Dietary Alteration of the Gut Microbiome and Its Impact on Weight and Fat Mass: A Systematic Review and Meta-Analysis
Abstract
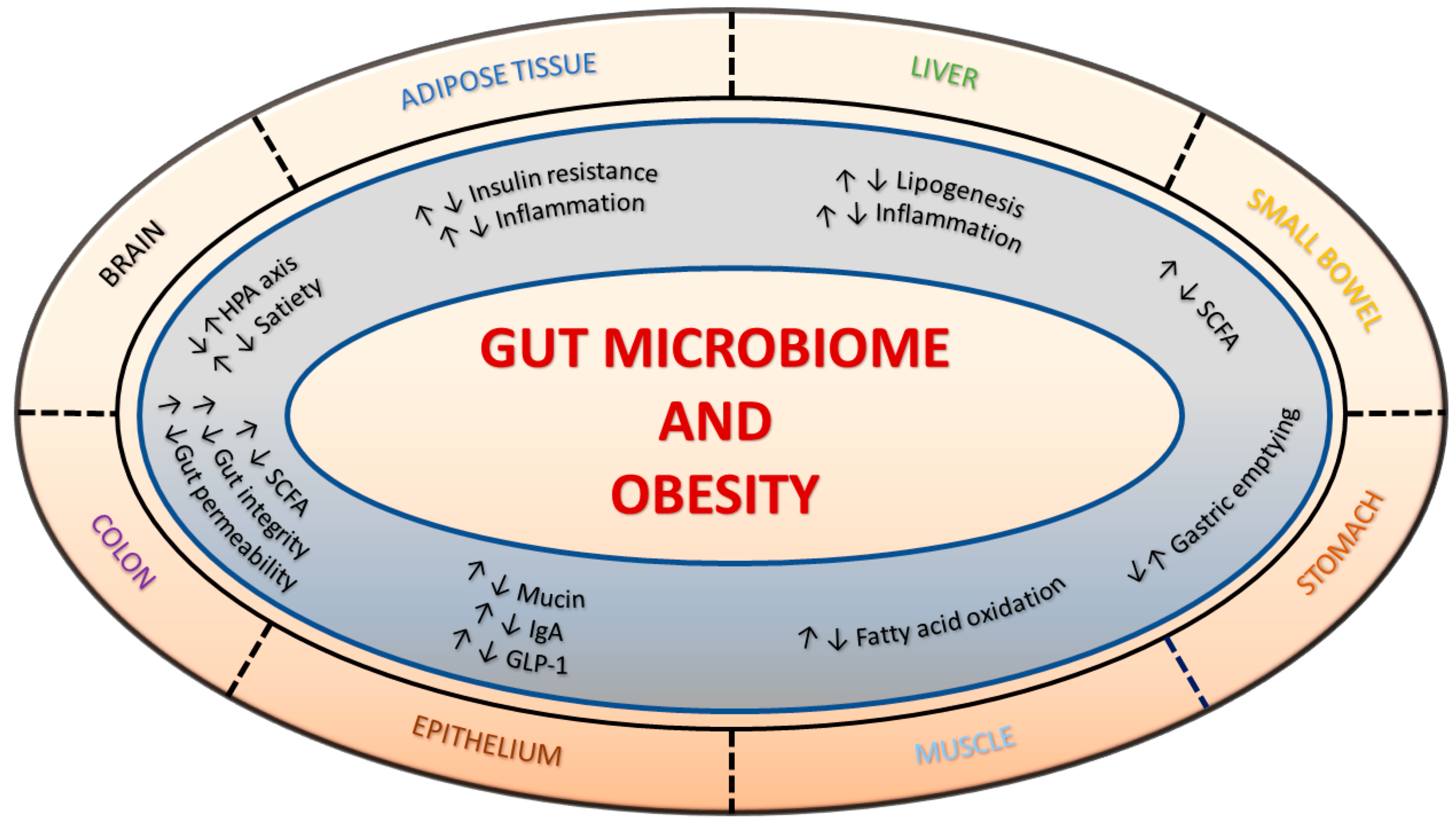
1. Introduction
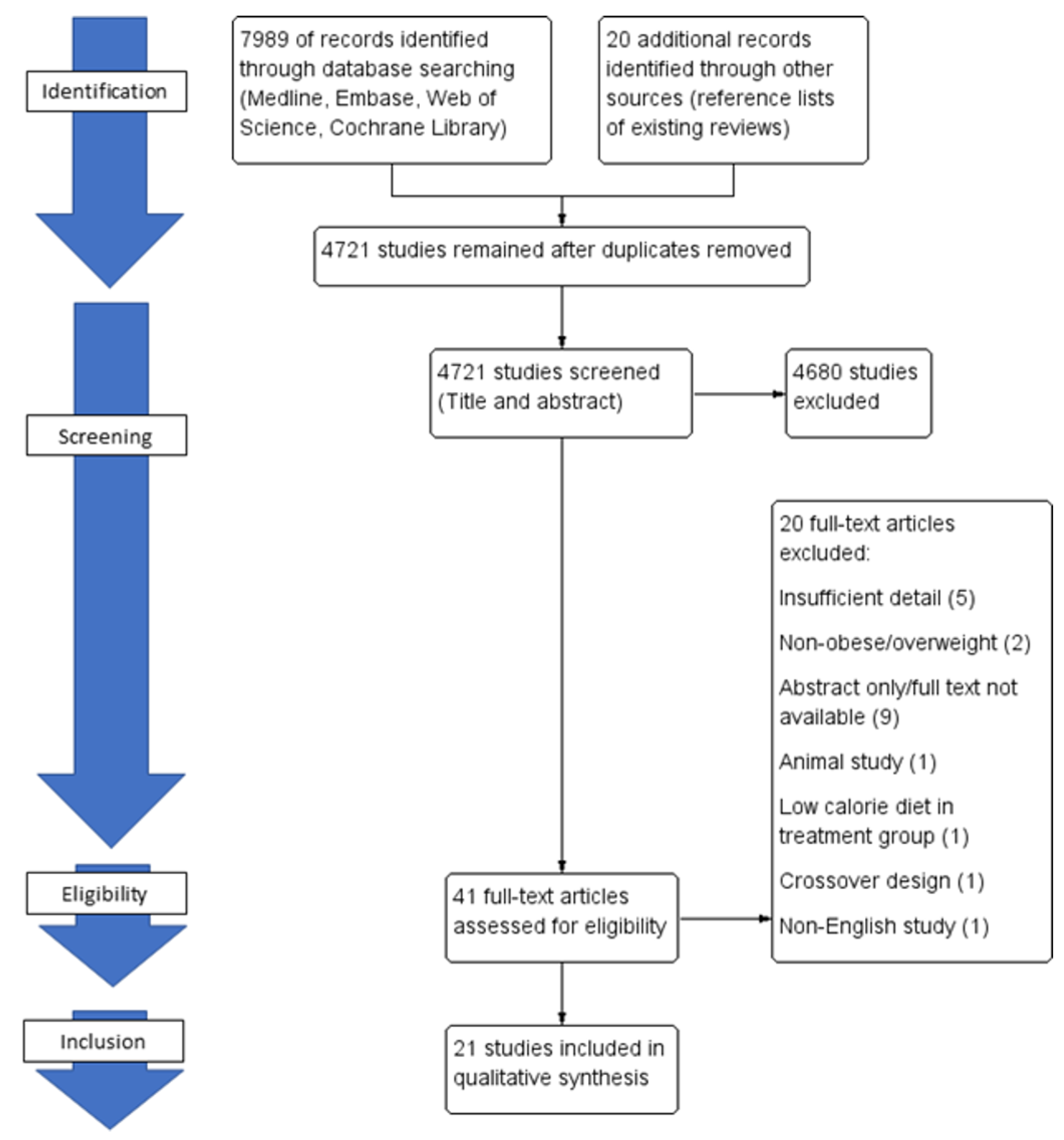
2. Methods
2.1. Protocol and Registration
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Data Extraction
2.5. Risk of Bias Assessment
2.6. Data Synthesis and Statistical Analysis
3. Results
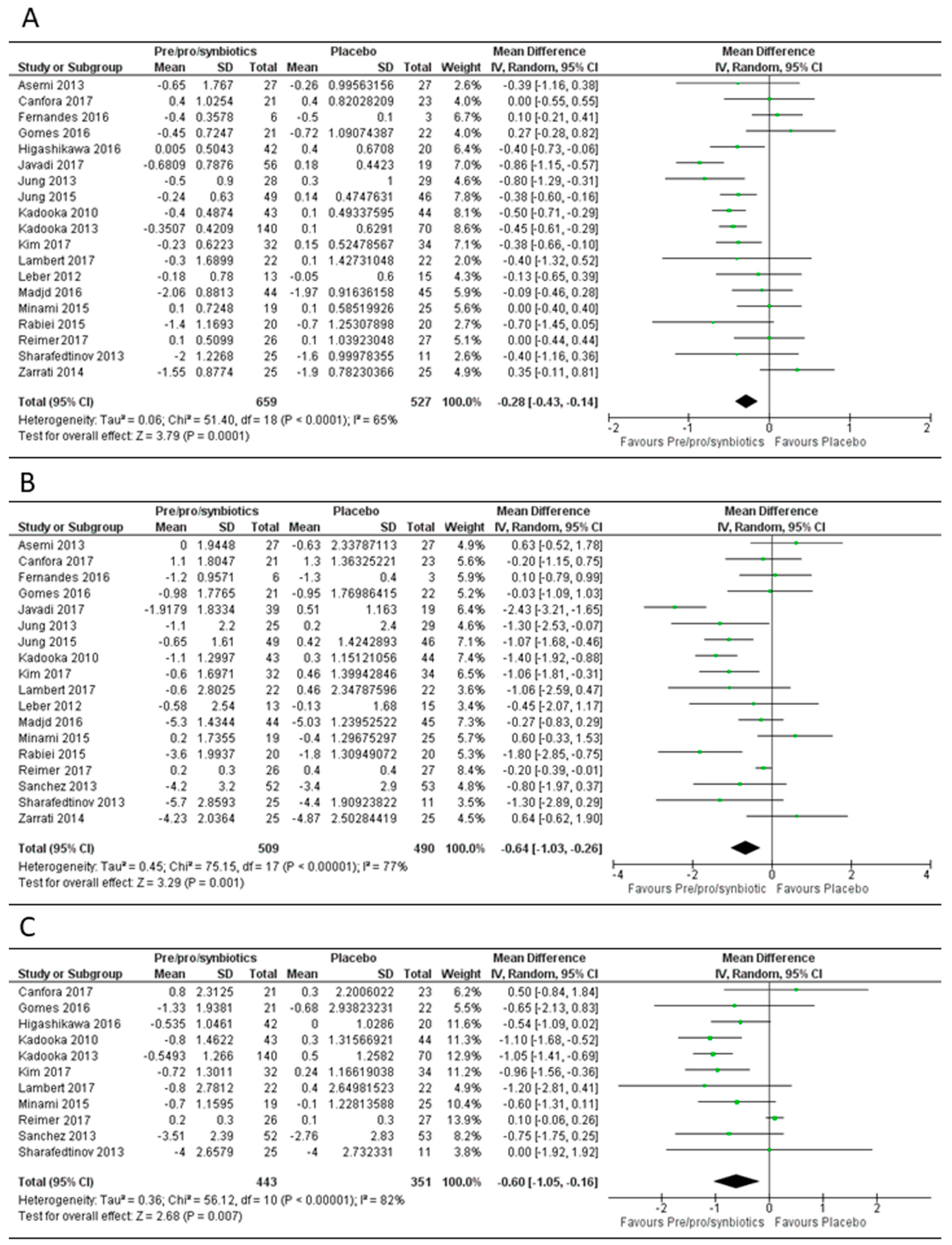
3.1. Overall Effect of Pre-, Pro- or Syn-Biotics on Body Mass Index, Body Weight and Fat Mass
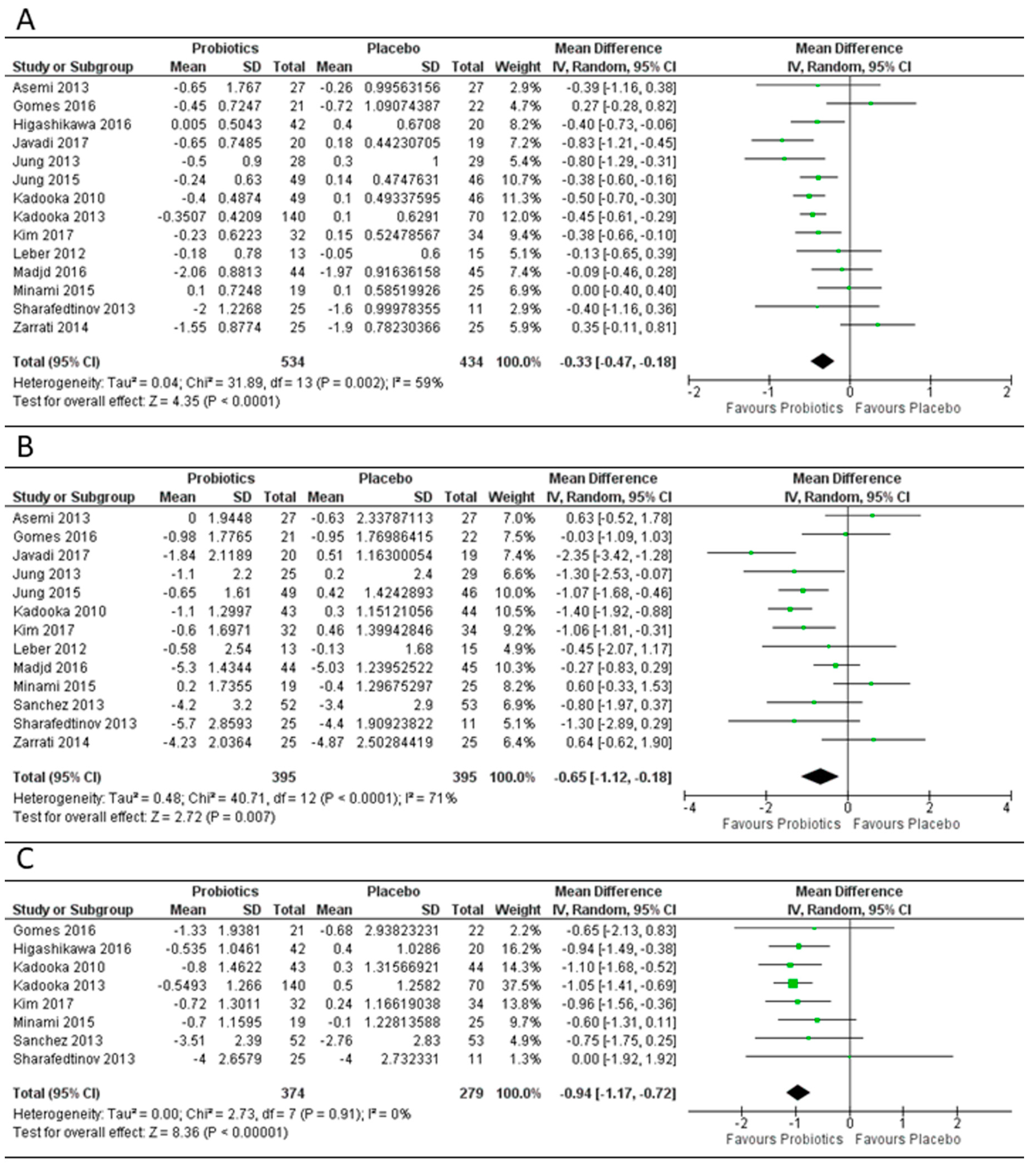
3.2. Effect of Probiotics on Body Mass Index, Weight and Fat Mass
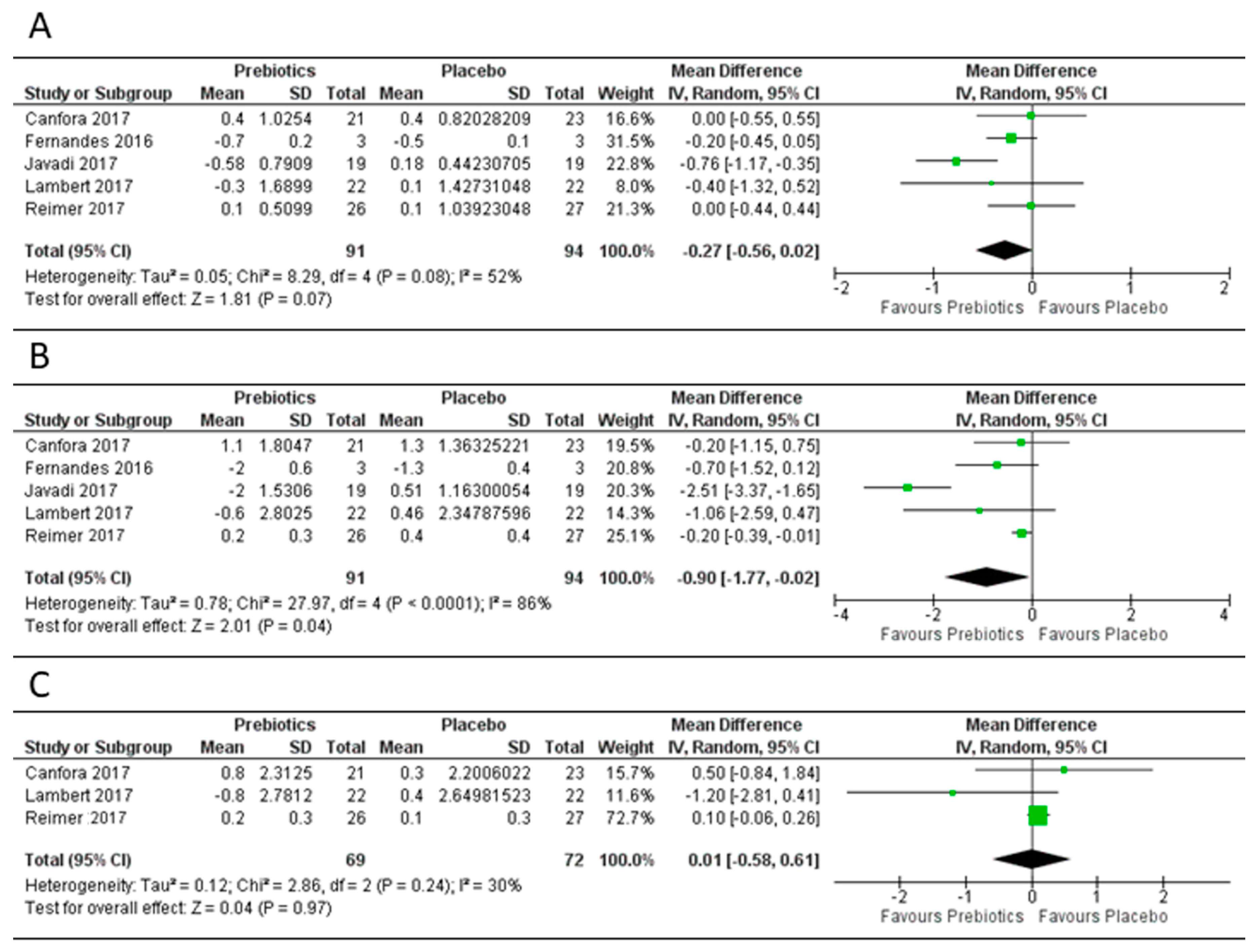
3.3. Effect of Prebiotics on Body Mass Index, Body Weight and Fat Mass
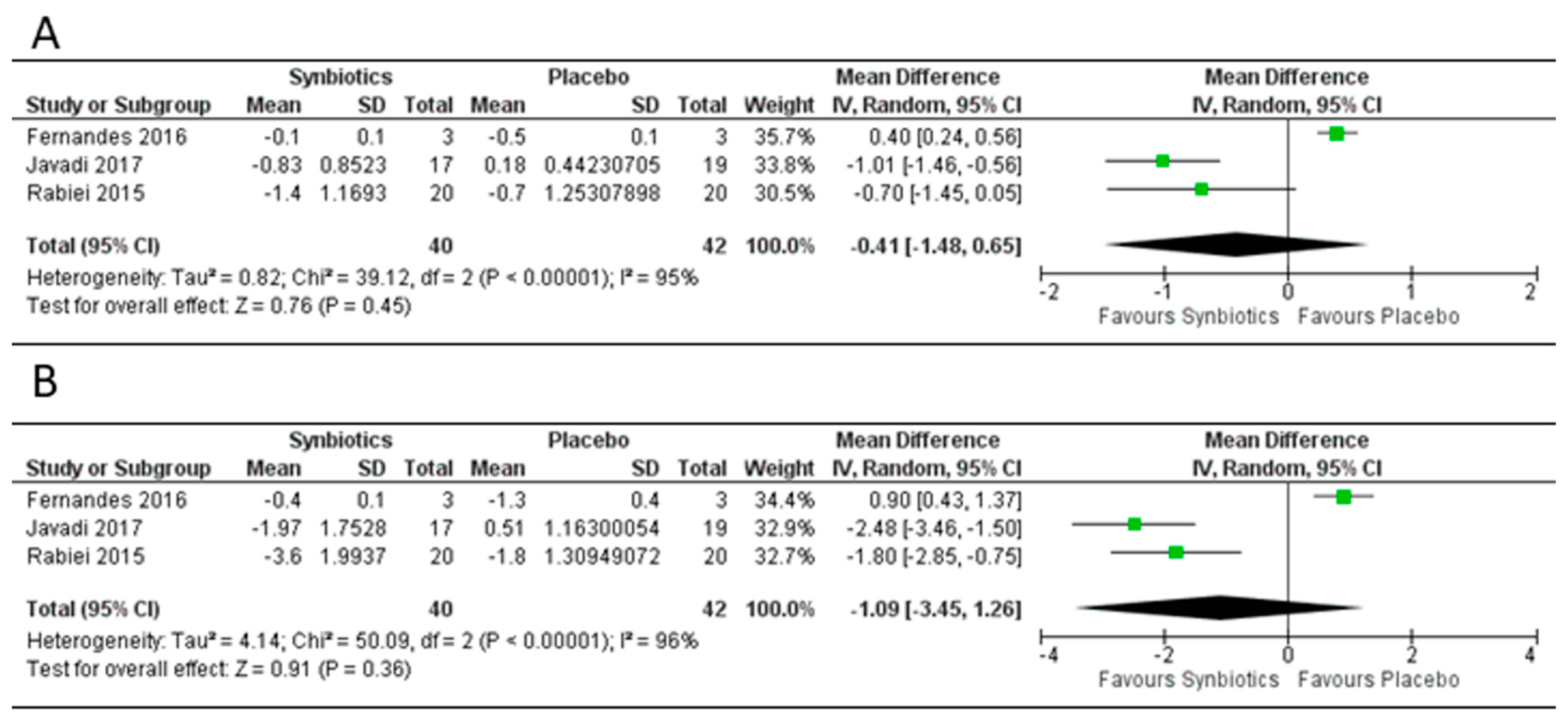
3.4. Effect of Synbiotics on Body Mass Index, Weight and Fat Mass
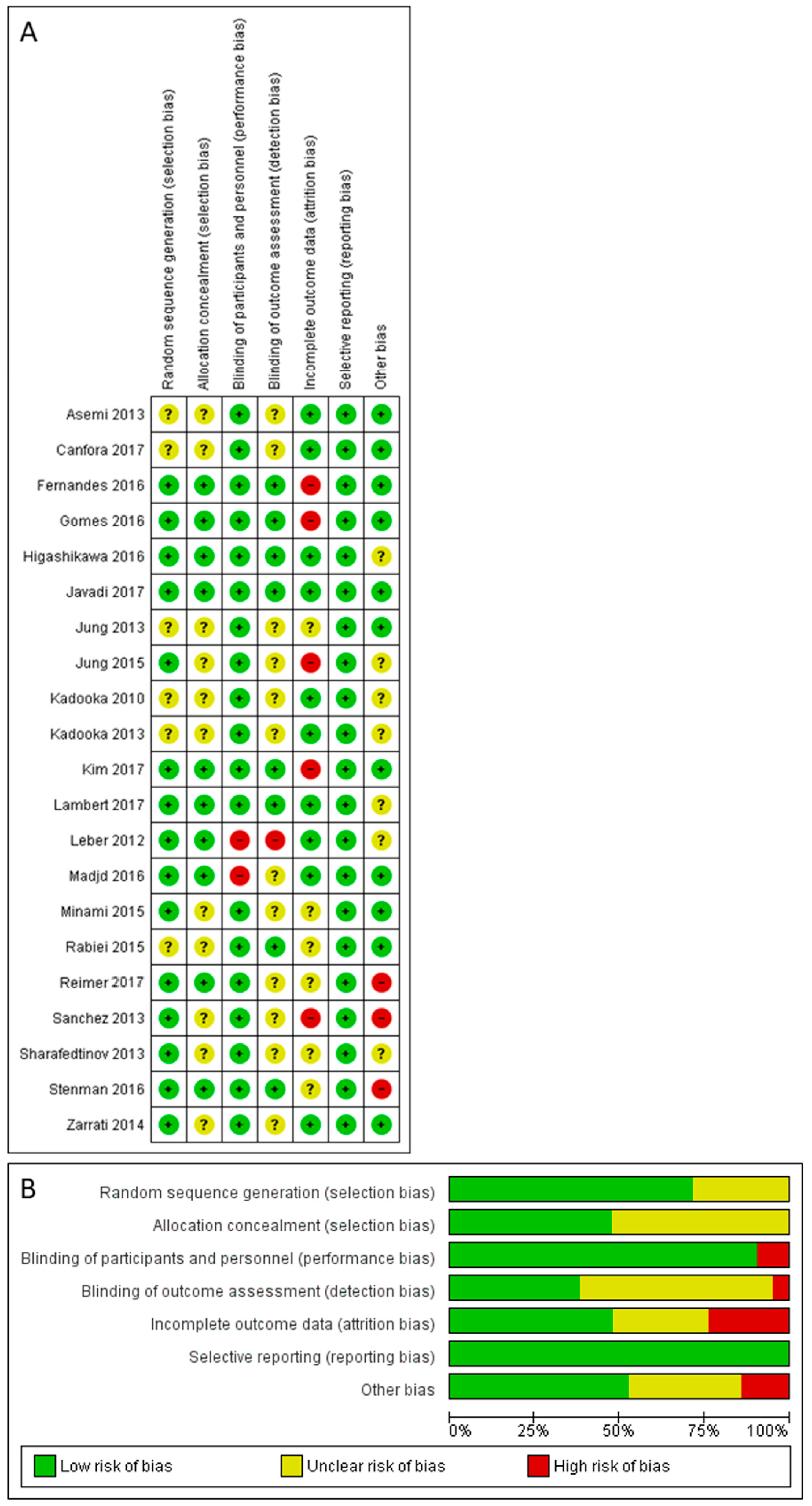
3.5. Risk of Bias Assessment
3.6. Publication Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- BMI Classification. Available online: http://apps.who.int/bmi/index.jsp?introPage=intro_3.htm (accessed on 9 September 2017).
- Kivimaki, M.; Kuosma, E.; Ferrie, J.E.; Luukkonen, R.; Nyberg, S.T.; Alfredsson, L.; Batty, G.D.; Brunner, E.J.; Fransson, E.; Goldberg, M.; et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: Pooled analysis of individual-level data for 120,813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health 2017, 2, e277–e285. [Google Scholar] [CrossRef]
- McMillan, D.C.; Sattar, N.; Lean, M.; McArdle, C.S. Obesity and cancer. BMJ 2006, 333, 1109–1111. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.J.; Armstrong, J.; Dorosty, A.R.; Emmett, P.M.; Ness, A.; Rogers, I.; Steer, C.; Sherriff, A. Early life risk factors for obesity in childhood: Cohort study. BMJ 2005, 330, 1357. [Google Scholar] [CrossRef] [PubMed]
- Baquero, F.; Nombela, C. The microbiome as a human organ. Clin. Microbiol. Infect. 2012, 18, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Okeke, F.; Roland, B.C.; Mullin, G.E. The role of the gut microbiome in the pathogenesis and treatment of obesity. Glob. Adv. Health Med. 2014, 3, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Raben, A.; Astrup, A.; Holst, J.J. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J. Clin. Investig. 1998, 101, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Mandard, S.; Zandbergen, F.; van Straten, E.; Wahli, W.; Kuipers, F.; Muller, M.; Kersten, S. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J. Biol. Chem. 2006, 281, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Planer, J.D.; Peng, Y.; Kau, A.L.; Blanton, L.V.; Ndao, I.M.; Tarr, P.I.; Warner, B.B.; Gordon, J.I. Development of the gut microbiota and mucosal IgA responses in twins and gnotobiotic mice. Nature 2016, 534, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Nieuwdorp, M.; Gilijamse, P.W.; Pai, N.; Kaplan, L.M. Role of the microbiome in energy regulation and metabolism. Gastroenterology 2014, 146, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- John, G.K.; Mullin, G.E. The gut microbiome and obesity. Curr. Oncol. Rep. 2016, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E. Obesity and the human microbiome. Curr. Opin. Gastroenterol. 2010, 26, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Bae, J.H. Probiotics for weight loss: A systematic review and meta-analysis. Nutr. Res. 2015, 35, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.K.; Renuka; Puniya, M.; Shandilya, U.K.; Dhewa, T.; Kumar, N.; Kumar, S.; Puniya, A.K.; Shukla, P. Gut microbiota modulation and its relationship with obesity using prebiotic fibers and probiotics: A review. Front. Microbiol. 2017, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, Y.; Fei, X. Effect of probiotics on body weight and body-mass index: A systematic review and meta-analysis of randomized, controlled trials. Int. J. Food Sci. Nutr. 2015, 67, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Dror, T.; Dickstein, Y.; Dubourg, G.; Paul, M. Microbiota manipulation for weight change. Microb. Pathog. 2017, 106, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Brahe, L.K.; Astrup, A.; Larsen, L.H. Can we prevent obesity-related metabolic diseases by dietary modulation of the gut microbiota? Adv. Nutr. 2016, 7, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Pineiro, M.; Asp, N.-G.; Reid, G.; Macfarlane, S.; Morelli, L.; Brunser, O.; Tuohy, K. FAO Technical Meeting on Prebiotics. J. Clin. Gastroenterol. 2008, 42, S156–S159. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.R.; Capurso, L.; Collins, K.; Cummings, J.; Delzenne, N.; Goulet, O.; Guarner, F.; Marteau, P.; Meier, R. Current level of consensus on probiotic science-Report of an expert meeting—London, 23 November 2009. Gut Microbes 2010, 1, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Angelakis, E.; Paul, M.; Armougom, F.; Leibovici, L.; Raoult, D. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb. Pathog. 2012, 53, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Kellow, N.J.; Coughlan, M.T.; Reid, C.M. Metabolic benefits of dietary prebiotics in human subjects: A systematic review of randomised controlled trials. Br. J. Nutr. 2014, 111, 1147–1161. [Google Scholar] [CrossRef] [PubMed]
- Mullin, G.; John, G.; Singh, R.; Nanavati, J.; Alammar, N. Dietary alteration of the gut microbiome and its impact on weight: A systematic review and meta-analysis. PROSPERO 2017 CRD42017075883. Available online: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017075883 (accessed on 5 December 2017).
- Medline (PubMed). National Library of Medicine. Available online: https://www.ncbi.nlm.nih.gov/pubmed/ (accessed on 15 March 2018).
- Elsevier. Embase. Available online: https://www.elsevier.com/solutions/embase-biomedical-research (accessed on 15 March 2018).
- Clarivate Analytics. Web of Science. Available online: http://www.webofknowledge.com (accessed on 15 March 2018).
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: West Sussex, UK, 2011. [Google Scholar]
- Fu, R.; Vandermeer, B.W.; Shamliyan, T.A.; O’Neil, M.E.; Yazdi, F.; Fox, S.H.; Morton, S.C. AHRQ methods for effective health carehandling continuous outcomes in quantitative synthesis. In Methods Guide for Effectiveness and Comparative Effectiveness Reviews; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2008. [Google Scholar]
- Fernandes, R.; Beserra, B.T.; Mocellin, M.C.; Kuntz, M.G.; da Rosa, J.S.; de Miranda, R.C.; Schreiber, C.S.; Frode, T.S.; Nunes, E.A.; Trindade, E.B. Effects of prebiotic and synbiotic supplementation on inflammatory markers and anthropometric indices after Roux-en-Y gastric bypass: A randomized, triple-blind, placebo-controlled pilot study. J. Clin. Gastroenterol. 2016, 50, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Zare, Z.; Shakeri, H.; Sabihi, S.; Esmaillzadeh, A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with Type 2 diabetes. Ann. Nutr. Metab. 2013, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; van der Beek, C.M.; Hermes, G.D.A.; Goossens, G.H.; Jocken, J.W.E.; Holst, J.J.; van Eijk, H.M.; Venema, K.; Smidt, H.; Zoetendal, E.G.; et al. Supplementation of diet with galacto-oligosaccharides increases bifidobacteria, but not insulin sensitivity, in obese prediabetic individuals. J. Pharm. Pharmacol. 2017, 153, 87–97.e3. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; de Sousa, R.G.; Botelho, P.B.; Gomes, T.L.; Prada, P.O.; Mota, J.F. The additional effects of a probiotic mix on abdominal adiposity and antioxidant status: A double-blind, randomized trial. Obesity 2017, 25, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Higashikawa, F.; Noda, M.; Awaya, T.; Danshiitsoodol, N.; Matoba, Y.; Kumagai, T.; Sugiyama, M. Antiobesity effect of Pediococcus pentosaceus LP28 on overweight subjects: A randomized, double-blind, placebo-controlled clinical trial. Eur. J. Clin. Nutr. 2016, 70, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Javadi, L.; Ghavami, M.; Khoshbaten, M.; Safaiyan, A.; Barzegari, A.; Gargari, B.P. The potential role of probiotics or/and prebiotic on serum lipid profile and insulin resistance in alcoholic fatty liver disease: A double blind randomized clinical trial. Crescent J. Med. Biol. Sci. 2017, 4, 131–138. [Google Scholar]
- Jung, S.; Lee, Y.J.; Kim, M.; Kim, M.; Kwak, J.H.; Lee, J.W.; Ahn, Y.T.; Sim, J.H.; Lee, J.H. Supplementation with two probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032, reduced body adiposity and Lp-PLA(2) activity in overweight subjects. J. Funct. Foods 2015, 19, 744–752. [Google Scholar] [CrossRef]
- Jung, S.P.; Lee, K.M.; Kang, J.H.; Yun, S.I.; Park, H.O.; Moon, Y.; Kim, J.Y. Effect of Lactobacillus gasseri BNR17 on overweight and obese adults: A randomized, double-blind clinical trial. Korean J. Fam. Med. 2013, 34, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Kadooka, Y.; Sato, M.; Imaizumi, K.; Ogawa, A.; Ikuyama, K.; Akai, Y.; Okano, M.; Kagoshima, M.; Tsuchida, T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010, 64, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Kadooka, Y.; Sato, M.; Ogawa, A.; Miyoshi, M.; Uenishi, H.; Ogawa, H.; Ikuyama, K.; Kagoshima, M.; Tsuchida, T. Effect of Lactobacillus gasseri SBT2055 in fermented milk on abdominal adiposity in adults in a randomised controlled trial. Br. J. Nutr. 2013, 110, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, M.; Kang, M.; Yoo, H.J.; Kim, M.S.; Ahn, Y.T.; Sim, J.H.; Jee, S.H.; Lee, J.H. Effects of weight loss using supplementation with Lactobacillus strains on body fat and medium-chain acylcarnitines in overweight individuals. Food Funct. 2017, 8, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.E.; Parnell, J.A.; Tunnicliffe, J.M.; Han, J.; Sturzenegger, T.; Reimer, R.A. Consuming yellow pea fiber reduces voluntary energy intake and body fat in overweight/obese adults in a 12-week randomized controlled trial. Clin. Nutr. 2017, 36, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Leber, B.; Tripolt, N.J.; Blattl, D.; Eder, M.; Wascher, T.C.; Pieber, T.R.; Stauber, R.; Sourij, H.; Oettl, K.; Stadlbauer, V. The influence of probiotic supplementation on gut permeability in patients with metabolic syndrome: An open label, randomized pilot study. Eur. J. Clin. Nutr. 2012, 66, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Madjd, A.; Taylor, M.A.; Mousavi, N.; Delavari, A.; Malekzadeh, R.; Macdonald, I.A.; Farshchi, H.R. Comparison of the effect of daily consumption of probiotic compared with low-fat conventional yogurt on weight loss in healthy obese women following an energy-restricted diet: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Minami, J.; Kondo, S.; Yanagisawa, N.; Odamaki, T.; Xiao, J.Z.; Abe, F.; Nakajima, S.; Hamamoto, Y.; Saitoh, S.; Shimoda, T. Oral administration of Bifidobacterium breve B-3 modifies metabolic functions in adults with obese tendencies in a randomised controlled trial. J. Nutr. Sci. 2015, 4, e17. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, S.; Shakerhosseini, R.; Saadat, N. The effects of symbiotic therapy on anthropometric measures, body composition and blood pressure in patient with metabolic syndrome: A triple blind RCT. Med. J. Islam. Repub. Iran 2015, 29, 213. [Google Scholar] [PubMed]
- Reimer, R.A.; Willis, H.J.; Tunnicliffe, J.M.; Park, H.; Madsen, K.L.; Soto-Vaca, A. Inulin-type fructans and whey protein both modulate appetite but only fructans alter gut microbiota in adults with overweight/obesity: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Darimont, C.; Drapeau, V.; Emady-Azar, S.; Philippe, L.; Ammon-Zuffrey, C.; Doré, J.; Tremblay, A. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Can. J. Diabetes 2013, 37, S269. [Google Scholar] [CrossRef][Green Version]
- Sharafedtinov, K.K.; Plotnikova, O.A.; Alexeeva, R.I.; Sentsova, T.B.; Songisepp, E.; Stsepetova, J.; Smidt, I.; Mikelsaar, M. Hypocaloric diet supplemented with probiotic cheese improves body mass index and blood pressure indices of obese hypertensive patients—A randomized double-blind placebo-controlled pilot study. Nutr. J. 2013, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Stenman, L.K.; Lehtinen, M.J.; Meland, N.; Christensen, J.E.; Yeung, N.; Saarinen, M.T.; Courtney, M.; Burcelin, R.; Lahdeaho, M.L.; Linros, J.; et al. Probiotic with or without fiber controls body fat mass, associated with serum zonulin, in overweight and obese adults-randomized controlled trial. PLoS ONE 2016, 13, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Zarrati, M.; Salehi, E.; Nourijelyani, K.; Mofid, V.; Zadeh, M.J.; Najafi, F.; Ghaflati, Z.; Bidad, K.; Chamari, M.; Karimi, M.; et al. Effects of probiotic yogurt on fat distribution and gene expression of proinflammatory factors in peripheral blood mononuclear cells in overweight and obese people with or without weight-loss diet. J. Am. Coll. Nutr. 2014, 33, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.E.; Versalovic, J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 2009, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.; Cornish, A.; Soper, P.; McKaigney, C.; Jijon, H.; Yachimec, C.; Doyle, J.; Jewell, L.; De Simone, C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001, 121, 580–591. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Shi, B. Gut microbiota as a potential target of metabolic syndrome: The role of probiotics and prebiotics. Cell Biosci. 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Begley, M.; Hill, C.; Gahan, C.G. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 2006, 72, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, L.E.M.; Koetsier, M.A.; van Deventer, S.J.H.; van Tol, E.A.F. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut 2003, 52, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.W.; de Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Backhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Raoult, D. The role of the manipulation of the gut microbiota in obesity. Curr. Infect. Dis. Rep. 2013, 15, 25–30. [Google Scholar] [CrossRef] [PubMed]
| Medline (PubMed) |
| Search hits 1892 (“Probiotics”[Mesh] OR probiotic*[tw] OR “Prebiotics”[Mesh] OR prebiotic*[tw] OR “Synbiotics”[Mesh] OR synbiotic*[tw] OR “Lactobacillus”[Mesh] OR lactobacillus[tw] OR “Yeast, Dried”[Mesh] OR “dried yeast”[tw]) AND (“Body Weight Changes”[Mesh] OR weight change*[tw] OR weight gain*[tw] OR weight loss*[tw] OR weight regulation*[tw] OR “weight modification”[tw] OR “Obesity”[Mesh] or obes*[tw] OR “Overweight”[Mesh] OR overweight[tw]) |
| Embase |
| Search hits 3126 (‘probiotic agent’/exp OR probiotic*:ti,ab OR ‘prebiotic agent’/exp OR prebiotic*:ti,ab OR ‘synbiotic agent’/exp OR synbiotic*:ti,ab OR ‘Lactobacillus’/exp OR lactobacillus:ti,ab OR ‘dried yeast’/exp OR ‘dried yeast’:ti,ab) AND (‘weight change’/exp OR ‘weight change*’:ti,ab OR ‘weight gain*’:ti,ab OR ‘weight loss*’:ti,ab OR ‘weight regulation*’:ti,ab OR ‘weight modification’:ti,ab OR ‘obesity’/exp or obes*:ti,ab OR overweight:ti,ab) |
| Web of Science |
| Search hits 2694 #1. TS = (probiotic* OR prebiotic* OR synbiotic* OR lactobacillus OR “dried yeast”) #2. TS = (“weight change*” OR “weight gain*” OR “weight loss*” OR “weight regulation*” OR “weight modification” OR obes* OR overweight) #3. #1 AND #2 |
| Cochrane Library |
| Search hits 277 #1 probiotic* or prebiotic* or synbiotic* or lactobacillus or “dried yeast”:ti,ab,kw #2 “weight change*” or “weight gain*” or “weight loss*” or “weight regulation*” or “weight modification” or obes* or overweight:ti,ab,kw #3 MeSH descriptor: [Probiotics] explode all trees #4 MeSH descriptor: [Prebiotics] explode all trees #5 MeSH descriptor: [Synbiotics] explode all trees #6 MeSH descriptor: [Lactobacillus] explode all trees #7 MeSH descriptor: [Yeast, Dried] explode all trees #8 #1 or #3 or #4 or #5 or #6 or #7 #9 MeSH descriptor: [Body Weight Changes] explode all trees #10 MeSH descriptor: [Obesity] explode all trees #11 MeSH descriptor: [Overweight] explode all trees #12 #2 or #9 or #10 or #11 #13 #8 and #12 |
| Author | Country | Year | Randomized | Blinding | Placebo Control | Overweight or Obese | Population | Probiotic/Prebiotic/Synbiotic | Single or Multi-Strain Probiotic/Prebiotic Agent Used | Daily Dose (in Billions, 109) CFU/Dose of Prebiotic in Grams | Duration (Weeks) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Asemi [39] | Iran | 2013 | Y | Y | Y | Both | T2DM | Probiotic | Multi | 39.2 × 109 | 8 |
| Canfora [40] | Netherlands | 2017 | Y | Y | Y | Both | Prediabetic | Prebiotic | GOS | 15 g | 12 |
| Fernandes [38] | Brazil | 2016 | Y | Y | Y | Obese | RYGB | Prebiotic | FOS | 6 g | 2 # |
| Fernandes [38] | Brazil | 2016 | Y | Y | Y | Obese | RYGB | Synbiotic | Multi + FOS | 4 × 109 + 6 g | 2 # |
| Gomes [41] | Brazil | 2016 | Y | Y | Y | Both | Healthy women | Probiotic | Multi | 20.0 × 109 | 8 |
| Higashikawa [42] | Japan | 2016 | Y | Y | Y | Overweight | Healthy | Probiotic | Single (Pediococcus pentosaceus LP28, heat killed) | 100 × 109 | 12 |
| Higashikawa [42] | Japan | 2016 | Y | Y | Y | Overweight | Healthy | Probiotic | Single (P. pentosaceus LP28, living) | 100 × 109 | 12 |
| Javadi [43] | Iran | 2017 | Y | Y | Y | Both | NAFLD | Probiotic | Multi | 0.02 × 109 | 12 |
| Javadi [43] | Iran | 2017 | Y | Y | Y | Both | NAFLD | Prebiotic | Inulin | 10 g | 12 |
| Javadi [43] | Iran | 2017 | Y | Y | Y | Both | NAFLD | Synbiotic | Multi + inulin | 0.02 × 109 + 10 g | 12 |
| Jung [44] | Korea | 2015 | Y | Y | Y | Overweight | Healthy | Probiotic | Multi | 10 × 109 | 12 |
| Jung [45] | Korea | 2013 | Y | Y | Y | Both | Healthy | Probiotic | Single (Lactobacillus gasseri BNR17) | 60 × 109 | 12 |
| Kadooka [46] | Japan | 2010 | Y | Y | Y | Overweight | Healthy | Probiotic | Single (L. gasseri SBT2055) | 50 × 109 | 12 |
| Kadooka [46] | Japan | 2010 | Y | Y | Y | Overweight | Healthy | Probiotic | Single (L. gasseri SBT2055) | 50 × 109 | 8 |
| Kadooka [46] | Japan | 2010 | Y | Y | Y | Overweight | Healthy | Probiotic | Single (L. gasseri SBT2055) | 50 × 109 | 4 |
| Kadooka [47] | Japan | 2013 | Y | Y | Y | Overweight | Healthy | Probiotic | Single * (L.gasseri SBT2055) | 0.08 × 109 | 12 |
| Kadooka [47] | Japan | 2013 | Y | Y | Y | Overweight | Healthy | Probiotic | Single * (L.gasseri SBT2055) | 0.007 × 109 | 12 |
| Kim [48] | Korea | 2017 | Y | Y | Y | Overweight | Healthy | Probiotic | Multi | 5 × 109 | 12 |
| Lambert [49] | Canada | 2017 | Y | Y | Y | Both | Healthy | Prebiotic | Yellow pea fiber | 5 g | 12 |
| Leber [50] | Austria | 2012 | Y | N | N | Both | Metabolic syndrome | Probiotic | Single (L. casei Shirota) | 19.5 × 109 | 12 |
| Madjd [51] | Iran | 2016 | Y | Y | Y | Both | Healthy women | Probiotic | Multi | 0.01 × 109 | 12 |
| Minami [52] | Japan | 2015 | Y | Y | Y | Overweight | Healthy | Probiotic | Single (Bifidobacterium breve B-3) | 50 × 109 | 12 |
| Minami [52] | Japan | 2015 | Y | Y | Y | Overweight | Healthy | Probiotic | Single (B. breve B-3) | 50 × 109 | 8 |
| Minami [52] | Japan | 2015 | Y | Y | Y | Overweight | Healthy | Probiotic | Single (B. breve B-3) | 50 × 109 | 4 |
| Rabiei [53] | Iran | 2015 | Y | Y | Y | Both | Metabolic syndrome | Synbiotic | Multi + FOS | 0.2 × 109 + NA | 12 |
| Rabiei [53] | Iran | 2015 | Y | Y | Y | Both | Metabolic syndrome | Synbiotic | Multi + FOS | 0.2 × 109+NA | 6 |
| Reimer [54] | Canada | 2017 | Y | Y | Y | Both | Healthy | Prebiotic | Oligofructose + Inulin | 6 g + 2 g | 12 |
| Sanchez [55] | Canada | 2013 | Y | Y | Y | Both | Healthy | Probiotic | Single (L. rhamnosus CGMCC1.3724) | 0.32 × 109 | 12 |
| Sharafedtinov [56] | Estonia | 2013 | Y | Y | Y | Obese | Metabolic syndrome | Probiotic | Single (L. plantarum TENSIA) | 150 × 109 | 3 |
| Stenman [57] | Finland | 2016 | Y | Y | Y | Both | Healthy | Probiotic | Single (B. animalis ssp. lactis 420) | 10 × 109 | 24 |
| Stenman [57] | Finland | 2016 | Y | Y | Y | Both | Healthy | Prebiotic | Polydextrose | 12 g | 24 |
| Stenman [57] | Finland | 2016 | Y | Y | Y | Both | Healthy | Synbiotic | Single + polydextrose | 10 × 109 + 12 g | 24 |
| Zarrati [58] | Iran | 2014 | Y | Y | Y | Both | Healthy | Probiotic | Multi | 0.03 × 109 | 8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
John, G.K.; Wang, L.; Nanavati, J.; Twose, C.; Singh, R.; Mullin, G. Dietary Alteration of the Gut Microbiome and Its Impact on Weight and Fat Mass: A Systematic Review and Meta-Analysis. Genes 2018, 9, 167. https://doi.org/10.3390/genes9030167
John GK, Wang L, Nanavati J, Twose C, Singh R, Mullin G. Dietary Alteration of the Gut Microbiome and Its Impact on Weight and Fat Mass: A Systematic Review and Meta-Analysis. Genes. 2018; 9(3):167. https://doi.org/10.3390/genes9030167
Chicago/Turabian StyleJohn, George Kunnackal, Lin Wang, Julie Nanavati, Claire Twose, Rajdeep Singh, and Gerard Mullin. 2018. "Dietary Alteration of the Gut Microbiome and Its Impact on Weight and Fat Mass: A Systematic Review and Meta-Analysis" Genes 9, no. 3: 167. https://doi.org/10.3390/genes9030167
APA StyleJohn, G. K., Wang, L., Nanavati, J., Twose, C., Singh, R., & Mullin, G. (2018). Dietary Alteration of the Gut Microbiome and Its Impact on Weight and Fat Mass: A Systematic Review and Meta-Analysis. Genes, 9(3), 167. https://doi.org/10.3390/genes9030167





