Identification of Key Genes and Pathways Associated with Frailty and Exercise Effects Using a Network and Evolutionary Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Microarray Data Acquisition and Sample Description
2.2. Differentially Expressed Gene Screening
2.3. WGCNA
2.4. Gene Annotation and Enrichment Analysis
2.5. Construction of a Human PPI Network
2.6. PPI Subnetworks Among Proteins Encoded by Frailty- and Exercise-Associated Genes
2.7. Enrichment Analysis of HAR, PS, and Aging Genes
3. Results
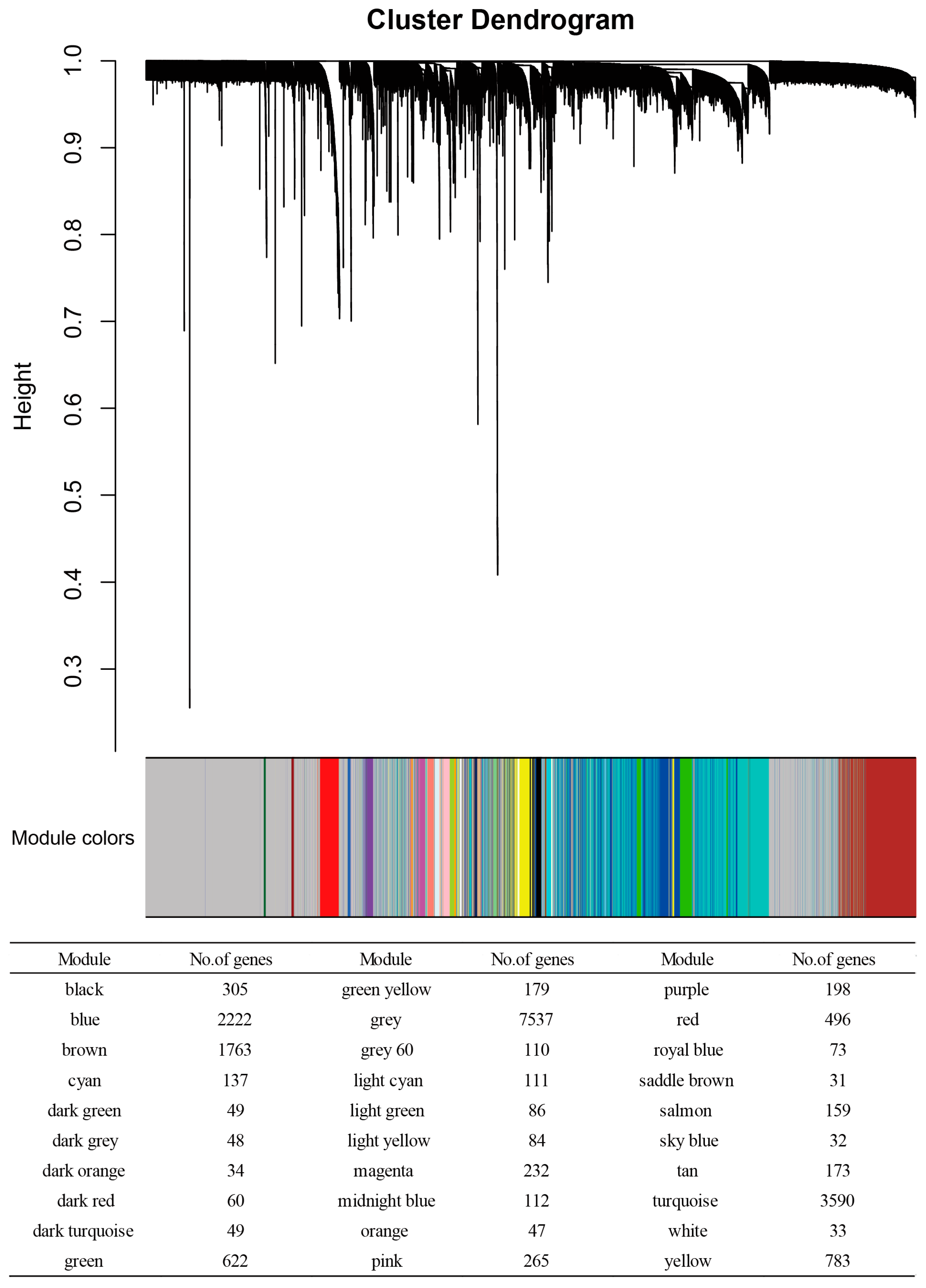
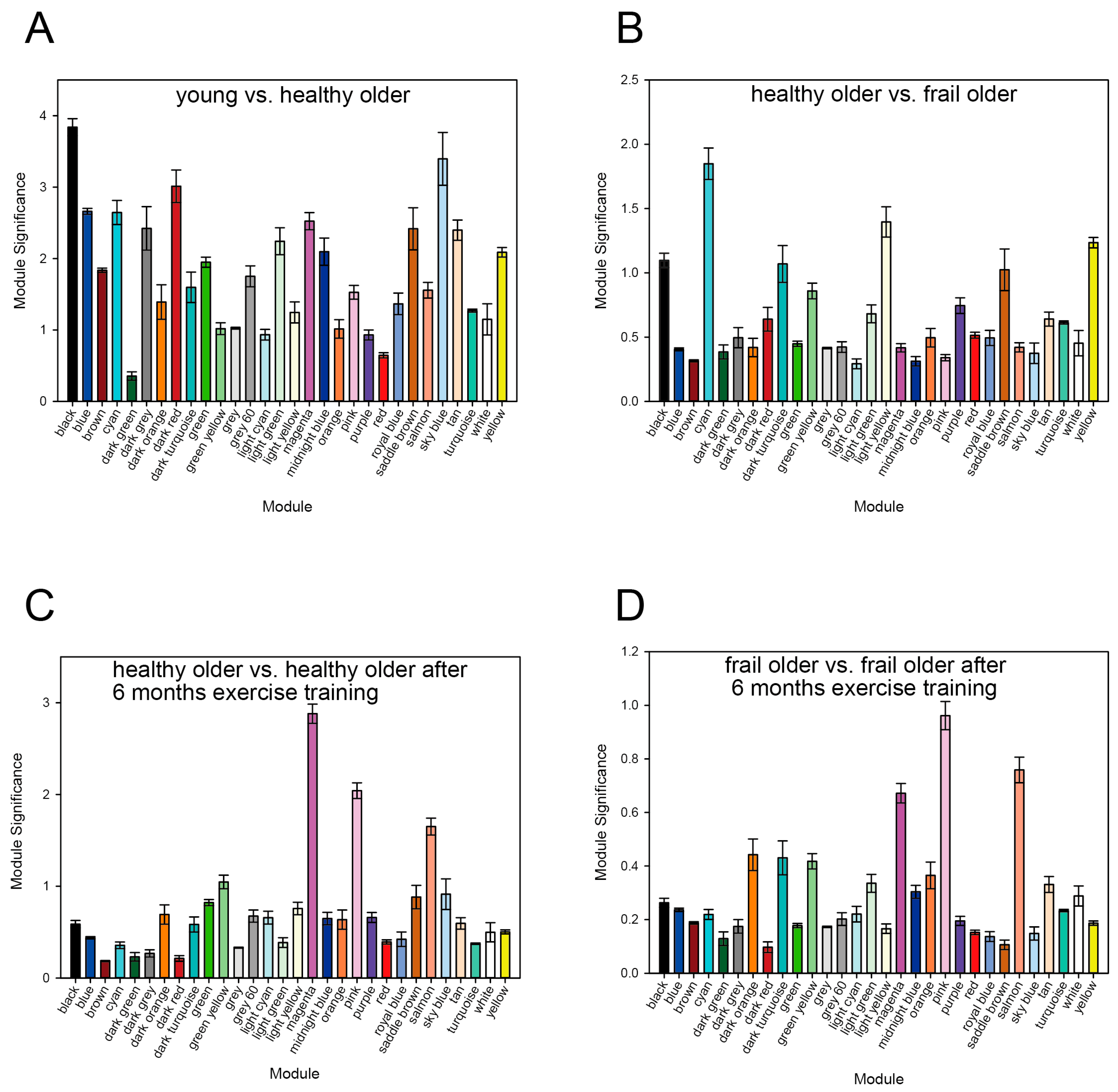
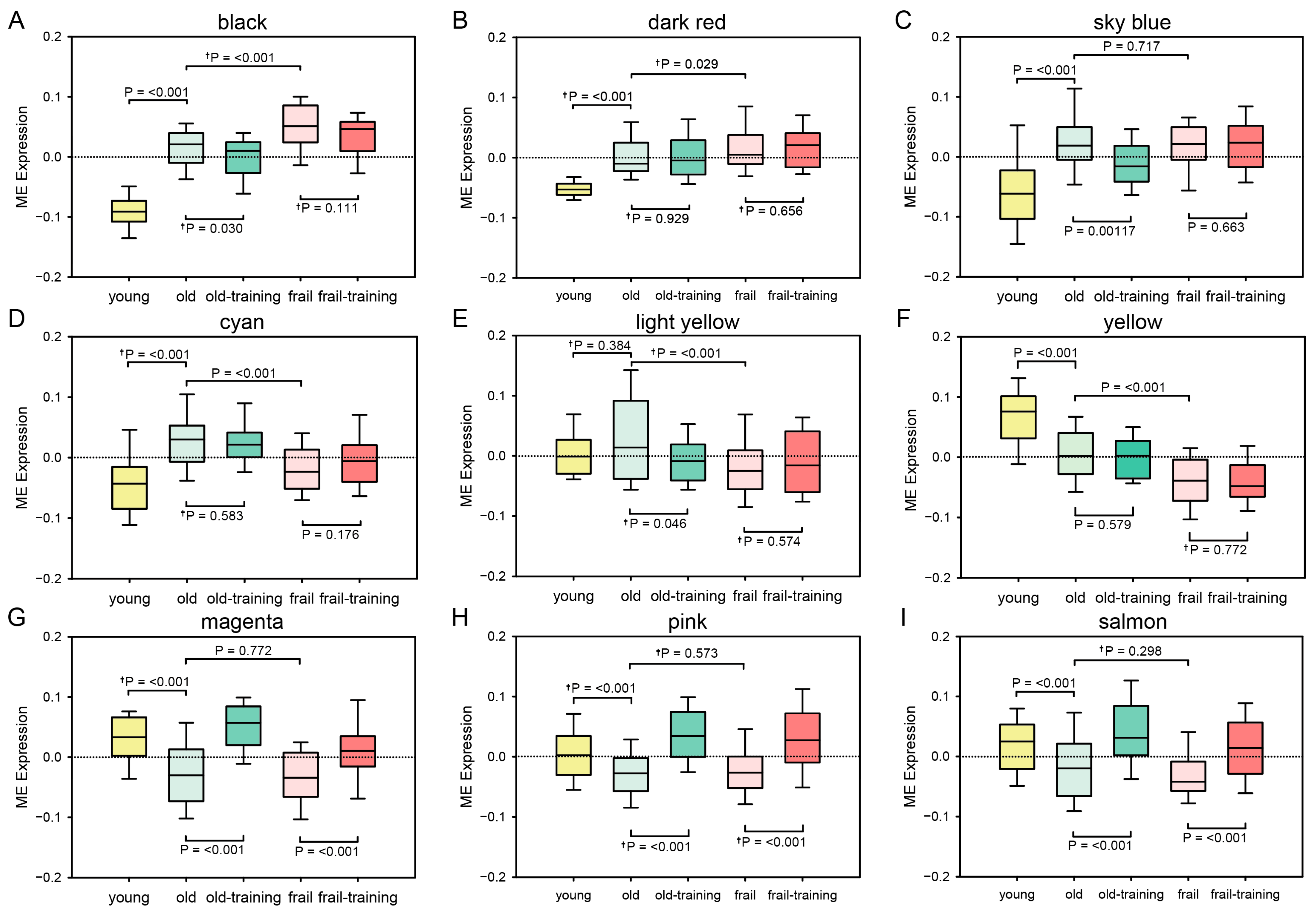
3.1. WGCNA Results
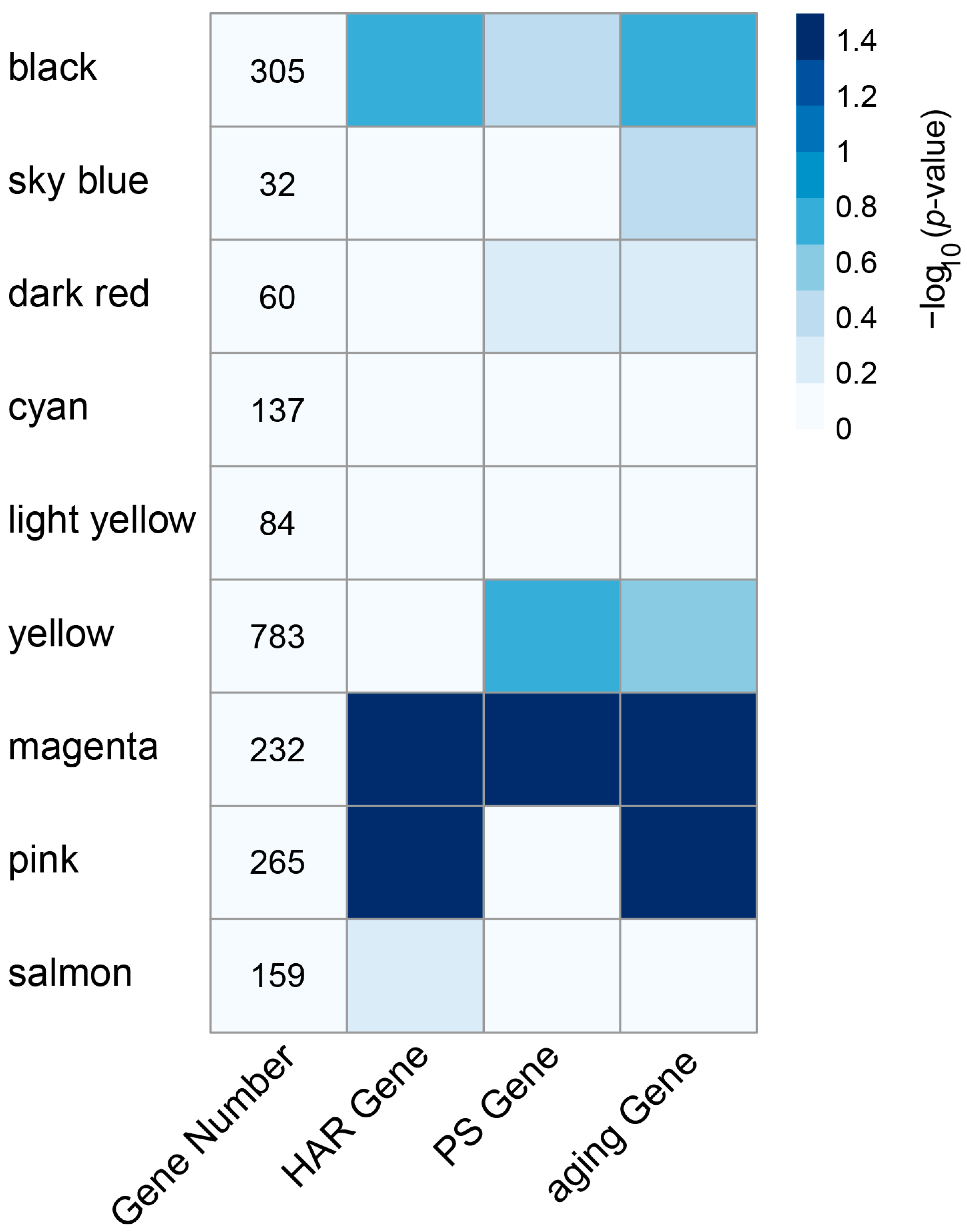
3.2. Enrichment of HAR, PS, and Aging Genes in Each Module
3.3. GO Analysis
3.4. PPI Analysis and the Identification of Densely Connected Clusters
3.5. Enrichment of HAR, PS, and Aging Genes in the PPI Clusters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Chen, X.; Mao, G.; Leng, S.X. Frailty syndrome: An overview. Clin. Interv. Aging 2014, 9, 433–441. [Google Scholar] [CrossRef]
- Morley, J.E. Frailty and sarcopenia in elderly. Wien. Klin. Wochenschr. 2016, 128, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.A.; Segev, D.L.; Pronovost, P.J.; Syin, D.; Bandeen-Roche, K.; Patel, P.; Takenaga, R.; Devgan, L.; Holzmueller, C.G.; Tian, J.; et al. Frailty as a predictor of surgical outcomes in older patients. J. Am. Coll. Surg. 2010, 210, 901–908. [Google Scholar] [CrossRef]
- Kojima, G.; Iliffe, S.; Jivraj, S.; Walters, K. Association between frailty and quality of life among community-dwelling older people: A systematic review and meta-analysis. J. Epidemiol. Community Health 2016, 70, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.L. The frailty syndrome: Definition and natural history. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef]
- Doody, P.; Lord, J.M.; Greig, C.A.; Whittaker, A.C. Frailty: Pathophysiology, Theoretical and Operational Definition(s), Impact, Prevalence, Management and Prevention, in an Increasingly Economically Developed and Ageing World. Gerontology 2023, 69, 927–945. [Google Scholar] [CrossRef]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of deficits as a proxy measure of aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.M.; Song, X.; Rockwood, K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J. Am. Geriatr. Soc. 2004, 52, 1929–1933. [Google Scholar] [CrossRef]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 2021, 50, 96–104. [Google Scholar] [CrossRef]
- Vina, J.; Tarazona-Santabalbina, F.J.; Perez-Ros, P.; Martinez-Arnau, F.M.; Borras, C.; Olaso-Gonzalez, G.; Salvador-Pascual, A.; Gomez-Cabrera, M.C. Biology of frailty: Modulation of ageing genes and its importance to prevent age-associated loss of function. Mol. Aspects Med. 2016, 50, 88–108. [Google Scholar] [CrossRef]
- Perazza, L.R.; Brown-Borg, H.M.; Thompson, L.V. Physiological systems in promoting frailty. Compr. Physiol. 2022, 12, 3575–3620. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Tong, C.; Ho, F.; Woo, J. Effects of a multicomponent frailty prevention program in prefrail community-dwelling older persons: A randomized controlled trial. J. Am. Med. Dir. Assoc. 2020, 21, 294.e1–294.e10. [Google Scholar] [CrossRef]
- Angulo, J.; El Assar, M.; Alvarez-Bustos, A.; Rodriguez-Manas, L. Physical activity and exercise: Strategies to manage frailty. Redox Biol. 2020, 35, 101513. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Lopez, L.; Maseda, A.; de Labra, C.; Regueiro-Folgueira, L.; Rodriguez-Villamil, J.L.; Millan-Calenti, J.C. Nutritional determinants of frailty in older adults: A systematic review. BMC Geriatr. 2017, 17, 108. [Google Scholar] [CrossRef]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Merchant, R.A.; Chen, M.Z.; Tan, L.W.L.; Lim, M.Y.; Ho, H.K.; van Dam, R.M. Singapore Healthy Older People Everyday (HOPE) Study: Prevalence of frailty and associated factors in older adults. J. Am. Med. Dir. Assoc. 2017, 18, 734.e9–734.e14. [Google Scholar] [CrossRef]
- Bonaga, B.; Sanchez-Jurado, P.M.; Martinez-Reig, M.; Ariza, G.; Rodriguez-Manas, L.; Gnjidic, D.; Salvador, T.; Abizanda, P. Frailty, Polypharmacy, and health outcomes in older adults: The Frailty and Dependence in Albacete Study. J. Am. Med. Dir. Assoc. 2018, 19, 46–52. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Fernandes, A.; Aguilar-Pimentel, J.A.; de Angelis, M.H.; Guedes, J.R.; Brito, M.A.; Ortolano, S.; Pani, G.; Athanasopoulou, S.; Gonos, E.S.; et al. Towards frailty biomarkers: Candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res. Rev. 2018, 47, 214–277. [Google Scholar] [CrossRef]
- Chu, X.Y.; Quan, Y.; Zhang, H.Y. Human accelerated genome regions with value in medical genetics and drug discovery. Drug Discov. Today 2020, 25, 821–827. [Google Scholar] [CrossRef]
- Doan, R.N.; Bae, B.I.; Cubelos, B.; Chang, C.; Hossain, A.A.; Al-Saad, S.; Mukaddes, N.M.; Oner, O.; Al-Saffar, M.; Balkhy, S.; et al. Mutations in human accelerated regions disrupt cognition and social behavior. Cell 2016, 167, 341–354.e12. [Google Scholar] [CrossRef]
- Srinivasan, S.; Bettella, F.; Hassani, S.; Wang, Y.; Witoelar, A.; Schork, A.J.; Thompson, W.K.; Collier, D.A.; Desikan, R.S.; Melle, I.; et al. Probing the association between early evolutionary markers and schizophrenia. PLoS ONE 2017, 12, e0169227. [Google Scholar] [CrossRef]
- Xu, K.; Schadt, E.E.; Pollard, K.S.; Roussos, P.; Dudley, J.T. Genomic and network patterns of schizophrenia genetic variation in human evolutionary accelerated regions. Mol. Biol. Evol. 2015, 32, 1148–1160. [Google Scholar] [CrossRef]
- Bufill, E.; Blesa, R.; Augusti, J. Alzheimer’s disease: An evolutionary approach. J. Anthropol. Sci. 2013, 91, 135–157. [Google Scholar] [CrossRef]
- Bektas, A.; Schurman, S.H.; Sen, R.; Ferrucci, L. Aging, inflammation and the environment. Exp. Gerontol. 2018, 105, 10–18. [Google Scholar] [CrossRef]
- Seo, A.Y.; Leeuwenburgh, C. The role of genome instability in frailty: Mitochondria versus Nucleus. Nestle Nutr. Inst. Workshop Ser. 2015, 83, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Booth, L.N.; Brunet, A. The aging epigenome. Mol. Cell 2016, 62, 728–744. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Sas, K.; Szabo, E.; Vecsei, L. Mitochondria, oxidative stress and the kynurenine system, with a focus on ageing and neuroprotection. Molecules 2018, 23, 191. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, D.E. Is exercise really medicine? An evolutionary perspective. Curr. Sports Med. Rep. 2015, 14, 313–319. [Google Scholar] [CrossRef]
- Hangelbroek, R.W.; Fazelzadeh, P.; Tieland, M.; Boekschoten, M.V.; Hooiveld, G.J.; van Duynhoven, J.P.; Timmons, J.A.; Verdijk, L.B.; de Groot, L.C.; van Loon, L.J.; et al. Expression of protocadherin gamma in skeletal muscle tissue is associated with age and muscle weakness. J. Cachexia Sarcopenia Muscle 2016, 7, 604–614. [Google Scholar] [CrossRef]
- Kallio, M.A.; Tuimala, J.T.; Hupponen, T.; Klemela, P.; Gentile, M.; Scheinin, I.; Koski, M.; Kaki, J.; Korpelainen, E.I. Chipster: User-friendly analysis software for microarray and other high-throughput data. BMC Genom. 2011, 12, 507. [Google Scholar] [CrossRef]
- Zhang, B.; Horvath, S. A general framework for weighted gene co-expression network analysis. Stat. Appl. Genet. Mol. Biol. 2005, 4, 17. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pages, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Razick, S.; Magklaras, G.; Donaldson, I.M. iRefIndex: A consolidated protein interaction database with provenance. BMC Bioinform. 2008, 9, 405. [Google Scholar] [CrossRef]
- Huttlin, E.L.; Bruckner, R.J.; Paulo, J.A.; Cannon, J.R.; Ting, L.; Baltier, K.; Colby, G.; Gebreab, F.; Gygi, M.P.; Parzen, H.; et al. Architecture of the human interactome defines protein communities and disease networks. Nature 2017, 545, 505–509. [Google Scholar] [CrossRef]
- Menche, J.; Sharma, A.; Kitsak, M.; Ghiassian, S.D.; Vidal, M.; Loscalzo, J.; Barabasi, A.L. Disease networks. Uncovering disease-disease relationships through the incomplete interactome. Science 2015, 347, 1257601. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Bader, G.D.; Hogue, C.W. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8 (Suppl. S4), S11. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Z.; Sun, M.; Liu, Q.; Wei, M.; Gao, H. Identification of cancer/testis antigen 2 gene as a potential hepatocellular carcinoma therapeutic target by hub gene screening with topological analysis. Oncol. Lett. 2019, 18, 4778–4788. [Google Scholar] [CrossRef]
- Murga-Moreno, J.; Coronado-Zamora, M.; Bodelon, A.; Barbadilla, A.; Casillas, S. PopHumanScan: The online catalog of human genome adaptation. Nucleic Acids Res. 2019, 47, D1080–D1089. [Google Scholar] [CrossRef]
- MacArthur, J.; Bowler, E.; Cerezo, M.; Gil, L.; Hall, P.; Hastings, E.; Junkins, H.; McMahon, A.; Milano, A.; Morales, J.; et al. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog). Nucleic Acids Res. 2017, 45, D896–D901. [Google Scholar] [CrossRef]
- Pinero, J.; Bravo, A.; Queralt-Rosinach, N.; Gutierrez-Sacristan, A.; Deu-Pons, J.; Centeno, E.; Garcia-Garcia, J.; Sanz, F.; Furlong, L.I. DisGeNET: A comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017, 45, D833–D839. [Google Scholar] [CrossRef]
- Pollard, K.S.; Salama, S.R.; King, B.; Kern, A.D.; Dreszer, T.; Katzman, S.; Siepel, A.; Pedersen, J.S.; Bejerano, G.; Baertsch, R.; et al. Forces shaping the fastest evolving regions in the human genome. PLoS Genet. 2006, 2, e168. [Google Scholar] [CrossRef]
- Kondoh, K.; Akahori, H.; Muto, Y.; Terada, T. Identification of key genes and pathways associated with preeclampsia by a WGCNA and an evolutionary approach. Genes 2022, 13, 2134. [Google Scholar] [CrossRef]
- Takahashi, Y.; Terada, T.; Muto, Y. Systems level analysis and identification of pathways and key genes associated with delirium. Genes 2020, 11, 1225. [Google Scholar] [CrossRef]
- Ohsawa, S.; Umemura, T.; Terada, T.; Muto, Y. Network and evolutionary analysis of human epigenetic regulators to unravel disease associations. Genes 2020, 11, 1457. [Google Scholar] [CrossRef]
- Wei, Y.; de Lange, S.C.; Scholtens, L.H.; Watanabe, K.; Ardesch, D.J.; Jansen, P.R.; Savage, J.E.; Li, L.; Preuss, T.M.; Rilling, J.K.; et al. Genetic mapping and evolutionary analysis of human-expanded cognitive networks. Nat. Commun. 2019, 10, 4839. [Google Scholar] [CrossRef]
- Sharma, A.; Menche, J.; Huang, C.C.; Ort, T.; Zhou, X.; Kitsak, M.; Sahni, N.; Thibault, D.; Voung, L.; Guo, F.; et al. A disease module in the interactome explains disease heterogeneity, drug response and captures novel pathways and genes in asthma. Hum. Mol. Genet. 2015, 24, 3005–3020. [Google Scholar] [CrossRef]
- Kelley, J.L.; Swanson, W.J. Positive selection in the human genome: From genome scans to biological significance. Annu. Rev. Genom. Hum. Genet. 2008, 9, 143–160. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Domenech, E.; Vina, J. Moderate exercise is an antioxidant: Ipregulation of antioxidant genes by training. Free Radic. Biol. Med. 2008, 44, 126–131. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Cobley, J.N.; Paschalis, V.; Veskoukis, A.S.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Principles for integrating reactive species into in vivo biological processes: Examples from exercise physiology. Cell. Signal. 2016, 28, 256–271. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Redox basis of exercise physiology. Redox Biol. 2020, 35, 101499. [Google Scholar] [CrossRef]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Mavropalias, G.; Boppart, M.; Usher, K.M.; Grounds, M.D.; Nosaka, K.; Blazevich, A.J. Exercise builds the scaffold of life: Muscle extracellular matrix biomarker responses to physical activity, inactivity, and aging. Biol. Rev. Camb. Philos. Soc. 2023, 98, 481–519. [Google Scholar] [CrossRef]
- Basso, K.; Margolin, A.A.; Stolovitzky, G.; Klein, U.; Dalla-Favera, R.; Califano, A. Reverse engineering of regulatory networks in human B cells. Nat. Genet. 2005, 37, 382–390. [Google Scholar] [CrossRef]
- Crombach, A.; Hogeweg, P. Evolution of evolvability in gene regulatory networks. PLoS Comput. Biol. 2008, 4, e1000112. [Google Scholar] [CrossRef]
- Patel, S.; Leal, A.D.; Gorski, D.H. The homeobox gene Gax inhibits angiogenesis through inhibition of nuclear factor-kappaB-dependent endothelial cell gene expression. Cancer Res. 2005, 65, 1414–1424. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, C.; Feng, J.B.; Zhao, Y.X.; Wang, X.P.; Yang, J.M.; Zhang, M.X.; Wang, X.L.; Zhang, Y. Cross talk among Smad, MAPK, and integrin signaling pathways enhances adventitial fibroblast functions activated by transforming growth factor-beta1 and inhibited by Gax. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 725–731. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, C.; Zhao, Y.X.; Feng, J.B.; Liu, C.X.; Chen, W.Q.; Yao, G.H.; Zhang, M.; Wang, X.L.; Zhang, Y. Gax gene transfer inhibits vascular remodeling induced by adventitial inflammation in rabbits. Atherosclerosis 2010, 212, 398–405. [Google Scholar] [CrossRef]
- Rovelet-Lecrux, A.; Legallic, S.; Wallon, D.; Flaman, J.M.; Martinaud, O.; Bombois, S.; Rollin-Sillaire, A.; Michon, A.; Le Ber, I.; Pariente, J.; et al. A genome-wide study reveals rare CNVs. exclusive to extreme phenotypes of Alzheimer disease. Eur. J. Hum. Genet. 2012, 20, 613–617. [Google Scholar] [CrossRef]
- Otto, A.; Macharia, R.; Matsakas, A.; Valasek, P.; Mankoo, B.S.; Patel, K. A hypoplastic model of skeletal muscle development displaying reduced foetal myoblast cell numbers, increased oxidative myofibres and improved specific tension capacity. Dev. Biol. 2010, 343, 51–62. [Google Scholar] [CrossRef]
- Tanaka, O.; Kondo, H. Localization of mRNAs for three novel members (beta 3, beta 4 and gamma 2) of phospholipase C family in mature rat brain. Neurosci. Lett. 1994, 182, 17–20. [Google Scholar] [CrossRef]
- Kim, D.; Jun, K.S.; Lee, S.B.; Kang, N.G.; Min, D.S.; Kim, Y.H.; Ryu, S.H.; Suh, P.G.; Shin, H.S. Phospholipase C isozymes selectively couple to specific neurotransmitter receptors. Nature 1997, 389, 290–293. [Google Scholar] [CrossRef]
- Chen, X.; Du, Y.; Broussard, G.J.; Kislin, M.; Yuede, C.M.; Zhang, S.; Dietmann, S.; Gabel, H.; Zhao, G.; Wang, S.S.; et al. Transcriptomic mapping uncovers Purkinje neuron plasticity driving learning. Nature 2022, 605, 722–727. [Google Scholar] [CrossRef]
- Shimomura, Y.; Wajid, M.; Ishii, Y.; Shapiro, L.; Petukhova, L.; Gordon, D.; Christiano, A.M. Disruption of P2RY5, an orphan G protein-coupled receptor, underlies autosomal recessive woolly hair. Nat. Genet. 2008, 40, 335–339. [Google Scholar] [CrossRef]
- Sokolov, E.; Eheim, A.L.; Ahrens, W.A.; Walling, T.L.; Swet, J.H.; McMillan, M.T.; Simo, K.A.; Thompson, K.J.; Sindram, D.; McKillop, I.H. Lysophosphatidic acid receptor expression and function in human hepatocellular carcinoma. J. Surg. Res. 2013, 180, 104–113. [Google Scholar] [CrossRef]
- Ishii, S.; Hirane, M.; Fukushima, K.; Tomimatsu, A.; Fukushima, N.; Tsujiuchi, T. Diverse effects of LPA4, LPA5 and LPA6 on the activation of tumor progression in pancreatic cancer cells. Biochem. Biophys. Res. Commun. 2015, 461, 59–64. [Google Scholar] [CrossRef]
- Ketscher, A.; Jilg, C.A.; Willmann, D.; Hummel, B.; Imhof, A.; Russeler, V.; Holz, S.; Metzger, E.; Muller, J.M.; Schule, R. LSD1 controls metastasis of androgen-independent prostate cancer cells through PXN and LPAR6. Oncogenesis 2014, 3, e120. [Google Scholar] [CrossRef]
- Kometani, K.; Yamada, T.; Sasaki, Y.; Yokosuka, T.; Saito, T.; Rajewsky, K.; Ishiai, M.; Hikida, M.; Kurosaki, T. CIN85 drives B cell responses by linking BCR signals to the canonical NF-kappaB pathway. J. Exp. Med. 2011, 208, 1447–1457. [Google Scholar] [CrossRef]
- Dawkins, E.; Small, D.H. Insights into the physiological function of the beta-amyloid precursor protein: Beyond Alzheimer’s disease. J. Neurochem. 2014, 129, 756–769. [Google Scholar] [CrossRef]
- Park, S.Y.; Kang, J.Y.; Lee, T.; Nam, D.; Jeon, C.J.; Kim, J.B. SPON1 can reduce amyloid beta and reverse cognitive impairment and memory dysfunction in Alzheimer’s disease mouse model. Cells 2020, 9, 1275. [Google Scholar] [CrossRef]
- Maltais, M.; De Souto Barreto, P.; Hooper, C.; Payoux, P.; Rolland, Y.; Vellas, B.; Group, M.D.S. Association Between brain beta-amyloid and frailty in older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 74, 1747–1752. [Google Scholar] [CrossRef]
- De la Rosa, A.; Olaso-Gonzalez, G.; Arc-Chagnaud, C.; Millan, F.; Salvador-Pascual, A.; Garcia-Lucerga, C.; Blasco-Lafarga, C.; Garcia-Dominguez, E.; Carretero, A.; Correas, A.G.; et al. Physical exercise in the prevention and treatment of Alzheimer’s disease. J. Sport Health Sci. 2020, 9, 394–404. [Google Scholar] [CrossRef]
- Lin, D.; Wu, S.; Li, W.; Ye, P.; Pan, X.; Zheng, T.; Gao, F. A cross-tissue transcriptome-wide association study identifies new susceptibility genes for frailty. Front. Genet. 2024, 15, 1404456. [Google Scholar] [CrossRef]
- Perez, K.; Ciotlos, S.; McGirr, J.; Limbad, C.; Doi, R.; Nederveen, J.P.; Nilsson, M.I.; Winer, D.A.; Evans, W.; Tarnopolsky, M.; et al. Single nuclei profiling identifies cell specific markers of skeletal muscle aging, frailty, and senescence. Aging 2022, 14, 9393–9422. [Google Scholar] [CrossRef]
- Prohaska, A.; Racimo, F.; Schork, A.J.; Sikora, M.; Stern, A.J.; Ilardo, M.; Allentoft, M.E.; Folkersen, L.; Buil, A.; Moreno-Mayar, J.V.; et al. Human disease variation in the light of population genomics. Cell 2019, 177, 115–131. [Google Scholar] [CrossRef]
- Byars, S.G.; Voskarides, K. Antagonistic pleiotropy in human disease. J. Mol. Evol. 2020, 88, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Corbett, S.; Courtiol, A.; Lummaa, V.; Moorad, J.; Stearns, S. The transition to modernity and chronic disease: Mismatch and natural selection. Nat. Rev. Genet. 2018, 19, 419–430. [Google Scholar] [CrossRef] [PubMed]
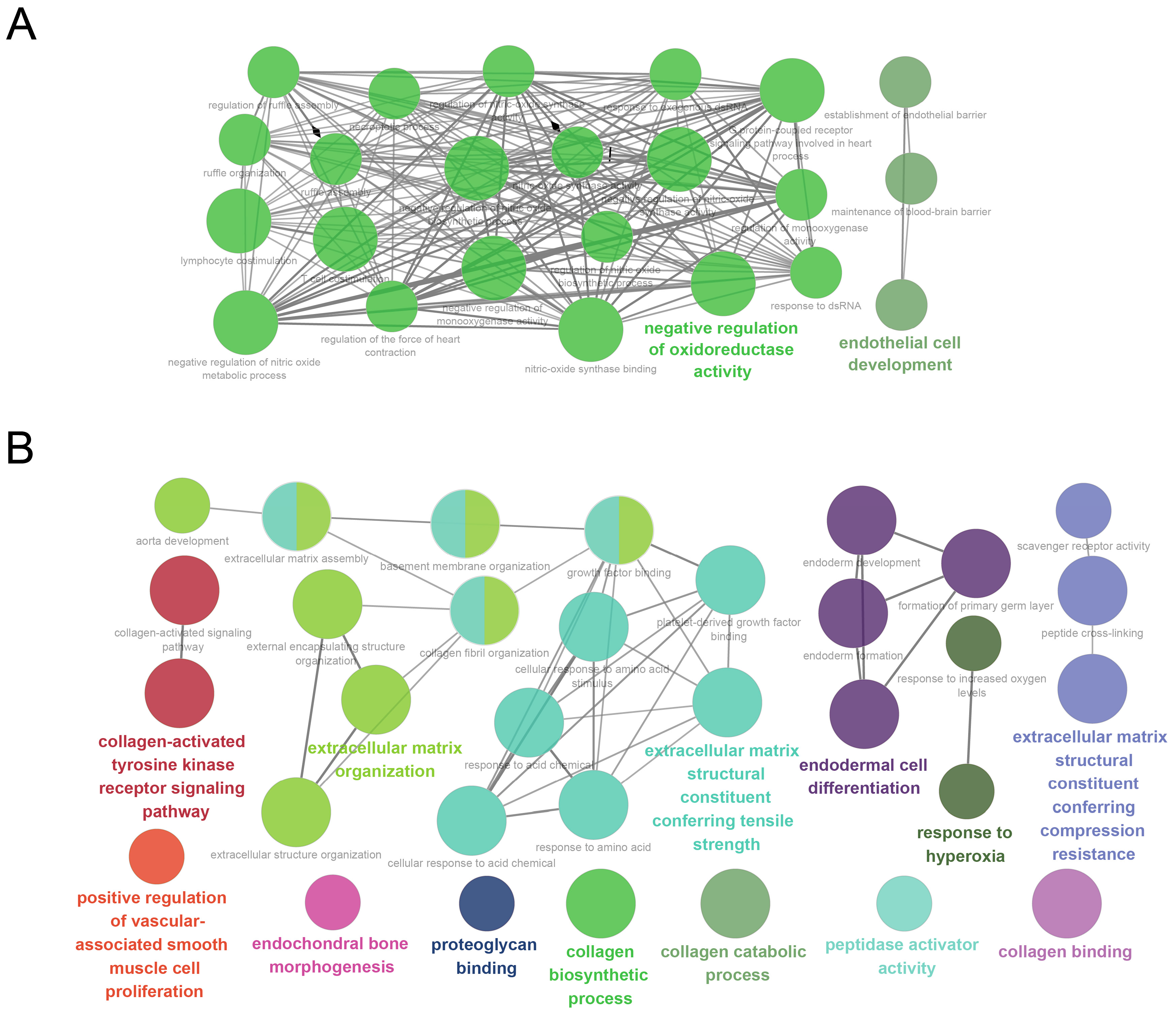
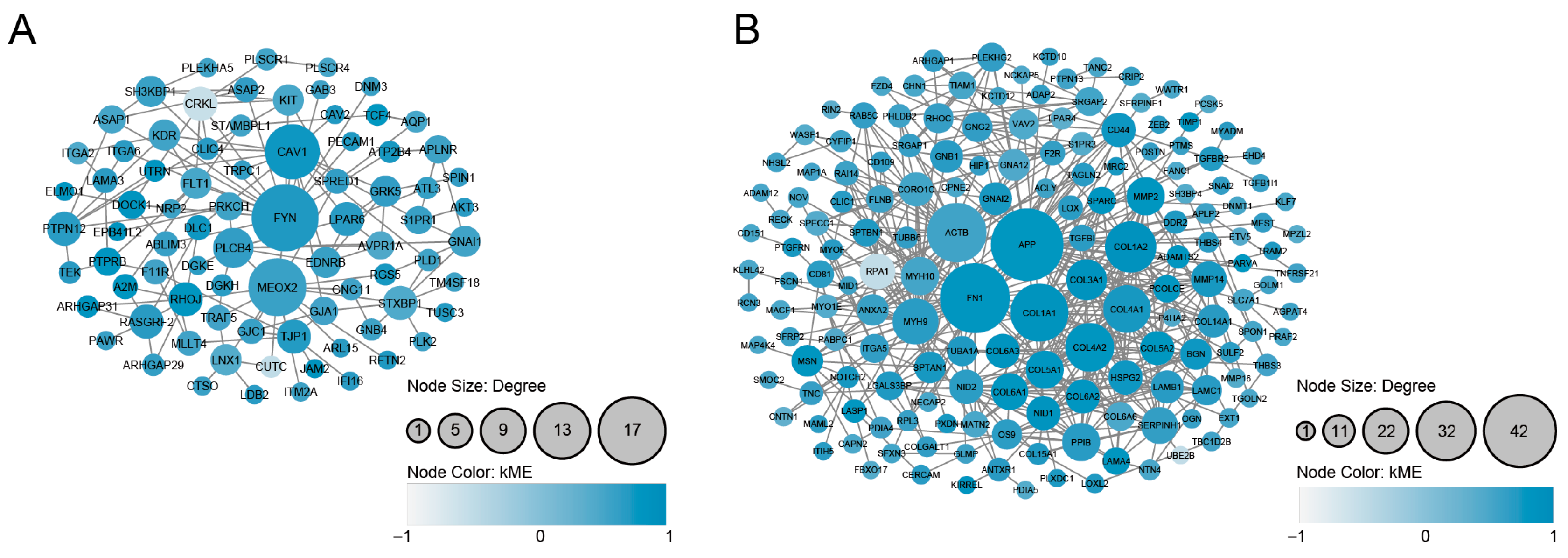
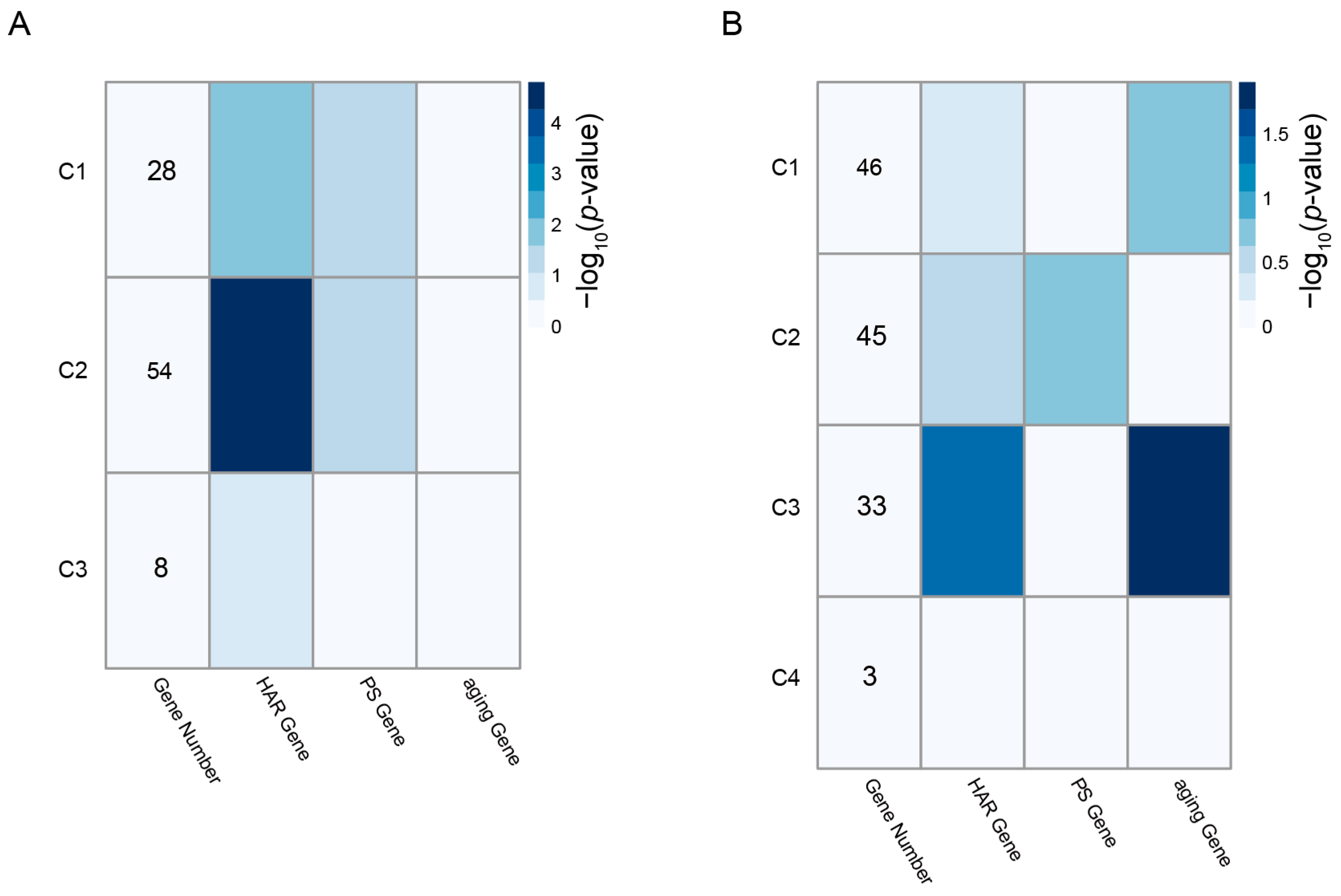
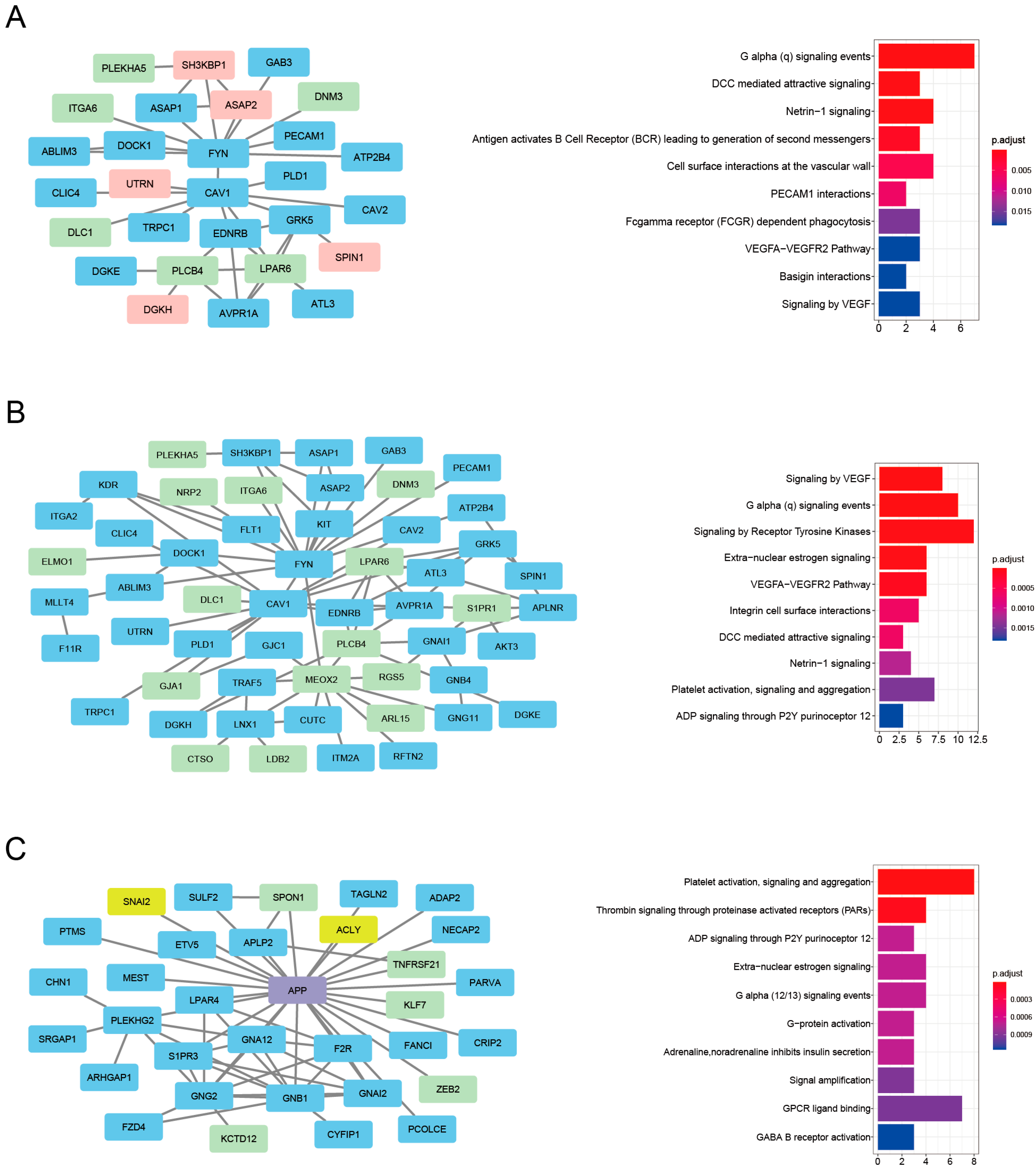
| Scheme | Gene Full Name | Module | Cluster | Degree | Betweenness | HAR Gene | PS Gene | Aging Gene |
|---|---|---|---|---|---|---|---|---|
| PLCB4 | Phospholipase C Beta 4 | magenta | cluster1,2 | 8 | 675.80101 | ○ | ||
| LPAR6 | Lysophosphatidic Acid Receptor 6 | magenta | cluster1,2 | 6 | 189.06032 | ○ | ||
| SH3KBP1 | SH3 Domain Containing Kinase Binding Protein 1 | magenta | cluster1 | 5 | 161.16667 | ○ | ||
| MEOX2 | Mesenchyme Homeobox 2 | magenta | cluster2 | 14 | 2686.26898 | ○ | ||
| APP | Amyloid Beta Precursor Protein | pink | cluster3 | 42 | 9368.80272 | ○ | ○ | |
| SPON1 | Spondin 1 | pink | cluster3 | 3 | 2.4 | ○ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naito, K.; Akahori, H.; Muto, Y.; Terada, T. Identification of Key Genes and Pathways Associated with Frailty and Exercise Effects Using a Network and Evolutionary Approach. Genes 2025, 16, 976. https://doi.org/10.3390/genes16080976
Naito K, Akahori H, Muto Y, Terada T. Identification of Key Genes and Pathways Associated with Frailty and Exercise Effects Using a Network and Evolutionary Approach. Genes. 2025; 16(8):976. https://doi.org/10.3390/genes16080976
Chicago/Turabian StyleNaito, Kyoko, Hiromichi Akahori, Yoshinori Muto, and Tomoyoshi Terada. 2025. "Identification of Key Genes and Pathways Associated with Frailty and Exercise Effects Using a Network and Evolutionary Approach" Genes 16, no. 8: 976. https://doi.org/10.3390/genes16080976
APA StyleNaito, K., Akahori, H., Muto, Y., & Terada, T. (2025). Identification of Key Genes and Pathways Associated with Frailty and Exercise Effects Using a Network and Evolutionary Approach. Genes, 16(8), 976. https://doi.org/10.3390/genes16080976





