Fitness Costs of the Glutathione S-Transferase Epsilon 2 (L119F-GSTe2) Mediated Metabolic Resistance to Insecticides in the Major African Malaria Vector Anopheles Funestus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Sample Collection
2.2. Life Traits Experiments
2.2.1. Fecundity and Fertility
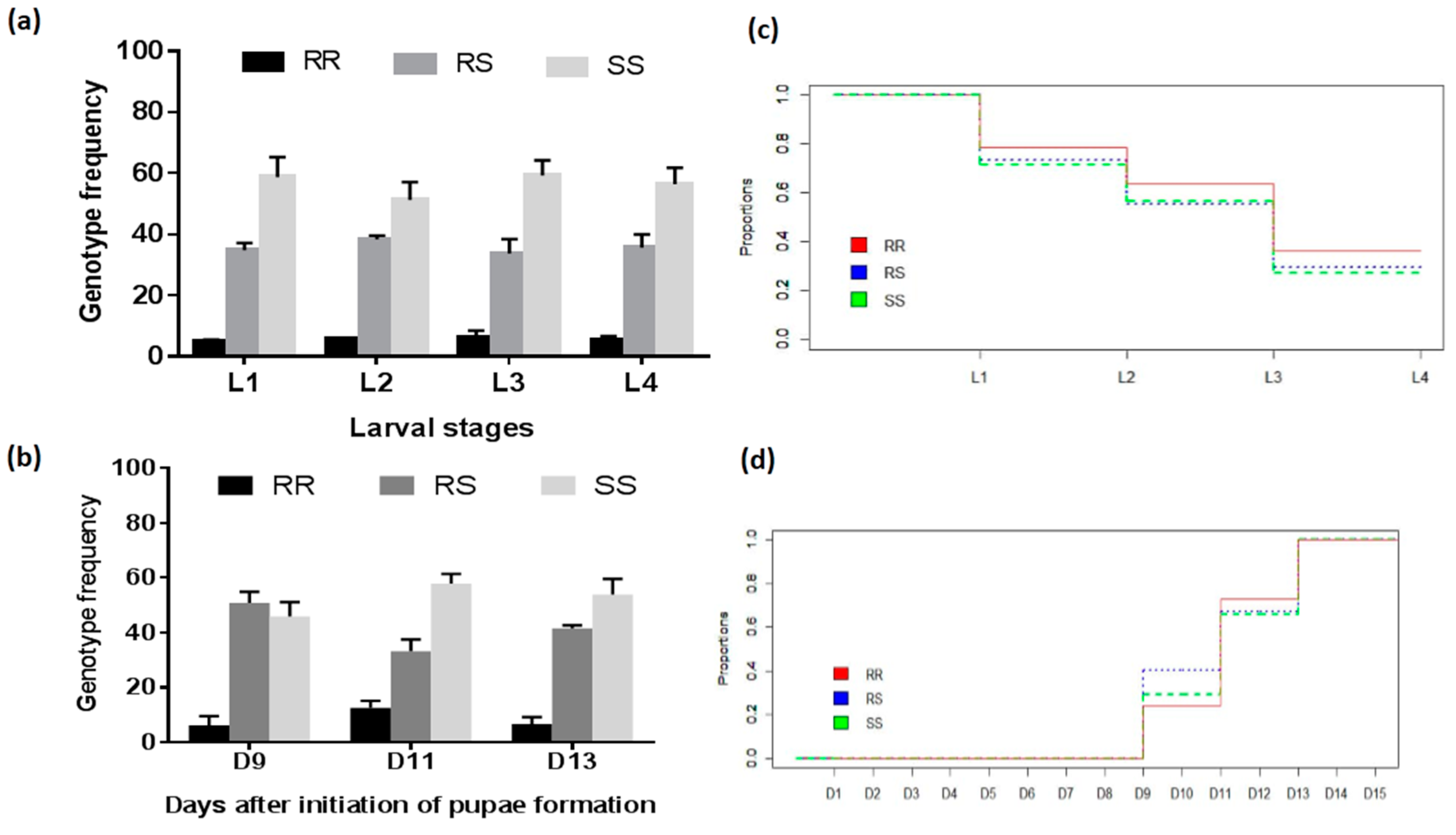
2.2.2. Development of Larvae and Pupae
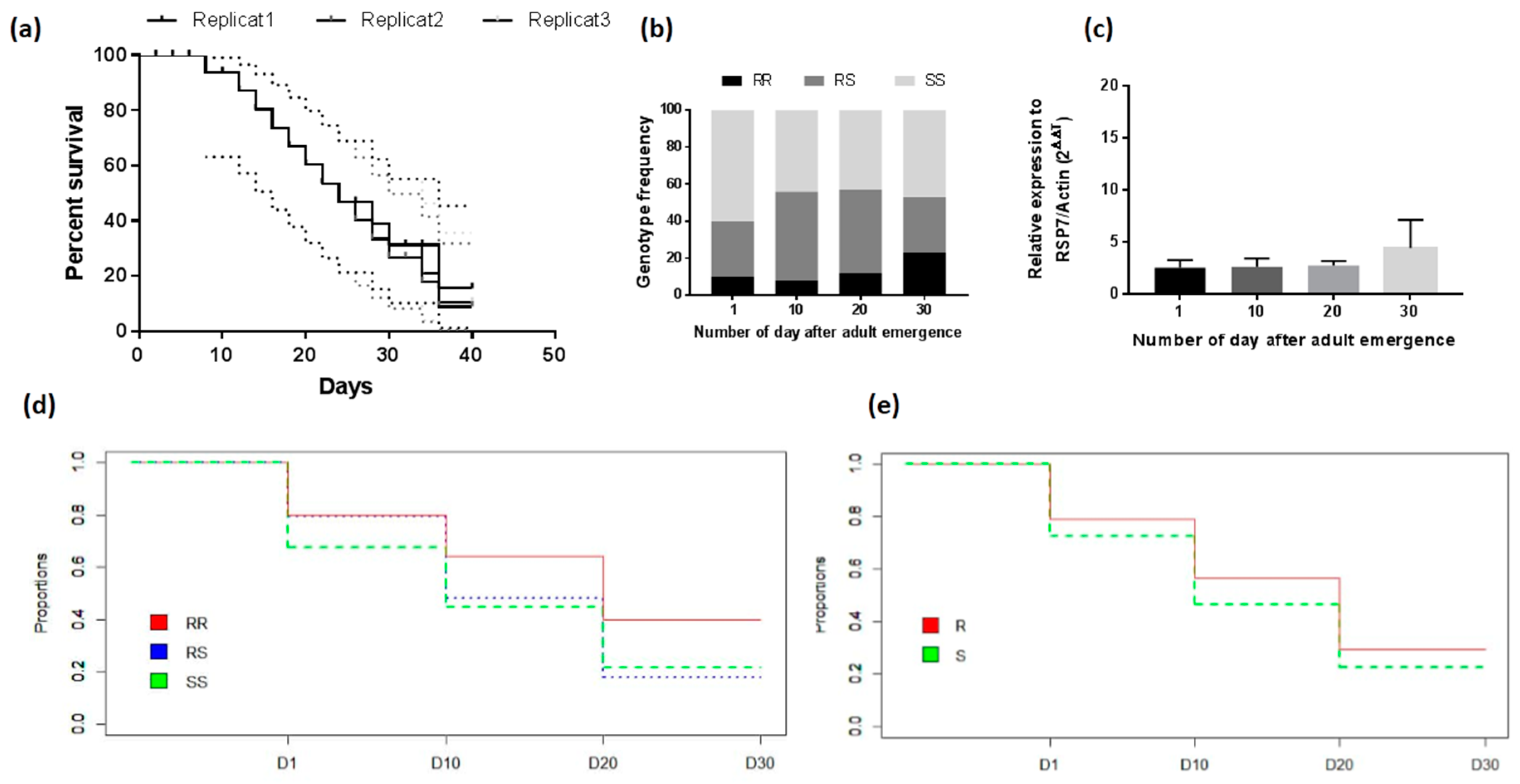
2.2.3. Longevity of the Adult Mosquitoes
2.3. DNA Extraction and Species Identification
2.4. Detection of Plasmodium Parasite in F0 Field-Collected Mosquitoes
2.5. Genotyping of L119F-GSTe2 Mutation
2.6. Gene Expression Profile of GSTe2 and Longevity Adult of Adult Mosquitoes Using Quantitative Reverse Transcription Polymerase Chain Reaction
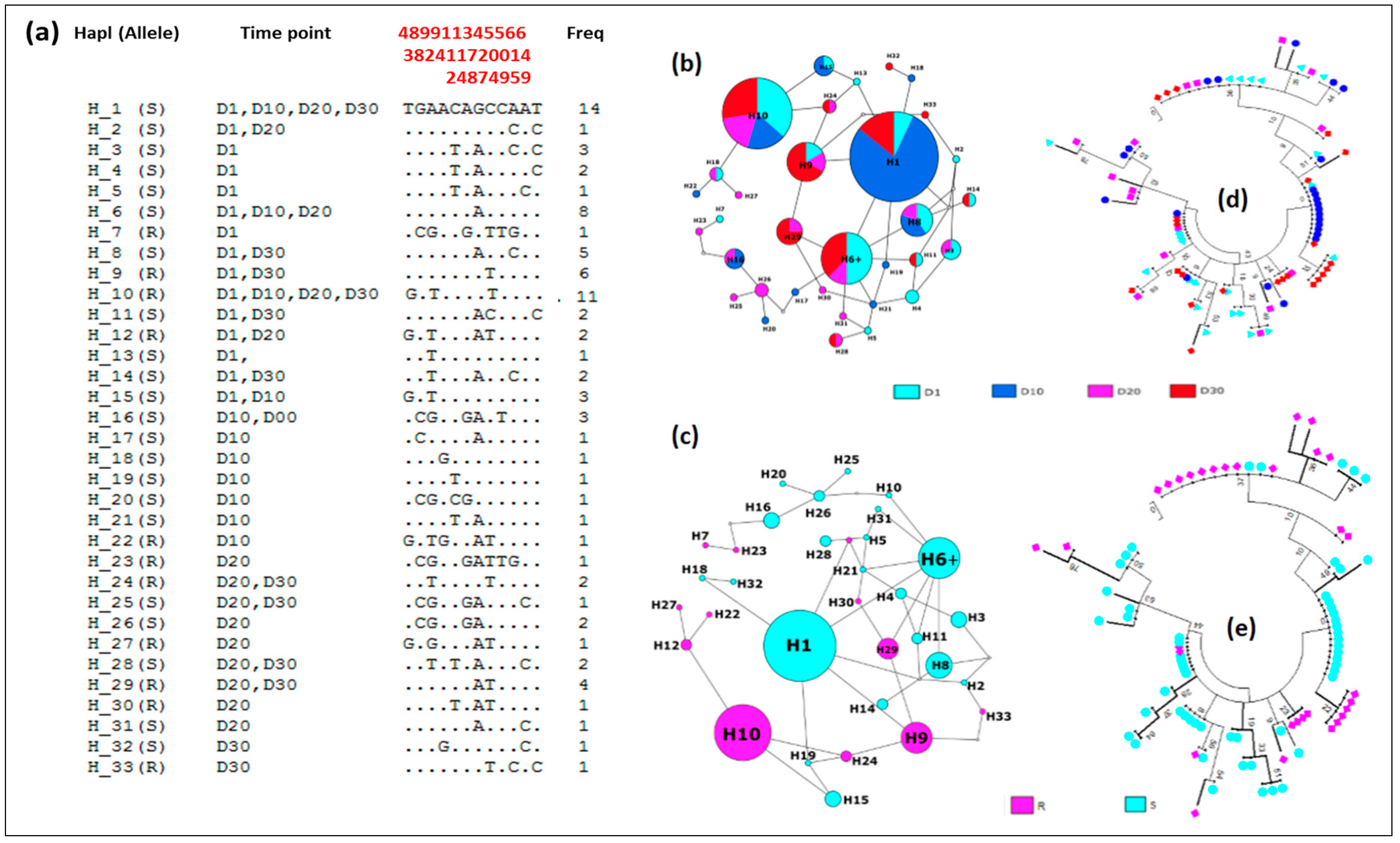
2.7. Genetic Diversity of GSTe2 Gene and Adult Longevity
2.8. Data Analysis
3. Results
3.1. Field Collection and Species Identification
3.2. Infection of An. funestus by Plasmodium Parasite
3.3. Genotyping of the L119F-GSTe2 in Field-Collected Mosquitoes
3.4. Assessment of the Association between the L119F-GSTe2 Mutation and the Life Traits of An. funestus
3.4.1. Association between L119F-GSTe2 and Fecundity/Fertility of Female Mosquitoes
3.4.2. Assessment of the Association between the L119F-GSTe2 Mutation and Larval Development
3.4.3. Assessment of the Association between L119F-GSTe2 Mutation and Adult Longevity
3.4.4. Association between GSTe2 Polymorphism and Longevity
Genetic Diversity of the GSTe2
3.4.5. Distribution of Haplotypes and Phylogeny
4. Discussion
4.1. Association between L119F Resistance Marker and Adult Longevity
4.2. Association between L119F Resistance Marker and Larvae/Pupae Formation
4.3. Association between L119F Resistance Marker and Fecundity/Fertility
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Plan for Insecticide Resistance Management in Malaria Vectors (GPIRM); World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.E.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207. [Google Scholar] [CrossRef] [PubMed]
- Zaim, M.; Aitio, A.; Nakashima, N. Safety of pyrethroid-treated mosquito nets. Med. Vet. Entomol. 2000, 14, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berticat, C.; Boquien, G.; Raymond, M.; Chevillon, C. Insecticide resistance genes induce a mating competition cost in Culex pipiens mosquitoes. Genet. Res. 2002, 79, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Platt, N.; Kwiatkowska, R.M.; Irving, H.; Diabate, A.; Dabire, R.; Wondji, C.S. Target-site resistance mutations (kdr and RDL), but not metabolic resistance, negatively impact male mating competiveness in the malaria vector Anopheles gambiae. Heredity 2015, 115, 243–252. [Google Scholar] [CrossRef]
- Rowland, M. Behaviour and fitness of γHCH/dieldrin resistant and susceptible female Anopheles gambiae and An. stephensi mosquitoes in the absence of insecticide. Med. Vet. Entomol. 1991, 5, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Rowland, M. Activity and mating competitiveness of γ HCH/dieldrin resistant and susceptible male and virgin female Anopheles gambiae and An. stephensi mosquitoes, with assessment of an insecticide-rotation strategy. Med. Vet. Entomol. 1991, 5, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.P.; Linss, J.G.; Lima-Camara, T.N.; Belinato, T.A.; Peixoto, A.A.; Lima, J.B.; Valle, D.; Martins, A.J. Assessing the effects of Aedes aegypti kdr mutations on pyrethroid resistance and its fitness cost. PLoS ONE 2013, 8, e60878. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.J.; Ribeiro, C.D.; Bellinato, D.F.; Peixoto, A.A.; Valle, D.; Lima, J.B. Effect of insecticide resistance on development, longevity and reproduction of field or laboratory selected Aedes aegypti populations. PLoS ONE 2012, 7, e31889. [Google Scholar] [CrossRef]
- Riveron, J.M.; Tchouakui, M.; Mugenzi, L.; Menze, B.D.; Chiang, M.-C.; Wondji, C.S. Insecticide resistance in malaria vectors: An update at a global scale. In Towards Malaria Elimination—A Leap Forward; IntechOpen: London, UK, 2018. [Google Scholar]
- Hemingway, J. The molecular basis of two contrasting metabolic mechanisms of insecticide resistance. Insect. Biochem. Mol. Biol. 2000, 30, 1009–1015. [Google Scholar] [CrossRef]
- Martinez-Torres, D.; Chandre, F.; Williamson, M.; Darriet, F.; Berge, J.B.; Devonshire, A.L.; Guillet, P.; Pasteur, N.; Pauron, D. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect. Mol. Biol. 1998, 7, 179–184. [Google Scholar] [CrossRef]
- Riveron, J.M.; Yunta, C.; Ibrahim, S.S.; Djouaka, R.; Irving, H.; Menze, B.D.; Ismail, H.M.; Hemingway, J.; Ranson, H.; Albert, A.; et al. A single mutation in the GSTe2 gene allows tracking of metabolically based insecticide resistance in a major malaria vector. Genome Biol. 2014, 15, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Gillies, M.; De Meillon, B. The Anophelinae of Africa south of the Sahara (Ethiopian zoogeographical region). Inst. Med. Res. 1968, 54, 343. [Google Scholar]
- Coetzee, M.; Koekemoer, L.L. Molecular systematics and insecticide resistance in the major African malaria vector Anopheles funestus. Annu. Rev. Entomol. 2013, 58, 393–412. [Google Scholar] [CrossRef] [PubMed]
- Dia, I.; Guelbeogo, M.W.; Ayala, D. Advances and perspectives in the study of the malaria mosquito Anopheles funestus. In Anopheles Mosquitoes—New Insights into Malaria Vectors; Manguin, S., Ed.; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Hargreaves, K.; Koekemoer, L.L.; Brooke, B.D.; Hunt, R.H.; Mthembu, J.; Coetzee, M. Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med. Vet. Entomol. 2000, 14, 181–189. [Google Scholar] [CrossRef]
- Brooke, B.D.; Kloke, G.; Hunt, R.H.; Koekemoer, L.L.; Temu, E.A.; Taylor, M.E.; Small, G.; Hemingway, J.; Coetzee, M. Bioassay and biochemical analyses of insecticide resistance in southern African Anopheles funestus (Diptera: Culicidae). Bull. Entomol. Res. 2001, 91, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Casimiro, S.L.; Hemingway, J.; Sharp, B.L.; Coleman, M. Monitoring the operational impact of insecticide usage for malaria control on Anopheles funestus from Mozambique. Malar. J. 2007, 6, 142. [Google Scholar] [CrossRef] [PubMed]
- Cuamba, N.; Morgan, J.C.; Irving, H.; Steven, A.; Wondji, C.S. High level of pyrethroid resistance in an Anopheles funestus population of the Chokwe district in Mozambique. PLoS ONE 2010, 5, e11010. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.H.; Edwardes, M.; Coetzee, M. Pyrethroid resistance in southern African Anopheles funestus extends to Likoma island in Lake Malawi. Parasit. Vectors 2010, 3, 122. [Google Scholar] [CrossRef]
- Wondji, C.S.; Coleman, M.; Kleinschmidt, I.; Mzilahowa, T.; Irving, H.; Ndula, M.; Rehman, A.; Morgan, J.; Barnes, K.G.; Hemingway, J. Impact of pyrethroid resistance on operational malaria control in Malawi. Proc. Natl. Acad. Sci. USA 2012, 109, 19063–19070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, J.C.; Irving, H.; Okedi, L.M.; Steven, A.; Wondji, C.S. Pyrethroid resistance in an Anopheles funestus population from Uganda. PLoS ONE 2010, 5, e11872. [Google Scholar] [CrossRef]
- Mulamba, C.; Riveron, J.M.; Ibrahim, S.S.; Irving, H.; Barnes, K.G.; Mukwaya, L.G.; Birungi, J.; Wondji, C.S. Widespread pyrethroid and DDT resistance in the major malaria vector Anopheles funestus in East Africa is driven by metabolic resistance mechanisms. PLoS ONE 2014, 9, e110058. [Google Scholar] [CrossRef] [PubMed]
- Lwetoijera, D.W.; Harris, C.; Kiware, S.S.; Dongus, S.; Devine, G.J.; McCall, P.J.; Majambere, S. Increasing role of Anopheles funestus and Anopheles arabiensis in malaria transmission in the Kilombero Valley, Tanzania. Malar. J. 2014, 13, 331. [Google Scholar] [CrossRef] [PubMed]
- Wondji, C.S.; Dabire, R.K.; Tukur, Z.; Irving, H.; Djouaka, R.; Morgan, J.C. Identification and distribution of a GABA receptor mutation conferring dieldrin resistance in the malaria vector Anopheles funestus in Africa. Insect. Biochem. Mol. Biol. 2011, 41, 484–491. [Google Scholar] [CrossRef]
- Menze, B.D.; Riveron, J.M.; Ibrahim, S.S.; Irving, H.; Antonio-Nkondjio, C.; Awono-Ambene, P.H.; Wondji, C.S. Multiple insecticide resistance in the malaria vector Anopheles funestus from northern Cameroon is mediated by metabolic resistance alongside potential target site insensitivity mutations. PLoS ONE 2016, 11, e0163261. [Google Scholar] [CrossRef]
- Menze, B.D.; Wondji, M.J.; Tchapga, W.; Tchoupo, M.; Riveron, J.M.; Wondji, C.S. Bionomics and insecticides resistance profiling of malaria vectors at a selected site for experimental hut trials in central Cameroon. Malar. J. 2018. [Google Scholar] [CrossRef]
- Djouaka, R.; Irving, H.; Tukur, Z.; Wondji, C.S. Exploring mechanisms of multiple insecticide resistance in a population of the malaria vector Anopheles funestus in Benin. PLoS ONE 2011, 6, e27760. [Google Scholar] [CrossRef] [PubMed]
- Djouaka, R.J.; Atoyebi, S.M.; Tchigossou, G.M.; Riveron, J.M.; Irving, H.; Akoton, R.; Kusimo, M.O.; Bakare, A.A.; Wondji, C.S. Evidence of a multiple insecticide resistance in the malaria vector Anopheles funestus in South West Nigeria. Malar. J. 2016, 15, 565. [Google Scholar] [CrossRef] [PubMed]
- Okoye, P.N.; Brooke, B.D.; Koekemoer, L.L.; Hunt, R.H.; Coetzee, M. Characterisation of DDT, pyrethroid and carbamate resistance in Anopheles funestus from Obuasi, Ghana. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 591–598. [Google Scholar] [CrossRef]
- Riveron, J.M.; Osae, M.; Egyir-Yawson, A.; Irving, H.; Ibrahim, S.S.; Wondji, C.S. Multiple insecticide resistance in the major malaria vector Anopheles funestus in southern Ghana: Implications for malaria control. Parasit. Vectors 2016, 9, 504. [Google Scholar] [CrossRef] [PubMed]
- Samb, B.; Konate, L.; Irving, H.; Riveron, J.M.; Dia, I.; Faye, O.; Wondji, C.S. Investigating molecular basis of lambda-cyhalothrin resistance in an Anopheles funestus population from Senegal. Parasit. Vectors 2016, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J. Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics 1984, 107, 611–634. [Google Scholar]
- Koekemoer, L.L.; Kamau, L.; Hunt, R.H.; Coetzee, M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am. J. Trop. Med. Hyg. 2002, 66, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Nikou, D.; Blagborough, A.M.; Vontas, J.; Sinden, R.E.; Williamson, M.S.; Field, L.M. PCR-based detection of Plasmodium in Anopheles mosquitoes: A comparison of a new high-throughput assay with existing methods. Malar. J. 2008, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Tchouakui, M.; Ndo, C.; Kuicheu, C.K.; Amvongo-Adjia, N.; Wondji, M.J.; Tchoupo, M.; Kusimo, M.O.; Riveron, J.M.; Wondji, C.S. A glutathione S-transferase mediated metabolic resistance to insecticides is associated with higher Plasmodium infection in the African malaria vector Anopheles funestus. Sci. Rep. 2018. in review. [Google Scholar]
- Kwiatkowska, R.M.; Platt, N.; Poupardin, R.; Irving, H.; Dabire, R.K.; Mitchell, S.; Jones, C.M.; Diabaté, A.; Ranson, H.; Wondji, C.S. Dissecting the mechanisms responsible for the multiple insecticide resistance phenotype in Anopheles gambiae s.s., M form, from Vallée du Kou, Burkina Faso. Gene 2013, 519, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Riveron, J.M.; Irving, H.; Ndula, M.; Barnes, K.G.; Ibrahim, S.S.; Paine, M.J.; Wondji, C.S. Directionally selected cytochrome p450 alleles are driving the spread of pyrethroid resistance in the major malaria vector Anopheles funestus. Proc. Natl. Acad. Sci. USA 2013, 110, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Hall, T. Bioedit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Thompson, J.; Higgins, D.; Gibson, T. CLUSTALW: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- McCarroll, L.; Hemingway, J. Can insecticide resistance status affect parasite transmission in mosquitoes? Insect. Biochem. Mol. Biol. 2002, 32, 1345–1351. [Google Scholar] [CrossRef]
- McElwee, J.J.; Schuster, E.; Blanc, E.; Piper, M.D.; Thomas, J.H.; Patel, D.S.; Selman, C.; Withers, D.J.; Thornton, J.M.; Partridge, L.; et al. Evolutionary conservation of regulated longevity assurance mechanisms. Genome Biol. 2007, 8, R132. [Google Scholar] [CrossRef]
- Vontas, J.G.; Small, G.J.; Hemingway, J. Glutathione S-transferases as antioxidant defence agents confer pyrethroid resistance in Nilaparvata lugens. Biochem. J. 2001, 357, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Mittapalli, O.; Neal, J.J.; Shukle, R.H. Antioxidant defense response in a galling insect. Proc. Natl. Acad. Sci. USA 2007, 104, 1889–1894. [Google Scholar] [CrossRef] [Green Version]
- Djouaka, R.; Akoton, R.; Tchigossou, G.M.; Atoyebi, S.M.; Irving, H.; Kusimo, M.O.; Djegbe, I.; Riveron, J.M.; Tossou, E.; Yessoufou, A.; et al. Mapping the distribution of Anopheles Funestus across Benin highlights a sharp contrast of susceptibility to insecticides and infection rate to Plasmodium between southern and northern populations. Wellcome Open Res. 2016, 1, 28. [Google Scholar] [CrossRef] [PubMed]
- Ndo, C.; Kopya, E.; Donbou, M.A.; Njiokou, F.; Awono-Ambene, P.; Wondji, C. Elevated Plasmodium infection rates and high pyrethroid resistance in major malaria vectors in a forested area of Cameroon highlight challenges of malaria control. Parasit. Vectors 2018, 11, 157. [Google Scholar] [CrossRef]
- Riveron, J.M.; Watsenga, F.; Irving, H.; Irish, S.R.; Wondji, C.S. High plasmodium infection rate and reduced bed net efficacy in multiple insecticide-resistant malaria vectors in Kinshasa, democratic republic of Congo. J. Infect. Dis. 2018, 217, 320–328. [Google Scholar] [CrossRef]
- Protopopoff, N.; Mosha, J.F.; Lukole, E.; Charlwood, J.D.; Wright, A.; Mwalimu, C.D.; Manjurano, A.; Mosha, F.W.; Kisinza, W.; Kleinschmidt, I.; et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: A cluster, randomised controlled, two-by-two factorial design trial. Lancet 2018, 391, 1577–1588. [Google Scholar]
- Charlesworth, B. Evolution in Age-Structured Populations, 2nd ed.; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Agnew, P.; Koella, J. Life history interactions with environmental conditions in a host–parasite relationship and the parasite’s mode of transmission. Evol. Ecol. 1999, 13, 67–91. [Google Scholar] [CrossRef]
- Foster, S.P.; Young, S.; Williamson, M.S.; Duce, I.; Denholm, I.; Devine, G.J. Analogous pleiotropic effects of insecticide resistance genotypes in peach-potato aphids and houseflies. Heredity 2003, 91, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.; Trumble, J. Inheritance and fitness consequences of resistance to fenvalerate in Spodoptera exigua (Lepidoptera: Noctuidae). J. Econ. Entomol. 1991, 84, 1638–1644. [Google Scholar] [CrossRef]
- Bouvier, J.C.; Bues, R.; Boivin, T.; Boudinhon, L.; Beslay, D.; Sauphanor, B. Deltamethrin resistance in the codling moth (Lepidoptera: Tortricidae): Inheritance and number of genes involved. Heredity 2001, 87, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Mebrahtu, Y.B.; Norem, J.; Taylor, M. Inheritance of larval resistance to permethrin in Aedes aegypti and association with sex ratio distortion and life history variation. Am. J. Trop. Med. Hyg. 1997, 56, 456–465. [Google Scholar] [CrossRef] [PubMed]
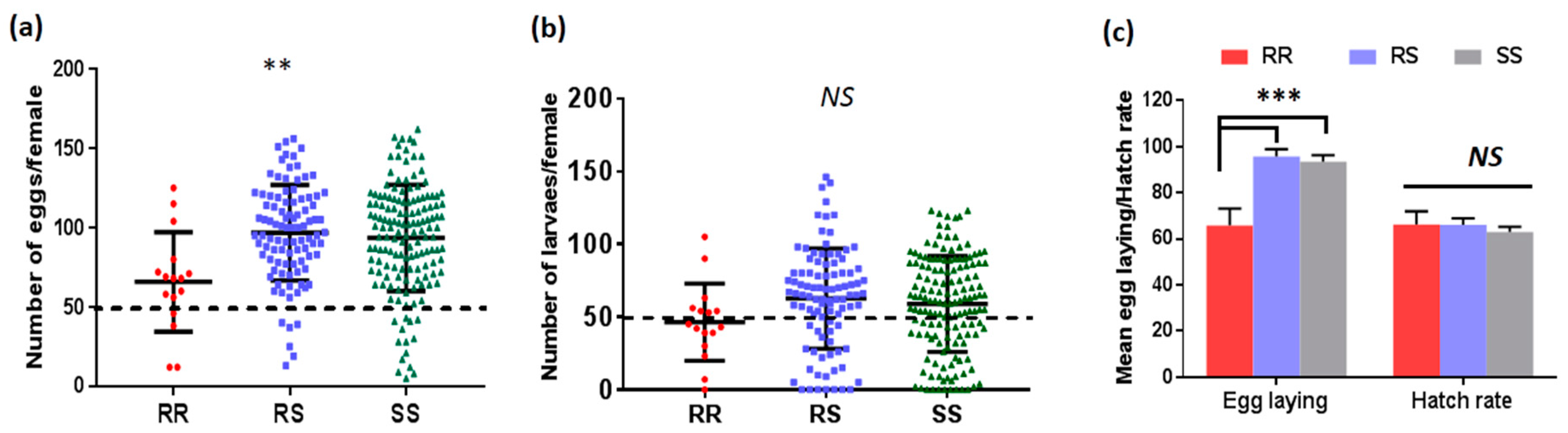
| Genotypes | L119F-GSTe2 and Oviposition | |
|---|---|---|
| Odds Ratio | p-Value | |
| SS vs. RR | 2.93 (1.66–5.18) | 0.0001 * |
| SS vs. RS | 2.06 (1.45–2.92) | 0.000001 * |
| RS vs. RR | 2.68 (1.51–4.77) | 0.0002 * |
| Genotypes | Pupae D9 vs. Pupae D11 | Pupae D11 vs. Pupae D11 | ||
|---|---|---|---|---|
| Odds Ratio | p-Value | Odds Ratio | p-Value | |
| RS vs. RR | 5.26 (2.24–12.34) | <0.0001 * | 1.04 (0.73–1.49) | 0.42 |
| RS vs. SS | 1.39 (0.89–2.17) | 0.08 | 1.38 (0.98–1.87) | 0.03 * |
| SS vs. RR | 9.66 (4.17–22.40) | <0.0001* | 1.40 (1.01–1.95) | 0.02 * |
| Genotypes | D1 x D10 | D10 x D20 | D20 x D30 | |||
|---|---|---|---|---|---|---|
| Odds Ratio | p | Odds Ratio | p | Odds Ratio | p | |
| RR vs. RS | 3.75 (1.21–11.29) | 0.019 | 3.83 (1.56–9.41) | 0.0023 | 2.2 (1.04–4.64) | 0.050 S |
| RR vs. SS | 7.5 (2.64–21.28) | 0.000006 | 3.83 (1.56–9.41) | 0.0059 | 2.1 (0.98–4.45) | 0.13 |
| RS vs. SS | 1.30 (0.75–2.24) | 0.41 | 1.04 (0.57–1.90) | 1 | 1.61 (0.80–3.22) | 0.22 |
| 2n | S | h | hd | π | D | D* | |
|---|---|---|---|---|---|---|---|
| D1 | 24 | 11 | 15 | 0.95 | 0.005 | −0.11 ns | −0.96 ns |
| D10 | 24 | 10 | 11 | 0.79 | 0.003 | −0.44 ns | 0.97 ns |
| D20 | 16 | 11 | 14 | 0.98 | 0.006 | 0.21 ns | 0.41 ns |
| D30 | 24 | 9 | 12 | 0.92 | 0.003 | 0.02 ns | 0.28 ns |
| TOTAL | 88 | 12 | 33 | 0.94 | 0.004 | 0.44 ns | 1.55 ns |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tchouakui, M.; Riveron, J.M.; Djonabaye, D.; Tchapga, W.; Irving, H.; Soh Takam, P.; Njiokou, F.; Wondji, C.S. Fitness Costs of the Glutathione S-Transferase Epsilon 2 (L119F-GSTe2) Mediated Metabolic Resistance to Insecticides in the Major African Malaria Vector Anopheles Funestus. Genes 2018, 9, 645. https://doi.org/10.3390/genes9120645
Tchouakui M, Riveron JM, Djonabaye D, Tchapga W, Irving H, Soh Takam P, Njiokou F, Wondji CS. Fitness Costs of the Glutathione S-Transferase Epsilon 2 (L119F-GSTe2) Mediated Metabolic Resistance to Insecticides in the Major African Malaria Vector Anopheles Funestus. Genes. 2018; 9(12):645. https://doi.org/10.3390/genes9120645
Chicago/Turabian StyleTchouakui, Magellan, Jacob M. Riveron, Doumani Djonabaye, Williams Tchapga, Helen Irving, Patrice Soh Takam, Flobert Njiokou, and Charles S. Wondji. 2018. "Fitness Costs of the Glutathione S-Transferase Epsilon 2 (L119F-GSTe2) Mediated Metabolic Resistance to Insecticides in the Major African Malaria Vector Anopheles Funestus" Genes 9, no. 12: 645. https://doi.org/10.3390/genes9120645
APA StyleTchouakui, M., Riveron, J. M., Djonabaye, D., Tchapga, W., Irving, H., Soh Takam, P., Njiokou, F., & Wondji, C. S. (2018). Fitness Costs of the Glutathione S-Transferase Epsilon 2 (L119F-GSTe2) Mediated Metabolic Resistance to Insecticides in the Major African Malaria Vector Anopheles Funestus. Genes, 9(12), 645. https://doi.org/10.3390/genes9120645






