Regulation of Structure-Specific Endonucleases in Replication Stress
Abstract
1. Introduction
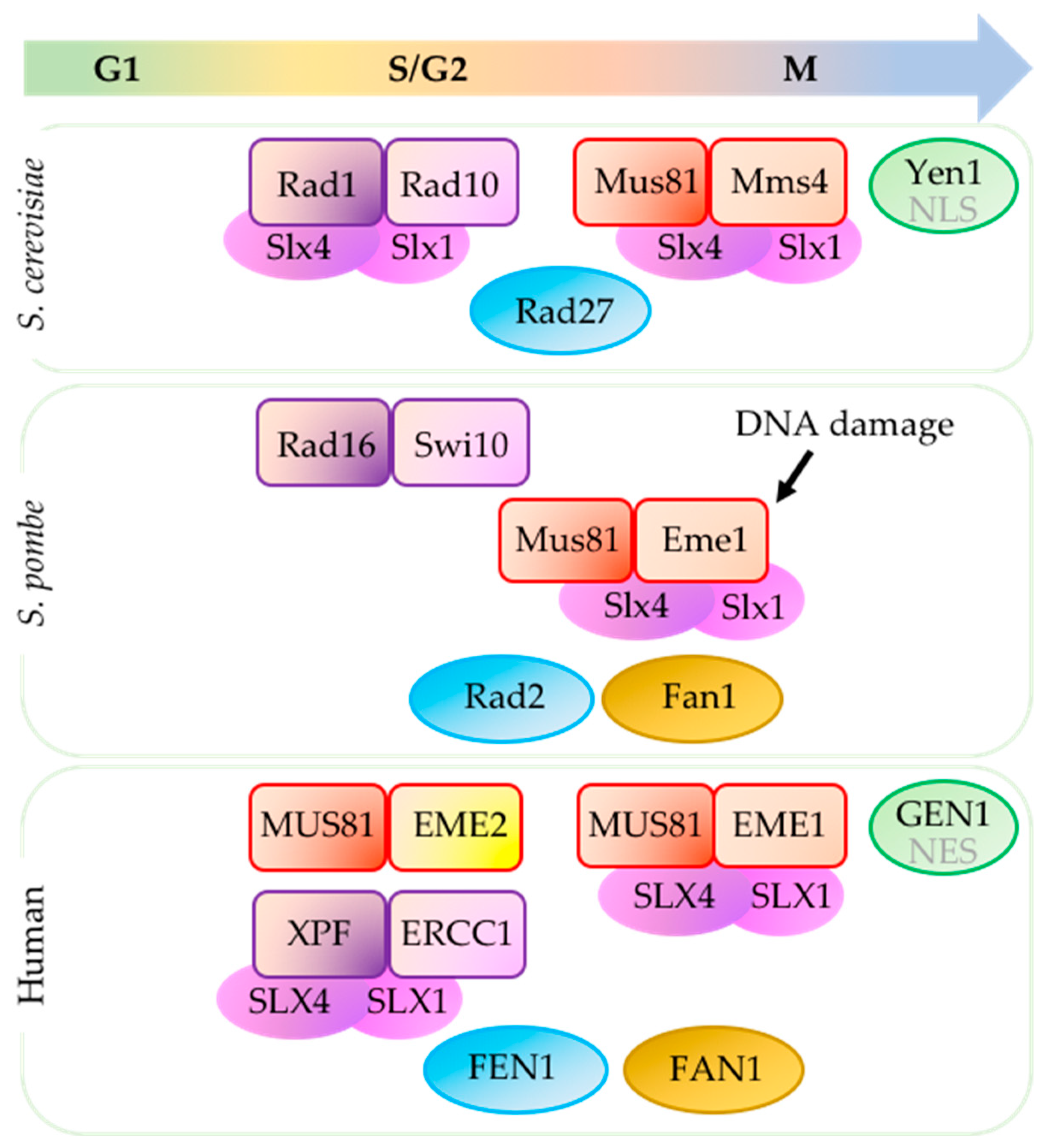
2. Mus81-Essential Meiotic Endonuclease 1 (Schizosaccharomyces pombe)/Mus81-Mms4 (Saccharomyces cerevisiae)/MUS81-EME1/2 (Human)
2.1. Mus81 Processes Replication and Recombination Intermediates
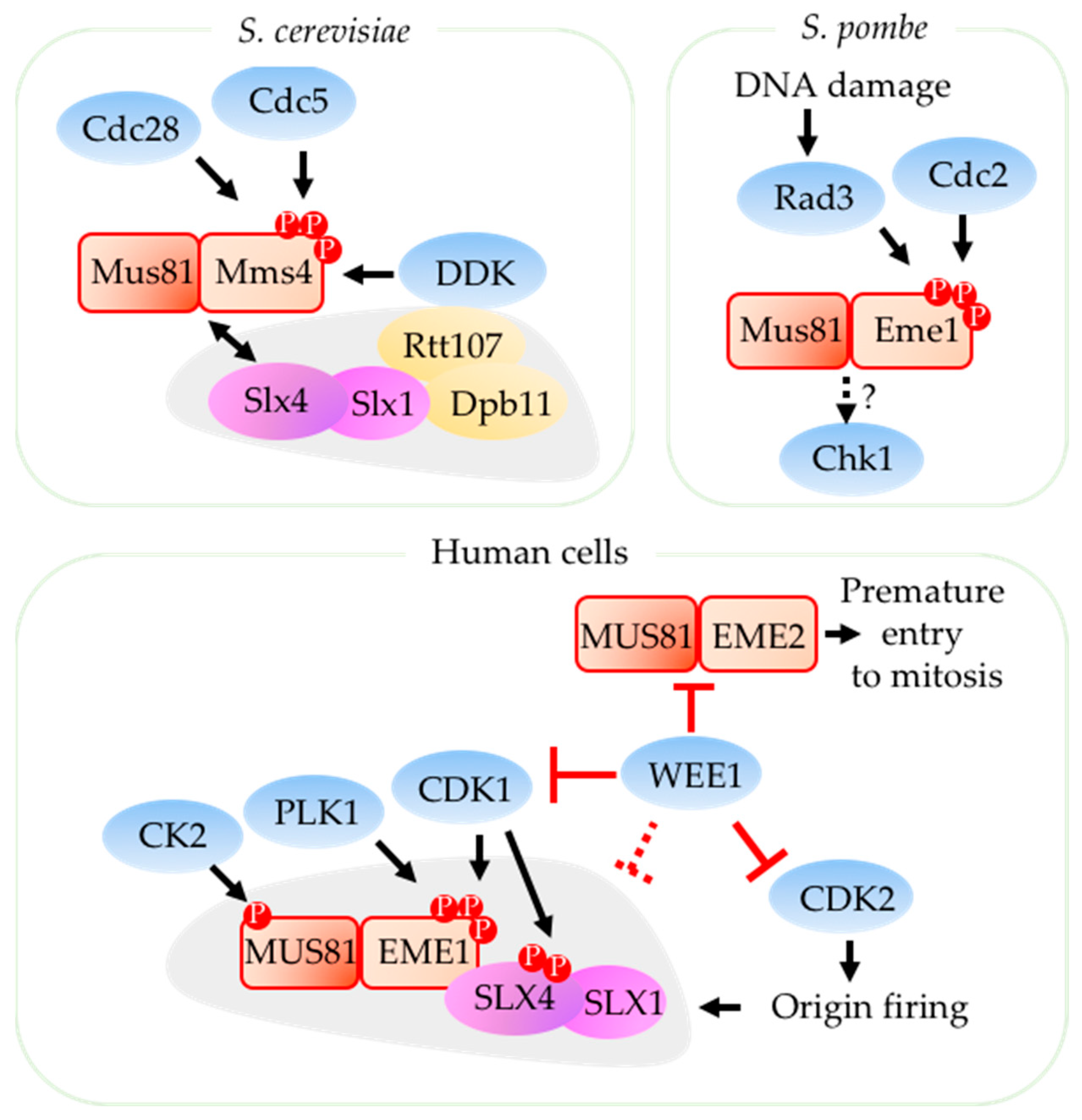
2.2. Regulation of Mus81 by Cell Cycle Kinases
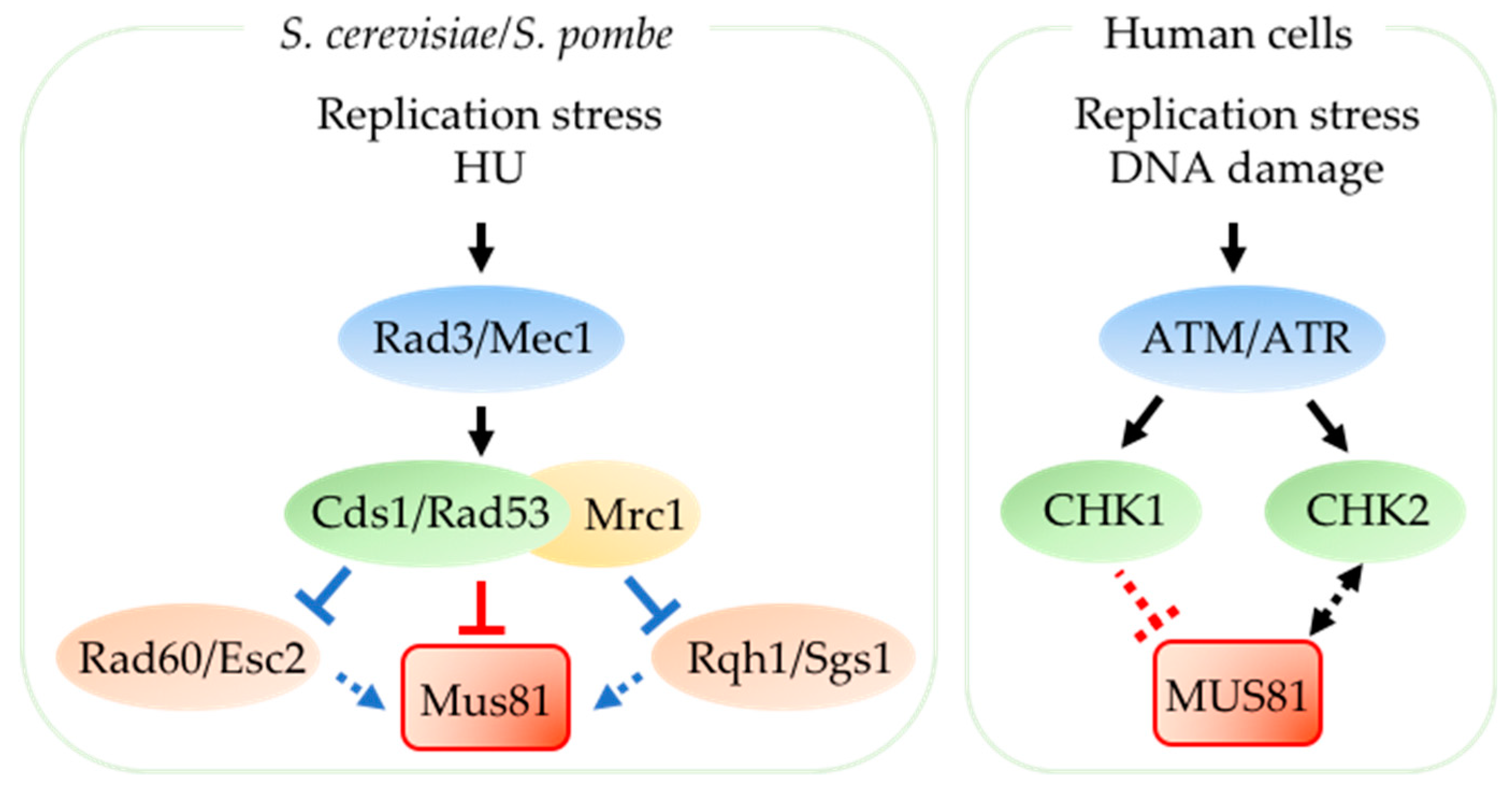
2.3. Mus81 is Regulated by the Replication Checkpoint during Replication Stress
2.4. Other Regulators of Mus81 Recruitment and Activity
3. Rad16-Swi10 (Schizosaccharomyces pombe)/Rad1-Rad10 (Saccharomycescerevisiae)/Xeroderma Pigmentosum Group F Complementing Protein (XPF)-Excision Repair Cross-Complementing Group 1 (ERCC1) (Human)
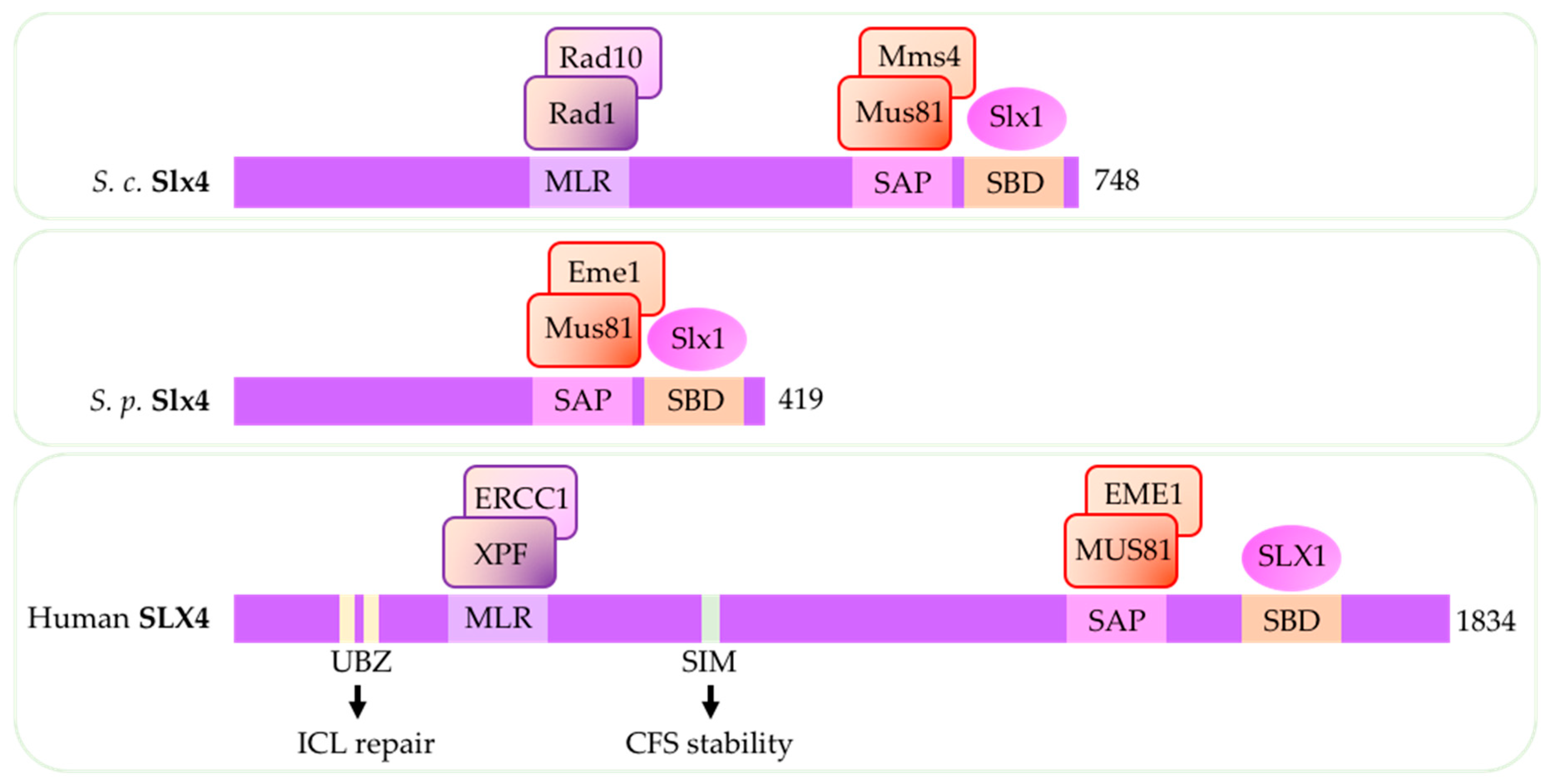
4. Structure-Specific Endonuclease Subunit Slx4 (Schizosaccharomyces pombe)/Slx4 (Saccharomyces cerevisiae)/SLX4 (Human)
5. Other Structure-Specific Endonuclease in Replication Stress
5.1. Rad2 (Schizosaccharomyces pombe)/Rad27 (Saccharomyces cerevisiae)/FEN1 (Flap Endonuclease 1) (Human)
5.2. Fan1 (Schizosaccharomyces pombe)/Absent in Saccharomyces cerevisiae/FAN1 (Fanconi-Associated Nuclease I) (Human)
5.3. Absent in S. pombe/Yen1/GEN1
6. Concluding Remarks
- How do regulation and roles of MUS81 and XPF differ between mitosis and meiosis?
- What molecular brakes exist that allow SSEs to process aberrant replication structures without deleterious DNA breakage?
- How does chromatin structure or components influence SSE recruitment and activity?
- How do SSEs coordinate or communicate with other SSEs and other DNA-remodeling enzymes?
- Do SSE activities contribute to checkpoint activation and cell cycle arrest? If so, what is the molecular mechanism?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zeman, M.K.; Cimprich, K.A. Causes and consequences of replication stress. Nat. Cell Biol. 2014, 16, 2–9. [Google Scholar] [CrossRef]
- Técher, H.; Koundrioukoff, S.; Nicolas, A.; Debatisse, M. The impact of replication stress on replication dynamics and DNA damage in vertebrate cells. Nat. Rev. Genet. 2017, 18, 535–550. [Google Scholar] [CrossRef]
- Mirkin, E.V.; Mirkin, S.M. Replication Fork Stalling at Natural Impediments. Microbiol. Mol. Biol. Rev. 2007, 71, 13–35. [Google Scholar] [CrossRef] [PubMed]
- Magdalou, I.; Lopez, B.S.; Pasero, P.; Lambert, S.A.E. The causes of replication stress and their consequences on genome stability and cell fate. Semin. Cell Dev. Biol. 2014, 30, 154–164. [Google Scholar] [CrossRef]
- Tubbs, A.; Nussenzweig, A. Endogenous DNA Damage as a Source of Genomic Instability in Cancer. Cell 2017, 168, 644–656. [Google Scholar] [CrossRef]
- Macheret, M.; Halazonetis, T.D. DNA Replication Stress as a Hallmark of Cancer. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, B.; Ben-Zimra, M.; Simon, I. Perturbations in the replication program contribute to genomic instability in cancer. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Dehé, P.M.; Gaillard, P.H.L. Control of structure-specific endonucleases to maintain genome stability. Nat. Rev. Mol. Cell Biol. 2017, 18, 315–330. [Google Scholar] [CrossRef]
- Ait Saada, A.; Lambert, S.A.E.; Carr, A.M. Preserving replication fork integrity and competence via the homologous recombination pathway. DNA Repair (Amst). 2018, 71, 135–147. [Google Scholar] [CrossRef]
- Bétous, R.; Goullet de Rugy, T.; Pelegrini, A.L.; Queille, S.; de Villartay, J.P.; Hoffmann, J.S. DNA replication stress triggers Rapid DNA Replication Fork Breakage by Artemis and XPF. PLoS Genet. 2018, 14, 1–16. [Google Scholar] [CrossRef]
- Kim, Y. Nuclease Delivery: Versatile Functions of SLX4/FANCP in Genome Maintenance. Mol. Cells 2014, 37, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, I.M.; Hain, K.; Déclais, A.C.; Gardiner, M.; Toh, G.W.; Sanchez-Pulido, L.; Heuckmann, J.M.; Toth, R.; Macartney, T.; Eppink, B.; Kanaar, R.; Ponting, C.P.; Lilley, D.M.J.; Rouse, J. Coordination of Structure-Specific Nucleases by Human SLX4/BTBD12 Is Required for DNA Repair. Mol. Cell 2009, 35, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.L.; Bergstralh, D.T.; Kohl, K.P.; LaRocque, J.R.; Moore, C.B.; Sekelsky, J. Drosophila MUS312 and the Vertebrate Ortholog BTBD12 Interact with DNA Structure-Specific Endonucleases in DNA Repair and Recombination. Mol. Cell 2009, 35, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Fekairi, S.; Scaglione, S.; Chahwan, C.; Taylor, E.R.; Tissier, A.; Coulon, S.; Dong, M.Q.; Ruse, C.; Yates, J.R.; Russell, P.; Fuchs, R.P.; McGowan, C.H.; Gaillard, P.H.L. Human SLX4 Is a Holliday Junction Resolvase Subunit that Binds Multiple DNA Repair/Recombination Endonucleases. Cell 2009, 138, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, J.M.; Smogorzewska, A.; Sowa, M.E.; O’Connell, B.C.; Gygi, S.P.; Elledge, S.J.; Harper, J.W. Mammalian BTBD12/SLX4 Assembles A Holliday Junction Resolvase and Is Required for DNA Repair. Cell 2009, 138, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Princz, L.N.; Gritenaite, D.; Pfander, B. The Slx4-Dpb11 scaffold complex: Coordinating the response to replication fork stalling in S-phase and the subsequent mitosis. Cell Cycle 2015, 14, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Ohouo, P.Y.; Bastos De Oliveira, F.M.; Liu, Y.; Ma, C.J.; Smolka, M.B. DNA-repair scaffolds dampen checkpoint signalling by counteracting the adaptor Rad9. Nature 2013, 493, 120–125. [Google Scholar] [CrossRef]
- Balint, A.; Kim, T.; Gallo, D.; Cussiol, J.R.; Bastos de Oliveira, F.M.; Yimit, A.; Ou, J.; Nakato, R.; Gurevich, A.; Shirahige, K.; Smolka, M.B.; Zhang, Z.; Brown, G.W. Assembly of Slx4 signaling complexes behind DNA replication forks. EMBO J. 2015, 34, 2182–2197. [Google Scholar] [CrossRef] [PubMed]
- Gritenaite, D.; Princz, L.N.; Szakal, B.; Bantele, S.C.S.; Wendeler, L.; Schilbach, S.; Habermann, B.H.; Matos, J.; Lisby, M.; Branzei, D.; Pfander, B. A cell cycle-regulated Slx4 – Dpb11 complex promotes the resolution of DNA repair intermediates linked to stalled replication. Genes Dev. 2014, 28, 1604–1619. [Google Scholar] [CrossRef]
- Cussiol, J.R.; Jablonowski, C.M.; Yimit, A.; Brown, G.W.; Smolka, M.B. Dampening DNA damage checkpoint signalling via coordinated BRCT domain interactions. EMBO J. 2015, 34, e201490834. [Google Scholar] [CrossRef] [PubMed]
- Mastro, T.L.; Forsburg, S.L. Increased meiotic crossovers and reduced genome stability in absence of Schizosaccharomyces pombe Rad16 (XPF). Genetics 2014, 198, 1457–1472. [Google Scholar] [CrossRef] [PubMed]
- Eissler, C.L.; Mazón, G.; Powers, B.L.; Savinov, S.N.; Symington, L.S.; Hall, M.C. The Cdk/Cdc14 Module Controls Activation of the Yen1 Holliday Junction Resolvase to Promote Genome Stability. Mol. Cell 2014, 54, 80–93. [Google Scholar] [CrossRef] [PubMed]
- García-Luis, J.; Clemente-Blanco, A.; Aragón, L.; Machín, F. Cdc14 targets the Holliday junction resolvase Yen1 to the nucleus in early anaphase. Cell Cycle 2014, 13, 1392–1399. [Google Scholar] [CrossRef]
- Blanco, M.G.; Matos, J.; West, S.C. Dual Control of Yen1 Nuclease Activity and Cellular Localization by Cdk and Cdc14 Prevents Genome Instability. Mol. Cell 2014, 54, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.W.; West, S.C. Spatial control of the GEN1 Holliday junction resolvase ensures genome stability. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, J.M.; Harper, J.W. GEN1/Yen1 and the SLX4 complex: Solutions to the problem of Holliday junction resolution. Genes Dev. 2010, 24, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Boddy, M.N.; Russell, P. DNA replication checkpoint. Curr. Biol. 2001, 11, R953–R956. [Google Scholar] [CrossRef]
- Ciccia, A.; McDonald, N.; West, S.C. Structural and Functional Relationships of the XPF/MUS81 Family of Proteins. Annu. Rev. Biochem. 2008, 77, 259–287. [Google Scholar] [CrossRef]
- Boddy, M.N.; Lopez-Girona, A.; Shanahan, P.; Interthal, H.; Heyer, W.D.; Russell, P. Damage tolerance protein Mus81 associates with the FHA1 domain of checkpoint kinase Cds1. Mol. Cell. Biol. 2000, 20, 8758–8766. [Google Scholar] [CrossRef]
- Doe, C.L.; Ahn, J.S.; Dixon, J.; Whitby, M.C. Mus81-Eme1 and Rqh1 involvement in processing stalled and collapsed replication forks. J. Biol. Chem. 2002, 277, 32753–32759. [Google Scholar] [CrossRef]
- Boddy, M.N.; Gaillard, P.L.; Mcdonald, W.H.; Shanahan, P.; Yates, J.R.; Russell, P.; Jolla, L. Mus81-Eme1 Are Essential Components of a Holliday Junction Resolvase. Cell 2001, 107, 537–548. [Google Scholar] [CrossRef]
- Chen, X.B.; Melchionna, R.; Denis, C.M.; Gaillard, P.H.L.; Blasina, A.; Van de Weyer, I.; Boddy, M.N.; Russell, P.; Vialard, J.; McGowan, C.H. Human Mus81-associated endonuclease cleaves Holliday junctions in vitro. Mol. Cell 2001, 8, 1117–1127. [Google Scholar] [CrossRef]
- Kaliraman, V.; Mullen, J.R.; Fricke, W.M.; Bastin-shanower, S.A.; Brill, S.J. Functional overlap between Sgs1 – Top3 and the Mms4 – Mus81 endonuclease. Genes Dev. 2001, 15, 2730–2740. [Google Scholar] [CrossRef] [PubMed]
- Oke, A.; Anderson, C.M.; Yam, P.; Fung, J.C. Controlling Meiotic Recombinational Repair – Specifying the Roles of ZMMs, Sgs1 and Mus81/Mms4 in Crossover Formation. PLoS Genet. 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- De Los Santos, T.; Loidl, J.; Larkin, B.; Hollingsworth, N.M. A role for MMS4 in the processing of recombination intermediates during meiosis in Saccharomyces cerevisiae. Genetics 2001, 159, 1511–1525. [Google Scholar]
- Roseaulin, L.; Yamada, Y.; Tsutsui, Y.; Russell, P.; Iwasaki, H.; Arcangioli, B. Mus81 is essential for sister chromatid recombination at broken replication forks. EMBO J. 2008, 27, 1378–1387. [Google Scholar] [CrossRef]
- Sanchez, A.; Gadaleta, M.C.; Limbo, O.; Russell, P. Lingering single-strand breaks trigger Rad51-independent homology-directed repair of collapsed replication forks in the polynucleotide kinase/phosphatase mutant of fission yeast. PLoS Genet. 2017, 13, 1–20. [Google Scholar] [CrossRef]
- Hanada, K.; Budzowska, M.; Davies, S.L.; Van Drunen, E.; Onizawa, H.; Beverloo, H.B.; Maas, A.; Essers, J.; Hickson, I.D.; Kanaar, R. The structure-specific endonuclease Mus81 contributes to replication restart by generating double-strand DNA breaks. Nat. Struct. Mol. Biol. 2007, 14, 1096–1104. [Google Scholar] [CrossRef]
- Regairaz, M.; Zhang, Y.W.; Fu, H.; Agama, K.K.; Tata, N.; Agrawal, S.; Aladjem, M.I.; Pommier, Y. Mus81-mediated DNA cleavage resolves replication forks stalled by topoisomerase I-DNA complexes. J. Cell Biol. 2011, 195, 739–749. [Google Scholar] [CrossRef]
- Shimura, T.; Torres, M.J.; Martin, M.M.; Rao, V.A.; Pommier, Y.; Katsura, M.; Miyagawa, K.; Aladjem, M.I. Bloom’s Syndrome Helicase and Mus81 are Required to Induce Transient Double-strand DNA Breaks in Response to DNA Replication Stress. J. Mol. Biol. 2008, 375, 1152–1164. [Google Scholar] [CrossRef]
- Fu, H.; Martin, M.M.; Regairaz, M.; Huang, L.; You, Y.; Lin, C.M.; Ryan, M.; Kim, R.; Shimura, T.; Pommier, Y.; Aladjem, M.I. The DNA repair endonuclease Mus81 facilitates fast DNA replication in the absence of exogenous damage. Nat. Commun. 2015, 6, 6746. [Google Scholar] [CrossRef] [PubMed]
- Lemaçon, D.; Jackson, J.; Quinet, A.; Brickner, J.R.; Li, S.; Yazinski, S.; You, Z.; Ira, G.; Zou, L.; Mosammaparast, N.; Vindigni, A. MRE11 and EXO1 nucleases degrade reversed forks and elicit MUS81-dependent fork rescue in BRCA2-deficient cells. Nat. Commun. 2017, 8, 860. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Broderick, R.; Bergoglio, V.; Zimmer, J.; Badie, S.; Niedzwiedz, W.; Hoffmann, J.S.; Tarsounas, M. MUS81 nuclease activity is essential for replication stress tolerance and chromosome segregation in BRCA2-deficient cells. Nat. Commun. 2017, 8, 15983. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.; Boddy, M.N.; Russell, P.; Wang, T.S. Replication checkpoint kinase Cds1 regulates Mus81 to preserve genome integrity during replication stress. Genes Dev. 2005, 19, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Morrow, C.A.; Nguyen, M.O.; Fower, A.; Wong, I.N.; Osman, F.; Bryer, C.; Whitby, M.C. Inter-Fork Strand Annealing causes genomic deletions during the termination of DNA replication. Elife 2017, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Neelsen, K.J.; Zanini, I.M.Y.; Herrador, R.; Lopes, M. Oncogenes induce genotoxic stress by mitotic processing of unusual replication intermediates. J. Cell Biol. 2013, 200, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Kim, J.J.; Choi, J.M.; Lee, J.H.; Cho, Y. Crystal structure of the Mus81 – Eme1 complex. Genes Dev. 2008, 22, 1093–1106. [Google Scholar] [CrossRef]
- Gallo-Fernández, M.; Saugar, I.; Ortiz-Bazán, M.Á.; Vázquez, M.V.; Tercero, J.A. Cell cycle-dependent regulation of the nuclease activity of Mus81-Eme1/Mms4. Nucleic Acids Res. 2012, 40, 8325–8335. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; West, S.C. Holliday junction resolution: Regulation in space and time. DNA Repair (Amst). 2014, 19, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Szakal, B.; Branzei, D. Premature Cdk1/Cdc5/Mus81 pathway activation induces aberrant replication and deleterious crossover. EMBO J. 2013, 32, 1155–1167. [Google Scholar] [CrossRef]
- Saugar, I.; Vázquez, M.V.; Gallo-Fernández, M.; Ortiz-Bazán, M.Á.; Segurado, M.; Calzada, A.; Tercero, J.A. Temporal regulation of the Mus81-Mms4 endonuclease ensures cell survival under conditions of DNA damage. Nucleic Acids Res. 2013, 41, 8943–8958. [Google Scholar] [CrossRef] [PubMed]
- Princz, L.N.; Wild, P.; Bittmann, J.; Aguado, F.J.; Blanco, M.G.; Matos, J.; Pfander, B. Dbf4-dependent kinase and the Rtt107 scaffold promote Mus81-Mms4 resolvase activation during mitosis. EMBO J. 2017, 36, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Dehé, P.M.; Coulon, S.; Scaglione, S.; Shanahan, P.; Takedachi, A.; Wohlschlegel, J.A.; Yates, J.R.; Llorente, B.; Russell, P.; Gaillard, P.H.L. Regulation of Mus81-Eme1 Holliday junction resolvase in response to DNA damage. Nat. Struct. Mol. Biol. 2013, 20, 598–603. [Google Scholar] [CrossRef]
- Sabatinos, S.A.; Ranatunga, N.S.; Yuan, J.; Green, M.D.; Forsburg, S.L.; Solomon, M.J. Replication stress in early S phase generates apparent micronuclei and chromosome rearrangement in fission yeast. Mol. Biol. Cell 2015, 26, 3439–3450. [Google Scholar] [CrossRef]
- Pepe, A.; West, S.C. MUS81-EME2 promotes replication fork restart. Cell Rep. 2014, 7, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Pfander, B.; Matos, J. Control of Mus81 nuclease during the cell cycle. FEBS Lett. 2017, 591, 2048–2056. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Wang, X.; Palmai-Pallag, T.; Shen, H.; Helleday, T.; Hickson, I.D.; Ying, S. Acute MUS81 depletion leads to replication fork slowing and a constitutive DNA damage response. Oncotarget 2015, 6, 37638–37646. [Google Scholar] [CrossRef]
- Wyatt, H.D.M.; Sarbajna, S.; Matos, J.; West, S.C. Coordinated actions of SLX1-SLX4 and MUS81-EME1 for holliday junction resolution in human cells. Mol. Cell 2013, 52, 234–247. [Google Scholar] [CrossRef]
- Castor, D.; Nair, N.; Déclais, A.C.; Lachaud, C.; Toth, R.; Macartney, T.J.; Lilley, D.M.J.; Arthur, J.S.C.; Rouse, J. Cooperative control of holliday junction resolution and DNA Repair by the SLX1 and MUS81-EME1 nucleases. Mol. Cell 2013, 52, 221–233. [Google Scholar] [CrossRef]
- Palma, A.; Pugliese, G.M.; Murfuni, I.; Marabitti, V.; Malacaria, E.; Rinalducci, S.; Minoprio, A.; Sanchez, M.; Mazzei, F.; Zolla, L.; Franchitto, A.; Pichierri, P. Phosphorylation by CK2 regulates MUS81/EME1 in mitosis and after replication stress. Nucleic Acids Res. 2018, 46, 5109–5124. [Google Scholar] [CrossRef]
- Domínguez-Kelly, R.; Martín, Y.; Koundrioukoff, S.; Tanenbaum, M.E.; Smits, V.A.J.; Medema, R.H.; Debatisse, M.; Freire, R. Wee1 controls genomic stability during replication by regulating the Mus81-Eme1 endonuclease. J. Cell Biol. 2011, 194, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Duda, H.; Arter, M.; Gloggnitzer, J.; Teloni, F.; Wild, P.; Blanco, M.G.; Altmeyer, M.; Matos, J. A Mechanism for Controlled Breakage of Underreplicated Chromosomes during Mitosis. Dev. Cell 2016, 39, 740–755. [Google Scholar] [CrossRef]
- Szmyd, R.; Niska-blakie, J.; Diril, M.K.; Nunes, P.R.; Tzelepis, K.; Lacroix, A.; Van Hul, N.; Joao, L.D.; Oliver, M.; Xavier, D. Premature activation of Cdk1 leads to mitotic events in S phase and embryonic lethality. Oncogene 2018. [Google Scholar] [CrossRef]
- Xu, Y.; Ning, S.; Wei, Z.; Xu, R.; Xu, X.; Xing, M.; Guo, R.; Xu, D. 53BP1 and BRCA1 control pathway choice for stalled replication restart. Elife 2017, 6, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, S.; Hasanova, Z.; Kanagaraj, R.; Chappidi, N.; Altmannova, V.; Menon, S.; Sedlackova, H.; Langhoff, J.; Surendranath, K.; Hühn, D.; Bhowmick, R.; Marini, V.; Ferrari, S.; Hickson, I.D.; Krejci, L.; Janscak, P. RECQ5 Helicase Cooperates with MUS81 Endonuclease in Processing Stalled Replication Forks at Common Fragile Sites during Mitosis. Mol. Cell 2017, 66, 658–671. [Google Scholar] [CrossRef]
- Beck, H.; Nähse, V.; Larsen, M.S.Y.; Groth, P.; Clancy, T.; Lees, M.; Jørgensen, M.; Helleday, T.; Syljuåsen, R.G.; Sørensen, C.S. Regulators of cyclin-dependent kinases are crucial for maintaining genome integrity in S phase. J. Cell Biol. 2010, 188, 629–638. [Google Scholar] [CrossRef]
- Beck, H.; Nahse-Kumpf, V.; Larsen, M.S.Y.; O’Hanlon, K.A.; Patzke, S.; Holmberg, C.; Mejlvang, J.; Groth, A.; Nielsen, O.; Syljuasen, R.G.; Sorensen, C.S. Cyclin-Dependent Kinase Suppression by WEE1 Kinase Protects the Genome through Control of Replication Initiation and Nucleotide Consumption. Mol. Cell. Biol. 2012, 32, 4226–4236. [Google Scholar] [CrossRef] [PubMed]
- Forment, J.V.; Blasius, M.; Guerini, I.; Jackson, S.P. Structure-specific DNA endonuclease Mus81/Eme1 generates DNA damage caused by Chk1 inactivation. PLoS ONE 2011, 6, e23517. [Google Scholar] [CrossRef]
- Murfuni, I.; Basile, G.; Subramanyam, S.; Malacaria, E.; Bignami, M.; Spies, M.; Franchitto, A.; Pichierri, P. Survival of the Replication Checkpoint Deficient Cells Requires MUS81-RAD52 Function. PLoS Genet. 2013, 9, e1003910. [Google Scholar] [CrossRef]
- Técher, H.; Koundrioukoff, S.; Carignon, S.; Wilhelm, T.; Millot, G.A.; Lopez, B.S.; Brison, O.; Debatisse, M. Signaling from Mus81-Eme2-Dependent DNA Damage Elicited by Chk1 Deficiency Modulates Replication Fork Speed and Origin Usage. Cell Rep. 2016, 14, 1114–1127. [Google Scholar] [CrossRef]
- Fragkos, M.; Naim, V. Rescue from replication stress during mitosis. Cell Cycle 2017, 16, 613–633. [Google Scholar] [CrossRef]
- Naim, V.; Wilhelm, T.; Debatisse, M.; Rosselli, F. ERCC1 and MUS81-EME1 promote sister chromatid separation by processing late replication intermediates at common fragile sites during mitosis. Nat. Cell Biol. 2013, 15, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Minocherhomji, S.; Chan, K.L.; Palmai-Pallag, T.; Chu, W.K.; Wass, T.; Mankouri, H.W.; Liu, Y.; Hickson, I.D. MUS81 promotes common fragile site expression. Nat. Cell Biol. 2013, 15, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, I.; Koepp, D.M. Degradation of Mrc1 promotes recombination-mediated restart of stalled replication forks. Nucleic Acids Res. 2017, 45, 2558–2570. [Google Scholar] [CrossRef]
- Sebesta, M.; Urulangodi, M.; Stefanovie, B.; Szakal, B.; Pacesa, M.; Lisby, M.; Branzei, D.; Krejci, L. Esc2 promotes Mus81 complex-activity via its SUMO-like and DNA binding domains. Nucleic Acids Res. 2017, 45, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Froget, B.; Blaisonneau, J.; Lambert, S.; Baldacci, G. Cleavage of Stalled Forks by Fission Yeast Mus81/Eme1 in Absence of DNA Replication Checkpoint. Mol. Biol. Cell 2008, 19, 445–456. [Google Scholar] [CrossRef]
- Feijoo, C.; Hall-Jackson, C.; Wu, R.; Jenkins, D.; Leitch, J.; Gilbert, D.M.; Smythe, C. Activation of mammalian Chk1 during DNA replication arrest: A role for Chk1 in the intra-S phase checkpoint monitoring replication origin firing. J. Cell Biol. 2001, 154, 913–923. [Google Scholar] [CrossRef]
- Kai, M.; Wang, T.S.F. Checkpoint responses to replication stalling: Inducing tolerance and preventing mutagenesis. Mutat. Res. 2003, 532, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Rhind, N.; Russell, P. Chk1 and Cds1: linchpins of the DNA damage and replication checkpoint pathways. J. Cell Sci. 2000, 113, 3889–3896. [Google Scholar]
- Mcgowan, C.H. Checking in on Cds1 (Chk2): a checkpoint kinase and tumor suppressor. BioEssays 2002, 24, 502–511. [Google Scholar] [CrossRef]
- Xu, Y.; Davenport, M.; Kelly, T.J. Two-stage mechanism for activation of the DNA replication checkpoint kinase Cds1 in fission yeast. Genes Dev. 2006, 20, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Miyabe, I.; Morishita, T.; Shinagawa, H.; Carr, A.M. Schizosaccharomyces pombe Cds1 Chk2 regulates homologous recombination at stalled replication forks through the phosphorylation of recombination protein Rad60. J. Cell Sci. 2009, 122, 3638–3643. [Google Scholar] [CrossRef] [PubMed]
- Boddy, M.N.; Shanahan, P.; McDonald, W.H.; Lopez-Girona, A.; Noguchi, E.; Yates, J.R., III; Russell, P. Replication checkpoint kinase Cds1 regulates recombinational repair protein Rad60. Mol. Cell. Biol. 2003, 23, 5939–5946. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Qian, Y.; Ni, X.; Xu, X.; Dong, X. Feedback regulation of methyl methanesulfonate and ultraviolet-sensitive gene clone 81 via ATM/Chk2 pathway contributes to the resistance of MCF-7 breast cancer cells to cisplatin. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef]
- Branzei, D.; Foiani, M. The DNA damage response during DNA replication. Curr. Opin. Cell Biol. 2005, 17, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Liu, V.F.; Bhaumik, D.; Wang, T.S. Mutator phenotype induced by aberrant replication. Mol. Cell. Biol. 1999, 19, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Huberman, J.A. Regulation of replication timing in fission yeast. EMBO J. 2001, 20, 6115–6126. [Google Scholar] [CrossRef]
- Shirahige, K.; Hori, Y.; Shiraishi, K.; Yamashita, M.; Takahashi, K.; Obuse, C.; Tsurimoto, T.; Yoshikawa, H. Regulation of DNA-replication origins during cell-cycle progression. Nature 1998, 395, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Santocanale, C.; Diffley, J.F.X. A Mec1-and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature 1998, 395, 615–618. [Google Scholar] [CrossRef]
- Rossi, S.E.; Ajazi, A.; Carotenuto, W.; Foiani, M.; Giannattasio, M. Rad53-Mediated Regulation of Rrm3 and Pif1 DNA Helicases Contributes to Prevention of Aberrant Fork Transitions under Replication Stress. Cell Rep. 2015, 13, 80–92. [Google Scholar] [CrossRef]
- Lucca, C.; Vanoli, F.; Cotta-Ramusino, C.; Pellicioli, A.; Liberi, G.; Haber, J.; Foiani, M. Checkpoint-mediated control of replisome-fork association and signalling in response to replication pausing. Oncogene 2004, 23, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Hsiao, J.; Fay, D.S.; Stern, D.F. Rad53 FHA domain associated with phosphorylated Rad9 in the DNA damage checkpoint. Science 1998, 281, 272–274. [Google Scholar] [CrossRef]
- Willis, N.; Rhind, N. Mus81, Rhp51(Rad51), and Rqh1 Form an Epistatic Pathway Required for the S-Phase DNA Damage Checkpoint. Mol. Cell. Biol. 2010, 82, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Phung, H.T.T.; Nguyen, H.L.H.; Vo, S.T.; Nguyen, D.H.; Le, M. Van Saccharomyces cerevisiae Mus81-Mms4 and Rad52 can cooperate in the resolution of recombination intermediates. Yeast 2018, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, S.K.; Kamileri, I.; Lugli, N.; Evangelou, K.; Da-Ré, C.; Huber, F.; Padayachy, L.; Tardy, S.; Nicati, N.L.; Barriot, S.; Ochs, F.; Lukas, C.; Lukas, J.; Gorgoulis, V.G.; Scapozza, L.; Halazonetis, T.D. Mammalian RAD52 Functions in Break-Induced Replication Repair of Collapsed DNA Replication Forks. Mol. Cell 2016, 64, 1127–1134. [Google Scholar] [CrossRef]
- Bhowmick, R.; Minocherhomji, S.; Hickson, I.D. RAD52 Facilitates Mitotic DNA Synthesis Following Replication Stress. Mol. Cell 2016, 64, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Sisakova, A.; Altmannova, V.; Sebesta, M.; Krejci, L. Role of PCNA and RFC in promoting Mus81-complex activity. BMC Biol. 2017, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Walter, J.C. Mechanism and regulation of incisions during DNA interstrand cross-link repair. DNA Repair (Amst). 2014, 19, 135–142. [Google Scholar] [CrossRef]
- Wehrkamp-Richter, S.; Hyppa, R.W.; Prudden, J.; Smith, G.R.; Boddy, M.N. Meiotic DNA joint molecule resolution depends on Nse5-Nse6 of the Smc5-Smc6 holocomplex. Nucleic Acids Res. 2012, 40, 9633–9646. [Google Scholar] [CrossRef]
- Copsey, A.; Tang, S.; Jordan, P.W.; Blitzblau, H.G.; Newcombe, S.; Chan, A.C.; Newnham, L.; Li, Z.; Gray, S.; Herbert, A.D.; Arumugam, P.; Hochwagen, A.; Hunter, N.; Hoffmann, E. Smc5/6 Coordinates Formation and Resolution of Joint Molecules with Chromosome Morphology to Ensure Meiotic Divisions. PLoS Genet. 2013, 9. [Google Scholar] [CrossRef]
- Minocherhomji, S.; Ying, S.; Bjerregaard, V.A.; Bursomanno, S.; Aleliunaite, A.; Wu, W.; Mankouri, H.W.; Shen, H.; Liu, Y.; Hickson, I.D. Replication stress activates DNA repair synthesis in mitosis. Nature 2015, 528, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, S.; Molnarova, L.; Richterova, J.; Huraiova, B.; Benko, Z.; Polakova, S.; Cipakova, I.; Sevcovicova, A.; Gaplovska-Kysela, K.; Mechtler, K.; Cipak, L.; Gregan, J. Mutations that prevent methylation of cohesin render sensitivity to DNA damage in S. pombe. J. Cell Sci. 2018, 131, jcs214924. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Chen, X.B.; McGowan, C.H. Mus81 Endonuclease Localizes to Nucleoli and to Regions of DNA Damage in Human S-phase Cells. Mol. Biol. Cell 2003, 14, 4826–4834. [Google Scholar] [CrossRef] [PubMed]
- Saugar, I.; Jiménez-Martín, A.; Tercero, J.A. Subnuclear Relocalization of Structure-Specific Endonucleases in Response to DNA Damage. Cell Rep. 2017, 20, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yang, F.; Lu, L.; Dai, W. Arsenic-induced sumoylation of Mus81 is involved in regulating genomic stability. Cell Cycle 2017, 16, 802–811. [Google Scholar] [CrossRef]
- Rondinelli, B.; Gogola, E.; Yücel, H.; Duarte, A.A.; Van De Ven, M.; Van Der Sluijs, R.; Konstantinopoulos, P.A.; Jonkers, J.; Ceccaldi, R.; Rottenberg, S.; D’Andrea, A.D. EZH2 promotes degradation of stalled replication forks by recruiting MUS81 through histone H3 trimethylation. Nat. Cell Biol. 2017, 19, 1371–1378. [Google Scholar] [CrossRef]
- Liang, C.C.; Zhan, B.; Yoshikawa, Y.; Haas, W.; Gygi, S.P.; Cohn, M.A. UHRF1 Is a sensor for DNA interstrand crosslinks and recruits FANCD2 to initiate the Fanconi Anemia pathway. Cell Rep. 2015, 10, 1947–1957. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Paramasivam, M.; Ghosal, G.; Chen, D.; Shen, X.; Huang, Y.; Akhter, S.; Legerski, R.; Chen, J.; Seidman, M.M.; Qin, J.; Li, L. UHRF1 contributes to DNA damage repair as a lesion recognition factor and nuclease scaffold. Cell Rep. 2015, 10, 1958–1967. [Google Scholar] [CrossRef]
- Guzder, S.N.; Sommers, C.H.; Prakash, L.; Prakash, S. Complex formation with damage recognition protein Rad14 is essential for Saccharomyces cerevisiae Rad1-Rad10 nuclease to perform its function in nucleotide excision repair in vivo. Mol. Cell. Biol. 2006, 26, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- De Laat, W.L.; Appeldoorn, E.; Sugasawa, K.; Weterings, E.; Jaspers, N.G.J.; Hoeijmakers, J.H.J. DNA-binding polarity of human replication protein A positions nucleases in nucleotide excision repair. Genes Dev. 1998, 12, 2598–2609. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, U.B.; McGouran, J.F.; Brolih, S.; Ptchelkine, D.; El-Sagheer, A.H.; Brown, T.; McHugh, P.J. RPA activates the XPF-ERCC1 endonuclease to initiate processing of DNA interstrand crosslinks. EMBO J. 2017, 36, 2047–2060. [Google Scholar] [CrossRef]
- Motycka, T.A.; Bessho, T.; Post, S.M.; Sung, P.; Tomkinson, A.E. Physical and Functional Interaction between the XPF/ERCC1 Endonuclease and hRad52. J. Biol. Chem. 2004, 279, 13634–13639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Liu, X.M.; Ding, Y.H.; Xiong, L.Y.; Ren, J.Y.; Zhou, Z.X.; Wang, H.T.; Zhang, M.J.; Yu, Y.; Dong, M.Q.; Du, L.L. Fission Yeast Pxd1 Promotes Proper DNA Repair by Activating Rad16XPF and Inhibiting Dna2. PLoS Biol. 2014, 12. [Google Scholar] [CrossRef]
- Li, F.; Dong, J.; Eichmiller, R.; Holland, C.; Minca, E.; Prakash, R.; Sung, P.; Yong Shim, E.; Surtees, J.A.; Eun Lee, S. Role of Saw1 in Rad1/Rad10 complex assembly at recombination intermediates in budding yeast. EMBO J. 2013, 32, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, L.; Bambara, R.A. Flap Endonuclease 1. Annu. Rev. Biochem. 2013, 82, 119–138. [Google Scholar] [CrossRef]
- Xu, X.; Shi, R.; Zheng, L.; Guo, Z.; Wang, L.; Zhou, M.; Zhao, Y.; Tian, B.; Truong, K.; Chen, Y.; Shen, B.; Hua, Y.; Xu, H. SUMO-1 modification of FEN1 facilitates its interaction with Rad9-Rad1-Hus1 to counteract DNA replication stress. J. Mol. Cell Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Lee, C.H.; Kang, Y.H.; Cho, I.T.; Nguyen, T.A.; Seo, Y.S. Genetic and functional interactions between Mus81-Mms4 and Rad27. Nucleic Acids Res. 2010, 38, 7611–7625. [Google Scholar] [CrossRef] [PubMed]
- Thu, H.P.T.; Nguyen, T.A.; Munashingha, P.R.; Kwon, B.; Van, Q.D.; Seo, Y.S. A physiological significance of the functional interaction between Mus81 and Rad27 in homologous recombination repair. Nucleic Acids Res. 2015, 43, 1684–1699. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Amangyeld, T.; Nguyen, T.A.; Munashingha, P.R.; Seo, Y.S. Human MUS81 complexes stimulate flap endonuclease 1. FEBS J. 2012, 279, 2412–2430. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bambara, R.A. Human bloom protein stimulates flap endonuclease 1 activity by resolving DNA secondary structure. J. Biol. Chem. 2005, 280, 5391–5399. [Google Scholar] [CrossRef] [PubMed]
- Brosh, R.M.; Von Kobbe, C.; Sommers, J.A.; Karmakar, P.; Opresko, P.L.; Piotrowski, J.; Dianova, I.; Dianov, G.L.; Bohr, V.A. Werner syndrome protein interacts with human Ap endonuclease 1 and stimulates its cleavage activity. EMBO J. 2001, 20, 5791–5801. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhou, M.; Chai, Q.; Parrish, J.; Xue, D.; Patrick, S.M.; Turchi, J.J.; Yannone, S.M.; Chen, D.; Shen, B. Novel function of the flap endonuclease 1 complex in processing stalled DNA replication forks. EMBO Rep. 2005, 6, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Lachaud, C.; Moreno, A.; Marchesi, F.; Toth, R.; Blow, J.J. Rouse Ubiquitinated Fancd2 recruits Fan1 to stalled replication forks to prevent genome instability. Science 2016, 351, 846–849. [Google Scholar] [CrossRef]
- Porro, A.; Berti, M.; Pizzolato, J.; Bologna, S.; Kaden, S.; Saxer, A.; Ma, Y.; Nagasawa, K.; Sartori, A.A.; Jiricny, J. FAN1 interaction with ubiquitylated PCNA alleviates replication stress and preserves genomic integrity independently of BRCA2. Nat. Commun. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- McNeil, E.M.; Melton, D.W. DNA repair endonuclease ERCC1-XPF as a novel therapeutic target to overcome chemoresistance in cancer therapy. Nucleic Acids Res. 2012, 40, 9990–10004. [Google Scholar] [CrossRef]
- Bastin-Shanower, S.A.; Fricke, W.M.; Mullen, J.R.; Brill, S.J. The mechanism of Mus81-Mms4 cleavage site selection distinguishes it from the homologous endonuclease Rad1-Rad10. Mol. Cell. Biol. 2003, 23, 3487–3496. [Google Scholar] [CrossRef] [PubMed]
- Brookman, K.W.; Lamerdin, J.E.; Thelen, M.P.; Hwang, M.; Reardon, J.T.; Sancar, A.; Zhou, Z.-Q.; Walter, C.A.; Parris, C.N.; Thompson, L.H. ERCC4 (XPF) Encodes a Human Nucleotide Excision Repair Protein with Eukaryotic Recombination Homologs. Mol. Cell. Biol. 1996, 16, 6553–6562. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Shinkura, R.; Shinkura, N.; Alt, F.W. Growth Retardation, Early Death, and DNA Repair Defects in Mice Deficient for the Nucleotide Excision Repair Enzyme XPF. Mol. Cell. Biol. 2004, 24, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Mazón, G.; Lam, A.F.; Ho, C.K.; Kupiec, M.; Symington, L.S. The Rad1-Rad10 nuclease promotes chromosome translocations between dispersed repeats. Nat. Struct. Mol. Biol. 2012, 19, 964–971. [Google Scholar] [CrossRef]
- Munoz-Galvan, S.; Tous, C.; Blanco, M.G.; Schwartz, E.K.; Ehmsen, K.T.; West, S.C.; Heyer, W.-D.; Aguilera, A. Distinct Roles of Mus81, Yen1, Slx1-Slx4, and Rad1 Nucleases in the Repair of Replication-Born Double-Strand Breaks by Sister Chromatid Exchange. Mol. Cell. Biol. 2012, 32, 1592–1603. [Google Scholar] [CrossRef]
- Saito, T.T.; Lui, D.Y.; Kim, H.M.; Meyer, K.; Colaiácovo, M.P. Interplay between Structure-Specific Endonucleases for Crossover Control during Caenorhabditis elegans Meiosis. PLoS Genet. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Narita, T.; Pham, V.T.; Iijima, J.; Hirota, K.; Keka, I.S.; Mohiuddin; Okawa, K.; Hori, T.; Fukagawa, T.; et al. Structure-specific endonucleases Xpf and Mus81 play overlapping but essential roles in DNA repair by homologous recombination. Cancer Res. 2013, 73, 4362–4371. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Seebode, C.; Smolorz, S.; Schubert, S.; Emmert, S. XPF knockout via CRISPR/Cas9 reveals that ERCC1 is retained in the cytoplasm without its heterodimer partner XPF. Cell. Mol. Life Sci. 2017, 74, 2081–2094. [Google Scholar] [CrossRef] [PubMed]
- Hodskinson, M.R.G.; Silhan, J.; Crossan, G.P.; Garaycoechea, J.I.; Mukherjee, S.; Johnson, C.M.; Schärer, O.D.; Patel, K.J. Mouse SLX4 Is a Tumor Suppressor that Stimulates the Activity of the Nuclease XPF-ERCC1 in DNA Crosslink Repair. Mol. Cell 2014, 54, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Klein Douwel, D.; Boonen, R.A.C.M.; Long, D.T.; Szypowska, A.A.; Räschle, M.; Walter, J.C.; Knipscheer, P. XPF-ERCC1 Acts in Unhooking DNA Interstrand Crosslinks in Cooperation with FANCD2 and FANCP/SLX4. Mol. Cell 2014, 54, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Lachaud, C.; Castor, D.; Hain, K.; Munoz, I.; Wilson, J.; MacArtney, T.J.; Schindler, D.; Rouse, J. Distinct functional roles for the two SLX4 ubiquitin-binding UBZ domains mutated in Fanconi anemia. J. Cell Sci. 2014, 127, 2811–2817. [Google Scholar] [CrossRef]
- Becker, J.R.; Gallo, D.; Leung, W.; Croissant, T.; Thu, Y.M.; Nguyen, H.D.; Starr, T.K.; Brown, G.W.; Bielinsky, A.-K. Flap endonuclease overexpression drives genome instability and DNA damage hypersensitivity in a PCNA-dependent manner. Nucleic Acids Res. 2018, 1–17. [Google Scholar] [CrossRef]
- Pedersen, R.T.; Kruse, T.; Nilsson, J.; Oestergaard, V.H.; Lisby, M. TopBP1 is required at mitosis to reduce transmission of DNA damage to G1 daughter cells. J. Cell Biol. 2015, 210, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.D.; Reddel, R.R. Assaying and investigating Alternative Lengthening of Telomeres activity in human cells and cancers. FEBS Lett. 2010, 584, 3800–3811. [Google Scholar] [CrossRef]
- Sarkar, J.; Wan, B.; Yin, J.; Vallabhaneni, H.; Horvath, K.; Kulikowicz, T.; Bohr, V.A.; Zhang, Y.; Lei, M.; Liu, Y. SLX4 contributes to telomere preservation and regulated processing of telomeric joint molecule intermediates. Nucleic Acids Res. 2015, 43, 5912–5923. [Google Scholar] [CrossRef] [PubMed]
- Sobinoff, A.P.; Allen, J.A.; Neumann, A.A.; Yang, S.F.; Walsh, M.E.; Henson, J.D.; Reddel, R.R.; Pickett, H.A. BLM and SLX4 play opposing roles in recombination-dependent replication at human telomeres. EMBO J. 2017, 36, e201796889. [Google Scholar] [CrossRef] [PubMed]
- Malacaria, E.; Franchitto, A.; Pichierri, P. SLX4 prevents GEN1-dependent DSBs during DNA replication arrest under pathological conditions in human cells. Sci. Rep. 2017, 7, 44464. [Google Scholar] [CrossRef] [PubMed]
- Guervilly, J.H.; Takedachi, A.; Naim, V.; Scaglione, S.; Chawhan, C.; Lovera, Y.; Despras, E.; Kuraoka, I.; Kannouche, P.; Rosselli, F.; Gaillard, P.H.L. The SLX4 complex is a SUMO E3 ligase that impacts on replication stress outcome and genome stability. Mol. Cell 2015, 57, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Garner, E.; Hallet, A.; Nguyen, H.D.; Rickman, K.A.; Gill, G.; Smogorzewska, A.; Zou, L. Noncovalent Interactions with SUMO and Ubiquitin Orchestrate Distinct Functions of the SLX4 Complex in Genome Maintenance. Mol. Cell 2015, 57, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Qian, L.; Liu, R.; Dai, H.; Zhou, M.; Zheng, L.; Shen, B. Nucleolar localization and dynamic roles of flap endonuclease 1 in ribosomal DNA replication and damage repair. Mol. Cell. Biol. 2008, 28, 4310–4319. [Google Scholar] [CrossRef] [PubMed]
- Saharia, A.; Teasley, D.C.; Duxin, J.P.; Dao, B.; Chiappinelli, K.B.; Stewart, S.A. FEN1 ensures telomere stability by facilitating replication fork re-initiation. J. Biol. Chem. 2010, 285, 27057–27066. [Google Scholar] [CrossRef]
- Teasley, D.C.; Parajuli, S.; Nguyen, M.; Moore, H.R.; Alspach, E.; Lock, Y.J.; Honaker, Y.; Saharia, A.; Piwnica-Worms, H.; Stewart, S.A. Flap endonuclease 1 limits telomere fragility on the leading strand. J. Biol. Chem. 2015, 290, 15133–15145. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Cho, Y. Structural and functional relationships of FAN1. DNA Repair (Amst). 2017, 56, 135–143. [Google Scholar] [CrossRef]
- Chaudhury, I.; Stroik, D.R.; Sobeck, A. FANCD2-Controlled Chromatin Access of the Fanconi-Associated Nuclease FAN1 Is Crucial for the Recovery of Stalled Replication Forks. Mol. Cell. Biol. 2014, 34, 3939–3954. [Google Scholar] [CrossRef]
- MacKay, C.; Déclais, A.C.; Lundin, C.; Agostinho, A.; Deans, A.J.; MacArtney, T.J.; Hofmann, K.; Gartner, A.; West, S.C.; Helleday, T.; Lilley, D.M.J.; Rouse, J. Identification of KIAA1018/FAN1, a DNA Repair Nuclease Recruited to DNA Damage by Monoubiquitinated FANCD2. Cell 2010, 142, 65–76. [Google Scholar] [CrossRef]
- Rao, T.; Longerich, S.; Zhao, W.; Aihara, H.; Sung, P.; Xiong, Y. Importance of homo-dimerization of Fanconi-associated nuclease 1 in DNA flap cleavage. DNA Repair (Amst). 2018, 64, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.N.; Usdin, K. FAN1 protects against repeat expansions in a Fragile X mouse model. DNA Repair (Amst). 2018, 69, 1–5. [Google Scholar] [CrossRef]
- Blanco, M.G.; Matos, J. Hold your horSSEs: Controlling structure-selective endonucleases MUS81 and Yen1/GEN1. Front. Genet. 2015, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.Y.; Lieber, M.R. Structure-Specific nuclease activities of Artemis and the Artemis: DNA-PKcs complex. Nucleic Acids Res. 2016, 44, 4991–4997. [Google Scholar] [CrossRef] [PubMed]
- Mayle, R.; Campbell, I.M.; Beck, C.R.; Yu, Y.; Wilson, M.; Shaw, C.A.; Bjergbaek, L.; Lupski, J.R.; Ira, G. Mus81 and converging forks limit the mutagenicity of replication fork breakage. Science 2015, 349, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Tanaka, H.; Horiuchi, T. Complex repeat structure promotes hyper-amplification and amplicon evolution through rolling-circle replication. Nucleic Acids Res. 2018, 46, 5097–5108. [Google Scholar] [CrossRef] [PubMed]
- Elango, R.; Sheng, Z.; Jackson, J.; Decata, J.; Ibrahim, Y.; Pham, N.T.; Liang, D.H.; Sakofsky, C.J.; Vindigni, A.; Lobachev, K.S.; Ira, G.; Malkova, A. Break-induced replication promotes formation of lethal joint molecules dissolved by Srs2. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, T.Y.; Su, S.C.; Yu, J.S.; Zhang, H.L.; Tan, G.Q.; Liu, J.W.; Wang, B.L. STC2 as a novel mediator for Mus81-dependent proliferation and survival in hepatocellular carcinoma. Cancer Lett. 2017, 388, 177–186. [Google Scholar] [CrossRef]
- Wu, F.; Su, S.C.; Tan, G.Q.; Yan, L.; Li, T.Y.; Zhang, H.L.; Yu, J.S.; Wang, B.L. Mus81 knockdown sensitizes colon cancer cells to chemotherapeutic drugs by activating CHK1 pathway. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.S.W.; Zhang, W.Y.L.; Tan, N.Y.J.; Khatoo, M.; Suter, M.A.; Tripathi, S.; Cheung, F.S.G.; Lim, W.K.; Tan, P.H.; Ngeow, J.; Gasser, S. The DNA Structure-Specific Endonuclease MUS81 Mediates DNA Sensor STING-Dependent Host Rejection of Prostate Cancer Cells. Immunity 2016, 44, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
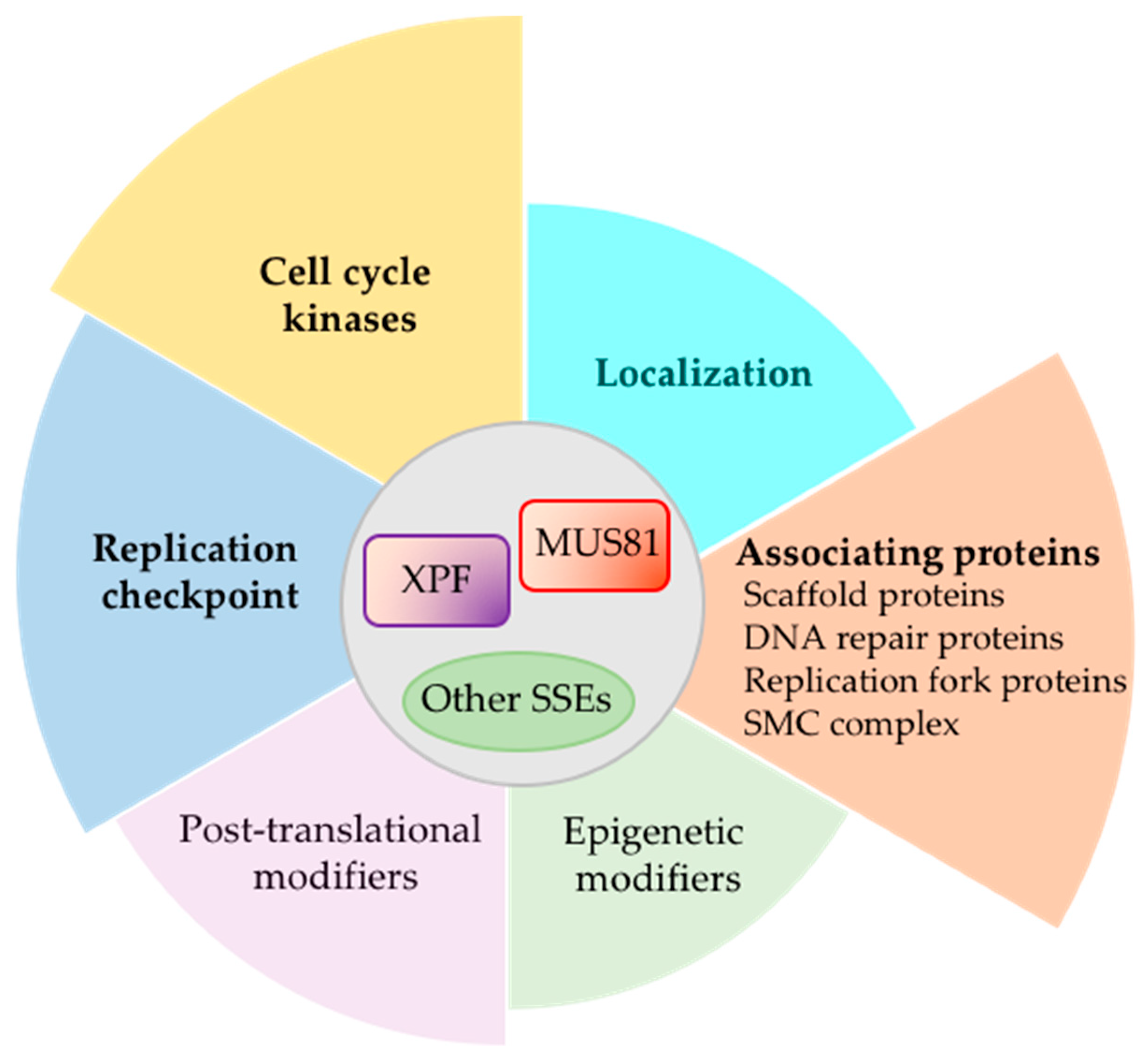
| Grouping of Regulatory Protein or Stimulus | Protein or Stimulus that Regulates SSE Activities | Organism | Effect on SSE | Reference |
|---|---|---|---|---|
| Mus81-Eme1 (S. p.)/Mus81-Mms4 (S. c.)/MUS81-EME1 (human) | ||||
| Players of DNA repair or replication | Rad52 | S. c., Human | Stimulate activity | [94,95,96] |
| Esc2 | S. c. | Stimulate activity | [75] | |
| RFC/PCNA | S. c. | Stimulate activity | [97] | |
| FANCD2 | Human | Promote recruitment & activity | Rev. in [98,99] | |
| RECQ5 helicase | Human | Promote recruitment to CFS | [65] | |
| SMC protein complex | Smc5/6 | S. c., Human | Stimulate activity | [99,100] |
| SMC2 | Human | Promote recruitment | [101] | |
| WAPL | Human | Promote recruitment | [101] | |
| Psm1 | S. p. | Stimulate activity | [102] | |
| Localization | Nucleolar | Human | Maintains repetitive nucleolar DNA | [103] |
| DNA damage-induced | S. c., Human | Maintains genome stability after DNA damage | [103,104] | |
| Post-translational modifier | SUMOylation | Human | Stimulate activity upon arsenic treatment | [105] |
| Epigenetic modifier | EZH2 | Human | Methylation on H3K27 at stalled replication fork stimulate recruitment | [106] |
| Scaffold protein | SLX4 | S. c., Human | Promote recruitment & activity | [11,12,13,14,15,60] |
| UHRF1 | Human | Promote recruitment | [107,108] | |
| Rad16-Swi10 (S. p.)/Rad1-Rad10 (S. c.)/XPF-ERCC1 (human) | ||||
| Players of DNA repair or replication | Rad14 | S. c. | Promote recruitment | [109] |
| RPA | Human | Stimulate activity | [110,111] | |
| Rad52 | Human | Stimulate activity | [110,112] | |
| FANCD2 | Human | Promote recruitment | Rev. in [98,99] | |
| Scaffold protein | Pxd1 | S. p. | Stimulate activity | [113] |
| SLX4 | S. c., Human | Promote recruitment & activity | [11,12,13,14,15] | |
| UHRF1 | Human | Promote recruitment | [107,108] | |
| DNA binding protein | Saw1 | S. c. | Promote recruitment | [114] |
| Rad2(S. p.)/Rad27(S. c.)/FEN1 (human) | ||||
| Players of DNA repair or replication | PCNA | Human | Promote recruitment & activity during Okazaki fragment maturation | Rev. in [115] |
| Rad9-Rad1-Hus1 complex | Human | Promotes activity during replication stress | [116] | |
| MUS81 | Human | Stimulate activity | [117,118,119] | |
| RECQ5 helicase WRN | Human | Promote recruitment & activity | [120,121,122] | |
| Post-translational modifier | SUMOylation | Human | Promotes association with Rad9-Rad1-Hus1 complex | [116] |
| Fan1(S. p.)/Absent in S. c./FAN1 (human) | ||||
| Players of DNA repair or replication | FANCD2 | Human | Promote recruitment | [123] |
| PCNA | Human | Promote recruitment | [124] | |
| Absent in S. p./Yen1 S. c./GEN1 (human) | ||||
| Localization | Cdc28 | S. c. | Nuclear exclusion at G1/S | [22,23,24] |
| Cdc14 | S. c. | Nuclear import at anaphase | [23] | |
| Nuclear Export Signal | Human | Nuclear exclusion until nuclear envelope breakdown | [25] | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.M.; Forsburg, S.L. Regulation of Structure-Specific Endonucleases in Replication Stress. Genes 2018, 9, 634. https://doi.org/10.3390/genes9120634
Kim SM, Forsburg SL. Regulation of Structure-Specific Endonucleases in Replication Stress. Genes. 2018; 9(12):634. https://doi.org/10.3390/genes9120634
Chicago/Turabian StyleKim, Seong Min, and Susan L. Forsburg. 2018. "Regulation of Structure-Specific Endonucleases in Replication Stress" Genes 9, no. 12: 634. https://doi.org/10.3390/genes9120634
APA StyleKim, S. M., & Forsburg, S. L. (2018). Regulation of Structure-Specific Endonucleases in Replication Stress. Genes, 9(12), 634. https://doi.org/10.3390/genes9120634





