Heterorhabditis bacteriophora Extracellular Vesicles Alter the Innate Immune Signaling in Drosophila melanogaster
Abstract
1. Introduction
2. Materials and Methods
2.1. Fly Stocks
2.2. Collection of Nematode Excreted–Secreted Products and Purification of Vesicles
2.3. Nanoparticle Tracking Analysis
2.4. Preparation of Dead Nematodes
2.5. Fly Survival Experiments
2.6. Fly Gene Transcriptional Expression
2.7. Statistical Analysis
3. Results
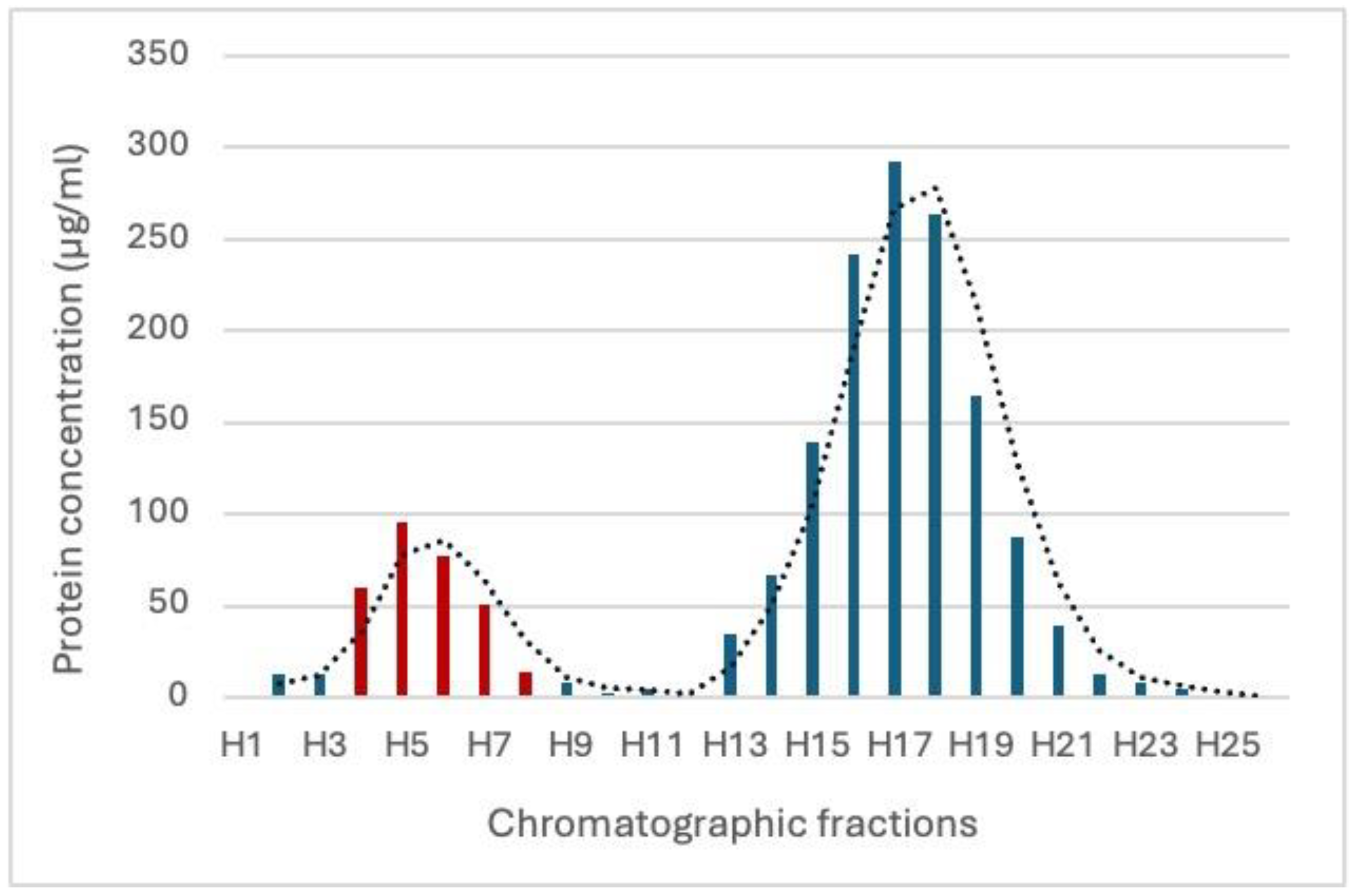
3.1. Characterization of Exosomal Vesicles from H. bacteriophora
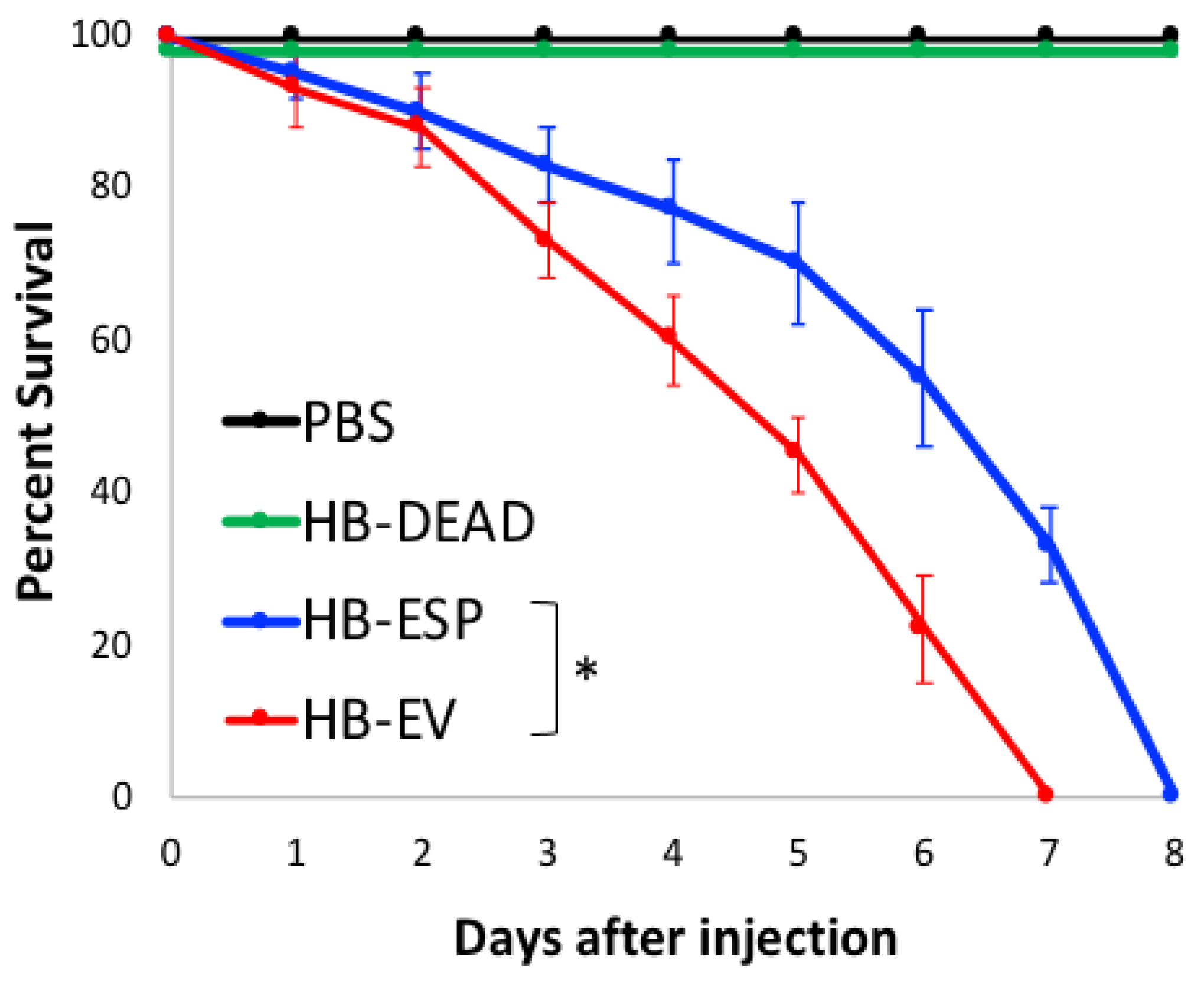
3.2. H. bacteriophora EVs Are Lethal to D. melanogaster Adult Flies
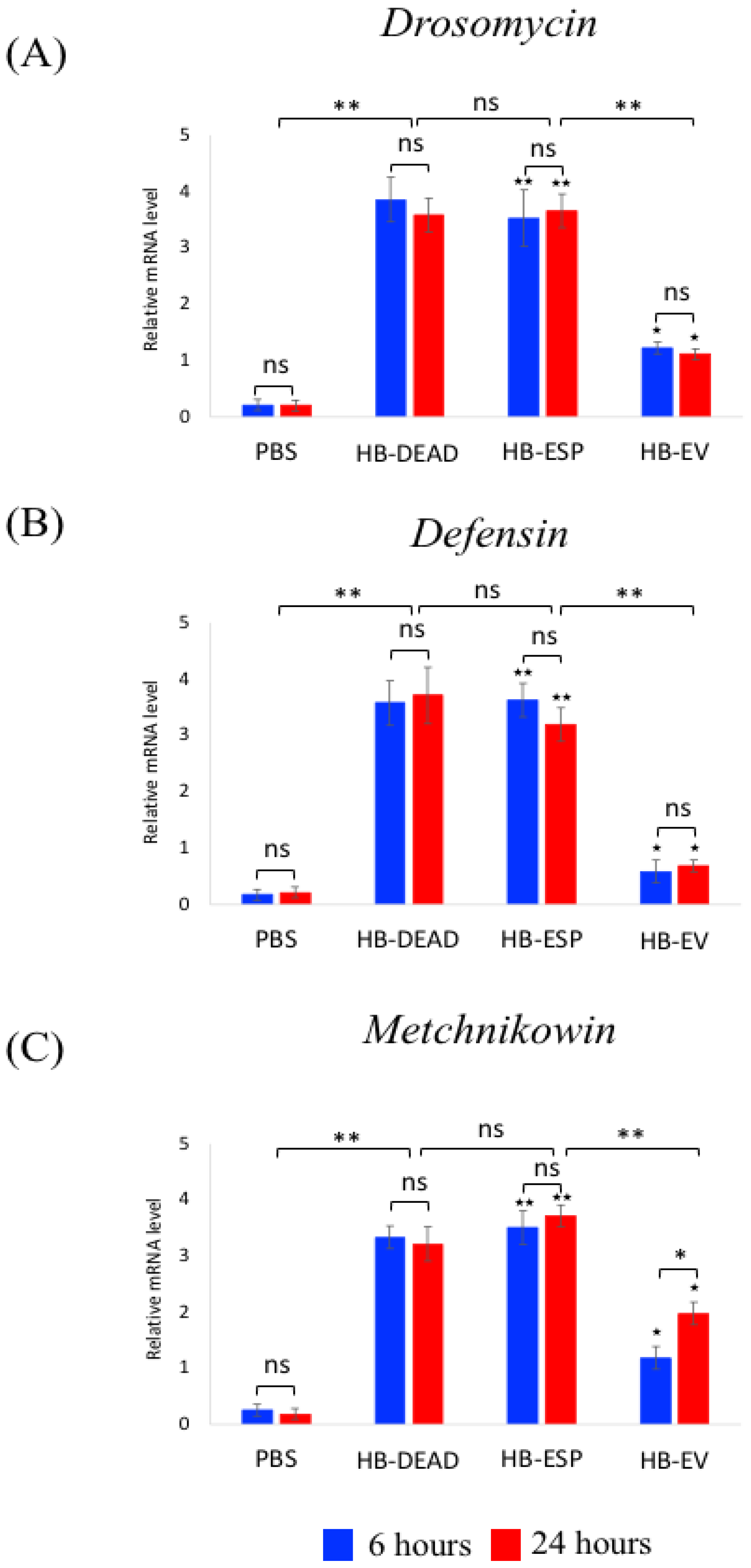
3.3. H. bacteriophora EVs Suppress the Expression of Attacin and Cecropin in Adult D. melanogaster
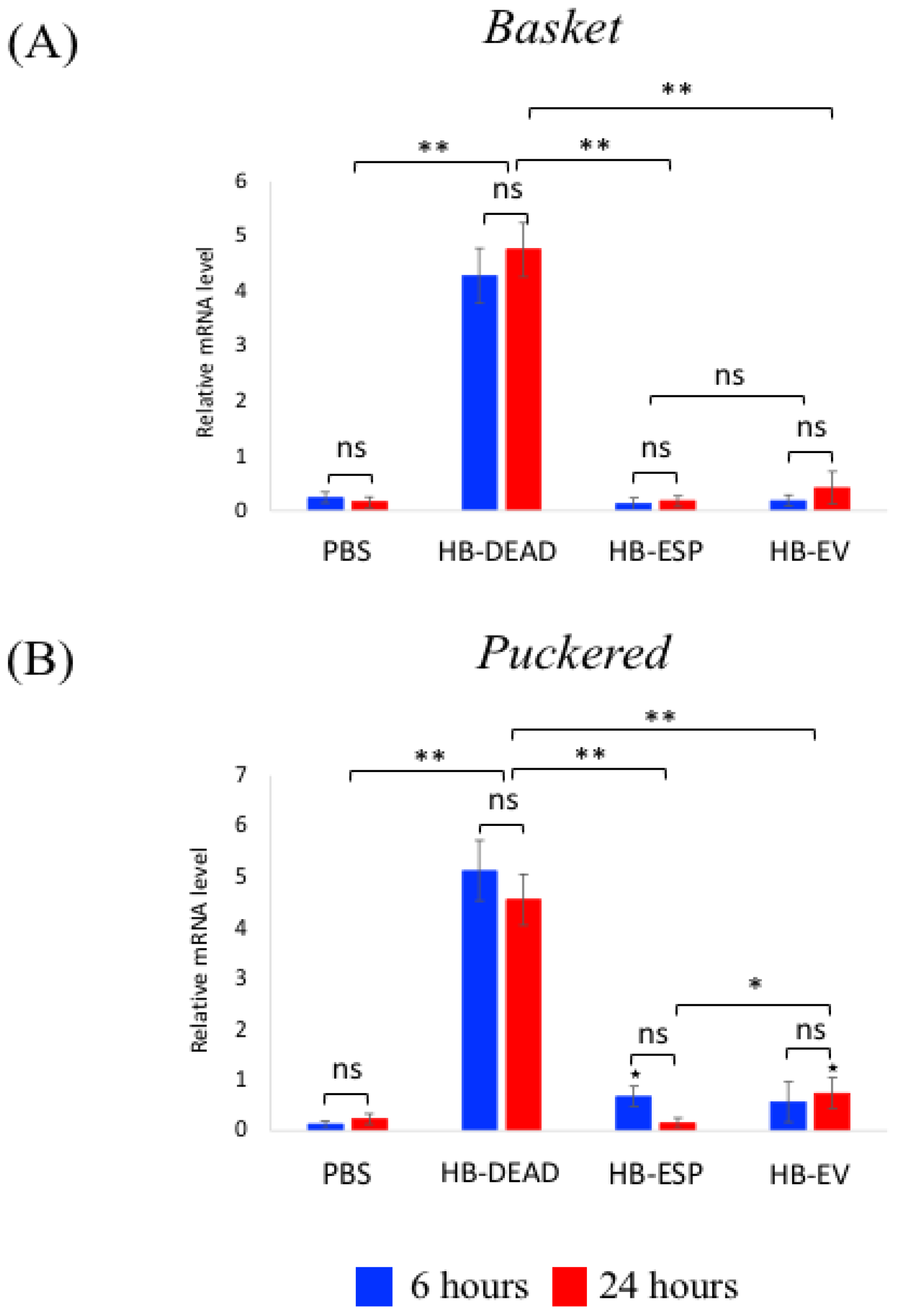
3.4. H. bacteriophora EVs Moderately Activate the JNK and Jak/Stat Signaling Pathways
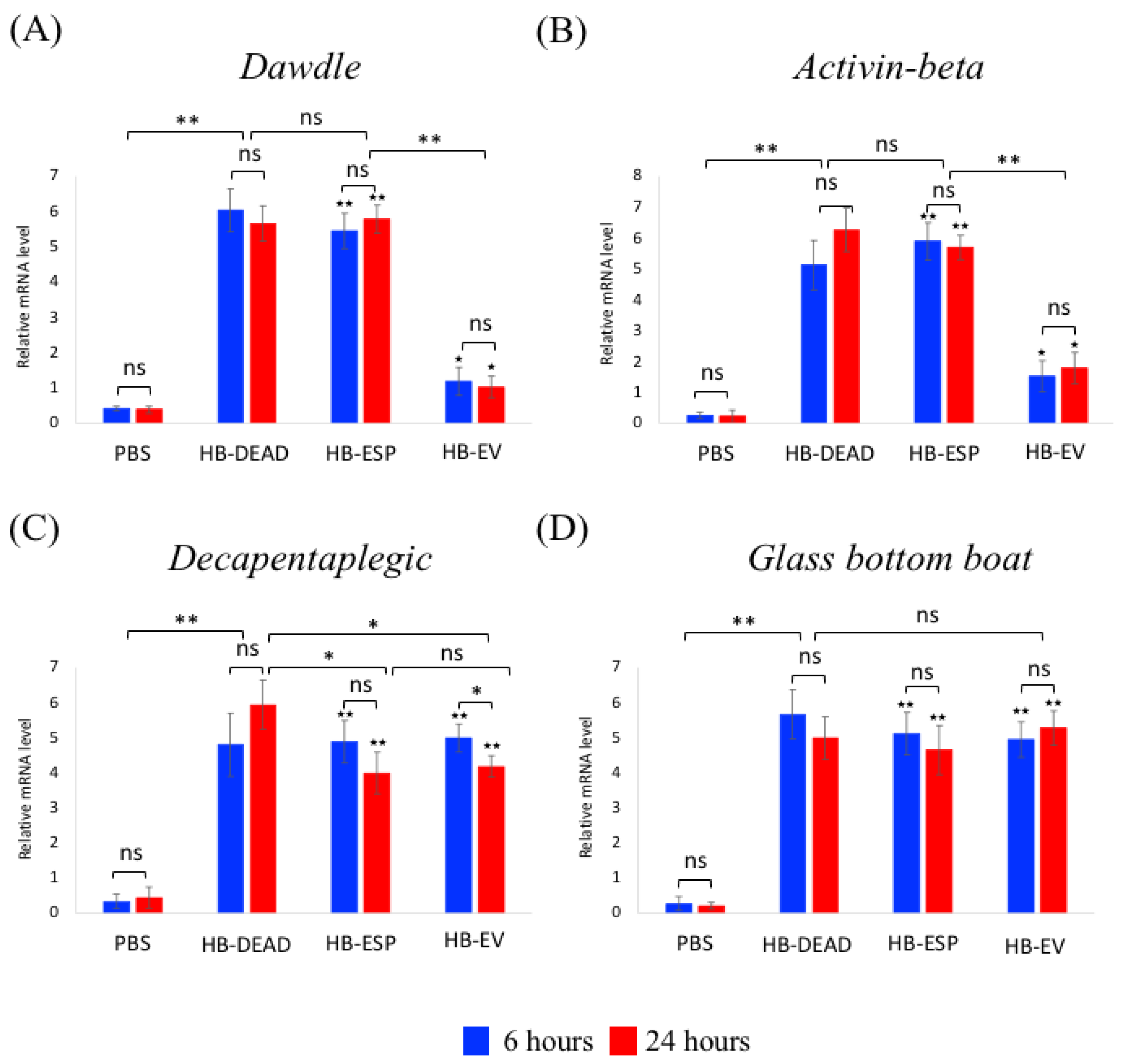
3.5. H. bacteriophora EVs Induce the TGF-β Signaling and Downregulate PPO3 Gene Expression in D. melanogaster Adults
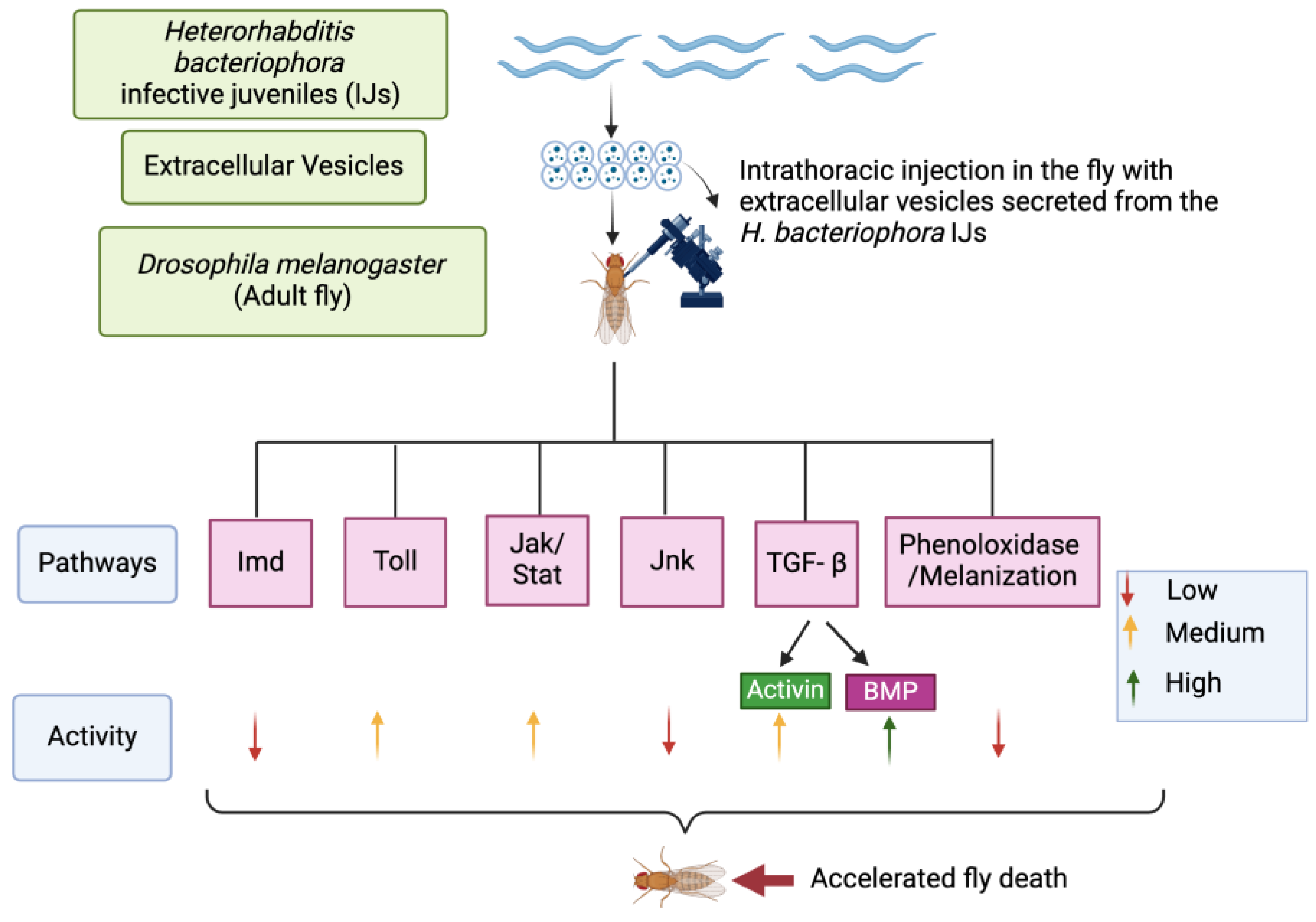
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dillman, A.R.; Sternberg, P.W. Entomopathogenic nematodes. Curr. Biol. 2012, 22, R430–R431. [Google Scholar] [CrossRef] [PubMed]
- Dillman, A.R.; Chaston, J.M.; Adams, B.J.; Ciche, T.A.; Goodrich-Blair, H.; Stock, S.P.; Sternberg, P.W. An entomopathogenic nematode by any other name. PLoS Pathog. 2012, 8, e1002527. [Google Scholar] [CrossRef] [PubMed]
- Tarasco, E.; Fanelli, E.; Salvemini, C.; El-Khoury, Y.; Troccoli, A.; Vovlas, A.; De Luca, F. Entomopathogenic nematodes and their symbiotic bacteria: From genes to field uses. Front. Insect Sci. 2023, 3, 1195254. [Google Scholar] [CrossRef] [PubMed]
- Parihar, R.D.; Dhiman, U.; Bhushan, A.; Gupta, P.K.; Gupta, P. Heterorhabditis and Photorhabdus Symbiosis: A Natural Mine of Bioactive Compounds. Front. Microbiol. 2022, 13, 790339. [Google Scholar] [CrossRef] [PubMed]
- Ciche, T. The biology and genome of Heterorhabditis bacteriophora. WormBook 2007, 13, 790339. [Google Scholar] [CrossRef]
- Clarke, D.J. Photorhabdus: A tale of contrasting interactions. Microbiology (Reading) 2020, 166, 335–348. [Google Scholar] [CrossRef]
- Vadnal, J.; Ratnappan, R.; Keaney, M.; Kenney, E.; Eleftherianos, I.; O’Halloran, D.; Hawdon, J.M. Identification of candidate infection genes from the model entomopathogenic nematode Heterorhabditis bacteriophora. BMC Genom. 2017, 18, 8. [Google Scholar] [CrossRef]
- Lara-Reyes, N.; Jiménez-Cortés, J.G.; Canales-Lazcano, J.; Franco, B.; Krams, I.; Contreras-Garduño, J. Insect Immune Evasion by Dauer and Nondauer Entomopathogenic Nematodes. J. Parasitol. 2021, 107, 115–124. [Google Scholar] [CrossRef]
- Bobardt, S.D.; Dillman, A.R.; Nair, M.G. The Two Faces of Nematode Infection: Virulence and Immunomodulatory Molecules From Nematode Parasites of Mammals, Insects and Plants. Front. Microbiol. 2020, 11, 577846. [Google Scholar] [CrossRef]
- Cooper, D.; Eleftherianos, I. Parasitic Nematode Immunomodulatory Strategies: Recent Advances and Perspectives. Pathogens 2016, 5, 58. [Google Scholar] [CrossRef]
- Kenney, E.; Hawdon, J.M.; O’Halloran, D.; Eleftherianos, I. Heterorhabditis bacteriophora Excreted-Secreted Products Enable Infection by Photorhabdus luminescens Through Suppression of the Imd Pathway. Front. Immunol. 2019, 10, 2372. [Google Scholar] [CrossRef] [PubMed]
- Eliáš, S.; Hurychová, J.; Toubarro, D.; Frias, J.; Kunc, M.; Dobeš, P.; Simões, N.; Hyršl, P. Bioactive Excreted/Secreted Products of Entomopathogenic Nematode Heterorhabditis bacteriophora Inhibit the Phenoloxidase Activity during the Infection. Insects 2020, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Coakley, G.; Maizels, R.M.; Buck, A.H. Exosomes and Other Extracellular Vesicles: The New Communicators in Parasite Infections. Trends Parasitol. 2015, 31, 477–489. [Google Scholar] [CrossRef]
- Macilla, A.; Jones, K.; Smith, T. Helminth extracellular vesicles: A new paradigm in parasite-host interactions. Rev. Parasitol. 2014, 12, 98–110. [Google Scholar]
- Maizels, R.M.; Yazdanbakhsh, M. Immune regulation by helminth parasites: Cellular and molecular mechanisms. Nat. Rev. Immunol. 2003, 3, 733–744. [Google Scholar] [CrossRef]
- Wang, L. Exosome-like vesicles derived by Schistosoma japonicum adult worms mediates M1 type immune activity of macrophage. Parasitol. Res. 2015, 114, 1865–1873. [Google Scholar] [CrossRef]
- Buck, A.H. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat. Commun. 2014, 5, 5488. [Google Scholar] [CrossRef] [PubMed]
- McSorley, H.J.; Blair, N.F.; Smith, K.A.; McKenzie, A.N.; Maizels, R.M. Blockade of IL-33 release and suppression of type 2 innate lymphoid cell responses by helminth secreted products in airway allergy. Mucosal Immunol. 2014, 7, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Romo, M.R.; Pérez-Martínez, D.; Castillo Ferre, C. Innate immunity in vertebrates: An overview. Immunology 2016, 148, 125–139. [Google Scholar] [CrossRef]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef]
- Buchon, N.; Silverman, N.; Cherry, S. Immunity in Drosophila melanogaster–from microbial recognition to whole-organism physiology. Nat. Rev. Immunol. 2014, 14, 796–810. [Google Scholar] [CrossRef]
- Yu, S.; Luo, F.; Xu, Y.; Zhang, Y.; Jin, L.H. Drosophila Innate Immunity Involves Multiple Signaling Pathways and Coordinated Communication Between Different Tissues. Front. Immunol. 2022, 13, 905370. [Google Scholar] [CrossRef] [PubMed]
- Peña, J.M.; Carillo, M.A.; Hallem, E.A. Variation in the susceptibility of Drosophila to different entomopathogenic nematodes. Infect. Immun. 2015, 83, 1130–1138. [Google Scholar] [CrossRef]
- Arefin, B.; Kucerova, L.; Dobes, P.; Markys, R.; Strnad, H.; Wang, Z.; Hyrsl, P.; Zurovec, M.; Theopold, U. Genome-wide transcriptional analysis of Drosophila larvae infected by entomopathogenic nematodes shows involvement of complement, recognition and extracellular matrix proteins. J. Innate Immun. 2014, 6, 192–204. [Google Scholar] [CrossRef]
- Hyrsl, P.; Dobes, P.; Wang, Z.; Hauling, T.; Wilhelmsson, C.; Theopold, U. Clotting factors and eicosanoids protect against nematode infections. J. Innate Immun. 2011, 3, 65–70. [Google Scholar] [CrossRef]
- Wang, Z.; Wilmhelmsson, C.; Hyrsl, P.; Loof, T.G.; Dobes, P.; Klupp, M.; Loseva, O.; Mörgelin, M.; Iklé, J.; Cripps, R.M.; et al. Pathogen entrapment by transglutaminase—A conserved early innate immune mechanism. PLoS Pathog. 2010, 6, e1000763. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.C.; Creasy, T.; Kumari, P.; Shetty, A.; Shokal, U.; Tallon, L.J.; Eleftherianos, I. Drosophila anti-nematode and antibacterial immune regulators revealed by RNA-Seq. BMC Genom. 2015, 16, 519. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Daugherty, S.; Shetty, A.C.; Eleftherianos, I. RNAseq Analysis of the Drosophila Response to the Entomopathogenic Nematode Steinernema. G3 (Bethesda) 2017, 7, 1955–1967. [Google Scholar] [CrossRef]
- Lima, A.K.; Dhillon, H.; Dillman, A.R. ShK-Domain-Containing Protein from a Parasitic Nematode Modulates Drosophila melanogaster Immunity. Pathogens 2022, 11, 1094. [Google Scholar] [CrossRef]
- Hallem, E.A.; Rengarajan, M.; Ciche, T.A.; Sternberg, P.W. Nematodes, bacteria, and flies: A tripartite model for nematode parasitism. Curr. Biol. 2007, 17, 898–904. [Google Scholar] [CrossRef]
- Castillo, J.C.; Shokal, U.; Eleftherianos, I. Immune gene transcription in Drosophila adult flies infected by entomopathogenic nematodes and their mutualistic bacteria. J. Insect Physiol. 2013, 59, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Campos-Herrera, R.; Barbercheck, M.; Hoy, C.W.; Stock, S.P. Entomopathogenic nematodes as a model for advancing the frontiers of ecology. J. Nematol. 2012, 44, 162–176. [Google Scholar] [PubMed]
- Lu, D.; Baiocchi, T.; Dillman, A.R. Genomics of Entomopathogenic Nematodes and Implications for Pest Control. Trends Parasitol. 2016, 32, 588–598. [Google Scholar] [CrossRef]
- Toubarro, D.; Avila, M.M.; Montiel, R.; Simões, N. A pathogenic nematode targets recognition proteins to avoid insect defenses. PLoS ONE 2013, 8, e75691. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Brun, S.; Vidal, S.; Spellamn, P.; Takahashi, K.; Tricoire, H.; Lemaitre, B. The MAPKKK Mekk1 regulates the expression of Turandot stress genes in response to septic injury in Drosophila. Genes Cells 2006, 11, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Ozakman, Y.; Raval, D.; Eleftherianos, I. Activin and BMP Signaling Activity Affects Different Aspects of Host Anti-Nematode Immunity in Drosophila. Front. Immunol. 2021, 12, 795331. [Google Scholar] [CrossRef]
- Ozakman, Y.; Pagadala, T.; Raval, D.; Eleftherianos, I. The Drosophila melanogaster Metabolic Response against Parasitic Nematode Infection Is Mediated by TGF-β Signaling. Microorganisms 2020, 8, 971. [Google Scholar] [CrossRef]
- Ozakman, Y.; Eleftherianos, I. TGF-β Signaling Interferes With the Drosophila Innate Immune and Metabolic Response to Parasitic Nematode Infection. Front. Physiol. 2019, 10, 716. [Google Scholar] [CrossRef]
- Patrnogic, J.; Heryanto, C.; Ozakman, Y.; Eleftherianos, I. Transcript analysis reveals the involvement of NF-κB transcription factors for the activation of TGF-β signaling in nematode-infected Drosophila. Immunogenetics 2019, 71, 501–510. [Google Scholar] [CrossRef]
- Patrnogic, J.; Heryanto, C.; Eleftherianos, I. Transcriptional up-regulation of the TGF-β intracellular signaling transducer Mad of Drosophila larvae in response to parasitic nematode infection. Innate Immun. 2018, 24, 349–356. [Google Scholar] [CrossRef]
- Patrnogic, J.; Heryanto, C.; Eleftherianos, I. Wounding-induced upregulation of the Bone-Morphogenic Protein signaling pathway in Drosophila promotes survival against parasitic nematode infection. Gene 2018, 673, 112–118. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Castillo, J.C.; Patrnogic, J. TGF-β signaling regulates resistance to parasitic nematode infection in Drosophila melanogaster. Immunobiology 2016, 221, 1362–1368. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Labaude, S.; Griffin, C.T. Transmission Success of Entomopathogenic Nematodes Used in Pest Control. Insects 2018, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Okakpu, O.K.; Dillman, A.R. Review of the role of parasitic nematode excretory/secretory proteins in host immunomodulation. J. Parasitol. 2022, 108, 199–208. [Google Scholar] [CrossRef]
- Ozakman, Y.; Eleftherianos, I. Nematode infection and antinematode immunity in Drosophila. Trends Parasitol. 2021, 37, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Somvanshi, V.S.; Gahoi, S.; Banakar, P.; Thakur, P.K.; Kumar, M.; Sajnani, M.; Pandey, P.; Rao, U. A transcriptomic insight into the infective juvenile stage of the insect parasitic nematode Heterorhabditis indica. BMC Genom. 2016, 17, 166. [Google Scholar] [CrossRef]
- Kenney, E.; Yaparla, A.; Hawdon, J.M.; O’Halloran, D.M.; Grayfer, L.; Eleftherianos, I. A putative UDP-glycosyltransferase from Heterorhabditis bacteriophora suppresses antimicrobial peptide gene expression and factors related to ecdysone signaling. Sci. Rep. 2020, 10, 12312. [Google Scholar] [CrossRef]
- Kenney, E.; Yaparla, A.; Hawdon, J.M.; O’Halloran, D.M.; Grayfer, L.; Eleftherianos, I. A putative lysozyme and serine carboxypeptidase from Heterorhabditis bacteriophora show differential virulence capacities in Drosophila melanogaster. Dev. Comp. Immunol. 2021, 114, 103820. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Kenney, E.; Eleftherianos, I. The Activin Branch Ligand Daw Regulates the Drosophila melanogaster Immune Response and Lipid Metabolism against the Heterorhabditis bacteriophora Serine Carboxypeptidase. Int. J. Mol. Sci. 2024, 25, 7970. [Google Scholar] [CrossRef] [PubMed]
- Ekengren, S.; Hultmark, D. A family of Turandot-related genes in the humoral stress response of Drosophila. Biochem. Biophys. Res. Commun. 2001, 284, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Takatsu, Y.; Nakamura, M.; Stapleton, M.; Danos, M.C.; Matsumoto, K.; O’Connor, M.B.; Shibuya, H.; Ueno, N. TAK1 participates in c-Jun N-terminal kinase signaling during Drosophila development. Mol. Cell Biol. 2000, 20, 3015–3326. [Google Scholar] [CrossRef]
- Peterson, A.J.; O’Connor, M.B. Strategies for exploring TGF-β signaling in Drosophila. Methods 2014, 68, 183–193. [Google Scholar] [CrossRef]
- Cooper, D.; Wuebbolt, C.; Heryanto, C.; Eleftherianos, I. The prophenoloxidase system in Drosophila participates in the anti-nematode immune response. Mol. Immunol. 2019, 109, 88–98. [Google Scholar] [CrossRef]
- Castillo, J.C.; Reynolds, S.E.; Eleftherianos, I. Insect immune responses to nematode parasites. Trends Parasitol. 2011, 27, 537–547. [Google Scholar] [CrossRef]
- Li, X.-Y.; Cowles, R.S.; Cowles, E.A.; Gaugler, R.; Cox-Foster, D.L. Relationship between the successful infection by entomopathogenic nematodes and the host immune response. Int. J. Parasitol. 2007, 37, 365–374. [Google Scholar] [CrossRef]
- Brivio, M.F.; Mastore, M. Nematobacterial Complexes and Insect Hosts: Different Weapons for the Same War. Insects 2018, 9, 117. [Google Scholar] [CrossRef]
- Dziedziech, A.; Shivankar, S.; Theopold, U. Drosophila melanogaster Responses against Entomopathogenic Nematodes: Focus on Hemolymph Clots. Insects 2020, 11, 62. [Google Scholar] [CrossRef]
- Chang, D.Z.; Lu, D.; Mortazavi, A.; Dillman, A.R. A core set of venom proteins is released by entomopathogenic nematodes in the genus Steinernema. PLoS Pathog. 2019, 15, e1007626. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Macchietto, M.; Chang, D.; Barros, M.M.; Baldwin, J.; Mortazavi, A.; Dillman, A.R. Activated entomopathogenic nematode infective juveniles release lethal venom proteins. PLoS Pathog. 2017, 13, e1006302. [Google Scholar] [CrossRef] [PubMed]




| Gene | Forward Primer 1 | Reverse Primer 1 |
|---|---|---|
| Attacin-A | CAATGGCAGACAATCTGG | ATTCCTGGGAAGTTGCTGTG |
| Cecropin-A1 | TCTTCGTTTTCGTCGCTCTC | CTTGTTGAGCGATTCCCAGT |
| Diptericin | ACCGCAGTACCCACTCAATC | CCCAAGTGCTGTCCATATCC |
| Drosomycin | TCTTCGTTTTCGTCGCTCTC | CTTGTTGAGCGATTCCCAGT |
| Metchnikowin | TCTTGGAGCGATTTTTCTGG | AATAAATTGGACCCGGTCTTG |
| Defensin | CGCATAGAAGCGAGCCACATG | GCAGTAGCCGCCTTTGAACC |
| Turandot A | GAAGATCGTGAGGCTGACAAC | GTCCTGGGCGTTTTTGATAA |
| Turandot M | GCTGGGAAAGGTAAATGCTG | AGGCGCTGTTTTTCTGTGAC |
| Basket | GACAGCTCAGCACCAACACT | GCTTGGCATGGGTTACATTT |
| Puckered | GGCCTACAAGCTGGTGAAAG | AGTTCAGATTGGGCGAGATG |
| Dawdle | GGTGGATCAGCAGAAGGACT | GCCACTGATCCAGTGTTTGA |
| Decapentaplegic | CCTTGGAGCCTCTGTCGAT | TGCACTCTGATCTGGGATTTT |
| Glass bottom boat | CCAGATGCAGACCCTGTACAT | CTGGTGCGATGATCCAGTC |
| Activin-β | ACGGCAAATTTTGACAAAGC | TTGGTATCATTCGTCCACCA |
| Prophenoloxidase 1 | CAACTGGCTTCGTTGAGTGA | CGGGCAGTTCCAATACAGTT |
| Prophenoloxidase 2 | CCCGCCTATACCGAGA | CGCACGTAGCCGAAAC |
| Prophenoloxidase 3 | GGCGAGCTGTTCTACT | GAGGATACGCCCTACTG |
| RpL32 | GATGACCATCCGCCCAGCA | CGGACCGACAGCTGCTTGGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toubarro, D.; Kenney, E.; Heryanto, C.; Mallick, S.; Simões, N.; Eleftherianos, I. Heterorhabditis bacteriophora Extracellular Vesicles Alter the Innate Immune Signaling in Drosophila melanogaster. Genes 2025, 16, 613. https://doi.org/10.3390/genes16060613
Toubarro D, Kenney E, Heryanto C, Mallick S, Simões N, Eleftherianos I. Heterorhabditis bacteriophora Extracellular Vesicles Alter the Innate Immune Signaling in Drosophila melanogaster. Genes. 2025; 16(6):613. https://doi.org/10.3390/genes16060613
Chicago/Turabian StyleToubarro, Duarte, Eric Kenney, Christa Heryanto, Sreeradha Mallick, Nelson Simões, and Ioannis Eleftherianos. 2025. "Heterorhabditis bacteriophora Extracellular Vesicles Alter the Innate Immune Signaling in Drosophila melanogaster" Genes 16, no. 6: 613. https://doi.org/10.3390/genes16060613
APA StyleToubarro, D., Kenney, E., Heryanto, C., Mallick, S., Simões, N., & Eleftherianos, I. (2025). Heterorhabditis bacteriophora Extracellular Vesicles Alter the Innate Immune Signaling in Drosophila melanogaster. Genes, 16(6), 613. https://doi.org/10.3390/genes16060613







