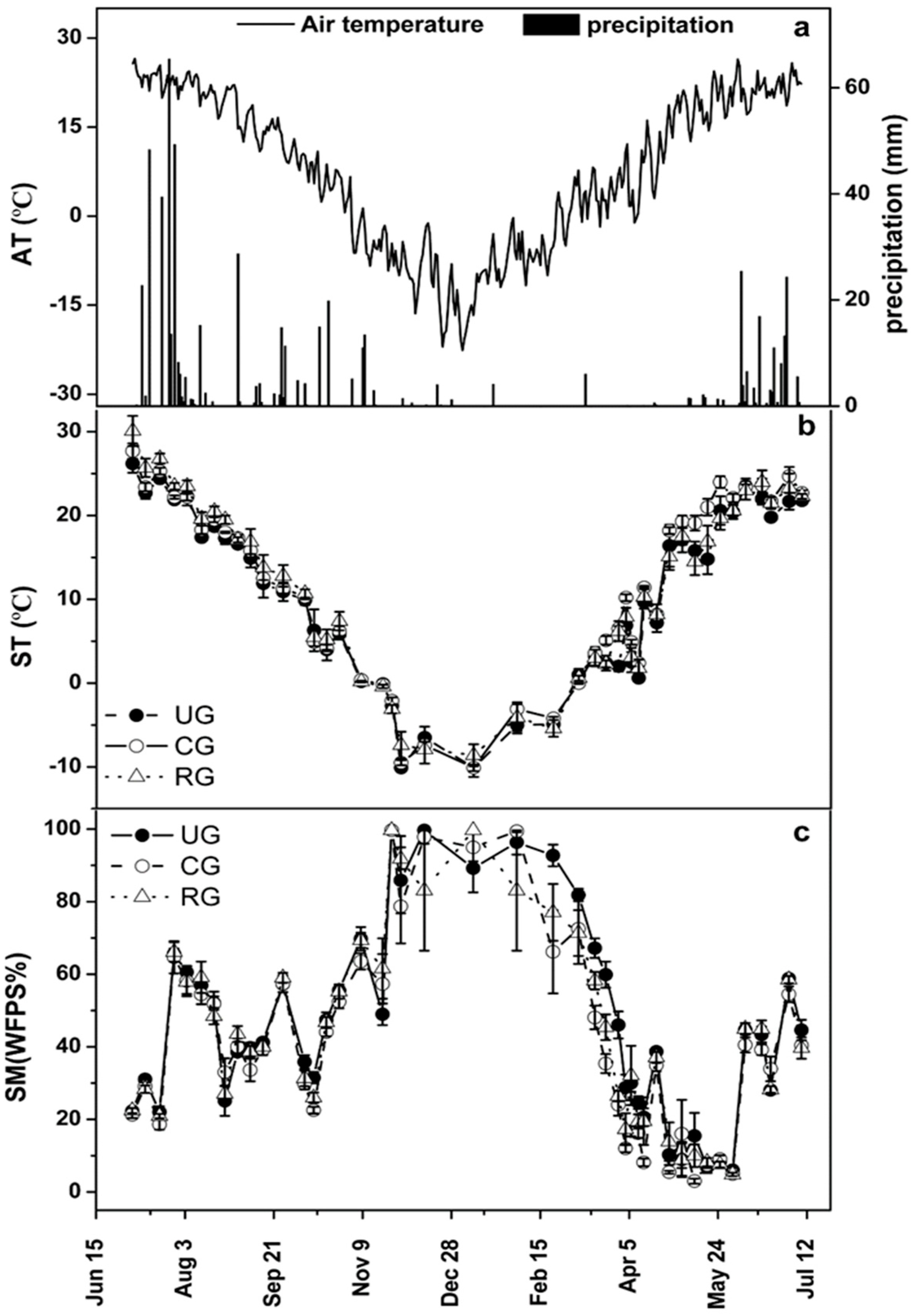
3.1. Environmental Factors
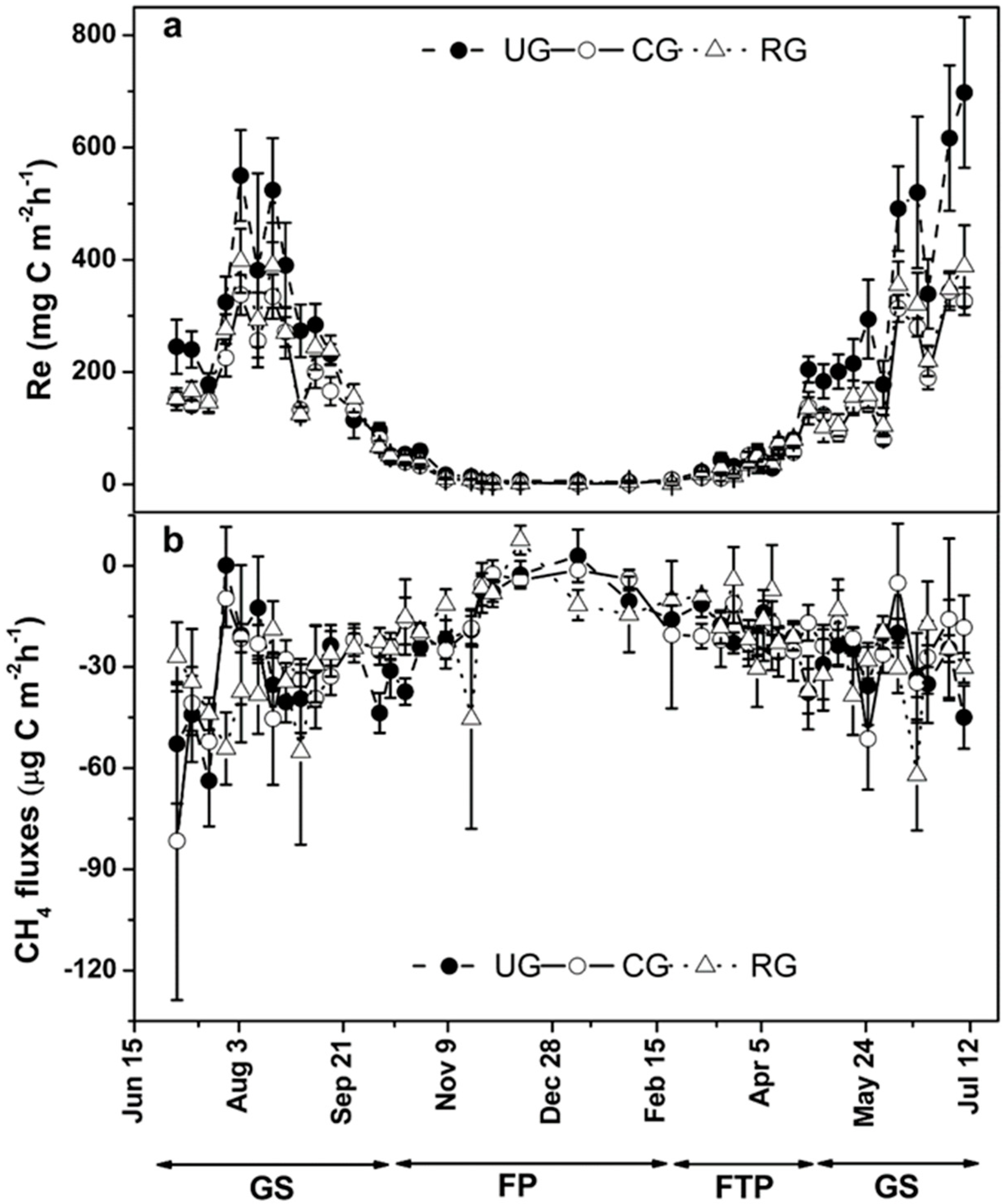
3.2. Ecosystem Respiration
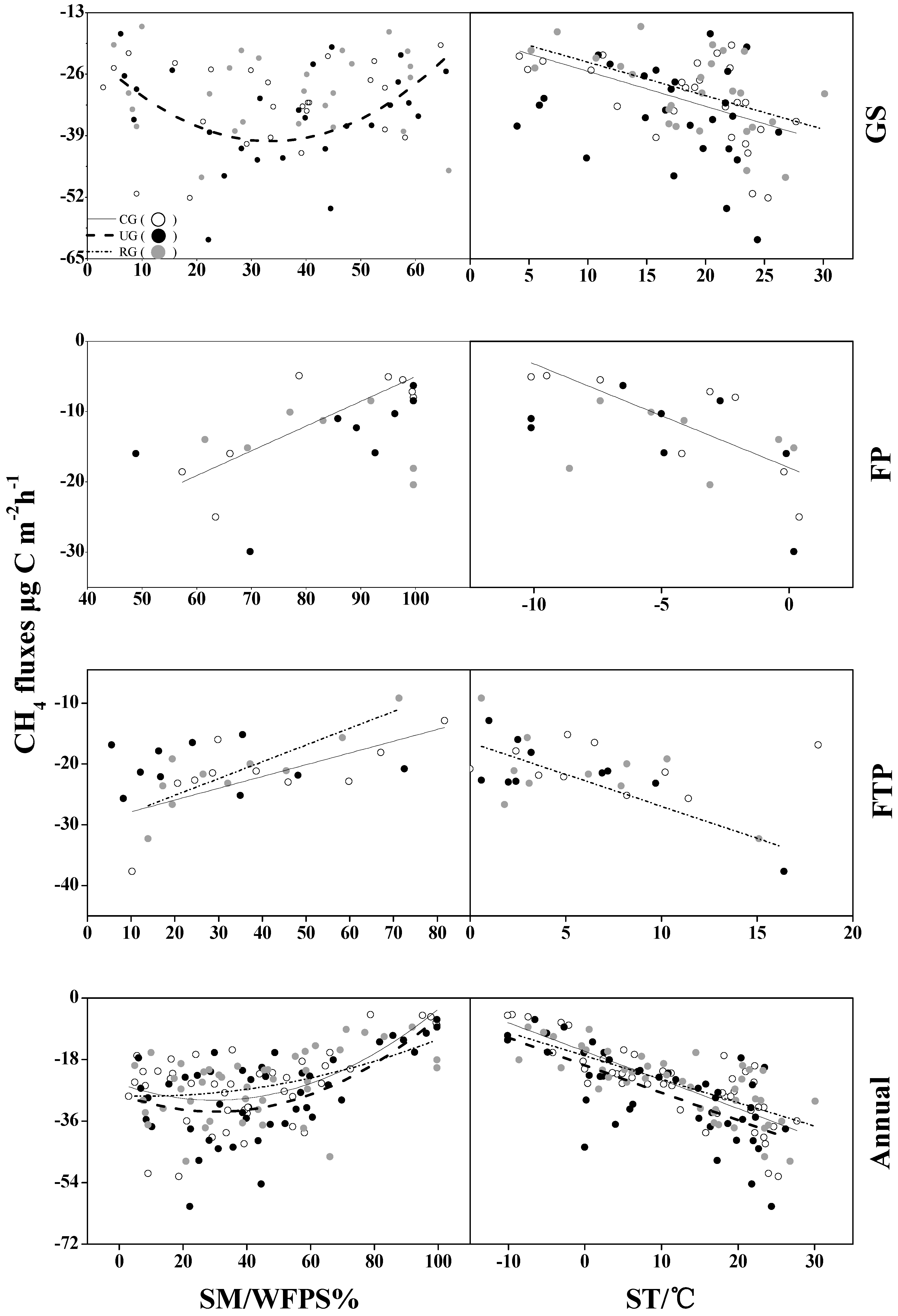
3.3. Methane Fluxes
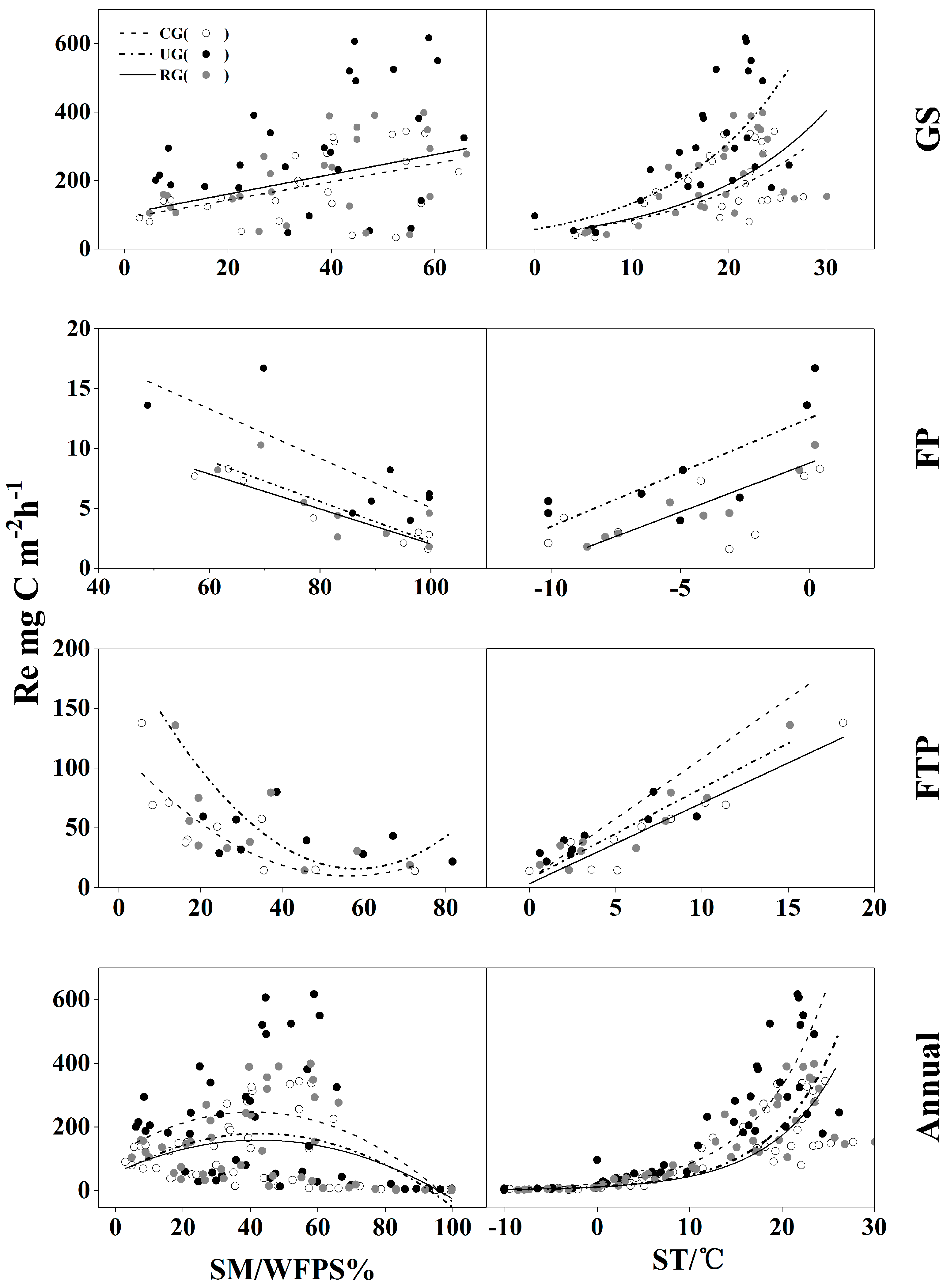
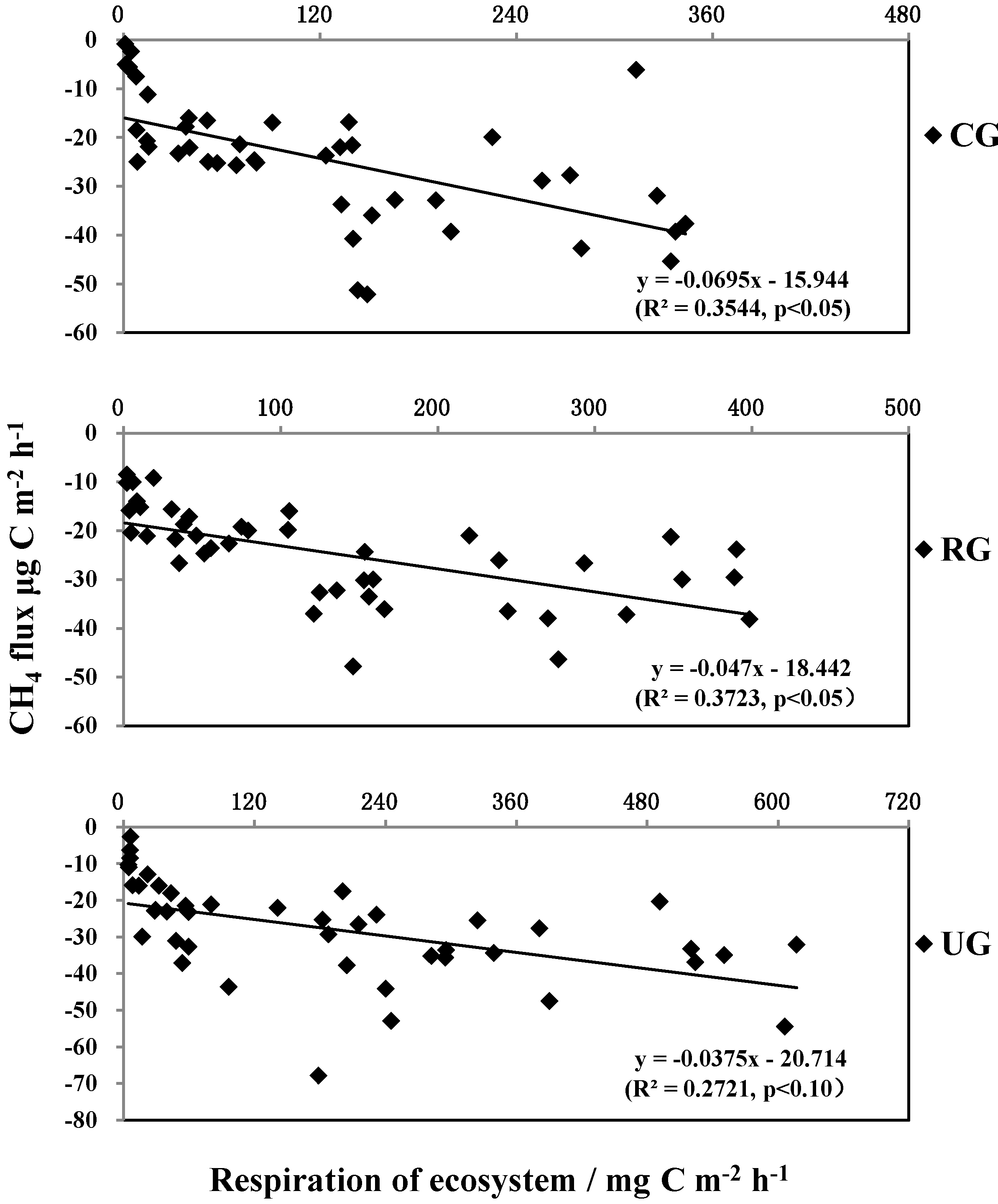
3.4. The Relationship between CH4 Flux and Ecosystem Respiration (Re)
4. Discussion
4.1. Ecosystem Respiration (Re)
4.2. CH4 Flux
4.3. Effects of Grazing on Ecosystem Respiration and CH4 Flux
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2013: The Physical Science Basis, in Contribution of Working Group I to the Fifth Assessment Report of the Inter-Governmental Panel on Climate Change. Chapter 6 Carbon and Other Biogeochemical Cycles. 2013, p. 467. Available online: https://www.ipcc.ch/report/ar5/wg1/ (accessed on 18 August 2017).
- Davidson, E.A. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat. Geosci. 2009, 2, 659–662. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Morgan, J.A.; Follett, R.F.; LeCain, D.R. Climate change reduces the net sink of CH4 and N2O in a semiarid grassland. Glob. Chang. Biol. 2013, 19, 1816–1826. [Google Scholar] [CrossRef] [PubMed]
- Galbally, I.E.; Kirstine, W.V.; Meyer, C.P.; Wang, Y.P. Soil–Atmosphere Trace Gas Exchange in Semiarid and Arid Zones. J. Environ. Qual. 2008, 37, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Schönbach, P.; Wolf, B.; Dickhöfer, U.; Wiesmeier, M.; Chen, W.; Wan, H.; Gierus, M.; Butterbach-Bahl, K.; Kögel-Knabner, I.; Susenbeth, A.; et al. Grazing effects on the greenhouse gas balance of a temperate steppe ecosystem. Nutr. Cycl. Agroecosyst. 2012, 93, 357–371. [Google Scholar] [CrossRef]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Wang, K.; Wang, T.; Xu, W. Overgrazing and soil carbon dynamics in eastern Inner Mongolia of China. Ecol. Res. 2006, 22, 135–142. [Google Scholar] [CrossRef]
- Schwenke, G.D.; McLeod, M.K.; Murphy, S.R.; Harden, S.; Cowie, A.L.; Lonergan, V.E. The potential for sown tropical perennial grass pastures to improve soil organic carbon in the North-West Slopes and Plains of New South Wales. Soil Res. 2013, 51, 726–737. [Google Scholar] [CrossRef]
- Wang, C.; Wang, G.; Liu, W.; Wang, Y.; Hu, L.; Ma, L. Effects of establishing an artificial grassland on vegetation characteristics and soil quality in a degraded meadow. Isr. J. Ecol. Evol. 2013, 59, 141–153. [Google Scholar] [CrossRef]
- Lodge, G.M.; King, K.L. Soil microbial biomass, labile and total carbon levels of grazed sown and native pastures in northern New South Wales. Aust. J. Agric. Res. 2006, 57, 837–845. [Google Scholar] [CrossRef]
- Wolf, B.; Chen, W.; Brüggemann, N.; Zheng, X.; Pumpanen, J.; Butterbach-Bahl, K. Applicability of the soil gradient method for estimating soil–atmosphere CO2, CH4, and N2O fluxes for steppe soils in Inner Mongolia. J. Plant Nutr. Soil Sci. 2011, 174, 359–372. [Google Scholar] [CrossRef]
- Laubach, J.; Hunt, J.E. Greenhouse-gas budgets for irrigated dairy pasture and a winter-forage kale crop. Agric. For. Meteorol. 2018, 258, 117–134. [Google Scholar] [CrossRef]
- Wu, X.; Yao, Z.; Brüggemann, N.; Shen, Z.Y.; Wolf, B.; Dannenmann, M.; Zheng, X.; Butterbach-Bahl, K. Effects of soil moisture and temperature on CO2 and CH4 soil–atmosphere exchange of various land use/cover types in a semi-arid grassland in Inner Mongolia, China. Soil Biol. Biochem. 2010, 42, 773–787. [Google Scholar] [CrossRef]
- Chen, W.; Wolf, B.; Zheng, X.; Yao, Z.; Butterbach-Bahl, K.; BrÜGgemann, N.; Liu, C.; Han, S.; Han, X. Annual methane uptake by temperate semiarid steppes as regulated by stocking rates, aboveground plant biomass and topsoil air permeability. Glob. Chang. Biol. 2011, 17, 2803–2816. [Google Scholar] [CrossRef]
- Liu, C.; Holst, J.; Yao, Z.; Brüggemann, N.; Butterbach-Bahl, K.; Han, S.; Han, X.; Tas, B.; Susenbeth, A.; Zheng, X. Growing season methane budget of an Inner Mongolian steppe. Atmos. Environ. 2009, 43, 3086–3095. [Google Scholar] [CrossRef]
- Nakano, T.; Shinoda, M. Modeling gross primary production and ecosystem respiration in a semiarid grassland of Mongolia. Soil Sci. Plant Nutr. 2014, 61, 106–115. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, X.; Chen, Q.; Wolf, B.; Butterbach-Bahl, K.; Brüggemann, N.; Lin, S. Effects of increasing precipitation and nitrogen deposition on CH4 and N2O fluxes and ecosystem respiration in a degraded steppe in Inner Mongolia, China. Geoderma 2013, 192, 335–340. [Google Scholar] [CrossRef]
- Jia, B.; Zhou, G.; Wang, Y.; Wang, F.; Wang, X. Effects of temperature and soil water-content on soil respiration of grazed and ungrazed Leymus chinensis steppes, Inner Mongolia. J. Arid Environ. 2006, 67, 60–76. [Google Scholar] [CrossRef]
- Liu, C.; Holst, J.; Brüggemann, N.; Butterbach-Bahl, K.; Yao, Z.; Yue, J.; Han, S.; Han, X.; Krümmelbein, J.; Horn, R.; et al. Winter-grazing reduces methane uptake by soils of a typical semi-arid steppe in Inner Mongolia, China. Atmos. Environ. 2007, 41, 5948–5958. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Y.; Mei, X.; Cui, X.; Zhou, X.; Huang, X. The sensitivity of temperate steppe CO2 exchange to the quantity and timing of natural interannual rainfall. Ecol. Inform. 2010, 5, 222–228. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, M.; Zheng, X.; Ji, B.; Du, R.; Wang, Y. Effects of environmental factors on N2O emission from and CH4 uptake by the typical grasslands in the Inner Mongolia. Chemosphere 2005, 58, 205–215. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, G.; Wang, Y. Environmental effects on net ecosystem CO2 exchange at half-hour and month scales over Stipa krylovii steppe in northern China. Agric. For. Meteorol. 2008, 148, 714–722. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.; Ji, B.; Du, R.; Chen, Z.; Wang, G.; Wang, Y.; Wan, X. Diurnal and seasonal variation in methane and nitrous oxide fluxes in meadow steppe of Inner Mongolia. Zhiwu Shengtai Xuebao 2003, 27, 792–796. [Google Scholar] [CrossRef]
- Kroon, P.S.; Hensen, A.; Bulk, W.C.M.v.d.; Jongejan, P.A.C.; Vermeulen, A.T. The importance of reducing the systematic error due to non-linearity in N2O flux measurements by static chambers. Nutr. Cycl. Agroecosyst. 2008, 82, 175–186. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, X.; Zhou, Z.; Han, S.; Wang, Y.; Wang, K.; Liang, W.; Li, M.; Chen, D.; Yang, Z. Nitrous oxide and nitric oxide emissions from an irrigated cotton field in Northern China. Plant Soil 2010, 332, 123–134. [Google Scholar] [CrossRef]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Chem. Erde Geochem. 2016, 76, 327–352. [Google Scholar] [CrossRef]
- Chang, C.T.; Sabate, S.; Sperlich, D.; Poblador, S.; Sabater, F.; Gracia, C. Does soil moisture overrule temperature dependence of soil respiration in Mediterranean riparian forests? Biogeosciences 2014, 11, 6173–6185. [Google Scholar] [CrossRef]
- Fu, Y.; Zheng, Z.; Yu, G.; Hu, Z.; Sun, X.; Shi, P.; Wang, Y.; Zhao, X. Environmental influences on carbon dioxide fluxes over three grassland ecosystems in China. Biogeosciences 2009, 6, 2879–2893. [Google Scholar] [CrossRef]
- Dore, S.; Hymus, G.J.; Johnson, D.P.; Hinkle, C.R.; Valentini, R.; Drake, B.G. Cross validation of open-top chamber and eddy covariance measurements of ecosystem CO2 exchange in a Florida scrub-oak ecosystem. Glob. Chang. Biol. 2003, 9, 84–95. [Google Scholar] [CrossRef]
- Janssens, I.A.; Kowalski, A.S.; Ceulemans, R. Forest floor CO2 fluxes estimated by eddy covariance and chamber-based model. Agric. For. Meteorol. 2001, 106, 61–69. [Google Scholar] [CrossRef]
- Yan, L.; Chen, S.; Huang, J.; Lin, G. Increasing water and nitrogen availability enhanced net ecosystem CO2 assimilation of a temperate semiarid steppe. Plant Soil 2011, 349, 227–240. [Google Scholar] [CrossRef]
- Cheng, J.; Lee, X.; Theng, B.K.G.; Fang, B.; Yang, F.; Wang, B.; Zhang, L. Spatial Variability of CO2, CH4, and N2O Fluxes during Midsummer in the Steppe of Northern China. Pol. J. Environ. Stud. 2014, 23, 319–328. [Google Scholar]
- Euskirchen, E.S.; Bret-Harte, M.S.; Shaver, G.R.; Edgar, C.W.; Romanovsky, V.E. Long-Term Release of Carbon Dioxide from Arctic Tundra Ecosystems in Alaska. Ecosystems 2017, 20, 960–974. [Google Scholar] [CrossRef]
- Lange, S.F.; Allaire, S.E.; Cuellar Castillo, M.A.; Dutilleul, P. N2O and CO2 dynamics in a pasture soil across the frozen period. Can. J. Soil Sci. 2016, 97, 497–511. [Google Scholar] [CrossRef]
- Bai, Y.; Li, X.; Wen, W.; Mi, X.; Li, R.; Huang, Q.; Zhang, M. CO2, CH4 and N2O flux changes in degraded grassland soil of Inner Mongolia, China. J. Arid Land 2018, 10, 347–361. [Google Scholar] [CrossRef]
- Sun, W.; Sun, Z.; Mou, X.; Sun, W.J. Short-Term Study on Variations of Carbon Dioxide and Methane Emissions from Intertidal Zone of the Yellow River Estuary during Autumn and Winter. Wetlands 2018, 1–20. [Google Scholar] [CrossRef]
- Scala Júnior, N.L.; Simas, F.N.B.; Mendon, E.D.S.; Souza, J.V.; Panosso, A.R.; Schaefer, C.E.; Gilkes, R.J.; Prakonkep, N. Spatial and temporal variability of soil C-CO2 emissions and its relation with soil temperature in King George Island, Maritime Antarctica. Polar Sci. 2010, 479–487. [Google Scholar] [CrossRef]
- Wagle, P.; Kakani, V.G. Confounding Effects of Soil Moisture on the Relationship Between Ecosystem Respiration and Soil Temperature in Switchgrass. Bioenergy Res. 2014, 7, 789–798. [Google Scholar] [CrossRef]
- Xu, L.; Baldocchi, D.D.; Tang, J. How soil moisture, rain pulses, and growth alter the response of ecosystem respiration to temperature. Glob. Biogeochem. Cycle 2004, 18. [Google Scholar] [CrossRef]
- Geng, Y.; Luo, G.; Yuan, G. CH4 uptake flux of Leymus chinensis steppe during rapid growth season in Inner Mongolia, China. Sci. China-Earth Sci. 2010, 53, 977–983. [Google Scholar] [CrossRef]
- Holst, J.; Liu, C.; Yao, Z.; Brüggemann, N.; Zheng, X.; Giese, M.; Butterbach-Bahl, K. Fluxes of nitrous oxide, methane and carbon dioxide during freezing–thawing cycles in an Inner Mongolian steppe. Plant Soil 2008, 308, 105–117. [Google Scholar] [CrossRef]
- Luo, G.J.; Kiese, R.; Wolf, B.; Butterbach-Bahl, K. Effects of soil temperature and moisture on methane uptake and nitrous oxide emissions across three different ecosystem types. Biogeosciences 2013, 10, 3205–3219. [Google Scholar] [CrossRef]
- Zhang, L.; Huo, Y.; Guo, D.; Wang, Q.; Bao, Y.; Li, L. Effects of Multi-nutrient Additions on GHG Fluxes in a Temperate Grassland of Northern China. Ecosystems 2014, 17, 657–672. [Google Scholar] [CrossRef]
- Tang, S.; Wang, C.; Wilkes, A.; Zhou, P.; Jiang, Y.; Han, G.; Zhao, M.; Huang, D.; Schönbach, P. Contribution of grazing to soil atmosphere CH4 exchange during the growing season in a continental steppe. Atmos. Environ. 2013, 67, 170–176. [Google Scholar] [CrossRef]
- Mosier, A.R.; Parton, W.J.; Valentine, D.W.; Ojima, D.S.; Schimel, D.S.; Delgado, J.A. CH4 and N2O fluxes in the Colorado shortgrass steppe: 1. Impact of landscape and nitrogen addition. Glob. Biogeochem. Cycle 1996, 10, 387–399. [Google Scholar] [CrossRef]
- Wang, C.; Tang, S.; Wilkes, A.; Jiang, Y.; Han, G.; Huang, D. Effect of stocking rate on soil-atmosphere CH4 flux during spring freeze-thaw cycles in a northern desert steppe, China. PLoS ONE 2012, 7, e36794. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Bardgett, R.D.; Smith, P.; Reay, D.S. Microorganisms and climate change: Terrestrial feedbacks and mitigation options. Nat. Rev. Microbiol. 2010, 8, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, F.A.; Morgan, J.A.; von Fischer, J.C.; Follett, R.F. Elevated CO2 and warming effects on CH4 uptake in a semiarid grassland below optimum soil moisture. J. Geophys. Res. 2011, 116. [Google Scholar] [CrossRef]
- Zhao, J.; Peichl, M.; Nilsson, M.B. Enhanced winter soil frost reduces methane emission during the subsequent growing season in a boreal peatland. Glob. Chang. Biol. 2016, 22, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Ma, L.; Johnson, D.A. Methane uptake by four land-use types in the agro-pastoral region of northern China. Atmos. Environ. 2015, 116, 12–21. [Google Scholar] [CrossRef]
- Roy Chowdhury, T.; Herndon, E.M.; Phelps, T.J.; Elias, D.A.; Gu, B.; Liang, L.; Wullschleger, S.D.; Graham, D.E. Stoichiometry and temperature sensitivity of methanogenesis and CO2 production from saturated polygonal tundra in Barrow, Alaska. Glob. Chang. Biol. 2015, 21, 722–737. [Google Scholar] [CrossRef]
- Kang, X.; Hao, Y.; Cui, X.; Chen, H.; Li, C.; Rui, Y.; Tian, J.; Kardol, P.; Zhong, L.; Wang, J.; et al. Effects of grazing on CO2 balance in a semiarid steppe: Field observations and modeling. J. Soils Sediments 2013, 13, 1012–1023. [Google Scholar] [CrossRef]
- Kang, X.; Hao, Y.; Li, C.; Cui, X.; Wang, J.; Rui, Y.; Niu, H.; Wang, Y. Modeling impacts of climate change on carbon dynamics in a steppe ecosystem in Inner Mongolia, China. J. Soils Sediments 2011, 11, 562–576. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, Y.; Zhai, X.; Wilkes, A.; Wang, C.; Wang, K. Effect of grazing on methane uptake from Eurasian steppe of China. BMC Ecol. 2018, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Da, W.; Xu, R.; Wang, Y.; Wang, Y.; Liu, Y.; Yao, T. Responses of CO2, CH4 and N2O fluxes to livestock exclosure in an alpine steppe on the Tibetan Plateau, China. Plant Soil 2012, 359, 45–55. [Google Scholar] [CrossRef]
- Gao, X.; Thomas, B.W.; Beck, R.; Thompson, D.J.; Zhao, M.; Willms, W.D.; Hao, X.J. Long-term grazing alters soil trace gas fluxes from grasslands in the foothills of the Rocky Mountains, Canada. Land Degrad. Dev. 2018, 29, 292–302. [Google Scholar] [CrossRef]
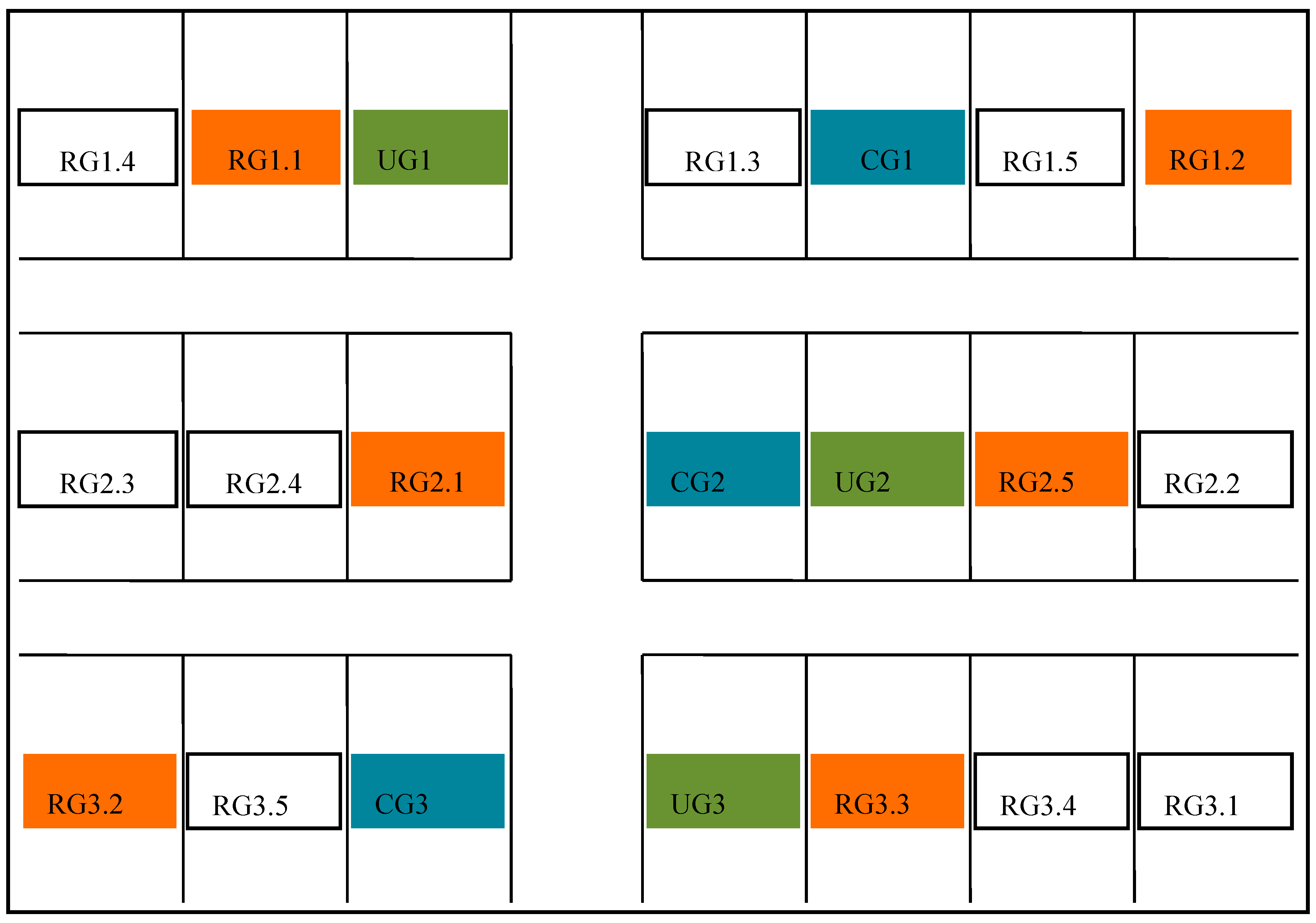
| Experiment Site | Number of Sampling Points | Duration of Grazing Period (d) | Grazing Rate (sheep ha−1) | pH | SOM 0–20 cm (g kg−1) | SAN 0–20 cm (g kg−1) | SBD (0–10 cm) (g/m3) |
|---|---|---|---|---|---|---|---|
| RG1.1 | 1 | 30 | 9 | 8.58 ± 0.11 | 13.54 ± 1.63 | 0.07 ± 0.01 | 1.51 |
| RG1.2 | 1 | 30 | 9 | 8.28 ± 0.16 | 12.84 ± 3.20 | 0.07 ± 0.02 | 1.51 |
| RG2.1 | 1 | 30 | 9 | 8.46 ± 0.14 | 9.92 ± 0.76 | 0.06 ± 0.02 | 1.40 |
| RG2.5 | 1 | 30 | 9 | 8.32 ± 0.17 | 9.45 ± 1.67 | 0.06 ± 0.02 | 1.40 |
| RG3.2 | 1 | 30 | 9 | 8.33 ± 0.03 | 14.93 ± 3.11 | 0.07 ± 0.01 | 1.55 |
| RG3.3 | 1 | 30 | 9 | 8.08 ± 0.57 | 13.36 ± 3.65 | 0.08 ± 0.03 | 1.55 |
| CG1 | 2 | 150 | 9 | 8.33 ± 0.47 | 16.9 ± 6.45 | 0.07 ± 0.02 | 1.65 |
| CG2 | 2 | 150 | 9 | 8.50 ± 0.27 | 10.63 ± 3.06 | 0.05 ± 0.01 | 1.46 |
| CG3 | 2 | 150 | 9 | 8.24 ± 0.30 | 12.03 ± 3.44 | 0.04 ± 0.04 | 1.49 |
| UG1 | 2 | - | - | 8.53 ± 0.13 | 12.08 ± 0.67 | 0.07 ± 0.01 | 1.39 |
| UG2 | 2 | - | - | 8.56 ± 0.11 | 10.01 ± 1.60 | 0.05 ± 0.01 | 1.56 |
| UG3 | 2 | - | - | 8.57 ± 0.13 | 16.20 ± 5.04 | 0.08 ± 0.02 | 1.46 |
| Treatment | Season | Re | CH4 Flux | ||||
|---|---|---|---|---|---|---|---|
| Mean a (mg C m−2 h−1) | CV | Cumulative (t C ha−1) | Mean a (μg C m−2 h−1) | CV | Cumulative (kg C ha−1) | ||
| CG | GS | 182.0 ± 98.1 | 0.54 | 8.10 | −31.7 ± 8.8 | 0.28 | −1.40 |
| FP | 4.6 ± 2.7 | 0.59 | 0.15 | −11.3 ± 7.6 | 0.67 | −0.29 | |
| FTP | 50.7 ± 37.3 | 0.74 | 0.81 | −20.36 ± 3.6 | 0.18 | −0.33 | |
| Annual | 119.9 ± 108.7 | 0.90 | 8.97 | −25.4 ± 11.1 | 0.44 | −2.02 | |
| UG | GS | 295.9 ± 170.6 | 0.58 | 12.96 | −34.6 ± 10.3 | 0.30 | −1.50 |
| FP | 8.1 ± 4.6 | 0.57 | 0.28 | −13.8 ± 7.3 | 0.53 | −0.39 | |
| FTP | 59.4 ± 54.0 | 0.91 | 1.08 | −21.9 ± 6.5 | 0.30 | −0.36 | |
| Annual | 189.8 ± 185.6 | 0.98 | 14.32 | −27.9 ± 12.3 | 0.44 | −2.25 | |
| RG | GS | 205.4 ± 114.0 | 0.56 | 9.00 | −29.5 ± 8.5 | 0.29 | −1.28 |
| FP | 5.0 ± 2.9 | 0.57 | 0.17 | −13.9 ± 4.3 | 0.31 | −0.43 | |
| FTP | 51.6 ± 36.6 | 0.91 | 0.83 | −21.3 ± 6.2 | 0.29 | −0.34 | |
| Annual | 134.0 ± 124.8 | 0.93 | 10.0 | −25.1 ± 9.5 | 0.38 | −2.05 | |
| Site | Season | Regression Equation | F | Sig. | R2 |
|---|---|---|---|---|---|
| CG | GS | y = −129.41 + 10.937x1 + 7.604x2 | 20.847 | <0.001 | 0.654 |
| FP | y = 16.861 − 0.363x2 | 51.601 | <0.001 | 0.910 | |
| FTP | y = 6.307 + 6.507x1 | 55.066 | <0.001 | 0.871 | |
| Annual | y = −78.973 + 10.635x1 + 4.377x2 | 55.665 | <0.001 | 0.766 | |
| UG | GS | y = −132.329 + 18.836x1 + 7.238x2 | 19.358 | <0.001 | 0.671 |
| FP | y = 20.954 − 2.231x3 | 11.806 | <0.05 | 0.730 | |
| FTP | y = −121.547 + 9.7x1 + 22.571x3 + 0.791x5 | 914.285 | <0.001 | 0.997 | |
| Annual | y = −111.769 + 18.622x1 + 5.723x2 | 61.013 | <0.001 | 0.795 | |
| RG | GS | y = −22.394 + 12.249x1 | 13.749 | <0.001 | 0.478 |
| FP | - | - | - | - | |
| FTP | y = 5.09 + 7.798x1 | 49.791 | <0.001 | 0.859 | |
| Annual | y = −79.807 + 12.077x1 + 4.061x2 | 34.881 | <0.001 | 0.708 |
| Site | Season | SM (WFPS, %) | ST (5 cm, °C) | |
|---|---|---|---|---|
| CG | GS (n = 26) | R2 | 0.201 * 89.561 + 6.497x | 0.485 ** 41.649e0.070x |
| f(x) | ||||
| FP (n = 8) | R2 | 0.915 ** 16.644 − 0.355x | 0.219 6.371 + 0.386x | |
| f(x) | ||||
| FTP (n = 10) | R2 | 0.546 * 115.598 − 9.128x + 0.19703x2 | 0.871 ** 3.4 + 6.712x | |
| f(x) | ||||
| Annual (n = 44) | R2 | 0.172 ** 56.275 + 11.706x − 0.333x2 | 0.840 ** 11.197e0.137x | |
| f(x) | ||||
| UG | GS (n = 26) | R2 | 0.093 172.168 + 8.242x | 0.602 ** 56.944e0.085x |
| f(x) | ||||
| FP (n = 8) | R2 | 0.564 ** 25.714 − 0.502x | 0.548 * 12.536 + 0.905x | |
| f(x) | ||||
| FTP (n = 10) | R2 | 0.432 * 208.147 − 16.069x + 0.336x2 | 0.83 ** 7.271 + 10.052x | |
| f(x) | ||||
| Annual (n = 44) | R2 | 0.135 * 113.743 + 15.923x − 0.476x2 | 0.882 ** 21.889e0.136x | |
| f(x) | ||||
| RG | GS (n = 26) | R2 | 0.187 * 102.403 + 7.022x | 0.506 ** 41.811e0.076x |
| f(x) | ||||
| FP (n = 8) | R2 | 0.576 * 19.125 − 0.412x | 0.861 ** 8.7856 + 0.817x | |
| f(x) | ||||
| FTP (n = 10) | R2 | 0.271 134.223 − 9.4x + 0.195x2 | 0.869 ** 7.526 + 7.539x | |
| f(x) | ||||
| Annual (n = 44) | R2 | 0.163 ** 49.172 + 14.775x − 0.419x2 | 0.850 ** 11.359e0.144x | |
| f(x) | ||||
| Site | Season | Regression Equation | F | Sig. | R2 |
|---|---|---|---|---|---|
| CG | GS | y = −17.092 − 0.766x1 | 9.051 | <0.01 | 0.277 |
| FP | y = −20.102 − 1.809x1 | 11.174 | <0.05 | 0.670 | |
| FTP | - | - | - | - | |
| Annual | y = −15.874 − 0.799x1 | 50.524 | <0.001 | 0.579 | |
| UG | GS | - | - | - | - |
| FP | - | - | - | - | |
| FTP | y = −5.427 − 0.805x1 − 0.213x4 | 22.313 | <0.01 | 0.859 | |
| Annual | y = −35.084 + 3.189x3 − 0.367x1 | 13.658 | <0.001 | 0.485 | |
| RG | GS | y = −13.105 − 0.987x1 | 16.377 | <0.001 | 0.490 |
| FP | - | - | - | - | |
| FTP | y = −29.6 + 0.567x2 | 7.814 | <0.05 | 0.460 | |
| Annual | y = −16.149 − 0.825x1 | 41.391 | <0.001 | 0.591 |
| Site | Season | SM (0–6 cm, WFPS %) | ST (5 cm, °C) | |
|---|---|---|---|---|
| CG | GS (n = 26) | R2 | 0.117 −21.475 − 1.563x + 0.055x2 | 0.268 ** −18.015 − 0.739x |
| f(x) | ||||
| FP (n = 8) | R2 | 0.635 * −40.182 + 0.855x | 0.557 * −17.965 − 1.476x | |
| f(x) | ||||
| FTP (n = 10) | R2 | 0.381 * −29.798 + 0.193x | −0.125 - | |
| f(x) | ||||
| Annual (n = 44) | R2 | 0.382 ** −25.066 − 0.785x + 0.032x2 | 0.639 ** −15.61 − 0.836x | |
| f(x) | ||||
| UG | GS (n = 26) | R2 | 0.193 * −20.791 − 2.805x + 0.102x2 | 0.014 −27.683 − 0.399x |
| f(x) | ||||
| FP (n = 8) | R2 | 0.258 −35.149 + 0.61x | 0.21 −18.905 − 1.047x | |
| f(x) | ||||
| FTP (n = 10) | R2 | −0.123 - | 0.255 −16.673 − 0.786x | |
| f(x) | ||||
| Annual (n = 44) | R2 | 0.374 ** −28.099 − 0.829x + 0.033x2 | 0.452 ** −19.671 − 0.804x | |
| f(x) | ||||
| RG | GS (n = 26) | R2 | −0.086 - | 0.257 ** −16.383 − 0.709x |
| f(x) | ||||
| FP (n = 8) | R2 | −0.057 | −0.157 | |
| f(x) | - | - | ||
| FTP (n = 10) | R2 | 0.697 ** −30.677 + 0.671x | 0.588 ** −16.464 − 1.051x | |
| f(x) | ||||
| Annual (n = 44) | R2 | 0.169 ** −28.545 − 0.08x + 0.012x2 | 0.558 ** −16.929 − 0.684x | |
| f(x) | ||||
| Site | Ecosystem Type | Re (mg C m−2 h−1) | Period | Source |
|---|---|---|---|---|
| Inner Mongolia (43°26′ N, 116°40′ E, 1189 m) | Temperate steppe | 47.7 | 2004 | [28] |
| 20.5 | 2005 | |||
| Inner Mongolia (42°27′ N, 116°41′ E, 1350 m) | Temperate steppe | 223.3 ± 9.9 | 2006.6–9 | [31] |
| 138.7 ± 6.9 | 2007.6–9 | |||
| Inner Mongolia (40°34′ N, 111°34′ E, 1055 m) | Sown pasture | 147.9 ± 21.3 | 2012.7–2013.7 | This study |
| 227.8 ± 34.7 | GS |
| Site | Ecosystem Type | SM (0–6 cm, v/v %) | ST (5 cm, °C) | CH4 Flux (μg C m−2 h−1) | Period | Source |
|---|---|---|---|---|---|---|
| Inner Mongolia (43°33′ N, 116°40′ E, 1268 m) | Temperate steppe | 13.5 ± 0.5 a | - | −38.7 | 2007.8–2008.8 | [42] |
| Inner Mongolia (43°38′ N, 116°42′ E) | Temperate steppe | 14.0 | 12.5 | −43.8 ± 2.5 a | 2007.10–2008.10 | [11] |
| Inner Mongolia (43°33′ N, 116°40′ E, 1250 m) | Temperate steppe | 17.2 | - | −66.6 ± 4.2 a | 2008.7–9 | [40] |
| Inner Mongolia (42°02′ N, 116°17′ E) | Temperate steppe | 8.2 | 16.9 | −60.7 | 2009.5–9 | [43] |
| 12.5 | 16.5 | −78.7 | 2010.5–9 | |||
| Inner Mongolia (43°11′–43°27′ N, 116°22′-117°00′ E) | Temperate steppe | 13.7 ± 0.8 a | 18.5 | −98.6 ± 10.4 a | 2010.5–10 | [44] |
| Inner Mongolia (40°34′ N, 111°34′ E, 1055 m) | Sown pasture | 18.1 ± 0.6 a | 11.1 ± 0.4 a | −26.1 ± 0.9 a | 2012.7–2013.7 | This study |
| 14.6 ± 0.2 a | 18.0 ± 0.8 a | −31.9 ± 1.5 a | GS |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, B.; Yue, H.; Zheng, X.; McDowell, W.H.; Zhao, Q.; Zhou, Z.; Yao, Z. Effects of Grazing Pattern on Ecosystem Respiration and Methane Flux in a Sown Pasture in Inner Mongolia, China. Atmosphere 2019, 10, 5. https://doi.org/10.3390/atmos10010005
Mei B, Yue H, Zheng X, McDowell WH, Zhao Q, Zhou Z, Yao Z. Effects of Grazing Pattern on Ecosystem Respiration and Methane Flux in a Sown Pasture in Inner Mongolia, China. Atmosphere. 2019; 10(1):5. https://doi.org/10.3390/atmos10010005
Chicago/Turabian StyleMei, Baoling, Hongyu Yue, Xunhua Zheng, William H. McDowell, Qingshan Zhao, Zaixing Zhou, and Zhisheng Yao. 2019. "Effects of Grazing Pattern on Ecosystem Respiration and Methane Flux in a Sown Pasture in Inner Mongolia, China" Atmosphere 10, no. 1: 5. https://doi.org/10.3390/atmos10010005
APA StyleMei, B., Yue, H., Zheng, X., McDowell, W. H., Zhao, Q., Zhou, Z., & Yao, Z. (2019). Effects of Grazing Pattern on Ecosystem Respiration and Methane Flux in a Sown Pasture in Inner Mongolia, China. Atmosphere, 10(1), 5. https://doi.org/10.3390/atmos10010005







