The Complete Mitochondrial Genome of Liobagrus huaiheensis (Teleostei: Siluriformes: Amblycipitidae): Characterization, Phylogenetic Placement, and Insights into Genetic Diversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. DNA Extraction, Library Construction, and Sequencing
2.3. Assembly and Annotation of the Mitochondrial Genome
2.4. Analysis of Mitochondrial Genome Sequence Characteristics
2.5. Phylogenetic Analysis Methods
2.6. Population-Level Genetic Diversity Analysis
3. Results and Discussion
3.1. Overall Characteristics of the Mitochondrial Genome of L. huaiheensis
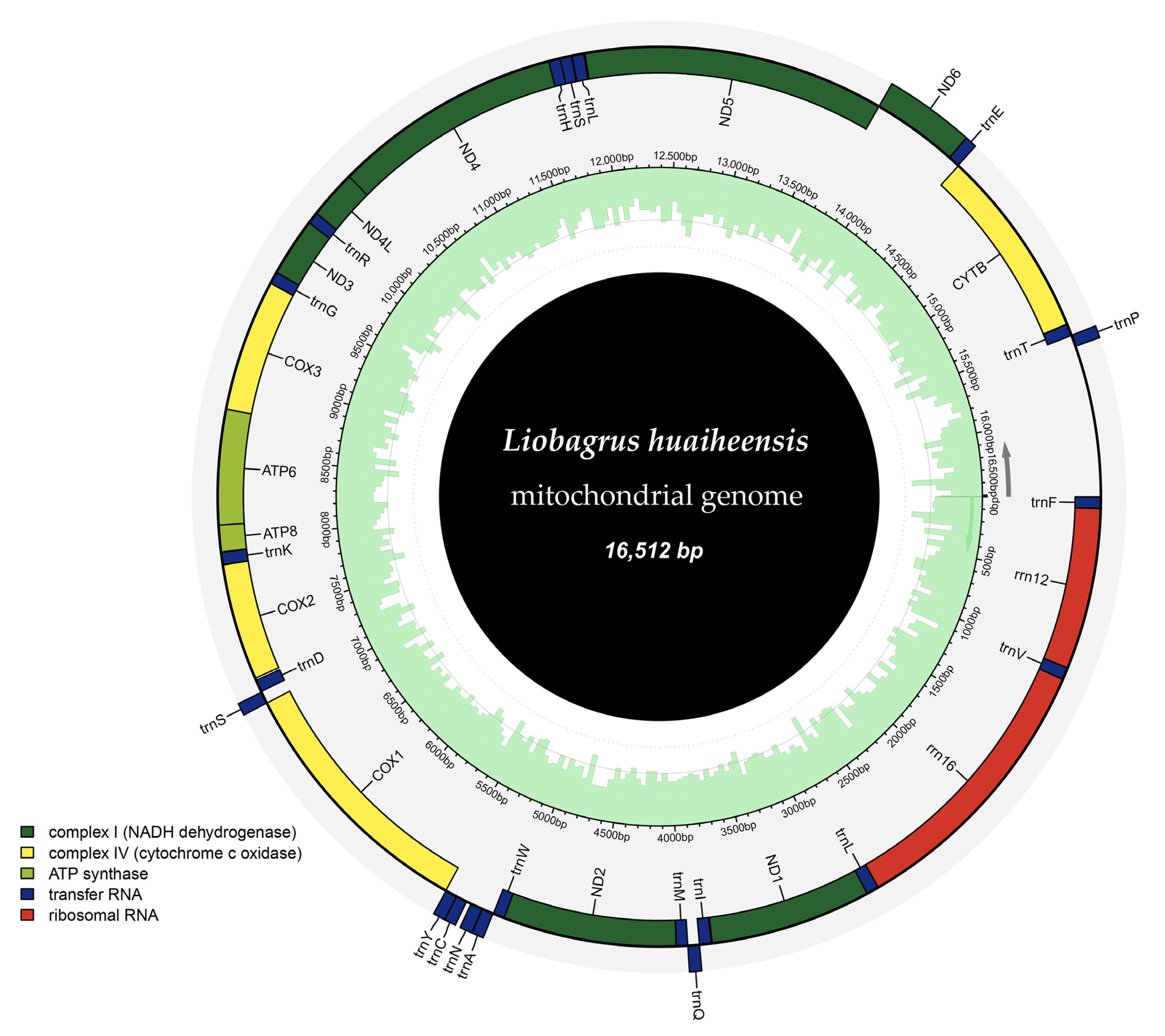
3.2. Nucleotide Composition and Base Bias Analysis
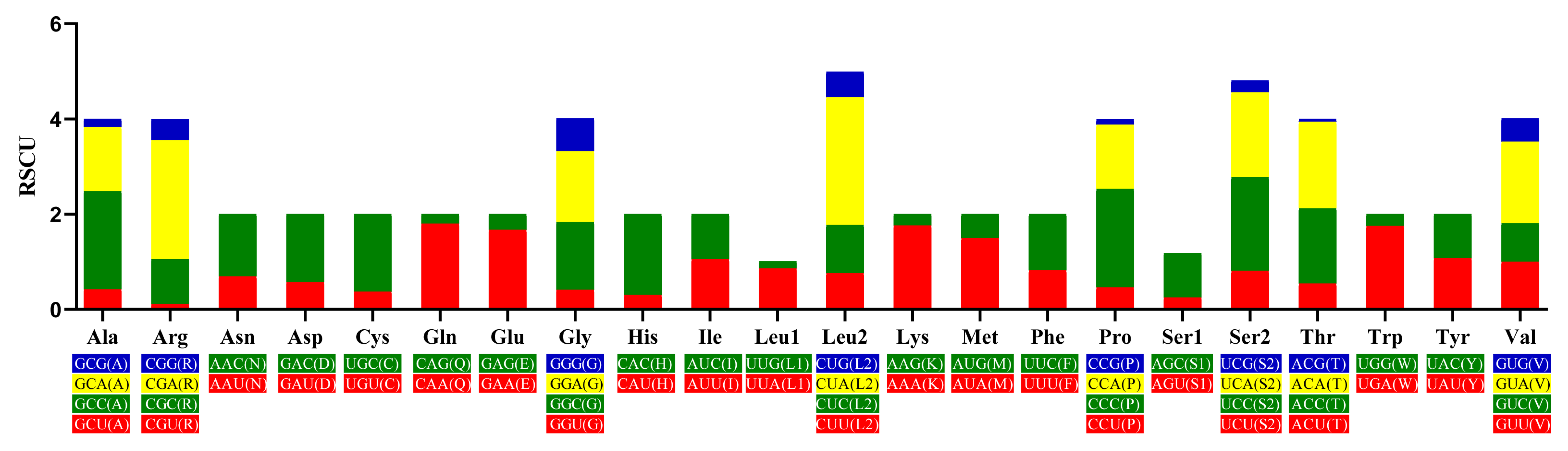
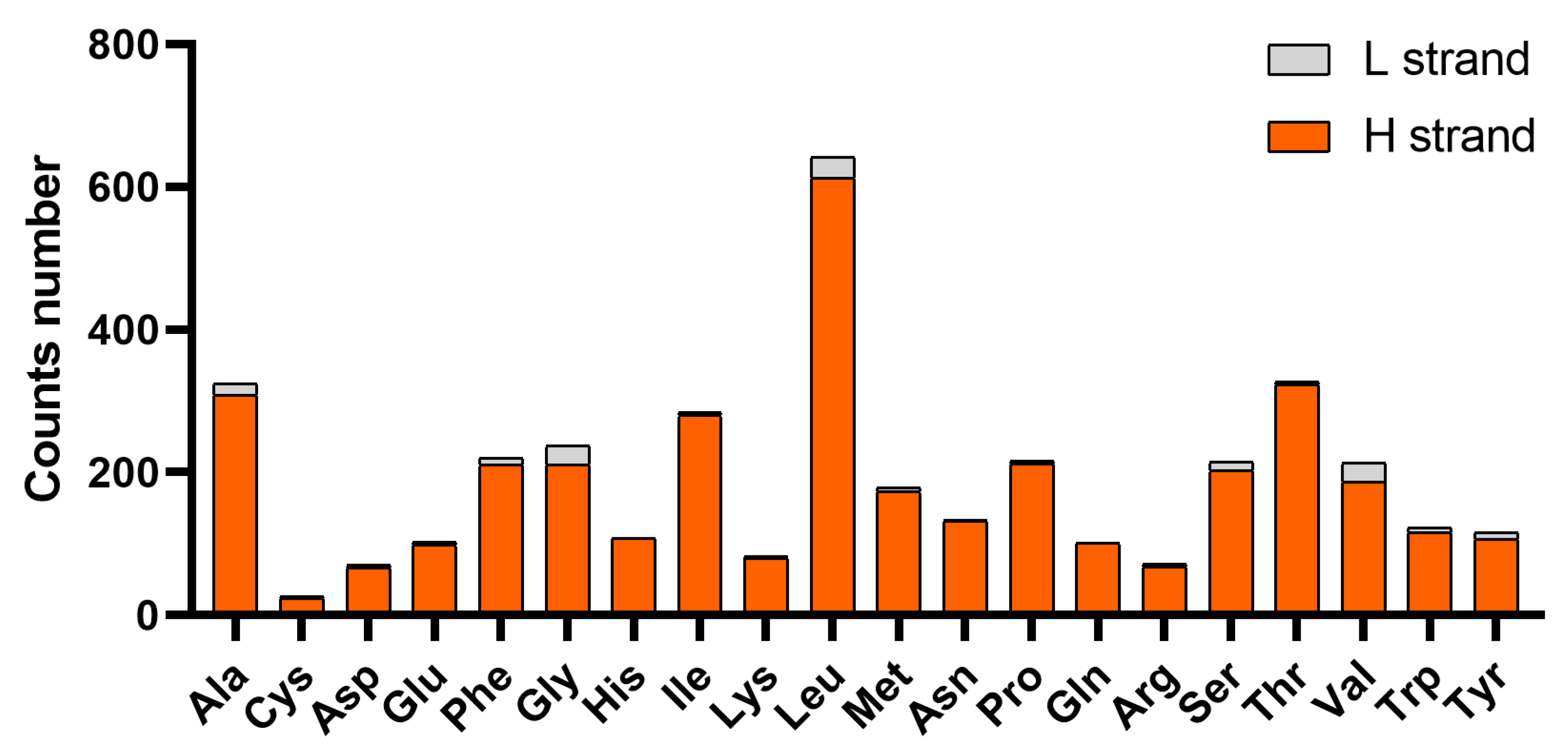
3.3. Protein-Coding Genes and Codon Usage Characteristics
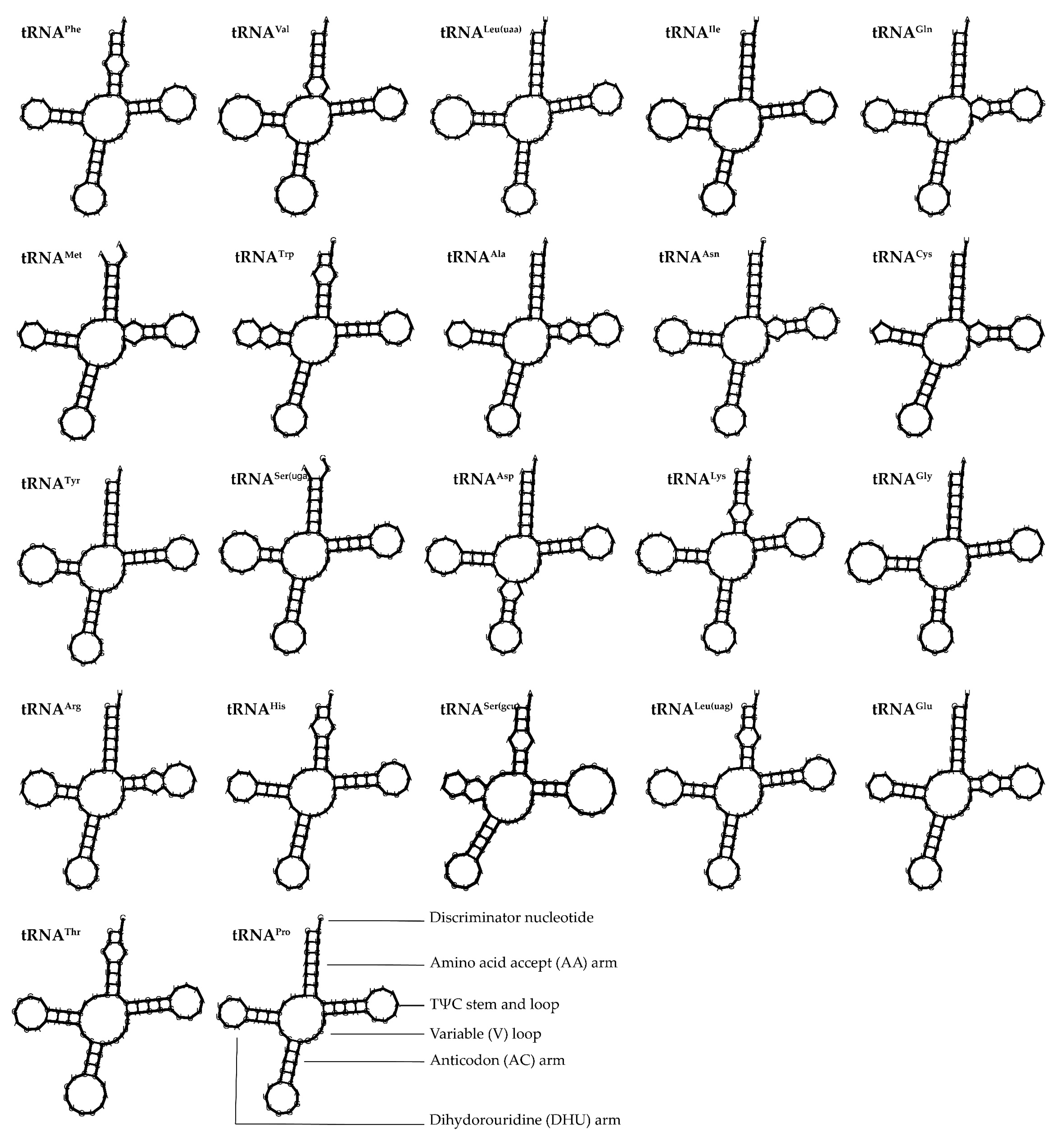
3.4. Characteristics of Ribosomal RNA and Transfer RNA Genes
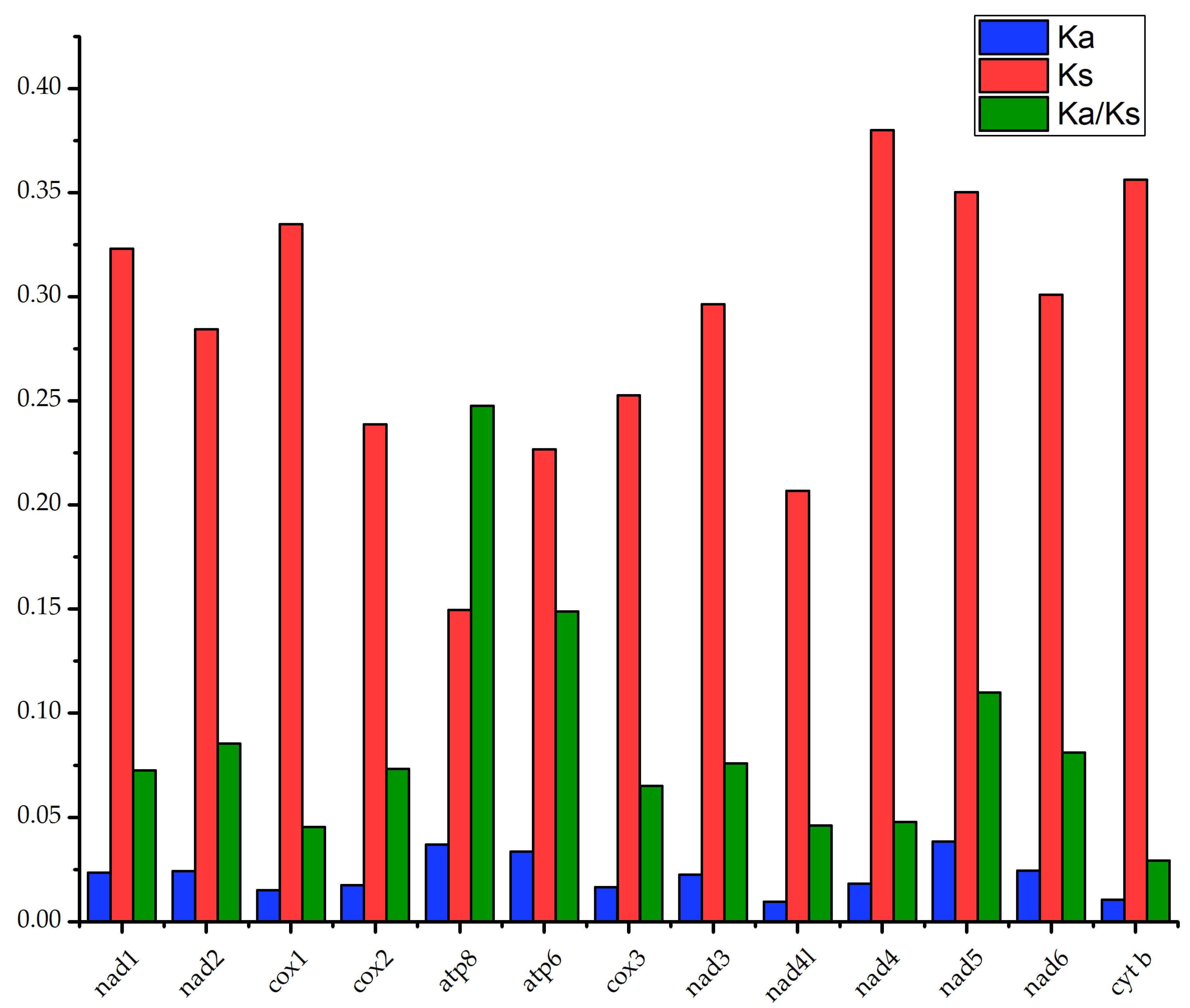
3.5. Selection Pressure Analysis
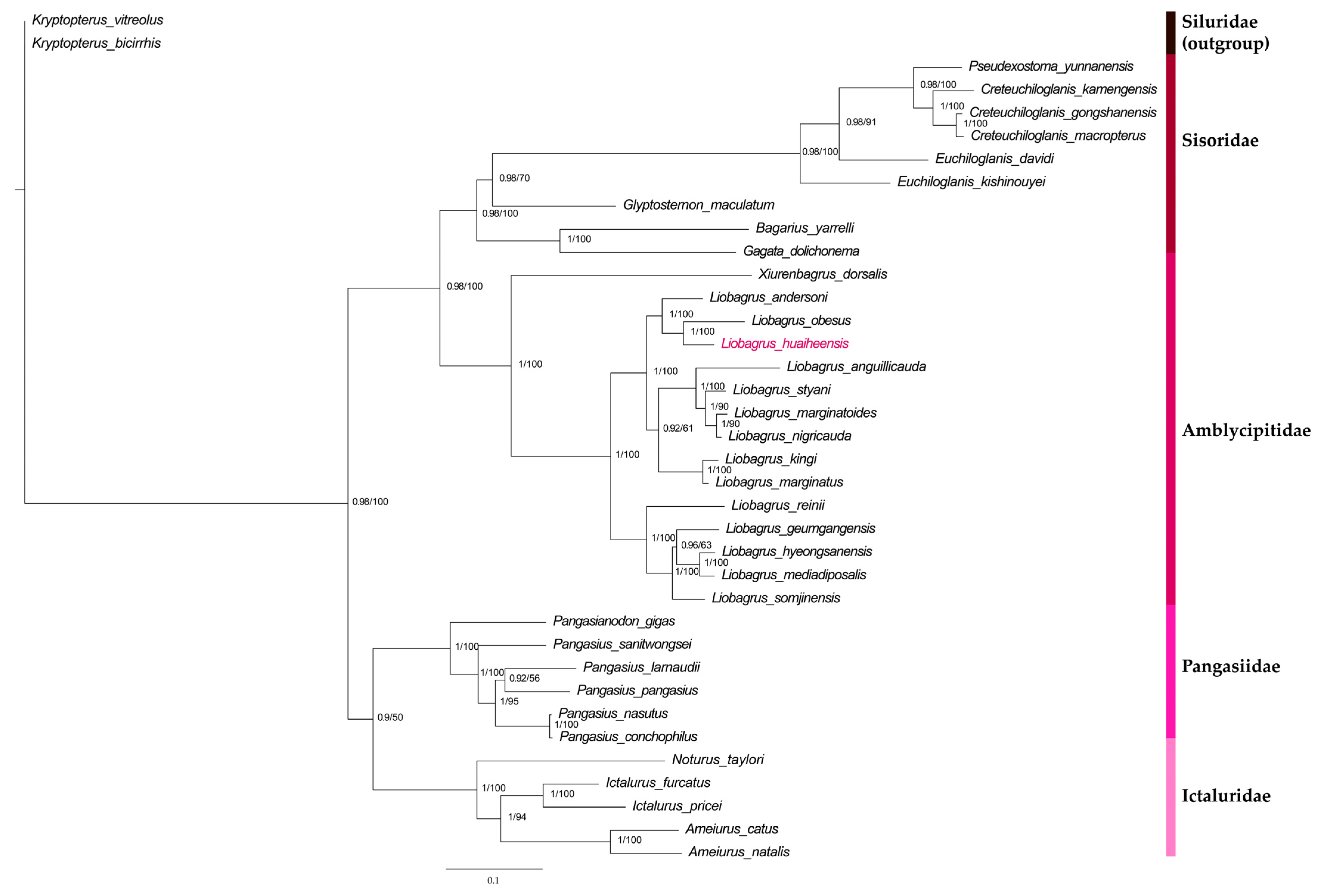
3.6. Results of Phylogenetic Analysis
3.7. Population Genetic Diversity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Z.; Wu, J.; Wen, A. Liobagrus huaiheensis, a New Species of Torrent Catfish (Teleostei: Siluriformes: Amblycipitidae) from the Huaihe River Basin in Central China. Zootaxa 2021, 4952, 391–400. [Google Scholar] [CrossRef]
- Li, Z.; Huang, R.J.; Tian, H.J.; Wu, K.J.; Wang, C.Z.; Meng, X.L.; Zhou, C.J.; Gu, Q.H.; Nie, G.X. Investigation of fishery resources in Xinyang Reaches of the Huai River. Henan Fish. 2015, 5, 22—24+30. [Google Scholar]
- Kim, S.-H.; Kim, H.-S.; Park, J.-Y. A New Species of Torrent Catfish, Liobagrus hyeongsanensis (Teleostei: Siluriformes: Amblycipitidae), from Korea. Zootaxa 2015, 4007, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.W.; Ren, S.J.; Zhang, E. Liobagrus chenghaiensis, a New Species of Catfish (Siluriformes: Amblycipitidae) from Yunnan, South China. Ichthyol. Exploit. Freshw. 2013, 23, 275–384. [Google Scholar]
- Xie, R.-X.; Zhang, E. Re-Description of the Catfish Species Liobagrus kingi Tchang, 1935 (Pisces: Amblycipitidae) from the Upper Chang-Jiang Basin, China. Zootaxa 2018, 4476, 69–76. [Google Scholar] [CrossRef]
- Roy, A. Molecular Markers in Phylogenetic Studies—A Review. J. Phylogenetics Evol. Biol. 2014, 2, 131. [Google Scholar] [CrossRef]
- Hurst, G.D.D.; Jiggins, F.M. Problems with Mitochondrial DNA as a Marker in Population, Phylogeographic and Phylogenetic Studies: The Effects of Inherited Symbionts. Proc. R. Soc. B Biol. Sci. 2005, 272, 1525–1534. [Google Scholar] [CrossRef]
- Duchêne, S.; Archer, F.I.; Vilstrup, J.; Caballero, S.; Morin, P.A. Mitogenome Phylogenetics: The Impact of Using Single Regions and Partitioning Schemes on Topology, Substitution Rate and Divergence Time Estimation. PLoS ONE 2011, 6, e27138. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Nadalin, F.; Vezzi, F.; Policriti, A. GapFiller: A de Novo Assembly Approach to Fill the Gap within Paired Reads. BMC Bioinform. 2012, 13, S8. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef] [PubMed]
- Birney, E.; Clamp, M.; Durbin, R. GeneWise and Genomewise. Genome Res. 2004, 14, 988–995. [Google Scholar] [CrossRef]
- Allio, R.; Schomaker-Bastos, A.; Romiguier, J.; Prosdocimi, F.; Nabholz, B.; Delsuc, F. MitoFinder: Efficient Automated Large-Scale Extraction of Mitogenomic Data in Target Enrichment Phylogenomics. Mol. Ecol. Resour. 2020, 20, 892–905. [Google Scholar] [CrossRef]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-Line: Integrating Search and Context for Analysis of Transfer RNA Genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Lu, Z.; Wang, S.; Jing-Yan Wang, J.; Gao, X. CMsearch: Simultaneous Exploration of Protein Sequence Space and Structure Space Improves Not Only Protein Homology Detection but Also Protein Structure Prediction. Bioinformatics 2016, 32, i332–i340. [Google Scholar] [CrossRef]
- Griffiths-Jones, S.; Moxon, S.; Marshall, M.; Khanna, A.; Eddy, S.R.; Bateman, A. Rfam: Annotating Non-Coding RNAs in Complete Genomes. Nucleic Acids Res. 2005, 33, D121–D124. [Google Scholar] [CrossRef]
- Donath, A.; Jühling, F.; Al-Arab, M.; Bernhart, S.H.; Reinhardt, F.; Stadler, P.F.; Middendorf, M.; Bernt, M. Improved Annotation of Protein-Coding Genes Boundaries in Metazoan Mitochondrial Genomes. Nucleic Acids Res. 2019, 47, 10543–10552. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Choudhuri, S.; Sau, K. CodonU: A Python Package for Codon Usage Analysis. IEEE/ACM Trans. Comput. Biol. Bioinform. 2024, 21, 36–44. [Google Scholar] [CrossRef]
- Sahyoun, A.H.; Bernt, M.; Stadler, P.F.; Tout, K. GC Skew and Mitochondrial Origins of Replication. Mitochondrion 2014, 17, 56–66. [Google Scholar] [CrossRef]
- Zhang, Z. KaKs_Calculator 3.0: Calculating Selective Pressure on Coding and Non-Coding Sequences. Genom. Proteom. Bioinform. 2022, 20, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A. AliView: A Fast and Lightweight Alignment Viewer and Editor for Large Datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef]
- Kim, P.; Kim, H.; Kim, S. Characterization of the Mitochondrial Complete Genome of Korean Indigenous Catfish, Liobagrus hyeongsanensis (Siluriformes: Amblycipitidae). Mitochondrial DNA Part B 2021, 6, 2383–2384. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New Methods for Selecting Partitioned Models of Evolution for Molecular and Morphological Phylogenetic Analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.H.; Song, H.Y. Complete Mitochondrial Genome of the Korean Torrent Catfish Liobagrus andersoni (Siluriformes, Amblycipitidae). Mitochondrial DNA Part B 2016, 1, 779–780. [Google Scholar] [CrossRef]
- Yun, S.; Park, J. Characterization of the Complete Mitochondrial Genome of a Newly Discovered Torrent Catfish, Liobagrus geumgangensis, and Their Phylogenetic Relationships. Genes Genom. 2024, 46, 1123–1131. [Google Scholar] [CrossRef]
- Jia, X.-Y.; Li, Y.-W.; Wang, D.-Q.; Tian, H.-W.; Xiong, X.; Li, S.-H.; Chen, D.-Q. The Complete Mitochondrial Genome of Liobagrus kingi (Teleostei, Siluriformes: Amblycipitidae). Mitochondrial DNA 2013, 24, 323–325. [Google Scholar] [CrossRef]
- Jia, X.-Y.; Li, Y.-W.; Wang, D.-Q.; Tian, H.-W.; Tu, B.; Xiong, X.; Li, S.-H.; Chen, D.-Q. The Mitogenome of Liobagrus marginatoides (Teleostei, Siluriformes:Amblycipitidae). Mitochondrial DNA 2013, 24, 645–647. [Google Scholar] [CrossRef]
- Li, Q.; Du, J.; Liu, Y.; Zhou, J.; Ke, H.; Liu, C.; Liu, G. The Complete Mitochondrial Genome of Liobagrus marginatus (Teleostei, Siluriformes: Amblycipitidae). Mitochondrial DNA 2014, 25, 120–121. [Google Scholar] [CrossRef]
- Park, C.E.; Kim, M.-C.; Kim, K.-H.; Park, H.C.; Shin, J.-H. The Complete Mitochondrial Genome Sequence of Liobagrus mediadiposalis (Teleostei, Siluriformes, Amblycipitidae). Mitochondrial DNA Part B 2017, 2, 879–880. [Google Scholar] [CrossRef]
- Jia, X.-Y.; Li, Y.-W.; Wang, D.-Q.; Li, S.-H.; Tian, H.-W.; Xiong, X.; Cheng, X.-F.; Chen, D.-Q. The Mitogenome of Liobagrus nigricauda (Teleostei, Siluriformes: Amblycipitidae). Mitochondrial DNA 2013, 24, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Kartavtsev, Y.P.; Jung, S.-O.; Lee, Y.-M.; Byeon, H.-K.; Lee, J.-S. Complete Mitochondrial Genome of the Bullhead Torrent Catfish, Liobagrus obesus (Siluriformes, Amblycipididae): Genome Description and Phylogenetic Considerations Inferred from the Cyt b and 16S rRNA Genes. Gene 2007, 396, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Han, J.-H.; An, S.L. Complete Mitochondrial Genome of Korean Catfish, Liobagrus somjinensis (Actinopterygii, Siluriformes, Amblycipitidae), from South Korea. Mitochondrial DNA Part B 2020, 5, 866–868. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Hu, S.; Bai, X.; Zhang, E. Complete Mitochondrial Genome of Liobagrus styani (Teleostei: Amblycipitidae). Mitochondrial DNA Part B 2017, 2, 15–16. [Google Scholar] [CrossRef]
- Du, M.; Zhou, C.J.; Niu, B.Z.; Liu, Y.H.; Li, N.; Ai, J.L.; Xu, G.L. The Complete Mitochondrial Genome of the Bagarius yarrelli from Honghe River. IOP Conf. Ser. Earth Environ. Sci. 2016, 41, 012031. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, H.; Zhang, P.; Wen, Z.; Wei, Q. The Complete Mitochondrial Genome of Euchiloglanis Davidi and Its Phylogenetic Implications. Mitochondrial DNA Part B 2019, 4, 1249–1250. [Google Scholar] [CrossRef]
- Jondeung, A.; Sangthong, P.; Zardoya, R. The Complete Mitochondrial DNA Sequence of the Mekong Giant Catfish (Pangasianodon gigas), and the Phylogenetic Relationships among Siluriformes. Gene 2007, 387, 49–57. [Google Scholar] [CrossRef]
- Halim, S.A.A.A.; Esa, Y.; Gan, H.M.; Zainudin, A.A.; Nor, S.A.M. The Complete Mitochondrial Genomes of Pangasius nasutus and P. conchophilus (Siluriformes: Pangasiidae). Mitochondrial DNA Part B 2023, 8, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Ye, X.; Lv, Y.; Teng, Z.; Gan, B.; Zou, H.; Mo, F.; Zhang, S. Complete Mitochondrial Genome and Phylogenetic Position of Pangasius sanitwongsei (Siluriformes: Pangasiidae). Mitochondrial DNA Part B 2020, 5, 945–946. [Google Scholar] [CrossRef]
- Ballesteros-Córdova, C.A.; Castañeda-Rivera, M.; Grijalva-Chon, J.M.; Castillo-Gámez, R.A.; Gutiérrez-Millán, L.E.; Camarena-Rosales, F.; Ruíz-Campos, G.; Varela-Romero, A. Complete Mitochondrial Genome of Ictalurus pricei (Teleostei: Ictaluridae) and Evidence of a Cryptic Ictalurus Species in Northwest Mexico. Mitochondrial DNA Part A 2016, 27, 4439–4441. [Google Scholar] [CrossRef] [PubMed]
- Kuang, T.; Shuai, F.; Li, X.; Chen, W.; Lek, S. Genetic Diversity and Population Structure of Hemibagrus guttatus (Bagridae, Siluriformes) in the Larger Subtropical Pearl River Based on COI and Cyt b Genes Analysis. Ann. Limnol. -Int. J. Lim. 2021, 57, 7. [Google Scholar] [CrossRef]
- Chen, Z.-G.; Guo, Y.-S.; Dai, Y.-T.; Huang, X.-C.; Huang, J.-H.; Jiang, J.; Ouyang, S.; Wen, A.-X.; Wu, X.-P. A New Species of Liobagrus hilgendorf, 1878 (Teleostei, Siluriformes, Amblycipitidae) from the Lower Changjiang River Basin in Southeast China. Zoosystematics Evol. 2024, 100, 555–563. [Google Scholar] [CrossRef]
- Chen, Z.-G.; Guo, Y.-S.; Wu, J.-Y.; Wen, A.-X. Liobagrus chengduensis, a New Species of Torrent Catfish (Teleostei: Siluriformes: Amblycipitidae) from the Upper Changjiang River Basin in Southwest China. Zool. Res. 2022, 43, 679–682. [Google Scholar] [CrossRef]
- Clewley, J.P. Macintosh Sequence Analysis Software. Mol. Biotechnol. 1995, 3, 221–224. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, T.; Li, H.; Deng, L. The Mitochondrial Genome of Linichthys laticeps (Cypriniformes: Cyprinidae): Characterization and Phylogeny. Genes 2023, 14, 1938. [Google Scholar] [CrossRef]
- Nakatani, M.; Miya, M.; Mabuchi, K.; Saitoh, K.; Nishida, M. Evolutionary History of Otophysi (Teleostei), a Major Clade of the Modern Freshwater Fishes: Pangaean Origin and Mesozoic Radiation. BMC Evol. Biol. 2011, 11, 177. [Google Scholar] [CrossRef]
- Grigoriev, A. Analyzing Genomes with Cumulative Skew Diagrams. Nucleic Acids Res. 1998, 26, 2286–2290. [Google Scholar] [CrossRef] [PubMed]
- Wolstenholme, D.R. Animal Mitochondrial DNA: Structure and Evolution. In International Review of Cytology; Wolstenholme, D.R., Jeon, K.W., Eds.; Academic Press: Cambridge, MA, USA, 1992; Volume 141, pp. 173–216. [Google Scholar]
- Clayton, D.A. Replication of Animal Mitochondrial DNA. Cell 1982, 28, 693–705. [Google Scholar] [CrossRef]
- Moritz, C.; Dowling, T.E.; Brown, W.M. Evolution of Animal Mitochondrial DNA: Relevance for Population Biology and Systematics. Annu. Rev. Ecol. Syst. 1987, 18, 269–292. [Google Scholar] [CrossRef]
- Burton, Z.F. The 3-Minihelix tRNA Evolution Theorem. J. Mol. Evol. 2020, 88, 234–242. [Google Scholar] [CrossRef]
- Yang, T.; Tan, C.; Zhao, L.; Hu, Z.; Su, C.; Li, F.; Ma, Y.; Zhang, W.; Hao, X.; Zou, W.; et al. The Complete Mitochondrial Genome of the Luciocyprinus langsoni (Cypriniformes: Cyprinidae): Characterization, Phylogeny, and Genetic Diversity Analysis. Genes 2024, 15, 1621. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, D.; Stepien, P.P.; Temperley, R.J.; Lightowlers, R.N.; Chrzanowska-Lightowlers, Z.M.A. Messenger RNA Stability in Mitochondria: Different Means to an End. Trends Genet. 2004, 20, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Xu, S.; Shu, Y.; Fang, J. The Complete Mitochondrial Genome of Red Costate Tiger Moth (Aloa lactinea [Cramer, 1777]), and Phylogenetic Analyses of the Subfamily Arctiinae. Genes 2025, 16, 554. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Z.; Liu, C.; Lee, X.; Zhu, K. Characterization of the Complete Mitochondrial Genome of the Spotted Catfish Arius maculatus (Thunberg, 1792) and Its Phylogenetic Implications. Genes 2022, 13, 2128. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Vang, S.; Yu, J.; Wong, G.K.-S.; Wang, J. Correlation Between Ka/Ks and Ks Is Related to Substitution Model and Evolutionary Lineage. J. Mol. Evol. 2009, 68, 414–423. [Google Scholar] [CrossRef]
- Nekrutenko, A.; Makova, K.D.; Li, W.-H. The K A/K S Ratio Test for Assessing the Protein-Coding Potential of Genomic Regions: An Empirical and Simulation Study. Genome Res. 2002, 12, 198–202. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Yu, J. Computing Ka and Ks with a Consideration of Unequal Transitional Substitutions. BMC Evol. Biol. 2006, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z. Taxonomic Study of Liobagrus (Siluriformes:Amblycipitidae) Destributed in Chinesemainland. Master’s Thesis, Sichuan Agricultural University, Ya’an, China, 2022. [Google Scholar]
- Gong, J.; Chen, B.; Li, B.; Zhou, Z.; Shi, Y.; Ke, Q.; Zhang, D.; Xu, P. Genetic Analysis of Whole Mitochondrial Genome of Lateolabrax maculatus (Perciformes: Moronidae) Indicates the Presence of Two Populations along the Chinese Coast. Zoologia 2020, 37, e49046. [Google Scholar] [CrossRef]
- Fu, Y.-X. Statistical Tests of Neutrality of Mutations Against Population Growth, Hitchhiking and Background Selection. Genetics 1997, 2, 915–925. [Google Scholar] [CrossRef] [PubMed]

| Family | Genus | Species | Genbank Accession No | Resource |
|---|---|---|---|---|
| Amblycipitidae | Liobagrus | Liobagrus andersoni | KX767082.1 | [28] |
| Liobagrus anguillicauda | JQ026256.1 | Unpublished | ||
| Liobagrus geumgangensis | NC_088753.1 | [29] | ||
| Liobagrus hyeongsanensis | MZ066608.1 | [24] | ||
| Liobagrus kingi | KC193779.1 | [30] | ||
| Liobagrus marginatoides | KC473938.1 | [31] | ||
| Liobagrus marginatus | KC757128.1 | [32] | ||
| Liobagrus mediadiposalis | KR075136.1 | [33] | ||
| Liobagrus nigricauda | KC316116.1 | [34] | ||
| Liobagrus obesus | JQ714035.1 | [35] | ||
| Liobagrus somjinensis | MN756661.1 | [36] | ||
| Liobagrus styani | KX096605.1 | [37] | ||
| Liobagrus reinii | AP012015.1 | Unpublished | ||
| Liobagrus huaiheensis | PV953861 | This study | ||
| Xiurenbagrus | Xiurenbagrus dorsalis | MN308285.1 | Unpublished | |
| Sisoridae | Bagarius | Bagarius yarrelli | JQ026260.1 | [38] |
| Gagata | Gagata dolichonema | JQ026250.1 | Unpublished | |
| Creteuchiloglanis | Creteuchiloglanis kamengensis | MN396886.1 | Unpublished | |
| Creteuchiloglanis gongshanensis | KP872697.1 | Unpublished | ||
| Creteuchiloglanis macropterus | KP872682.1 | Unpublished | ||
| Euchiloglanis | Euchiloglanis davidi | MK181572.1 | [39] | |
| Euchiloglanis kishinouyei | JQ026252.1 | Unpublished | ||
| Glyptosternon | Glyptosternon maculatum | JQ026251.1 | Unpublished | |
| Pseudexostoma | Pseudexostoma yunnanensis | JQ026258.1 | Unpublished | |
| Pangasiidae | Pangasianodon | Pangasianodon gigas | AY762971.1 | [40] |
| Pangasius | Pangasius nasutus | OQ078746.1 | [41] | |
| Pangasius sanitwongsei | MN809630.1 | [42] | ||
| Pangasius conchophilus | OQ078745.1 | [41] | ||
| Pangasius pangasius | KC572135.1 | Unpublished | ||
| Pangasius larnaudii | AP012018.1 | Unpublished | ||
| Siluridae | Kryptopterus | Kryptopterus vitreolus | KY710750.1 | Unpublished |
| Kryptopterus bicirrhis | KY569440.1 | Unpublished | ||
| Ictaluridae | Noturus | Noturus taylori | KP013089.1 | Unpublished |
| Ameiurus | Ameiurus catus | MG570433.1 | Unpublished | |
| Ameiurus natalis | MG570406.1 | Unpublished | ||
| Ictalurus | Ictalurus furcatus | KM576102.1 | Unpublished | |
| Ictalurus pricei | KJ496298.1 | [43] |
| Gene | Location | Strand | Gene Length (bp) | Intergenic Nucleotides | Overlapping Nucleotides | Codons | ||
|---|---|---|---|---|---|---|---|---|
| From | To | Start | Stop | |||||
| tRNAPhe | 1 | 69 | H | 69 | - | |||
| 12S rRNA | 70 | 1025 | H | 956 | ||||
| tRNAVal | 1026 | 1097 | H | 72 | ||||
| 16S rRNA | 1098 | 2763 | H | 1666 | ||||
| tRNALeu(tta) | 2764 | 2838 | H | 75 | ||||
| nad1 | 2839 | 3810 | H | 972 | 5 | ATG | TAG | |
| tRNAIle | 3816 | 3887 | H | 72 | 1 | |||
| tRNAGln | 3887 | 3957 | L | 71 | 1 | |||
| tRNAMet | 3957 | 4025 | H | 69 | ||||
| nad2 | 4026 | 5072 | H | 1047 | 2 | ATG | TAG | |
| tRNATrp | 5071 | 5139 | H | 69 | 2 | |||
| tRNAAla | 5142 | 5210 | L | 69 | 1 | |||
| tRNAAsn | 5212 | 5284 | L | 73 | 29 | |||
| tRNACys | 5314 | 5380 | L | 67 | ||||
| tRNATyr | 5381 | 5451 | L | 71 | 1 | |||
| cox1 | 5453 | 7003 | H | 1551 | GTG | TAG | ||
| tRNASer(tga) | 7004 | 7074 | L | 71 | 4 | |||
| tRNAAsp | 7079 | 7150 | H | 72 | 13 | |||
| cox2 | 7164 | 7854 | H | 691 | ATG | T- | ||
| tRNALys | 7855 | 7928 | H | 74 | 1 | |||
| atp8 | 7930 | 8097 | H | 168 | 10 | ATG | TAA | |
| atp6 | 8088 | 8771 | H | 684 | 1 | ATG | TAA | |
| cox3 | 8771 | 9555 | H | 785 | 1 | ATG | TA- | |
| tRNAGly | 9555 | 9627 | H | 73 | ||||
| nad3 | 9628 | 9976 | H | 349 | ATG | T- | ||
| tRNAArg | 9977 | 10,046 | H | 70 | ||||
| nad4l | 10,047 | 10,343 | H | 297 | 7 | ATG | TAA | |
| nad4 | 10,337 | 11,717 | H | 1381 | ATG | T- | ||
| tRNAHis | 11,718 | 11,787 | H | 70 | ||||
| tRNASer(gct) | 11,788 | 11,856 | H | 69 | 2 | |||
| tRNALeu(tag) | 11,859 | 11,931 | H | 73 | ||||
| nad5 | 11,932 | 13,755 | H | 1824 | 4 | ATG | TAA | |
| nad6 | 13,752 | 14,267 | L | 516 | ATG | TAG | ||
| tRNAGlu | 14,268 | 14,336 | L | 69 | 2 | |||
| cyt b | 14,339 | 15,476 | H | 1138 | ATG | T- | ||
| tRNAThr | 15,477 | 15,548 | H | 72 | 2 | |||
| tRNAPro | 15,547 | 15,616 | L | 70 | ||||
| CR | 15,617 | 16,512 | H | 896 | ||||
| Gene/Region | Size (bp) | Base Composition (%) | AT Skew | GC Skew | |||||
|---|---|---|---|---|---|---|---|---|---|
| A | T | C | G | A + T | G + C | ||||
| Genome | 16,512 | 30.87 | 24.87 | 28.85 | 15.41 | 55.74 | 44.26 | 0.10764 | −0.30366 |
| PCGs | 11,373 | 28.56 | 26.33 | 29.92 | 15.19 | 54.88 | 45.12 | 0.04069 | −0.32645 |
| nad1 | 969 | 27.97 | 25.39 | 32.92 | 13.73 | 53.36 | 46.65 | 0.04835 | −0.41136 |
| nad2 | 1044 | 32.95 | 22.51 | 32.28 | 12.26 | 55.46 | 44.54 | 0.18824 | −0.44948 |
| cox1 | 1548 | 26.16 | 28.10 | 28.23 | 17.51 | 54.26 | 45.74 | −0.03575 | −0.23437 |
| cox2 | 690 | 30.87 | 26.09 | 27.97 | 15.07 | 56.96 | 43.04 | 0.08392 | −0.29972 |
| atp8 | 165 | 37.58 | 21.82 | 29.09 | 11.52 | 59.40 | 40.61 | 0.26532 | −0.43265 |
| atp6 | 681 | 32.01 | 24.96 | 29.66 | 13.36 | 56.97 | 43.02 | 0.12375 | −0.37889 |
| cox3 | 783 | 25.67 | 25.80 | 31.03 | 17.50 | 51.47 | 48.53 | −0.00253 | −0.27880 |
| nad3 | 348 | 26.15 | 27.30 | 32.47 | 14.08 | 53.45 | 46.55 | −0.02152 | −0.39506 |
| nad4l | 294 | 23.47 | 24.49 | 35.03 | 17.01 | 47.96 | 52.04 | −0.02127 | −0.34627 |
| nad4 | 1380 | 30.36 | 24.78 | 31.38 | 13.48 | 55.14 | 44.86 | 0.10120 | −0.39902 |
| nad5 | 1821 | 30.75 | 25.65 | 31.30 | 12.30 | 56.40 | 43.60 | 0.09043 | −0.43578 |
| nad6 | 513 | 14.04 | 40.74 | 9.94 | 35.28 | 54.78 | 45.22 | −0.48740 | 0.56037 |
| cyt b | 1137 | 28.41 | 26.82 | 31.13 | 13.63 | 55.23 | 44.76 | 0.02879 | −0.39097 |
| First site | 3791 | 27.64 | 20.18 | 27.20 | 24.98 | 47.82 | 52.18 | 0.15600 | −0.04255 |
| Secondary site | 3791 | 18.81 | 40.60 | 27.41 | 13.19 | 59.41 | 40.60 | −0.36677 | −0.35025 |
| Tertiary site | 3791 | 39.22 | 18.20 | 35.16 | 7.41 | 57.42 | 42.57 | 0.36607 | −0.65187 |
| tRNA gene | 1560 | 28.65 | 27.44 | 21.15 | 22.76 | 56.09 | 43.91 | 0.02157 | 0.03667 |
| rRNA gene | 2602 | 34.44 | 21.10 | 24.44 | 20.02 | 55.54 | 44.46 | 0.24019 | −0.09942 |
| D-loop zone | 896 | 33.37 | 32.70 | 20.65 | 13.28 | 66.07 | 33.93 | 0.01014 | −0.21721 |
| Species | Whole Genome | PCGs | rRNA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Size | A + T | GC Skew | AT Skew | Size | A + T | GC Skew | AT Skew | Size | A + T | GC Skew | AT Skew | |
| (bp) | (%) | (bp) | (%) | (bp) | (%) | |||||||
| Liobagrus andersoni | 16,514 | 55.42 | −0.2904 | 0.0962 | 11,373 | 54.63 | −0.3085 | 0.0246 | 2620 | 55.11 | −0.0986 | 0.2396 |
| Liobagrus anguillicauda | 16,536 | 57.07 | −0.2813 | 0.0836 | 11,382 | 56.73 | −0.3003 | 0.0150 | 2622 | 55.95 | −0.0892 | 0.2243 |
| Liobagrus geumgangensis | 16,522 | 55.93 | −0.2873 | 0.0894 | 11,377 | 55.10 | −0.3019 | 0.0190 | 2624 | 55.34 | −0.1058 | 0.2383 |
| Liobagrus hyeongsanensis | 16,529 | 55.41 | −0.2806 | 0.0877 | 11,376 | 54.22 | −0.2945 | 0.0156 | 2626 | 56.02 | −0.1048 | 0.2373 |
| Liobagrus kingi | 16,483 | 54.10 | −0.2822 | 0.0967 | 11,376 | 53.02 | −0.2913 | 0.0211 | 2609 | 54.08 | −0.1135 | 0.2417 |
| Liobagrus marginatoides | 16,498 | 55.67 | −0.2870 | 0.0955 | 11,373 | 54.95 | −0.3020 | 0.0186 | 2602 | 55.00 | −0.0965 | 0.2383 |
| Liobagrus marginatus | 16,497 | 54.53 | −0.2797 | 0.0944 | 11,376 | 53.47 | −0.2942 | 0.0212 | 2570 | 54.09 | −0.1000 | 0.2388 |
| Liobagrus mediadiposalis | 16,534 | 55.17 | −0.2796 | 0.0907 | 11,376 | 53.89 | −0.2901 | 0.0183 | 2626 | 55.98 | −0.1038 | 0.2367 |
| Liobagrus nigricauda | 16,509 | 55.93 | −0.2852 | 0.0929 | 11,371 | 55.29 | −0.3025 | 0.0189 | 2618 | 55.42 | −0.0968 | 0.2378 |
| Liobagrus obesus | 16,506 | 54.42 | −0.2751 | 0.1069 | 11,376 | 53.46 | −0.2920 | 0.0276 | 2619 | 55.25 | −0.0973 | 0.2440 |
| Liobagrus somjinensis | 16,526 | 55.58 | −0.2824 | 0.0883 | 11,376 | 54.34 | −0.2980 | 0.0194 | 2624 | 56.10 | −0.0972 | 0.2323 |
| Liobagrus styani | 16,515 | 56.19 | −0.2886 | 0.0928 | 11,373 | 55.61 | −0.3064 | 0.0212 | 2621 | 55.67 | −0.0998 | 0.2378 |
| Liobagrus huaiheensis | 16,512 | 55.74 | −0.3037 | 0.1076 | 11,373 | 54.88 | −0.3264 | 0.0407 | 2602 | 55.53 | −0.0994 | 0.2401 |
| Amino Acid | Codon | Count | RSCU | Amino Acid | Codon | Count | RSCU |
|---|---|---|---|---|---|---|---|
| Phe | UUU | 90 | 0.82 | Tyr | UAU | 62 | 1.07 |
| Phe | UUC | 130 | 1.18 | Tyr | UAC | 54 | 0.93 |
| Leu | UUA | 92 | 0.86 | stop codon | UAA | 4 | 1.00 |
| Leu | UUG | 16 | 0.15 | stop codon | UAG | 4 | 1.00 |
| Leu | CUU | 81 | 0.76 | His | CAU | 16 | 0.30 |
| Leu | CUC | 108 | 1.01 | His | CAC | 92 | 1.70 |
| Leu | CUA | 287 | 2.68 | Gln | CAA | 91 | 1.80 |
| Leu | CUG | 58 | 0.54 | Gln | CAG | 10 | 0.20 |
| Ile | AUU | 150 | 1.05 | Asn | AAU | 46 | 0.69 |
| Ile | AUC | 135 | 0.95 | Asn | AAC | 87 | 1.31 |
| Met | AUA | 133 | 1.49 | Lys | AAA | 72 | 1.76 |
| Met | AUG | 46 | 0.51 | Lys | AAG | 10 | 0.24 |
| Val | GUU | 53 | 1.00 | Asp | GAU | 20 | 0.57 |
| Val | GUC | 43 | 0.81 | Asp | GAC | 50 | 1.43 |
| Val | GUA | 91 | 1.71 | Glu | GAA | 86 | 1.67 |
| Val | GUG | 26 | 0.49 | Glu | GAG | 17 | 0.33 |
| Ser | UCU | 29 | 0.81 | Cys | UGU | 5 | 0.37 |
| Ser | UCC | 70 | 1.96 | Cys | UGC | 22 | 1.63 |
| Ser | UCA | 64 | 1.79 | Trp | UGA | 107 | 1.75 |
| Ser | UCG | 9 | 0.25 | Trp | UGG | 15 | 0.25 |
| Pro | CCU | 25 | 0.46 | Arg | CGU | 2 | 0.11 |
| Pro | CCC | 112 | 2.07 | Arg | CGC | 17 | 0.94 |
| Pro | CCA | 73 | 1.35 | Arg | CGA | 45 | 2.50 |
| Pro | CCG | 6 | 0.11 | Arg | CGG | 8 | 0.44 |
| Thr | ACU | 44 | 0.54 | Ser | AGU | 9 | 0.25 |
| Thr | ACC | 129 | 1.58 | Ser | AGC | 33 | 0.93 |
| Thr | ACA | 149 | 1.82 | stop codon | AGA | 0 | 0 |
| Thr | ACG | 5 | 0.06 | stop codon | AGG | 0 | 0 |
| Ala | GCU | 34 | 0.42 | Gly | GGU | 24 | 0.41 |
| Ala | GCC | 167 | 2.06 | Gly | GGC | 84 | 1.42 |
| Ala | GCA | 109 | 1.35 | Gly | GGA | 88 | 1.49 |
| Ala | GCG | 14 | 0.17 | Gly | GGG | 41 | 0.69 |
| Population | Gene | Number of Haplotypes | Haplotype (Gene) Diversity | Average Number of Nucleotide Difference | Nucleotide Diversity | Tajima’s D | Fu’s Fs |
|---|---|---|---|---|---|---|---|
| Huai River | cox1 | 4 | 0.656 | 1.888 | 0.00171 | 0.02195 | 1.899 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.; Tan, C.; Zhao, L.; Liu, J.; Guo, X.; Yao, G.; Zhang, W.; Yang, T. The Complete Mitochondrial Genome of Liobagrus huaiheensis (Teleostei: Siluriformes: Amblycipitidae): Characterization, Phylogenetic Placement, and Insights into Genetic Diversity. Genes 2025, 16, 977. https://doi.org/10.3390/genes16080977
Su C, Tan C, Zhao L, Liu J, Guo X, Yao G, Zhang W, Yang T. The Complete Mitochondrial Genome of Liobagrus huaiheensis (Teleostei: Siluriformes: Amblycipitidae): Characterization, Phylogenetic Placement, and Insights into Genetic Diversity. Genes. 2025; 16(8):977. https://doi.org/10.3390/genes16080977
Chicago/Turabian StyleSu, Chaoqun, Chenxi Tan, Liangjie Zhao, Jiahui Liu, Xusheng Guo, Gaoyou Yao, Weizhao Zhang, and Tiezhu Yang. 2025. "The Complete Mitochondrial Genome of Liobagrus huaiheensis (Teleostei: Siluriformes: Amblycipitidae): Characterization, Phylogenetic Placement, and Insights into Genetic Diversity" Genes 16, no. 8: 977. https://doi.org/10.3390/genes16080977
APA StyleSu, C., Tan, C., Zhao, L., Liu, J., Guo, X., Yao, G., Zhang, W., & Yang, T. (2025). The Complete Mitochondrial Genome of Liobagrus huaiheensis (Teleostei: Siluriformes: Amblycipitidae): Characterization, Phylogenetic Placement, and Insights into Genetic Diversity. Genes, 16(8), 977. https://doi.org/10.3390/genes16080977






