Repetitive Fragile Sites: Centromere Satellite DNA as a Source of Genome Instability in Human Diseases
Abstract
:1. Current Overview of Human Centromeric DNA
2. Epigenetic Specification and Inheritance of Centromeres
3. Centromere Stability in Human Health and Disease
3.1. Human Immunodeficiency–Centromeric Instability–Facial Anomalies (ICF) Syndrome
3.2. Aging: Antagonistic Pleiotropy Applied to Centromeres
3.3. Cancer: The Multifaceted Role of Centromeres in Tumorigenesis
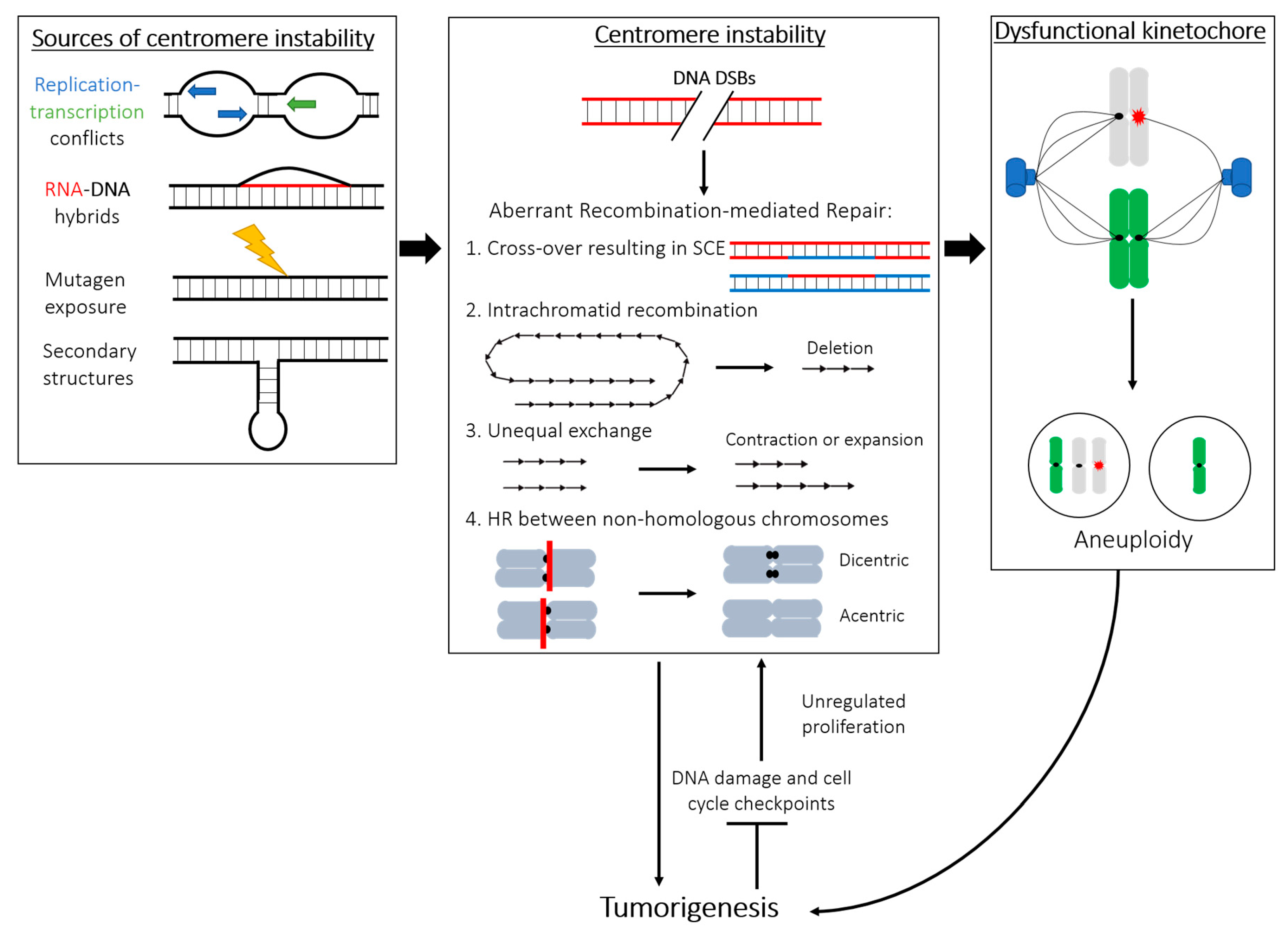
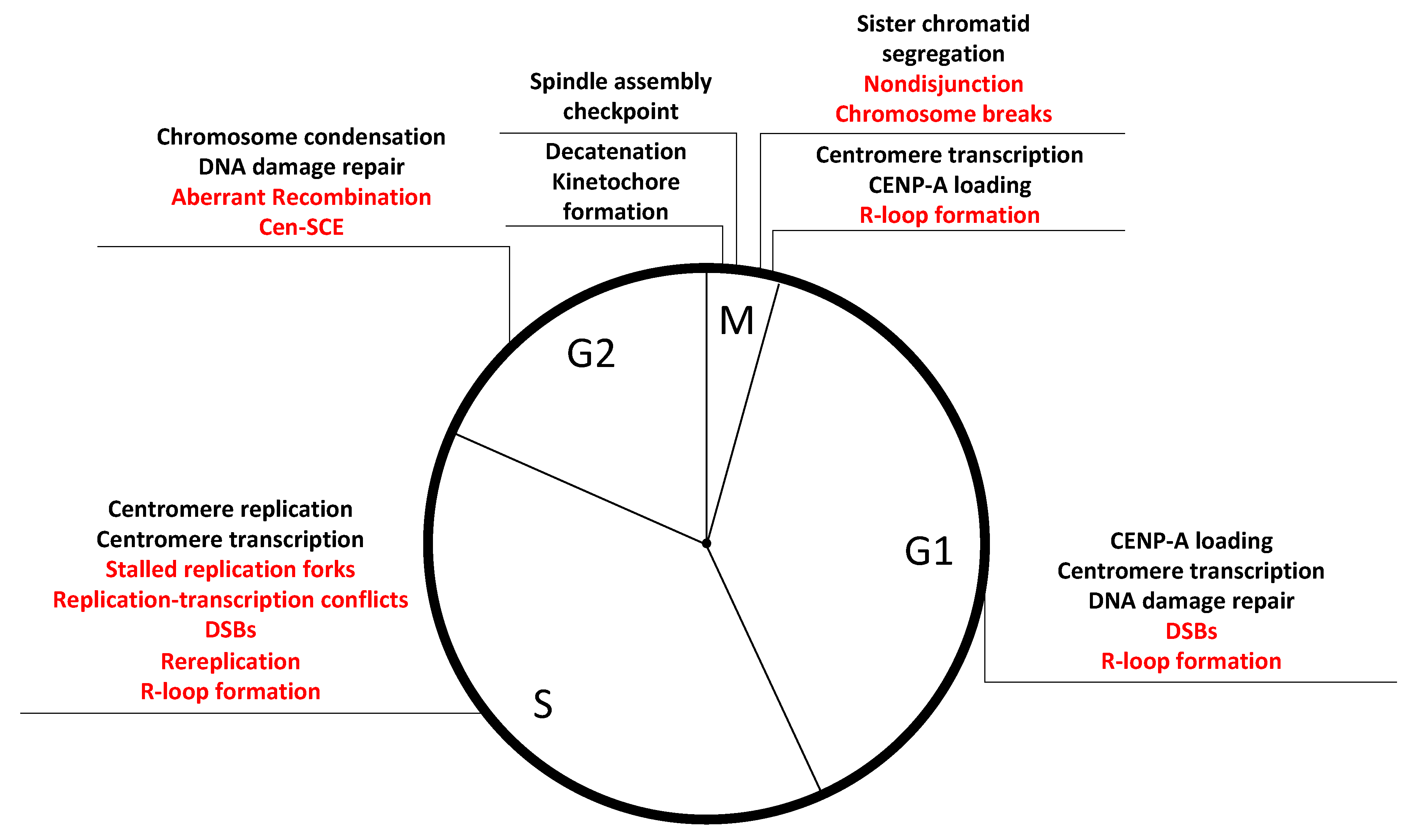
4. Sources of Instability within the Centromere DNA Repeats
4.1. Recombination and Repair at Centromeres: Errors in Copying and Mending Highly Repetitive DNA
4.2. Secondary Structures: Physical Hurdles and Barriers to DNA Repeats Stability
4.3. Repeating the Repeats: The Challenges of Centromere Replication
4.4. Breaking the Silence: Active Transcription of Centromere Alpha-Satellite Challenges Repeats Stability
4.5. Mitosis: A Tense Time for Centromeres
4.6. Transposable Elements (TEs) at the Centromere: Friends or Foes?
5. Future Directions: Novel Fragility of the Human Genome Specific to Centromeres
Acknowledgments
Conflicts of Interest
References
- Henikoff, S.; Dalal, Y. Centromeric chromatin: What makes it unique? Curr. Opin. Genet. Dev. 2005, 15, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Fukagawa, T. Assembly of kinetochores in vertebrate cells. Exp. Cell Res. 2004, 296, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.S.; Green, E.D.; Guttmacher, A.E.; Mark, S. A vision for the future of genomics research. Nature 2003, 422, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Koren, S.; Miga, K.H.; Quick, J.; Rand, A.C.; Sasani, T.A.; Tyson, J.R.; Beggs, A.D.; Dilthey, A.T.; Fiddes, I.T.; et al. Nanopore sequencing and assembly of a human genome with ultra-long reads. Nat. Biotechnol. 2018, 36, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Miga, K.H.; Newton, Y.; Jain, M.; Altemose, N.; Willard, H.F.; Kent, E.J. Centromere reference models for human chromosomes X and Y satellite arrays. Genome Res. 2014, 24, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Schneider, V.A.; Graves-Lindsay, T.; Howe, K.; Bouk, N.; Chen, H.-C.; Kitts, P.A.; Murphy, T.D.; Pruitt, K.D.; Thibaud-Nissen, F.; Albracht, D.; et al. Evaluation of GRCh38 and de novo haploid genome assemblies demonstrates the enduring quality of the reference assembly. Genome Res. 2017, 27, 849–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkan, C.; Ventura, M.; Archidiacono, N.; Rocchi, M.; Sahinalp, S.C.; Eichler, E.E. Organization and Evolution of Primate Centromeric DNA from Whole-Genome Shotgun Sequence Data. PLoS Comput. Biol. 2007, 3, 1807–1818. [Google Scholar] [CrossRef] [PubMed]
- Warburton, P.E.; Haaf, T.; Gosden, J.; Lawson, D.; Willard, H.F. Characterization of a Chromosome-Specific Chimpanzee Alpha Satellite Subset: Evolutionary Relationship to Subsets on Human Chromosomes. Genomics 1996, 33, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Aldrup-Macdonald, M.E.; Kuo, M.E.; Sullivan, L.L.; Chew, K.; Sullivan, B.A. Genomic variation within alpha satellite DNA influences centromere location on human chromosomes with metastable epialleles. Genome Res. 2016, 26, 1301–1311. [Google Scholar] [CrossRef] [Green Version]
- Fowler, C.; Drinkwater, R.; Burgoyne, L.; Skinner, J. Hypervariable lengths of human DNA associated with a human satellite HI sequence found in the 3.4kb Y-specific fragment. Nucleic Acids Res. 1987, 15, 3929. [Google Scholar] [CrossRef]
- van Dekken, H.; Arkesteijn, G.J.A.; Visser, J.W.M.; Bauman, J.G.J. Flow Cytometric Quantification of Human Chromosome Specific Repetitive DNA Sequences by Single and Bicolor Fluorescent In Situ Hybridization to Lymphocyte Interphase- Nuclei. Cytometry 1990, 11, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Altemose, N.; Miga, K.H.; Maggioni, M.; Willard, H.F. Genomic Characterization of Large Heterochromatic Gaps in the Human Genome Assembly. PLoS Comput. Biol. 2014, 10, e1003628. [Google Scholar] [CrossRef] [PubMed]
- Iwata-Otsubo, A.; Dawicki-McKenna, J.M.; Akera, T.; Falk, S.J.; Chmatal, L.; Yang, K.; Sullivan, B.A.; Schultz, R.M.; Lampson, M.A.; Black, B.E. Expanded satellite repeats amplify a discrete CENP-A nucleosome assembly site on chromosomes that drive in female meiosis. Curr. Biol. 2018, 27, 2365–2373. [Google Scholar] [CrossRef]
- Lampson, M.A.; Black, B.E. Cellular and Molecular Mechanisms of Centromere Drive. Cold Spring Harb. Symp. Quant. Biol. 2017, 82, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T. Regulation of ribosomal RNA gene copy number and its role in modulating genome integrity and evolutionary adaptability in yeast. Cell. Mol. Life Sci. 2011, 68, 1395–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, S.J.; O’Neill, R.J. Transposable elements: Genome innovation, chromosome diversity, and centromere conflict. Chromosome Res. 2018, 26, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.; Frommer, M.; Paul, C.; Vincent, P.C. Sequence relationships of three human satellite DNAs. J. Mol. Biol. 1986, 187, 145–155. [Google Scholar] [CrossRef]
- Plohl, M.; Meštrović, N.; Mravinac, B. Centromere identity from the DNA point of view. Chromosoma 2014, 123, 313–325. [Google Scholar] [CrossRef] [Green Version]
- McNulty, S.M.; Sullivan, B.A. Alpha satellite DNA biology: Finding function in the recesses of the genome. Chromosome Res. 2018, 26, 115–138. [Google Scholar] [CrossRef]
- Haaf, T.; Mater, A.; Wienberg, J.; Ward, D. Presence and abundance of CENP-B box sequences in great ape subsets of primate-specific α-satellite DNA. J. Mol. Evol. 1995, 41, 487–491. [Google Scholar] [CrossRef]
- Ohzeki, J.; Nakano, M.; Okada, T.; Masumoto, H. CENP-B box is required for de novo centromere chromatin assembly on human alphoid DNA. J. Cell Biol. 2002, 159, 765–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Castro, A.V.; Shamanski, F.L.; Meneses, J.J.; Lovato, T.L.; Vogel, K.G.; Moyzis, R.K.; Pedersen, R. Centromeric protein B null mice are viable with no apparent abnormalities. Dev. Biol. 1998, 201, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Gieni, R.S.; Chan, G.K.T.; Hendzel, M.J. Epigenetics regulate centromere formation and kinetochore function. J. Cell. Biochem. 2008, 104, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Blower, M.D.; Sullivan, B.A.; Karpen, G.H.; Jolla, L. Conserved Organization of Centromeric Chromatin in Flies and Humans. Dev. Cell 2002, 2, 319–330. [Google Scholar] [CrossRef]
- Malik, H.S.; Henikoff, S. Adaptive Evolution of Cid, a Centromere-Specific Histone in Drosophila. Genetics 2001, 157, 1293–1298. [Google Scholar] [PubMed]
- Fukagawa, T.; Earnshaw, W.C. The Centromere: Chromatin Foundation for the Kinetochore Machinery. Dev. Cell 2014, 30, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Burrack, L.S.; Berman, J. Neocentromeres and epigenetically inherited features of centromeres. Chromosome Res. 2013, 20, 607–619. [Google Scholar] [CrossRef]
- Bergmann, J.H.; Rodriquez, M.G.; Martins, N.M.; Kimura, H.; Kelly, D.A.; Masumoto, H.; Larionov, V.; Jansen, L.E.T.; Earnshaw, W.C. Epigenetic engineering shows H3K4me2 is required for HJURP targeting and CENP-A assembly on a synthetic human kinetochore. EMBO J. 2011, 30, 328–340. [Google Scholar] [CrossRef]
- Lam, A.L.; Boivin, C.D.; Bonney, C.F.; Rudd, M.K.; Sullivan, B.A. Human centromeric chromatin is a dynamic chromosomal domain that can spread over noncentromeric DNA. Proc. Natl. Acad. Sci. USA 2006, 103, 4186–4191. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, S.A.; Vagnarelli, P.; Dong, Y.; Hori, T.; McEwen, B.F.; Fukagawa, T.; Flors, C.; Earnshaw, W.C. A super-resolution map of the vertebrate kinetochore. Proc. Natl. Acad. Sci. USA 2010, 107, 10484–10489. [Google Scholar] [CrossRef] [Green Version]
- Hori, T.; Shang, W.H.; Toyoda, A.; Misu, S.; Monma, N.; Ikeo, K.; Molina, O.; Vargiu, G.; Fujiyama, A.; Kimura, H.; et al. Histone H4 Lys 20 Monomethylation of the CENP-A Nucleosome Is Essential for Kinetochore Assembly. Dev. Cell 2014, 29, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.O.; Panchenko, T.; Shabanowitz, J.; Lehman, S.M.; Bai, D.L.; Hunt, D.F.; Black, B.E.; Foltz, D.R. Identification of the Post-translational Modifications Present in Centromeric Chromatin. Mol. Cell. Proteom. 2016, 15, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.A.; Karpen, G.H. Centromeric chromatin exhibits a histone modification pattern that is distinct from both euchromatin and heterochromatin. Nat. Struct. Mol. Biol. 2004, 11, 1076–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmann, J.H.; Jakubsche, J.N.; Martins, N.M.; Kagansky, A.; Nakano, M.; Kimura, H.; Kelly, D.A.; Turner, B.M.; Masumoto, H.; Larionov, V.; et al. Epigenetic engineering: Histone H3K9 acetylation is compatible with kinetochore structure and function. J. Cell Sci. 2011, 125, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Saksouk, N.; Simboeck, E.; Déjardin, J. Constitutive heterochromatin formation and transcription in mammals. Epigenet. Chromatin 2015, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.C.; Karpen, G.H. Epigenetic regulation of heterochromatic DNA stability. Curr. Opin. Genet. Dev. 2010, 18, 204–211. [Google Scholar] [CrossRef]
- Jaco, I.; Vera, E.; Blasco, M.A. Centromere mitotic recombination in mammalian cells. J. Cell Biol. 2008, 181, 885–892. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Zhou, X.; Wang, W.; Deng, W.; Fang, J.; Hu, H.; Wang, Z.; Li, S.; Cui, L.; Shen, J.; et al. Dynamic Phosphorylation of CENP-A at Ser68 Orchestrates Its Cell-Cycle-Dependent Deposition at Centromeres. Dev. Cell 2015, 32, 68–81. [Google Scholar] [CrossRef]
- Niikura, Y.; Kitagawa, R.; Ogi, H.; Abdulle, R.; Pagala, V.; Kitagawa, K. CENP-A K124 Ubiquitylation Is Required for CENP-A Deposition at the Centromere. Dev. Cell 2015, 32, 589–603. [Google Scholar] [CrossRef] [Green Version]
- Bui, M.; Pitman, M.; Nuccio, A.; Roque, S.; Gregory, P.; Asp, D.; Lazar, A.N.; Papoian, G.A.; Dalal, Y. Internal modifications in the CENP—A nucleosome modulate centromeric dynamics. Epigenet. Chromatin 2017, 10, 17. [Google Scholar] [CrossRef]
- Bui, M.; Dimitriadis, E.K.; Hoischen, C.; An, E.; Quenetnet, D.; Giebe, S.; Nita-lazar, A.; Diekmann, S.; Dalal, Y. Cell-Cycle-Dependent Structural Transitions in the Human CENP-A Nucleosome In Vivo. Cell 2012, 150, 317–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, S.; Foltz, D.R. Posttranslational modifications of CENP-A: Marks of distinction. Chromosoma 2018, 127, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Jansen, L.E.T.; Black, B.E.; Foltz, D.R.; Cleveland, D.W. Propagation of centromeric chromatin requires exit from mitosis. J. Cell Biol. 2007, 176, 795–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, S.; Almouzni, G. Chromatin Dynamics during the Cell Cycle. Nat. Rev. Genet. 2017, 18, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Dunleavy, E.M.; Roche, D.; Tagami, H.; Lacoste, N.; Ray-Gallet, D.; Nakamura, Y.; Daigo, Y.; Nakatani, Y.; Almouzni-Pettinotti, G. HJURP Is a Cell-Cycle-Dependent Maintenance and Deposition Factor of CENP-A at Centromeres. Cell 2009, 137, 485–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellone, B.G.; Grive, K.J.; Shteyn, V.; Bowers, S.R.; Oderberg, I. Assembly of Drosophila Centromeric Chromatin Proteins during Mitosis. PLoS Genet. 2011, 7, e1002068. [Google Scholar] [CrossRef] [PubMed]
- Dunleavy, E.M.; Almouzni, G.; Karpen, G.H. H3.3 is deposited at centromeres in S phase as a placeholder for newly assembled CENP-A in G1 phase. Nucleus 2011, 2, 146–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nechemia-Arbely, Y.; Miga, K.H.; Shoshani, O.; Aslanian, A.; McMahon, M.A.; Lee, A.Y.; Fachinetti, D.; Yates, J.R., III; Ren, B.; Cleveland, D.W. DNA replication-mediated error correction of ectopic CENP-A deposition maintains centromere identity. bioRxiv 2018. [Google Scholar] [CrossRef]
- Zasadzińska, E.; Huang, J.; Bailey, A.O.; Guo, L.Y.; Lee, N.S.; Srivastava, S.; Wong, K.A.; French, B.T.; Black, B.E.; Foltz, D.R. Inheritance of CENP-A Nucleosomes during DNA Replication Requires HJURP. Dev. Cell 2018, 47, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.L.; Marshall, O.J.; Saffery, R.; Kim, B.W.; Earle, E.; Choo, K.H.A.; Wong, L.H. Active transcription and essential role of RNA polymerase II at the centromere during mitosis. Proc. Natl. Acad. Sci. USA 2012, 109, 1979–1984. [Google Scholar] [CrossRef] [Green Version]
- Bobkov, G.O.M.; Gilbert, N.; Heun, P. Centromere transcription allows CENP-A to transit from chromatin association to stable incorporation. J. Cell Biol. 2018, 217, 1957–1972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fachinetti, D.; Diego Folco, H.; Nechemia-Arbely, Y.; Valente, L.P.; Nguyen, K.; Wong, A.J.; Zhu, Q.; Holland, A.J.; Desai, A.; Jansen, L.E.T.; et al. A two-step mechanism for epigenetic specification of centromere identity and function. Nat. Cell Biol. 2013, 15, 1056–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKinley, K.L.; Cheeseman, I.M. The molecular basis for centromere identity and function. Nat. Rev. Mol. Cell Biol. 2016, 17, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Giunta, S.; Funabiki, H. Integrity of the human centromere DNA repeats is protected by CENP-A, CENP-C, and CENP-T. Proc. Natl. Acad. Sci. USA 2017, 114, 1928–1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giunta, S. Centromere Chromosome Orientation Fluorescent in situ Hybridization (Cen-CO-FISH) Detects Sister Chromatid Exchange at the Centromere in Human Cells. Bio-Protocol 2018, 8. [Google Scholar] [CrossRef]
- Knutsen, T.; Gobu, V.; Knaus, R.; Padilla-nash, H.; Augustus, M.; Strausberg, R.L.; Kirsch, I.R.; Sirotkin, K.; Ried, T. The Interactive Online SKY/M-FISH & CGH Database and the Entrez Cancer Chromosomes Search Database: Linkage of Chromosomal Aberrations with the Genome Sequence. Genes Chromosomes Cancer 2005, 44, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.-L.; Bestor, T.H.; Bourc’his, D.; Hsieh, C.-L.; Tommerup, N.; Bugge, M.; Hulten, M.; Qu, X.; Russo, J.J.; Viegas-Pequignot, E. Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature 1999, 402, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, P.E.; Ito, Y.; Grillo, G.; Wang, J.; Velasco, G.; Nitta, H.; Unoki, M.; Yoshihara, M.; Suyama, M.; Sun, Y.; et al. Mutations in CDCA7 and HELLS cause immunodeficiency–centromeric instability–facial anomalies syndrome. Nat. Commun. 2015, 6, 7870. [Google Scholar] [CrossRef] [Green Version]
- De Greef, J.C.; Wang, J.; Balog, J.; Den Dunnen, J.T.; Frants, R.R.; Straasheijm, K.R.; Aytekin, C.; Van Der Burg, M.; Duprez, L.; Ferster, A.; et al. Mutations in ZBTB24 Are Associated with Immunodeficiency, Centromeric Instability, and Facial Anomalies Syndrome Type 2. Am. J. Hum. Genet. 2011, 88, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Wijmenga, C.; Hansen, R.S.; Gimelli, G.; Björck, E.J.; Davies, E.G.; Valentine, D.; Belohradsky, B.H.; Van Dongen, J.J.; Smeets, D.F.C.M.; Van Den Heuvel, W.J.; et al. Genetic Variation in ICF Syndrome: Evidence for Genetic Heterogeneity. Hum. Mutat. 2000, 16, 509–517. [Google Scholar] [CrossRef]
- Miniou, P.; Jeanpierre, M.; Blanquet, V.; Sibella, V.; Bonneau, D.; Herbelin, C.; Fischer, A.; Niveleau, A.; Viegas-Péquignot, E. Abnormal methylation pattern in constitutive and facultative (X inactive chromosome) heterochromatin of ICF patients. Hum. Mol. Genet. 1994, 3, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Hagleitner, M.M.; Lankester, A.; Maraschio, P.; Hulte, M.; Fryns, J.P.; Schuetz, C.; Gimelli, G.; Davies, E.G.; Gennery, A.; Belohradsky, B.H.; et al. Clinical spectrum of immunodeficiency, centromeric instability and facial dysmorphism (ICF syndrome). J. Med. Genet. 2008, 45, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Unoki, M.; Funabiki, H.; Velasco, G.; Francastel, C.; Sasaki, H. CDCA7 and HELLS mutations undermine nonhomologous end joining in centromeric instability syndrome. J. Clin. Investig. 2018. [Google Scholar] [CrossRef] [PubMed]
- Jenness, C.; Giunta, S.; Müller, M.M.; Kimura, H.; Muir, T.W.; Funabiki, H. HELLS and CDCA7 comprise a bipartite nucleosome remodeling complex defective in ICF syndrome. Proc. Natl. Acad. Sci. USA 2018, 115, E876–E885. [Google Scholar] [CrossRef] [PubMed]
- Velasco, G.; Grillo, G.; Touleimat, N.; Ferry, L.; Chantalat, S.; Ivkovic, I.; Ribierre, F.; Picard, C.; Francastel, C. Comparative methylome analysis of ICF patients identifies heterochromatin loci that require ZBTB24, CDCA7 and HELLS for their methylated state. Hum. Mol. Genet. 2018, 27, 2409–2424. [Google Scholar] [CrossRef] [PubMed]
- Maestroni, L.; Matmati, S.; Coulon, S. Solving the telomere replication problem. Genes 2017, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- d’Adda di Fagagna, F.; Teo, S.-H.; Jackson, S.P. Functional links between telomeres and proteins of the DNA-damage response. Genes Dev. 2004, 18, 1781–1799. [Google Scholar] [CrossRef] [Green Version]
- Nakagome, Y.; Abe, T.; Misawa, S.; Takeshita, T.; Iinuma, K. The “loss” of centromeres from chromosomes of aged women. Am. J. Hum. Genet. 1984, 36, 398–404. [Google Scholar]
- Hédouin, S.; Grillo, G.; Ivkovic, I.; Velasco, G.; Francastel, C. CENP-A chromatin disassembly in stressed and senescent murine cells. Nat. Sci. Rep. 2016, 7, 42520. [Google Scholar] [CrossRef]
- Lee, S.H.; Itkin-Ansari, P.; Levine, F. CENP-A, a protein required for chromosome segregation in mitosis, declines with age in islet but not exocrine cells. Aging 2010, 2, 785–790. [Google Scholar] [CrossRef] [Green Version]
- Maehara, K.; Takahashi, K.; Saitoh, S. CENP-A Reduction Induces a p53-Dependent Cellular Senescence Response To Protect Cells from Executing Defective Mitoses. Mol. Cell. Biol. 2010, 30, 2090–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, E.C.; Manning, B.; Zhang, H.; Lawrence, J.B. Higher-order unfolding of satellite heterochromatin is a consistent and early event in cell senescence. J. Cell Biol. 2013, 203, 929–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukášová, E.; Kovařík, A.; Kozubek, S. Consequences of Lamin B1 and Lamin B Receptor Downregulation in Senescence. Cells 2018, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Xi, R.; Luquette, L.J.; Park, R.W.; Johnson, M.D.; Park, P.J. Functional genomic analysis of chromosomal aberrations in a compendium of 8000 cancer genomes. Genome Res. 2013, 23, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Ricke, R.M.; van Deursen, J.M. Aneuploidy in health, disease, and aging. J. Cell Biol. 2013, 201, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitelman, F.; Mertens, F.; Johansson, B. A breakpoint map of recurrent chromosomal rearangements in human neoplasia. Nat. Genet. 1997, 15, 417–474. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Nash, H.M.; Heselmeyer-Haddad, K.; Wangsa, D.; Zhang, H.; Ghadimi, B.M.; Macville, M.; Augustus, M.; Schröck, E.; Hilgenfeld, E.; Ried, T. Jumping translocations are common in solid tumor cell lines and result in recurrent fusions of whole chromosome arms. Genes Chromosome Cancer 2001, 30, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Martínez-A, C.; van Wely, K.H.M. Centromere fission, not telomere erosion, triggers chromosomal instability in human carcinomas. Carcinogenesis 2011, 32, 796–803. [Google Scholar] [CrossRef] [Green Version]
- Thompson, S.L.; Compton, D.A. Chromosome missegregation in human cells arises through specific types of kinetochore-microtubule attachment errors. Proc. Natl. Acad. Sci. USA 2011, 108, 17974–17978. [Google Scholar] [CrossRef] [Green Version]
- Takemura, H.; Rao, V.A.; Sordet, O.; Furuta, T.; Miao, Z.H.; Meng, L.H.; Zhang, H.; Pommier, Y. Defective Mre11-dependent activation of Chk2 by ataxia telangiectasia mutated in colorectal carcinoma cells in response to replication-dependent DNA double strand breaks. J. Biol. Chem. 2006, 281, 30814–30823. [Google Scholar] [CrossRef]
- Fournier, A.; Mcleer-florin, A.; Lefebvre, C.; Duley, S.; Debernardi, A.; Rousseaux, S.; De Fraipont, F.; Figeac, M.; Kerckaert, J.; De Vos, J.; et al. 1q12 chromosome translocations form aberrant heterochromatic foci associated with changes in nuclear architecture and gene expression in B cell lymphoma. EMBO Mol. Med. 2010, 2, 159–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrlich, M. DNA hypomethylation in cancer cells. Epigenomics 2009, 1, 239–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bersani, F.; Lee, E.; Kharchenko, P.V.; Xu, A.W.; Liu, M.; Xega, K.; MacKenzie, O.C.; Brannigan, B.W.; Wittner, B.S.; Jung, H.; et al. Pericentromeric satellite repeat expansions through RNA-derived DNA intermediates in cancer. Proc. Natl. Acad. Sci. USA 2015, 112, 15148–15153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomonaga, T.; Matsushita, K.; Yamaguchi, S.; Oohashi, T.; Shimada, H.; Ochiai, T.; Yoda, K.; Nomura, F. Overexpression and mistargeting of centromere protein-A in human primary colorectal cancer. Cancer Res. 2003, 63, 3511–3516. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Schillaci, T.; Lentini, L.; Di Leonardo, A. CENPA overexpression promotes genome instability in pRb-depleted human cells. Mol. Cancer 2009, 8, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhu, Z.; Zhang, S.; Yu, D.; Yu, H.; Liu, L.; Cao, X.; Wang, L.; Gao, H.; Zhu, M. ShRNA-Targeted Centromere Protein A Inhibits Hepatocellular Carcinoma Growth. PLoS ONE 2011, 6, e17794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mao, J.; Zhu, W.; Jain, A.K.; Liu, K.; Brown, J.B.; Karpen, G.H. Centromere and kinetochore gene misexpression predicts cancer patient survival and response to radiotherapy and chemotherapy. Nat. Commun. 2016, 7, 12619. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Clermont, P.L.; Jiao, W.; Helgason, C.D.; Gout, P.W.; Wang, Y.; Qu, S. Elevated expression of the centromere protein-A(CENP-A)-encoding gene as a prognostic and predictive biomarker in human cancers. Int. J. Cancer 2016, 139, 899–907. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.-J.; Guo, J.-J.; Lv, T.-J.; Jin, H.-Y.; Ding, J.-X.; Feng, W.-W.; Zhang, Y.; Hua, K.-Q. Prognostic value of centromere protein-A expression in patients with epithelial ovarian cancer. Tumor Biol. 2013, 34, 2971–2975. [Google Scholar] [CrossRef]
- Wu, Q.; Qian, Y.-M.; Zhao, X.-L.; Wang, S.-M.; Feng, X.-J.; Chen, X.-F.; Zhang, S.-H. Expression and prognostic significance of centromere protein A in lung adenocarcinoma. Lung Cancer 2012, 77, 407–414. [Google Scholar] [CrossRef]
- Lacoste, N.; Woolfe, A.; Tachiwana, H.; Garea, A.V.; Barth, T.; Cantaloube, S.; Kurumizaka, H.; Imhof, A.; Almouzni, G. Mislocalization of the Centromeric Histone Variant CenH3/CENP-A in Human Cells Depends on the Chaperone DAXX. Mol. Cell 2014, 53, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Athwal, R.K.; Walkiewicz, M.P.; Baek, S.; Fu, S.; Bui, M.; Camps, J.; Ried, T.; Sung, M.-H.; Dalal, Y. CENP-A nucleosomes localize to transcription factor hotspots and subtelomeric sites in human cancer cells. Epigenet. Chromatin 2015, 8, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nye, J.; Sturgill, D.; Athwal, R.; Dalal, Y. HJURP antagonizes CENP-A mislocalization driven by the H3.3 chaperones HIRA and DAXX. PLoS ONE 2018, 13, e0205948. [Google Scholar] [CrossRef] [PubMed]
- Nechemia-Arbely, Y.; Fachinetti, D.; Miga, K.H.; Sekulic, N.; Soni, G.V.; Kim, D.H.; Wong, A.K.; Lee, A.Y.; Nguyen, K.; Dekker, C.; et al. Human centromeric CENP-A chromatin is a homotypic, octameric nucleosome at all cell cycle points. J. Cell Biol. 2017, 216, 607–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, F.; van’t Veer, L.J.; Bogaerts, J.; Slaets, L.; Viale, G.; Delaloge, S.; Pierga, J.-Y.; Brain, E.; Causeret, S.; DeLorenzi, M.; et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N. Engl. J. Med. 2016, 375, 717–729. [Google Scholar] [CrossRef] [Green Version]
- Choo, K.H.A. Why Is the Centromere So Cold? Genome Res. 1998, 8, 81–82. [Google Scholar] [CrossRef] [Green Version]
- McFarlane, R.J.; Humphrey, T.C. A role for recombination in centromere function. Trends Genet. 2010, 26, 209–213. [Google Scholar] [CrossRef]
- Talbert, P.B.; Henikoff, S. Centromeres convert but don’t cross. PLoS Biol. 2010, 8, e1000326. [Google Scholar] [CrossRef]
- Durfy, S.J.; Willard, H.F. Concerted Evolution of Primate Alpha Satellite DNA Evidence for an Ancestral Sequence Shared by Gorilla and Human X Chromosome α Satellite. J. Mol. Biol. 1990, 216, 555–566. [Google Scholar] [CrossRef]
- Lee, H.; Hayden, K.E.; Willard, H.F. Organization and Molecular Evolution of CENP-A–Associated Satellite DNA Families in a Basal Primate Genome. Genome Biol. Evol. 2011, 3, 1136–1149. [Google Scholar] [CrossRef] [Green Version]
- Hayden, K.E.; Strome, E.D.; Merrett, S.L.; Lee, H.-R.; Rudd, M.K.; Willard, H.F. Sequences Associated with Centromere Competency in the Human Genome. Mol. Cell. Biol. 2013, 33, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, C.; Li, L.; Vogelstein, B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science 2017, 355, 1330–1334. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlesworth, B.; Sniegowski, P.; Stephan, W. The evolutionary dynamics of repetitive DNA in eukaryotes. Nature 1994, 371, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Kasinathan, S.; Henikoff, S. Non-B-Form DNA Is Enriched at Centromeres. Mol. Biol. Evol. 2018, 35, 949–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Bacolla, A.; Wang, G.; Vasquez, K.M. Non-B DNA structure-induced genetic instability and evolution. Cell. Mol. Life Sci. 2010, 67, 43–62. [Google Scholar] [CrossRef]
- Zhu, L.M.; Chou, S.H.; Reid, B.R. A single G-to-C change causes human centromere TGGAA repeats to fold back into hairpins. Proc. Natl. Acad. Sci. USA 1996, 93, 12159–12164. [Google Scholar] [CrossRef]
- Ohno, M.; Fukagawa, T.; Lee, J.S.; Ikemura, T. Triplex-forming DNAs in the human interphase nucleus visualized in situ by polypurine/polypyrimidine DNA probes and antitriplex antibodies. Chromosoma 2002, 111, 201–213. [Google Scholar] [CrossRef]
- Jonstrup, A.T.; Thomsen, T.; Wang, Y.; Knudsen, B.R.; Koch, J.; Andersen, A.H. Hairpin structures formed by alpha satellite DNA of human centromeres are cleaved by human topoisomerase IIα. Nucleic Acids Res. 2008, 36, 6165–6174. [Google Scholar] [CrossRef]
- Garavís, M.; Escaja, N.; Gabelica, V.; Villasante, A.; González, C. Centromeric Alpha-Satellite DNA Adopts Dimeric i-Motif Structures Capped by at Hoogsteen Base Pairs. Chemistry 2015, 21, 9816–9824. [Google Scholar] [CrossRef]
- Garavís, M.; Méndez-Lago, M.; Gabelica, V.; Whitehead, S.L.; González, C.; Villasante, A. The structure of an endogenous Drosophila centromere reveals the prevalence of tandemly repeated sequences able to form i-motifs. Sci. Rep. 2015, 5, 13307. [Google Scholar] [CrossRef] [PubMed]
- Aze, A.; Sannino, V.; Soffientini, P.; Bachi, A.; Costanzo, V. Centromeric DNA replication reconstitution reveals DNA loops and ATR checkpoint suppression. Nat. Cell Biol. 2016, 18, 684–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabeche, L.; Nguyen, H.D.; Buisson, R.; Zou, L. A mitosis-specific and R loop–driven ATR pathway promotes faithful chromosome segregation. Science 2018, 359, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, B.; Jin, W.; Wu, X.; Zhou, M.; Liu, V.Z.; Goel, A.; Shen, Z.; Zheng, L.; Shen, B. hDNA2 nuclease/helicase promotes centromeric DNA replication and genome stability. EMBO J. 2018, 37, e96729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romeo, F.; Falbo, L.; Costanzo, V. Replication, checkpoint suppression and structure of centromeric DNA. Nucleus 2016, 7, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Broderick, R.; Bergoglio, V.; Zimmer, J.; Badie, S.; Niedzwiedz, W.; Hoffmann, J.-S.; Tarsounas, M. MUS81 nuclease activity is essential for replication stress tolerance and chromosome segregation in BRCA2-deficient cells. Nat. Commun. 2016, 8, 15983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, J.; Suzuki, K.; Qu, J.; Wang, P.; Zhou, J.; Liu, X.; Ren, R.; Xu, X.; Ocampo, A.; et al. A Werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging. Science 2015, 348, 1160–1163. [Google Scholar] [CrossRef] [Green Version]
- Sidorova, J.M.; Li, N.; Folch, A.; Monnat, R.J.; Sidorova, J.M.; Li, N.; Folch, A.; Monnat, R.J.J. The RecQ helicase WRN is required for normal replication fork arrest progression after DNA damage or replication fork arrest. Cell Cycle 2008, 7, 796–807. [Google Scholar] [CrossRef]
- Sfeir, A.; Kosiyatrakul, S.T.; Hockemeyer, D.; MacRae, S.L.; Karlseder, J.; Schildkraut, C.L.; de Lange, T. Mammalian Telomeres Resemble Fragile Sites and Require TRF1 for Efficient Replication. Cell 2009, 138, 90–103. [Google Scholar] [CrossRef] [Green Version]
- Zeitlin, S.G.; Baker, N.M.; Chapados, B.R.; Soutoglou, E.; Wang, J.Y.J.; Berns, M.W.; Cleveland, D.W. Double-strand DNA breaks recruit the centromeric histone CENP-A. Proc. Natl. Acad. Sci. USA 2009, 106, 15762–15767. [Google Scholar] [CrossRef] [Green Version]
- McNulty, S.M.; Sullivan, L.L.; Sullivan, B.A. Human Centromeres Produce Chromosome-Specific and Array-Specific α Satellite Transcripts that Are Complexed with CENP-A and CENP-C. Dev. Cell 2017, 42, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Wimberly, H.; Shee, C.; Thornton, P.C.; Sivaramakrishnan, P.; Rosenberg, S.M.; Hastings, P.J. R-loops and nicks initiate DNA breakage and genome instability in non-growing Escherichia coli. Nat. Commun. 2013, 4, 2115. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Jinks-Robertson, S. Transcription as a source of genome instability. Nat. Rev. Genet. 2012, 13, 204–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marayati, B.F.; Drayton, A.L.; Tucker, J.F.; Huckabee, R.H.; Anderson, A.M.; Pease, J.B.; Zeyl, C.W.; Zhang, K. Loss of Elongation-Like Factor 1 Spontaneously Induces Diverse, RNase H-Related Suppressor Mutations in Schizosaccharomyces pombe. Genetics 2018, 209, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, V.O.; Venkitaraman, A.R. RNA Processing and Genome Stability: Cause and Consequence. Mol. Cell 2016, 61, 496–505. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, S.; Rusanov, T.; Kent, T.; Chandramouly, G.; Pomerantz, R.T. How RNA transcripts coordinate DNA recombination and repair. Nat. Commun. 2018, 9, 1091. [Google Scholar] [CrossRef] [PubMed]
- Greenfeder, S.A.; Newlon, C.S. Replication forks pause at yeast centromeres. Mol. Cell. Biol. 1992, 12, 4056–4066. [Google Scholar] [CrossRef]
- Song, W.; Dominska, M.; Greenwell, P.W.; Petes, T.D. Genome-wide high-resolution mapping of chromosome fragile sites in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2014, 111, E2210–E2218. [Google Scholar] [CrossRef]
- Barlow, J.; Faryabi, R.B.; Callen, E.; Wong, N.; Malhowski, A.; Chen, H.T.; Gutierez-cruz, G.; Sun, H.; Mckinnon, P.; Casellas, R.; et al. A novel class of early replicating fragile sites that contribute to genome instability in B cell lymphomas. Cell 2013, 152, 620–632. [Google Scholar] [CrossRef]
- Ohkuni, K.; Kitagawa, K. Endogenous Transcription at the Centromere Facilitates Centromere Activity in Budding Yeast. Curr. Biol. 2011, 21, 1695–1703. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.; Bloom, K. Genetic Manipulation of Centromere Function. Mol. Cell. Biol. 1987, 7, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.L.; Yewdell, W.T.; Bell, J.C.; Mcnulty, S.M.; Duda, Z.; O’Neill, R.J.; Sullivan, B.A.; Straight, A.F. RNA-dependent stabilization of SUV39H1 at constitutive heterochromatin. eLife 2017, 6, e25299. [Google Scholar] [CrossRef] [PubMed]
- Giunta, S.; Belotserkovskaya, R.; Jackson, S.P. DNA damage signaling in response to double- strand breaks during mitosis. J. Cell Biol. 2010, 190, 197–207. [Google Scholar] [CrossRef]
- Giunta, S.; Jackson, S.P. Give me a break, but not in mitosis. Cell Cycle 2011, 10, 1215–1221. [Google Scholar] [CrossRef] [Green Version]
- Jullien, D.; Vagnarelli, P.; Earnshaw, W.C.; Adachi, Y. Kinetochore localisation of the DNA damage response component 53BP1 during mitosis. J. Cell Sci. 2002, 115, 71–79. [Google Scholar]
- Guererro, A.A.; Gamero, M.C.; Trachana, V.; Fütterer, A.; Pacios-bras, C.; Díaz-Concha, N.P.; Cigudose, J.C.; Martínez-A, C.; Wely, K.H.M. van Centromere-localized breaks indicate the generation of DNA damage by the mitotic spindle. Proc. Natl. Acad. Sci. USA 2010, 107, 4159–4164. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.; Korner, R.; Hofmann, K.; Nigg, E.A. PICH, a Centromere-Associated SNF2 Family ATPase, Is Regulated by Plk1 and Required for the Spindle Checkpoint. Cell 2007, 128, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.-C.; Schwarzbraun, T.; Speicher, M.R.; Nigg, E.A. Persistence of DNA threads in human anaphase cells suggests late completion of sister chromatid decatenation. Chromosoma 2008, 117, 123–135. [Google Scholar] [CrossRef]
- O’Keefe, R.T.; Henderson, S.C.; Spector, D.L. Dynamic organization of DNA replication in mammalian cell nuclei: Spatially and temporally defined replication of chromosome-specific α- satellite DNA sequences. J. Cell Biol. 1992, 116, 1095–1110. [Google Scholar] [CrossRef] [PubMed]
- Ten Hagen, K.G.; Gilbert, D.M.; Willard, H.F.; Cohen, S.N. Replication timing of DNA sequences associated with human centromeres and telomeres. Mol. Cell. Biol. 1990, 10, 6348–6355. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.-C.; Mayer, B.; Stemmann, O.; Nigg, E.A. Centromere DNA decatenation depends on cohesin removal and is required for mammalian cell division. J. Cell Sci. 2010, 123, 806–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicklas, R.B. The Forces that Move Chromosomes in Mitosis. Annu. Rev. Biophys. Biophys. Chem. 1988, 17, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, I.M.; Desai, A. Molecular architecture of the kinetochore—Microtubule interface. Nat. Rev. Mol. Cell Biol. 2008, 9, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Kline, S.L.; Cheeseman, I.M.; Hori, T.; Fukagawa, T.; Desai, A. The human Mis12 complex is required for kinetochore assembly and proper chromosome segregation. J. Biol. Chem. 2006, 173, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendiburo, M.J.; Padeken, J.; Fülöp, S.; Schepers, A.; Heun, P. Drosophila CENH3 Is Sufficient for Centromere Formation. Science 2011, 334, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.; Van Der Burg, M.; Szuhai, K.; Kops, G.J.P.L.; Medema, R.H. Chromosome Segregation Errors as a Cause of DNA Damage and Structural Chromosome Aberrations. Science 2011, 333, 1895–1898. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, S.; Kawabe, A.; Kobayashi, A.; Ito, T.; Aizu, T.; Shin-i, T.; Toyoda, A.; Fujiyama, A.; Tarutani, Y.; Kakutani, T. Centromere-targeted de novo integrations of an LTR retrotransposon of Arabidopsis lyrata. Genes Dev. 2012, 26, 705–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birchler, J.A.; Presting, G.G. Retrotransposon insertion targeting: A mechanism for homogenization of centromere sequences on nonhomologous chromosomes. Genes Dev. 2012, 26, 638–640. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Yamazaki, T.; Miki, H.; Ogonuki, N.; Inoue, K.; Ogura, A.; Baba, T. Centromeric DNA hypomethylation as an epigenetic signature discriminates between germ and somatic cell lineages. Dev. Biol. 2007, 312, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Fultz, D.; Choudury, S.G.; Slotkin, R.K. Silencing of active transposable elements in plants. Curr. Opin. Plant Biol. 2015, 27, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [PubMed]
- Mills, R.E.; Bennett, E.A.; Iskow, R.C.; Devine, S.E. Which transposable elements are active in the human genome? Trends Genet. 2007, 23, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.F.A.; Riggs, A.D. Tiggers and other DNA transposon fossils in the human genome. Proc. Natl. Acad. Sci. USA 1996, 93, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Kipling, D.; Warburton, P.E. Centromeres, CENP-B and Tigger too. Trends Genet. 1997, 13, 141–145. [Google Scholar] [CrossRef]
- Garsed, D.W.; Marshall, O.J.; Corbin, V.D.A.; Hsu, A.; DiStefano, L.; Schröder, J.; Li, J.; Feng, Z.P.; Kim, B.W.; Kowarsky, M.; et al. The Architecture and Evolution of Cancer Neochromosomes. Cancer Cell 2014, 26, 653–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasson, D.; Alonso, A.; Cheung, F.; Tepperberg, J.H.; Papenhausen, P.R.; Engelen, J.J.M.; Warburton, P.E. Formation of novel CENP-A domains on tandem repetitive DNA and across chromosome breakpoints on human chromosome 8q21 neocentromeres. Chromosoma 2011, 120, 621–632. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Black, E.M.; Giunta, S. Repetitive Fragile Sites: Centromere Satellite DNA as a Source of Genome Instability in Human Diseases. Genes 2018, 9, 615. https://doi.org/10.3390/genes9120615
Black EM, Giunta S. Repetitive Fragile Sites: Centromere Satellite DNA as a Source of Genome Instability in Human Diseases. Genes. 2018; 9(12):615. https://doi.org/10.3390/genes9120615
Chicago/Turabian StyleBlack, Elizabeth M., and Simona Giunta. 2018. "Repetitive Fragile Sites: Centromere Satellite DNA as a Source of Genome Instability in Human Diseases" Genes 9, no. 12: 615. https://doi.org/10.3390/genes9120615
APA StyleBlack, E. M., & Giunta, S. (2018). Repetitive Fragile Sites: Centromere Satellite DNA as a Source of Genome Instability in Human Diseases. Genes, 9(12), 615. https://doi.org/10.3390/genes9120615




