Halotolerant Mycorrhizal Symbiosis Enhances Tolerance in Limonium Species Under Long-Term Salinity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Set Up
2.2. Stomatal Conductance and Chlorophyll Fluorescence
2.3. Ultrastructure of Leaf Epidermal Cells
2.4. Quantitative qRT-PCR
2.5. Statistical Analysis
3. Results
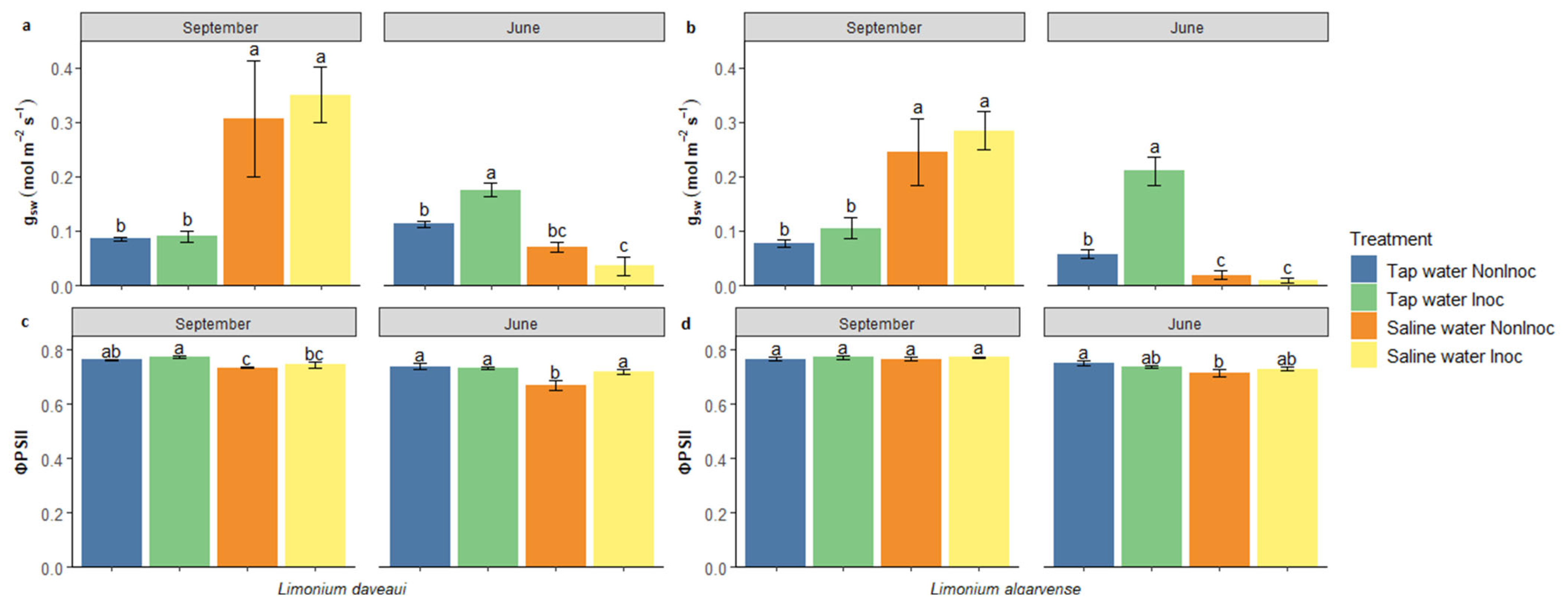
3.1. Physiological Status
3.2. Leaf Cell Composition
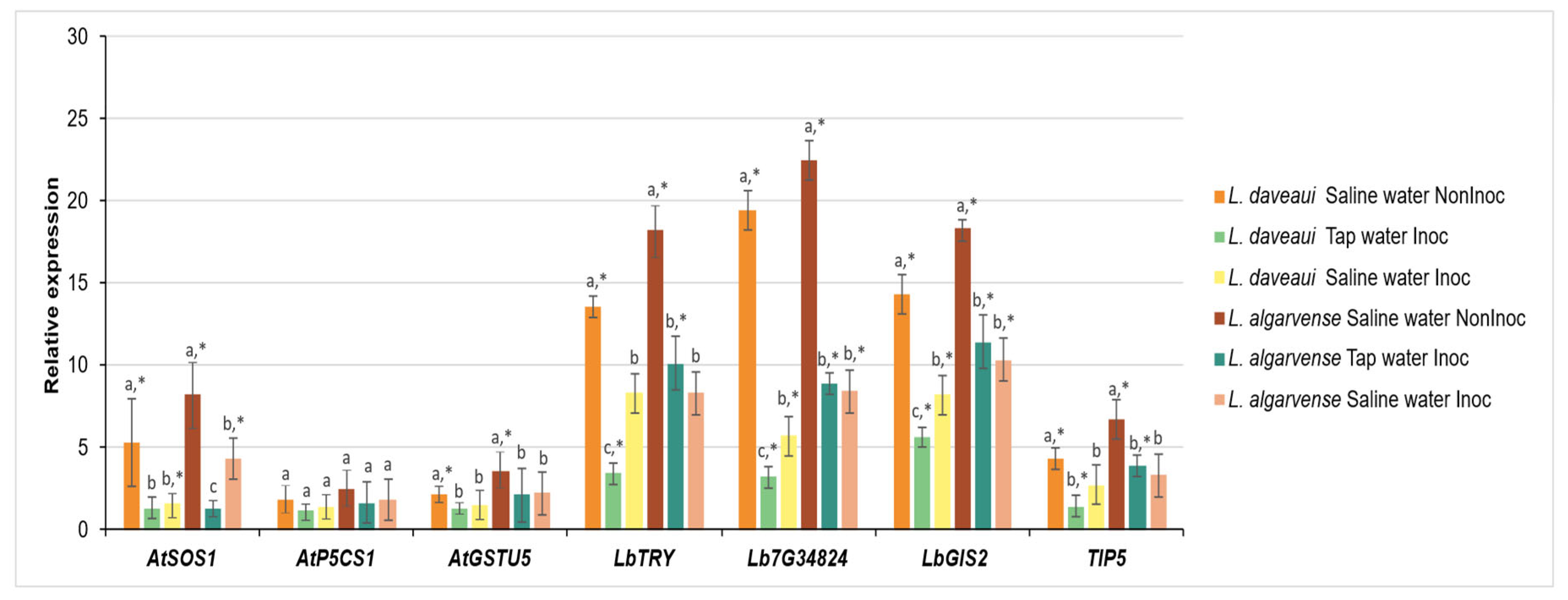
3.3. Gene Expression
4. Discussion
4.1. Halotolerant AMF Inoculation Enhances Stomatal Conductance and Photosynthesis
4.2. AMF Inoculation Improves Salinity Tolerance by Altering Leaf Cell Composition
4.3. Halotolerant AMF Inoculation Differentially Reduces the Expression of Salt-Related Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olsson, L.; Barbosa, H.; Bhadwal, S.; Cowie, A.; Delusca, K.; Flores-Renteria, D.; Hermans, K.; Jobbagy, E.; Kurz, W.; Li, D.; et al. Land Degradation. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Calvo Buendia, E., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019; pp. 345–436. [Google Scholar]
- Stavi, I.; Thevs, N.; Priori, S. Soil salinity and sodicity in drylands: A review of causes, effects, monitoring, and restoration measures. Front. Environ. Sci. 2021, 9, 330. [Google Scholar] [CrossRef]
- Daliakopoulos, I.N.; Tsanis, I.K.; Koutroulis, A.; Kourgialas, N.N.; Varouchakis, A.E.; Karatzas, G.P.; Ritsema, C.J. The threat of soil salinity: A European scale review. Sci. Total Environ. 2016, 573, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Ondrasek, G.; Rengel, Z. Environmental salinization processes: Detection, implications & solutions. Sci. Total Environ. 2021, 754, 142432. [Google Scholar]
- Kumar, A.; Mann, A.; Lata, C.; Kumar, N.; Sharma, P.C. Salinity-induced physiological and molecular responses of halophytes. In Research Developments in Saline Agriculture; Dagar, J.C., Ed.; Springer Nature: Singapore, 2019; pp. 331–356. [Google Scholar]
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef]
- Morton, M.J.; Awlia, M.; Al-Tamimi, N.; Saade, S.; Pailles, Y.; Negrão, S.; Tester, M. Salt stress under the scalpel–dissecting the genetics of salt tolerance. Plant J. 2019, 97, 148–163. [Google Scholar] [CrossRef]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Samadi, M.; Kazemeini, S.A.; Ozturk, M.; Ludwiczak, A.; Piernik, A. ROS homeostasis and antioxidants in the halophytic plants and seeds. Plants 2023, 12, 3023. [Google Scholar] [CrossRef]
- Aronson, J.A. Salt-Tolerant Plants of the World; University of Arizona: Tucson, AZ, USA, 1989. [Google Scholar]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Yuan, F.; Xu, Y.; Leng, B.; Wang, B. Beneficial effects of salt on halophyte growth: Morphology, cells, and genes. Open Life Sci. 2019, 14, 191–200. [Google Scholar] [CrossRef]
- Mann, A.; Lata, C.; Kumar, N.; Kumar, A.; Kumar, A.; Sheoran, P. Halophytes as new model plant species for salt tolerance strategies. Front. Plant Sci. 2023, 14, 1137211. [Google Scholar] [CrossRef]
- Estrada, B.; Aroca, R.; Maathuis, F.J.; Barea, J.M.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal fungi native from a Mediterranean saline area enhance maize tolerance to salinity through improved ion homeostasis. Plant Cell Environ. 2013, 36, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Zahir, Z.A.; Nadeem, S.M.; Khan, M.Y.; Binyamin, R.; Waqas, M.R. Role of halotolerant microbes in plant growth promotion under salt stress conditions. In Saline Soil-Based Agriculture by Halotolerant Microorganisms; Kumar, M., Etesami, H., Kumar, V., Eds.; Springer: Singapore, 2019; pp. 209–253. [Google Scholar]
- Pan, J.; Peng, F.; Tedeschi, A.; Xue, X.; Wang, J.; Liao, J.; Zhang, Z.; Huang, C. Do halophytes and glycophytes differ in their interactions with arbuscular mycorrhizal fungi under salt stress? A meta-analysis. Bot. Stud. 2020, 61, 13. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2012, 32, 181. [Google Scholar] [CrossRef]
- Kapoor, R.; Evelin, H.; Mathur, P.; Giri, B. Arbuscular mycorrhiza: Approaches for abiotic stress tolerance in crop plants for sustainable agriculture. In Plant Acclimation to Environmental Stress; Tuteja, N., Singh, S.G., Eds.; Springer: New York, NY, USA, 2013; pp. 359–401. [Google Scholar]
- Navarro-Torre, S.; Garcia-Caparrós, P.; Nogales, A.; Abreu, M.M.; Santos, E.; Cortinhas, A.L.; Caperta, A.D. Sustainable agricultural management of saline soils in arid and semi-arid Mediterranean regions through halophytes, microbial and soil-based technologies. Environ. Exp. Bot. 2023, 212, 105397. [Google Scholar] [CrossRef]
- Sharma, K.; Gupta, S.; Thokchom, S.D.; Jangir, P.; Kapoor, R. Arbuscular mycorrhiza mediated regulation of polyamines and aquaporins during abiotic stress: Deep insights on the recondite players. Front. Plant Sci. 2021, 12, 642101. [Google Scholar] [CrossRef]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef]
- Ganie, S.A.; Molla, K.A.; Henry, R.J.; Bhat, K.V.; Mondal, T.K. Advances in understanding salt tolerance in rice. Theor. Appl. Genet. 2019, 132, 851–870. [Google Scholar] [CrossRef]
- Sandhu, D.; Pudussery, M.V.; Kumar, R.; Pallete, A.; Markley, P.; Bridges, W.C.; Sekhon, R.S. Characterization of natural genetic variation identifies multiple genes involved in salt tolerance in maize. Funct. Integr. Genom. 2020, 20, 261–275. [Google Scholar] [CrossRef]
- Ding, Y.E.; Fan, Q.F.; He, J.D.; Wu, H.H.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Effects of mycorrhizas on physiological performance and root TIPs expression in trifoliate orange under salt stress. Arch. Agron. Soil Sci. 2020, 66, 182–192. [Google Scholar] [CrossRef]
- Yuan, F.; Leng, B.; Wang, B. Progress in studying salt secretion from the salt glands in recretohalophytes: How do plants secrete salt? Front. Plant Sci. 2016, 7, 977. [Google Scholar] [CrossRef]
- Dassanayake, M.; Larkin, J.C. Making plants break a sweat: The structure, function, and evolution of plant salt glands. Front. Plant Sci. 2017, 8, 406. [Google Scholar] [CrossRef]
- Caperta, A.D.; Róis, A.S.; Teixeira, G.; Garcia-Caparrós, P.; Flowers, T.J. Secretory structures in plants: Lessons from the Plumbaginaceae on their origin, evolution and roles in stress tolerance. Plant Cell Environ. 2020, 43, 2912–2931. [Google Scholar] [CrossRef]
- Faraday, C.D.; Thomson, W.W. Functional aspects of the salt glands of the Plumbaginaceae. J. Exp. Bot. 1986, 37, 1129–1135. [Google Scholar] [CrossRef]
- Feng, Z.T.; Sun, Q.J.; Deng, Y.Q.; Sun, S.F.; Zhang, J.G.; Wang, B.S. Study on pathway and characteristics of ion secretion of salt glands of Limonium bicolor. Acta Physiol. Plant. 2014, 36, 2729–2741. [Google Scholar] [CrossRef]
- Chater, C.; Caine, R.S.; Fleming, A.J.; Gray, J.E. Origins and evolution of stomatal development. Plant Physiol. 2017, 174, 624–638. [Google Scholar] [CrossRef]
- Yang, C.; Ye, Z. Trichomes as models for studying plant cell differentiation. Cell. Mol. Life Sci. 2013, 70, 1937–1948. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, B.; Jiao, X.; Chen, M.; Wang, B.; Yuan, F. Coupled development of salt glands, stomata, and pavement cells in Limonium bicolor. Front. Plant Sci. 2021, 12, 745422. [Google Scholar] [CrossRef]
- Cortinhas, A.; Caperta, A.D.; Teixeira, G.; Carvalho, L.; Abreu, M.M. Harnessing sediments of coastal aquaculture ponds through technosols construction for halophyte cultivation using saline water irrigation. J. Environ. Manag. 2020, 261, 109907. [Google Scholar] [CrossRef] [PubMed]
- Cortinhas, A.; Ferreira, T.C.; Abreu, M.M.; Caperta, A.D. Conservation of a critically endangered endemic halophyte of west Portugal: A microcosm assay to assess the potential of soil technology for species reintroduction. Front. Ecol. Evol. 2021, 9, 604509. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, X.; Zhao, B.; Xu, X.; Shi, M.; Leng, B.; Dong, X.; Lu, C.; Feng, Z.; Guo, J.; et al. The genome of the recretohalophyte Limonium bicolor provides insights into salt gland development and salinity adaptation during terrestrial evolution. Mol. Plant 2022, 15, 1024–1044. [Google Scholar] [CrossRef]
- Zhao, B.; Gao, Y.; Ma, Q.; Wang, X.; Zhu, J.K.; Li, W.; Wang, B.; Yuan, F. Global dynamics and cytokinin participation of salt gland development trajectory in recretohalophyte Limonium bicolor. Plant Physiol. 2024, 195, 2094–2110. [Google Scholar] [CrossRef]
- Nogales, A.; Navarro-Torre, S.; Abreu, M.M.; Santos, E.S.; Cortinhas, A.; Fors, R.; Bailly, M.; Róis, A.S.; Caperta, A.D. Unravelling the combined use of soil and microbial technologies to optimize cultivation of halophyte Limonium algarvense (Plumbaginaceae) using saline soils and water. Soil Syst. 2023, 7, 74. [Google Scholar] [CrossRef]
- Gomes-Domingues, C. Do Halotolerant Mycorrhizae Contribute to Halophyte Limonium Species Growth in Saline Conditions? Master’s Thesis, Instituto Superior de Agronomia, University of Lisbon, Lisbon, Portugal, 2025. [Google Scholar]
- Róis, A.S.; Teixeira, G.; Sharbel, T.F.; Fuchs, J.; Martins, S.; Espírito-Santo, D.; Caperta, A.D. Male fertility versus sterility, cytotype, and DNA quantitative variation in seed production in diploid and tetraploid sea lavenders (Limonium sp., Plumbaginaceae) reveal diversity in reproduction modes. Sex. Plant Reprod. 2012, 25, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Balasooriya, B.L.W.K.; Samson, R.; Mbikwa, F.; Boeckx, P.; Van Meirvenne, M. Biomonitoring of urban habitat quality by anatomical and chemical leaf characteristics. Environ. Exp. Bot. 2009, 65, 386–394. [Google Scholar] [CrossRef]
- Marques, I.; Fernandes, I.; David, P.H.C.; Paulo, O.S.; Goulão, L.F.; Fortunato, A.S.; Lidon, F.C.; Damatta, F.M.; Ramalho, J.C.; Ribeiro-Barros, A.I. Transcriptomic leaf profiling reveals differential responses of the two most traded coffee species to elevated [CO2]. Int. J. Mol. Sci. 2020, 21, 9211. [Google Scholar] [CrossRef] [PubMed]
- Leng, B.; Wang, X.; Yuan, F.; Zhang, H.; Lu, C.; Chen, M.; Wang, B. Heterologous expression of the Limonium bicolor MYB transcription factor LbTRY in Arabidopsis thaliana increases salt sensitivity by modifying root hair development and osmotic homeostasis. Plant Sci. 2021, 302, 110704. [Google Scholar] [CrossRef]
- Zou, H.; Leng, B.; Gao, Y.; Wang, B.; Yuan, F. The MYB transcription factor LbCPC of Limonium bicolor negatively regulates salt gland development and salt tolerance. Environ. Exp. Bot. 2023, 209, 105310. [Google Scholar] [CrossRef]
- Caperta, A.D.; Fernandes, I.; Conceição, S.I.; Marques, I.; Róis, A.S.; Paulo, O.S. Ovule transcriptome analysis discloses deregulation of genes and pathways in sexual and apomictic Limonium species (Plumbaginaceae). Genes 2023, 14, 901. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3-new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Balnokin, Y.V.; Kurkova, E.B.; Myasoedov, N.A.; Lun’kov, R.V.; Shamsutdinov, N.Z.; Egorova, E.A.; Bukhov, N.G. Structural and functional state of thylakoids in a halophyte Suaeda altissima before and after disturbance of salt–water balance by extremely high concentrations of NaCl. Russ. J. Plant Physiol. 2004, 51, 815–821. [Google Scholar] [CrossRef]
- Zhang, S.; Song, J.; Wang, H.; Feng, G. Effect of salinity on photosynthesis and chloroplasts ultrastructure in cotyledons of desiccated seeds of halophytes or xerophyte growing in central Asia. J. Plant Ecol. 2010, 3, 259–267. [Google Scholar] [CrossRef]
- Zia, S.; Egan, T.P.; Khan, M.A. Growth and selective ion transport of Limonium stocksii Plumbaginaceae under saline conditions. Pak. J. Bot. 2008, 40, 697–709. [Google Scholar]
- Yin, D.; Zhang, J.; Jing, R.; Qu, Q.; Guan, H.; Zhang, L.; Dong, L. Effect of salinity on ion homeostasis in three halophyte species, Limonium bicolor, Vitex trifolia Linn. var. simplicifolia Cham and Apocynaceae venetum. Acta Physiol. Plant. 2018, 40, 40. [Google Scholar] [CrossRef]
- Buckley, T.N. Modeling stomatal conductance. Plant Physiol. 2017, 174, 572–582. [Google Scholar] [CrossRef]
- Hetherington, A.; Woodward, F. The role of stomata in sensing and driving environmental change. Nature 2023, 424, 901–908. [Google Scholar] [CrossRef]
- Chaves, M.; Pereira, J.S.; Maroco, J. Understanding plant response to drought-from genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Galmés, J.; Flexas, J.; Savé, R.; Medrano, H. Water relations and stomatal characteristics of Mediterranean plants with different growth forms and leaf habits: Responses to water stress and recovery. Plant Soil 2007, 290, 139–155. [Google Scholar] [CrossRef]
- Sade, N.; Gebremedhin, A.; Moshelion, M. Risk-taking plants: Anisohydric behavior as a stress-resistance trait. Plant Signal. Behav. 2012, 7, 767–770. [Google Scholar] [CrossRef]
- Liu, C.; Dai, Z.; Cui, M.; Lu, W.; Sun, H. Arbuscular mycorrhizal fungi alleviate boron toxicity in Puccinellia tenuiflora under the combined stresses of salt and drought. Environ. Pollut. 2018, 240, 557–565. [Google Scholar] [CrossRef]
- Mi, P.; Yuan, F.; Guo, J.; Han, G.; Wang, B. Salt glands play a pivotal role in the salt resistance of four recretohalophyte Limonium Mill. species. Plant Biol. 2021, 23, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Rozema, J.; Riphagen, I. Physiology and ecologic relevance of salt secretion by the salt gland of Glaux maritima L. Oecologia 1977, 29, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, Y.; Mi, P.; Wang, B.; Yuan, F. Salt-tolerance screening in Limonium sinuatum varieties with different flower colors. Sci. Rep. 2021, 11, 14562. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Liang, X.; Li, Y.; Yin, S.; Wang, B. Methyl jasmonate improves salinity tolerance in Limonium bicolor by enhancing photosynthesis and abaxial salt gland density. Funct. Plant Biol. 2018, 46, 82–92. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Tian, C.Y.; Feng, G.; Li, X.L.; Zhang, F.S. Different effects of arbuscular mycorrhizal fungal isolates from saline or non-saline soil on salinity tolerance of plants. Appl. Soil Ecol. 2004, 26, 143–148. [Google Scholar] [CrossRef]
- Rani, A.; Bhat, M.A.; Verma, R.K. Plant growth-promoting rhizobacteria improve salinity tolerance through alterations in antioxidative enzyme activities and gene expression in Oryza sativa. Plant Physiol. Rep. 2021, 26, 241–250. [Google Scholar]
- Singh, R.P.; Jha, P.N.; Shukla, R.B. Endophytic bacterial consortia mitigate salt stress in tomato by modulating growth, antioxidant responses, and stress gene expression. Environ. Exp. Bot. 2023, 210, 105967. [Google Scholar]
- Timmusk, S.; Behers, L.; Muthoni, J.; Muraya, A.; Aronsson, A.C. Perspectives and challenges of microbial application for crop improvement. Front. Plant Sci. 2022, 13, 837928. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Beattie, G.A. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microb. 2018, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Redman, R.S.; Kim, Y.O.; Woodward, C.J.D.A.; Greer, C.; Espino, L.; Doty, S.L.; Rodriguez, R.J. Increased fitness of rice plants to abiotic stress via habitat-adapted symbiosis: A strategy for mitigating impacts of climate change. PLoS ONE 2011, 6, e14823. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, F.; Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. Influence of salinity on the in vitro development of Glomus intraradices and on the in vivo physiological and molecular responses of mycorrhizal lettuce plants. Microb. Ecol. 2008, 55, 45–53. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes-Domingues, C.; Marques, I.; Simões Costa, M.C.; Caperta, A.D. Halotolerant Mycorrhizal Symbiosis Enhances Tolerance in Limonium Species Under Long-Term Salinity. Genes 2025, 16, 1084. https://doi.org/10.3390/genes16091084
Gomes-Domingues C, Marques I, Simões Costa MC, Caperta AD. Halotolerant Mycorrhizal Symbiosis Enhances Tolerance in Limonium Species Under Long-Term Salinity. Genes. 2025; 16(9):1084. https://doi.org/10.3390/genes16091084
Chicago/Turabian StyleGomes-Domingues, Catarina, Isabel Marques, Maria Cristina Simões Costa, and Ana D. Caperta. 2025. "Halotolerant Mycorrhizal Symbiosis Enhances Tolerance in Limonium Species Under Long-Term Salinity" Genes 16, no. 9: 1084. https://doi.org/10.3390/genes16091084
APA StyleGomes-Domingues, C., Marques, I., Simões Costa, M. C., & Caperta, A. D. (2025). Halotolerant Mycorrhizal Symbiosis Enhances Tolerance in Limonium Species Under Long-Term Salinity. Genes, 16(9), 1084. https://doi.org/10.3390/genes16091084







