Psychosocial Factors Involved in Genetic Testing for Rare Diseases: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Identifying the Research Question
2.2. Identifying the Relevant Literature
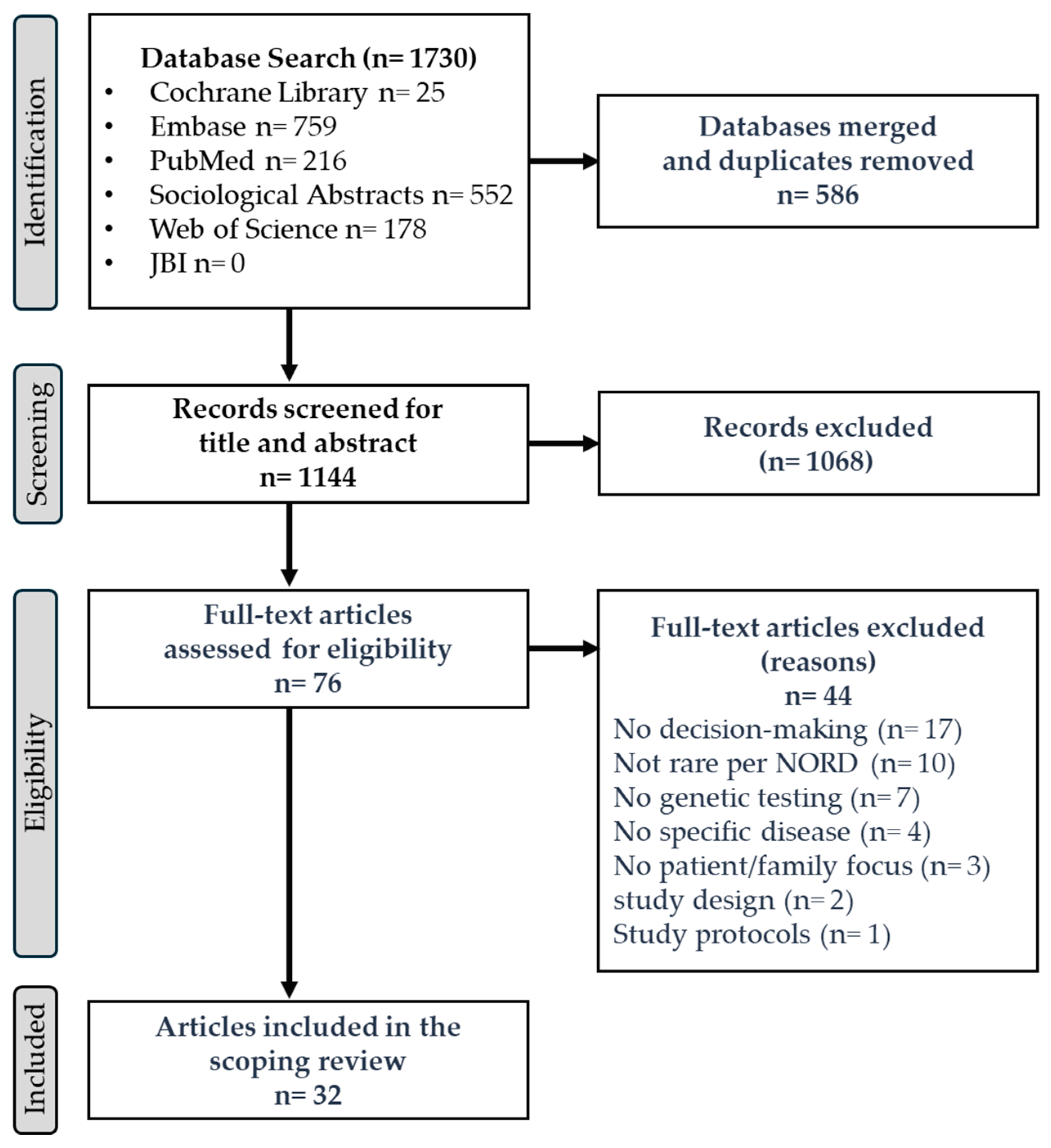
2.3. Selecting the Literature
2.4. Data Charting
2.5. Synthesis of Results
2.6. Analysis
2.7. Consultation
2.8. Mapping to the Theory of Planned Behavior
3. Results
3.1. Study Characteristics
3.2. Rare Disorders Under Investigation and Types of Genetic Testing
3.3. Qualitative Studies (n = 19)
3.4. Quantitative Studies (n = 10)
3.5. Mixed-Methods Studies (n = 3)
3.6. Promoters and Barriers to Genetic Testing
3.7. Lifespan Perspective on Genetic Testing Decisions
3.8. Findings Mapped to the Theory of Planned Behavior
4. Discussion
4.1. Logistical/Structural Barriers to Genetic Testing
4.2. Similarities in Psychosocial Factors Between Rare and Common (Tier 1) Genetic Diseases
4.3. Patient and Public Involvement Insights
4.4. Future Directions for Person-Centered Genetic Testing Decisional Support
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | artificial intelligence |
| GS | genomics sequencing |
| GT | genetic testing |
| HBOC | hereditary breast and ovarian cancer syndrome |
| NORD | National Organization of Rare Disorders |
| TPB | Theory of Planned Behavior |
| U.K. | United Kingdom |
| U.S. | United States |
Appendix A
Appendix A.1. Search Terms
References
- Nguengang Wakap, S.; Lambert, D.M.; Olry, A.; Rodwell, C.; Gueydan, C.; Lanneau, V.; Murphy, D.; Le Cam, Y.; Rath, A. Estimating cumulative point prevalence of rare diseases: Analysis of the Orphanet database. Eur. J. Hum. Genet. 2020, 28, 165–173. [Google Scholar] [CrossRef]
- Walewski, J.L.; Dan, D.; Nori, M. How many zebras are there, and where are they hiding in medical literature? A literature review of publications on rare diseases. Expert Opin. Orphan Drugs 2019, 7, 513–519. [Google Scholar] [CrossRef]
- The Lancet Global Health. The landscape for rare diseases in 2024. Lancet Glob. Health 2024, 12, e341. [Google Scholar] [CrossRef] [PubMed]
- Maron, J.L.; Kingsmore, S.; Gelb, B.D.; Vockley, J.; Wigby, K.; Bragg, J.; Stroustrup, A.; Poindexter, B.; Suhrie, K.; Kim, J.H.; et al. Rapid Whole-Genomic Sequencing and a Targeted Neonatal Gene Panel in Infants with a Suspected Genetic Disorder. JAMA 2023, 330, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.F.; Campbell, P.; Eberhardt, R.Y.; Aitken, S.; Perrett, D.; Brent, S.; Danecek, P.; Gardner, E.J.; Chundru, V.K.; Lindsay, S.J.; et al. Genomic Diagnosis of Rare Pediatric Disease in the United Kingdom and Ireland. N. Engl. J. Med. 2023, 388, 1559–1571. [Google Scholar] [CrossRef]
- Wojcik, M.H.; Lemire, G.; Berger, E.; Zaki, M.S.; Wissmann, M.; Win, W.; White, S.M.; Weisburd, B.; Wieczorek, D.; Waddell, L.B.; et al. Genome Sequencing for Diagnosing Rare Diseases. N. Engl. J. Med. 2024, 390, 1985–1997. [Google Scholar] [CrossRef]
- Manolio, T.A.; Rowley, R.; Williams, M.S.; Roden, D.; Ginsburg, G.S.; Bult, C.; Chisholm, R.L.; Deverka, P.A.; McLeod, H.L.; Mensah, G.A.; et al. Opportunities, resources, and techniques for implementing genomics in clinical care. Lancet 2019, 394, 511–520. [Google Scholar] [CrossRef]
- Genoff Garzon, M.C.; Rubin, L.R.; Lobel, M.; Stelling, J.; Pastore, L.M. Review of patient decision-making factors and attitudes regarding preimplantation genetic diagnosis. Clin. Genet. 2018, 94, 22–42. [Google Scholar] [CrossRef]
- Smith, H.S.; Bonkowski, E.S.; Deloge, R.B.; Gutierrez, A.M.; Recinos, A.M.; Lavelle, T.A.; Veenstra, D.L.; McGuire, A.L.; Pereira, S. Key drivers of family-level utility of pediatric genomic sequencing: A qualitative analysis to support preference research. Eur. J. Hum. Genet. 2023, 31, 445–452. [Google Scholar] [CrossRef]
- Vaynrub, A.; Salazar, B.; Feng, Y.E.; West, H.; Michel, A.; Umakanth, S.; Crew, K.D.; Kukafka, R. The breast cancer genetic testing experience: Probing the potential utility of an online decision aid in risk perception and decision making. BMC Cancer 2025, 25, 19. [Google Scholar] [CrossRef]
- Pozzar, R.A.; Seven, M. Interventions to support decision making in people considering germline genetic testing for BRCA 1/2 pathogenic and likely pathogenic variants: A scoping review. J. Genet. Couns. 2024, 33, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, Y.; Kim, S.; Katapodi, M.C. Informational needs of individuals from families harboring BRCA pathogenic variants: A systematic review and content analysis. Genet. Med. 2023, 25, 100001. [Google Scholar] [CrossRef] [PubMed]
- Holtzclaw Williams, P. Policy framework for rare disease health disparities. Policy Politics Nurs. Pract. 2011, 12, 114–118. [Google Scholar] [CrossRef]
- Skivington, K.; Matthews, L.; Simpson, S.A.; Craig, P.; Baird, J.; Blazeby, J.M.; Boyd, K.A.; Craig, N.; French, D.P.; McIntosh, E.; et al. A new framework for developing and evaluating complex interventions: Update of Medical Research Council guidance. Bmj 2021, 374, n2061. [Google Scholar] [CrossRef]
- Ajzen, I. The theory of planned behavior. Organ. Behav. Hum. Decis. Process. 1991, 50, 179–211. [Google Scholar] [CrossRef]
- Katapodi, M.C.; Pedrazzani, C.; Barnoy, S.; Dagan, E.; Fluri, M.; Jones, T.; Kim, S.; Underhill-Blazey, M.L.; Uveges, M.K.; Dwyer, A.A. ACCESS: An empirically-based framework developed by the International Nursing CASCADE Consortium to address genomic disparities through the nursing workforce. Front. Genet. 2023, 14, 1337366. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’malley, L. Scoping studies: Toward a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.; Colquhoun, H.; Kastner, M.; Levac, D.; Ng, C.; Sharpe, J.P.; Wilson, K.; et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med. Res. Methodol. 2016, 16, 15. [Google Scholar] [CrossRef]
- Taruscio, D.; Gahl, W.A. Rare diseases: Challenges and opportunities for research and public health. Nat. Rev. Dis. Primers 2024, 10, 13. [Google Scholar] [CrossRef]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Saunders, C.H.; Sierpe, A.; von Plessen, C.; Kennedy, A.M.; Leviton, L.C.; Bernstein, S.L.; Goldwag, J.; King, J.R.; Marx, C.M.; Pogue, J.A.; et al. Practical thematic analysis: A guide for multidisciplinary health services research teams engaging in qualitative analysis. BMJ 2023, 381, e074256. [Google Scholar] [CrossRef] [PubMed]
- Olesen, A.P.; Nor, S.N.; Amin, L. Attitudes Toward Pre-implantation Genetic Diagnosis (PGD) for Genetic Disorders Among Potential Users in Malaysia. Sci. Eng. Ethics 2016, 22, 133–146. [Google Scholar] [CrossRef]
- Alderfer, M.A.; Zelley, K.; Lindell, R.B.; Novokmet, A.; Mai, P.L.; Garber, J.E.; Nathan, D.; Scollon, S.; Chun, N.M.; Patenaude, A.F.; et al. Parent decision-making around the genetic testing of children for germline TP53 mutations. Cancer 2015, 121, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, L.; Delatycki, M.B.; Massie, J.; Hodgson, J.; Lewis, S. “Suddenly Having two Positive People who are Carriers is a Whole New Thing”—Experiences of Couples Both Identified as Carriers of Cystic Fibrosis Through a Population-Based Carrier Screening Program in Australia. J. Genet. Couns. 2015, 24, 987–1000. [Google Scholar] [CrossRef]
- Halverson, C.M.; Clift, K.E.; McCormick, J.B. Was it worth it? Patients’ perspectives on the perceived value of genomic-based individualized medicine. J. Community Genet. 2016, 7, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Pélissier, A.; Peyron, C.; Béjean, S. Next-generation sequencing in clinical practice: From the patients’ preferences to the informed consent process. Public Health 2016, 138, 157–159. [Google Scholar] [CrossRef]
- Seven, M.; Akyüz, A.; Eroglu, K.; Daack-Hirsch, S.; Skirton, H. Women’s knowledge and use of prenatal screening tests. J. Clin. Nurs. 2017, 26, 1869–1877. [Google Scholar] [CrossRef]
- Tsai, G.J.; Cameron, C.A.; Czerwinski, J.L.; Mendez-Figueroa, H.; Peterson, S.K.; Noblin, S.J. Attitudes Towards Prenatal Genetic Counseling, Prenatal Genetic Testing, and Termination of Pregnancy among Southeast and East Asian Women in the United States. J. Genet. Couns. 2017, 26, 1041–1058. [Google Scholar] [CrossRef]
- Ibisler, A.; Ocklenburg, S.; Stemmler, S.; Arning, L.; Epplen, J.T.; Saft, C.; Hoffjan, S. Prospective Evaluation of Predictive DNA Testing for Huntington’s Disease in a Large German Center. J. Genet. Couns. 2017, 26, 1029–1040. [Google Scholar] [CrossRef]
- Metcalfe, S.A.; Martyn, M.; Ames, A.; Anderson, V.; Archibald, A.D.; Couns, G.D.G.; Carter, R.; Cohen, J.; Cotter, M.; GenCouns, M.; et al. Informed decision making and psychosocial outcomes in pregnant and nonpregnant women offered population fragile X carrier screening. Genet. Med. 2017, 19, 1346–1355. [Google Scholar] [CrossRef]
- Mackley, M.P.; Blair, E.; Parker, M.; Taylor, J.C.; Watkins, H.; Ormondroyd, E. Views of rare disease participants in a UK whole-genome sequencing study towards secondary findings: A qualitative study. Eur. J. Hum. Genet. 2018, 26, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Halliday, J.L.; Muller, C.; Charles, T.; Norris, F.; Kennedy, J.; Lewis, S.; Meiser, B.; Donath, S.; Stark, Z.; McGillivray, G.; et al. Offering pregnant women different levels of genetic information from prenatal chromosome microarray: A prospective study. Eur. J. Hum. Genet. 2018, 26, 485–494. [Google Scholar] [CrossRef]
- Mayo-Gamble, T.L.; Middlestadt, S.E.; Lin, H.C.; Cunningham-Erves, J.; Barnes, P.; Jackson, P.B. Identifying Factors Underlying the Decision for Sickle Cell Carrier Screening Among African Americans Within Middle Reproductive Age. J. Genet. Couns. 2018, 27, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Stuttgen, K.M.; Bollinger, J.M.; Dvoskin, R.L.; McCague, A.; Shpritz, B.; Brandt, J.; Mathews, D.J.H. Perspectives on Genetic Testing and Return of Results from the First Cohort of Presymptomatically Tested Individuals At Risk of Huntington Disease. J. Genet. Couns. 2018, 27, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Warby, M.; Wakefield, C.E.; Vetsch, J.; Tucker, K.M. Families’ and health care professionals’ attitudes towards Li-Fraumeni syndrome testing in children: A systematic review. Clin. Genet. 2019, 95, 140–150. [Google Scholar] [CrossRef]
- Sullivan, H.K.; Bayefsky, M.; Wakim, P.G.; Huddleston, K.; Biesecker, B.B.; Hull, S.C.; Berkman, B.E. Noninvasive Prenatal Whole Genome Sequencing: Pregnant Women’s Views and Preferences. Obstet. Gynecol. 2019, 133, 525–532. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Huang, S.; Sun, L.; Zhao, S.; Zhong, Y.; Xiao, H.; Ding, X. Parents’ perceptions of diagnostic genetic testing for children with inherited retinal disease in China. Mol. Genet. Genomic. Med. 2019, 7, e916. [Google Scholar] [CrossRef]
- Schwartz, M.; Brandel, J.P.; Babonneau, M.L.; Boucher, C.; Schaerer, E.; Haik, S.; Laplanche, J.L.; Gargiulo, M.; Durr, A. Genetic Testing in Prion Disease: Psychological Consequences of the Decisions to Know or Not to Know. Front. Genet. 2019, 10, 895. [Google Scholar] [CrossRef]
- Mendes, A.; Paneque, M.; Clarke, A.; Sequeiros, J. Choosing not to know: Accounts of non-engagement with pre-symptomatic testing for Machado-Joseph disease. Eur. J. Hum. Genet. 2019, 27, 353–359. [Google Scholar] [CrossRef]
- Marshall, D.A.; MacDonald, K.V.; Heidenreich, S.; Hartley, T.; Bernier, F.P.; Gillespie, M.K.; McInnes, B.; Innes, A.M.; Armour, C.M.; Boycott, K.M. The value of diagnostic testing for parents of children with rare genetic diseases. Genet. Med. 2019, 21, 2798–2806. [Google Scholar] [CrossRef]
- Forbes Shepherd, R.; Werner-Lin, A.; Keogh, L.A.; Delatycki, M.B.; Forrest, L.E. “I need to know if I’m going to die young”: Adolescent and young adult experiences of genetic testing for Li-Fraumeni syndrome. J. Psychosoc. Oncol. 2021, 39, 54–73. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.; Hammond, J.; Hill, M.; Searle, B.; Hunter, A.; Patch, C.; Chitty, L.S.; Sanderson, S.C. Young people’s understanding, attitudes and involvement in decision-making about genome sequencing for rare diseases: A qualitative study with participants in the UK 100, 000 Genomes Project. Eur. J. Med. Genet. 2020, 63, 104043. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.A.; Lewis, S.; Massie, J.; Kirk, E.P.; Archibald, A.D.; Barlow-Stewart, K.; Boardman, F.K.; Halliday, J.; McClaren, B.; Delatycki, M.B. Which types of conditions should be included in reproductive genetic carrier screening? Views of parents of children with a genetic condition. Eur. J. Med. Genet. 2020, 63, 104075. [Google Scholar] [CrossRef]
- Dwyer, A.A.; Shen, H.; Zeng, Z.; Gregas, M.; Zhao, M. Framing Effects on Decision-Making for Diagnostic Genetic Testing: Results from a Randomized Trial. Genes 2021, 12, 941. [Google Scholar] [CrossRef] [PubMed]
- Wilsnack, C.; Young, J.L.; Merrill, S.L.; Groner, V.; Loud, J.T.; Bremer, R.C.; Greene, M.H.; Khincha, P.P.; Werner-Lin, A. Family Identity and Roles in the Context of Li-Fraumeni Syndrome: “No One’s Like Us Mutants”. Health Soc. Work. 2021, 46, 299–307. [Google Scholar] [CrossRef]
- Dwyer, A.A.; Uveges, M.K.; Dockray, S.; Smith, N. Exploring Rare Disease Patient Attitudes and Beliefs regarding Genetic Testing: Implications for Person-Centered Care. J. Pers. Med. 2022, 12, 477. [Google Scholar] [CrossRef]
- Gregersen, P.A.; Funding, M.; Alsner, J.; Olsen, M.H.; Overgaard, J.; Staffieri, S.E.; Lou, S.; Urbak, S.F. Genetic testing in adult survivors of retinoblastoma in Denmark: A study of the experience and impact of genetic testing many years after initial diagnosis. Eur. J. Med. Genet. 2022, 65, 104569. [Google Scholar] [CrossRef]
- Dubois, M.L.; Winters, P.D.; Rodrigue, M.A.; Gekas, J. Patient attitudes and preferences about expanded noninvasive prenatal testing. Front. Genet. 2023, 14, 976051. [Google Scholar] [CrossRef]
- Smith, H.S.; Bonkowski, E.S.; Hickingbotham, M.R.; Deloge, R.B.; Pereira, S. Framing the Family: A Qualitative Exploration of Factors That Shape Family-Level Experience of Pediatric Genomic Sequencing. Children 2023, 10, 774. [Google Scholar] [CrossRef]
- Peter, M.; Hammond, J.; Sanderson, S.C.; Gurasashvili, J.; Hunter, A.; Searle, B.; Patch, C.; Chitty, L.S.; Hill, M.; Lewis, C. Knowledge, attitudes and decision regret: A longitudinal survey study of participants offered genome sequencing in the 100,000 Genomes Project. Eur. J. Hum. Genet. 2023, 31, 1407–1413. [Google Scholar] [CrossRef]
- Konig, A.; Reinsch, S. “I am happy to be alive, but I prefer to have children without my chronic disease”: Chronically ill persons’ views on reproduction and genetic testing for their own condition. New Genet. Soc. 2024, 43, e2332299. [Google Scholar] [CrossRef]
- Thomas, S.P.; Fletcher, F.E.; Willard, R.; Ranson, T.M.; Bonham, V.L. Patient Perceptions on the Advancement of Noninvasive Prenatal Testing for Sickle Cell Disease among Black Women in the United States. AJOB Empir. Bioeth. 2024, 15, 154–163. [Google Scholar] [CrossRef]
- Tam, M.T.; Daboub, A.; Lou, H.; Robillard, J.M. Short Communication: Lived experience perspectives on genetic testing for a rare eye disease. J. Community Genet. 2024, 15, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.; Kahn, R.M.; Perez, L.; Brewer, J.; Ratner, S.; Li, X.; Yeoshoua, E.; Frey, M.K. Cascade genetic testing for hereditary cancer syndromes: A review of barriers and breakthroughs. Fam. Cancer 2024, 23, 111–120. [Google Scholar] [CrossRef]
- Korte, K.T.; Terry, S.F. Socioeconomic Barriers Surrounding Genetic Counseling. Genet. Test. Mol. Biomark. 2023, 27, 34–35. [Google Scholar] [CrossRef] [PubMed]
- Sarki, M.; Ming, C.; Aceti, M.; Fink, G.; Aissaoui, S.; Bürki, N.; Graffeo, R.; Heinimann, K.; Caiata Zufferey, M.; Monnerat, C.; et al. Relatives from Hereditary Breast and Ovarian Cancer and Lynch Syndrome Families Forgoing Genetic Testing: Findings from the Swiss CASCADE Cohort. J. Pers. Med. 2022, 12, 1740. [Google Scholar] [CrossRef]
- Hendricks-Sturrup, R.M.; Mazor, K.M.; Sturm, A.C.; Lu, C.Y. Barriers and Facilitators to Genetic Testing for Familial Hypercholesterolemia in the United States: A Review. J. Pers. Med. 2019, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Wiedower, J.; Smith, H.S.; Farrell, C.L.; Parker, V.; Rebek, L.; Davis, S.C. Payer perspectives on genomic testing in the United States: A systematic literature review. Genet. Med. 2025, 27, 101329. [Google Scholar] [CrossRef]
- Best, S.; Vidic, N.; An, K.; Collins, F.; White, S.M. A systematic review of geographical inequities for accessing clinical genomic and genetic services for non-cancer related rare disease. Eur. J. Hum. Genet. 2022, 30, 645–652. [Google Scholar] [CrossRef]
- Stacey, D.; Légaré, F.; Col, N.F.; Bennett, C.L.; Barry, M.J.; Eden, K.B.; Holmes-Rovner, M.; Llewellyn-Thomas, H.; Lyddiatt, A.; Thomson, R.; et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst. Rev. 2024, 1, CD001431. [Google Scholar] [CrossRef]
- Gorrie, A.; Gold, J.; Cameron, C.; Krause, M.; Kincaid, H. Benefits and limitations of telegenetics: A literature review. J. Genet. Couns. 2021, 30, 924–937. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.G.; Watts, I.; Beales, E.R.; Maudhoo, A.; Hayward, J.; Sheridan, E.; Rafi, I. Videoconferencing to deliver genetics services: A systematic review of telegenetics in light of the COVID-19 pandemic. Genet. Med. 2021, 23, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.T.W.; Chung, C.C.Y.; Hue, S.P.Y.; Chung, B.H.Y. The growing needs of genetic counselling-Feasibility in utilization of tele-genetic counselling in Asia and Hong Kong. Front. Genet. 2023, 14, 1239817. [Google Scholar] [CrossRef]
- Baroutsou, V.; Duong, V.; Signorini, A.; Saccilotto, R.; Ciorba, F.M.; Bürki, N.; Caiata-Zufferey, M.; Ryu, J.M.; Kim, S.W.; Lim, M.C.; et al. Acceptability and Usability of the Family Gene Toolkit for Swiss and Korean Families Harboring BRCA1/BRAC2 Pathogenic Variants: A Web-Based Platform for Cascade Genetic Testing. Cancers 2023, 15, 4485. [Google Scholar] [CrossRef]
- Cazzaniga, A.; Plebani, M.; Crimi, M. Genome access and other web-based IT solutions: Genetic counseling in the digital era. Front. Public Health 2022, 10, 1035316. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Haneda, E.; Hiroshima, Y.; Narimatsu, H. Preliminary Screening for Hereditary Breast and Ovarian Cancer Using an AI Chatbot as a Genetic Counselor: Clinical Study. J. Med. Internet Res. 2024, 26, e48914. [Google Scholar] [CrossRef]
- Afaya, A.; Kim, S.W.; Park, H.S.; Lim, M.C.; Jung, M.S.; Nam, E.J.; Park, J.S.; Jeong, J.; Ryu, J.M.; Kim, J.; et al. Psychosocial barriers and facilitators for cascade genetic testing in hereditary breast and ovarian cancer: A scoping review. Fam. Cancer 2024, 23, 121–132. [Google Scholar] [CrossRef]
- Baroutsou, V.; Underhill-Blazey, M.L.; Appenzeller-Herzog, C.; Katapodi, M.C. Interventions Facilitating Family Communication of Genetic Testing Results and Cascade Screening in Hereditary Breast/Ovarian Cancer or Lynch Syndrome: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 925. [Google Scholar] [CrossRef]
- Hesse-Biber, S.; Seven, M.; Jiang, J.; Schaik, S.V.; Dwyer, A.A. Impact of BRCA Status on Reproductive Decision-Making and Self-Concept: A Mixed-Methods Study Informing the Development of Tailored Interventions. Cancers 2022, 14, 1484. [Google Scholar] [CrossRef]
- Skrovanek, E.; Dunbar-Jacob, J.; Dunwoody, C.; Wesmiller, S. Integrative Review of Reproductive Decision Making of Women Who Are BRCA Positive. J. Obstet. Gynecol. Neonatal. Nurs. 2020, 49, 525–536. [Google Scholar] [CrossRef]
- Doll, E.S.; Lerch, S.P.; Schmalenberger, K.M.; Alex, K.; Kölker, S.; Brennenstuhl, H.; Pereira, S.; Smith, H.; Winkler, E.C.; Mahal, J.; et al. How do parents decide on genetic testing in pediatrics? A systematic review. Genet. Med. 2025, 27, 101390. [Google Scholar] [CrossRef] [PubMed]
- Hoskovec, J.M.; Bennett, R.L.; Carey, M.E.; DaVanzo, J.E.; Dougherty, M.; Hahn, S.E.; LeRoy, B.S.; O’Neal, S.; Richardson, J.G.; Wicklund, C.A. Projecting the Supply and Demand for Certified Genetic Counselors: A Workforce Study. J. Genet. Couns. 2018, 27, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Keels, J.N.; Thomas, J.; Calzone, K.A.; Badzek, L.; Dewell, S.; Murthy, V.; O’Shea, R.; Tonkin, E.T.; Dwyer, A.A. Consumer-oriented (patient and family) outcomes from nursing in genomics: A scoping review of the literature (2012–2022). Front. Genet. 2024, 15, 1481948. [Google Scholar] [CrossRef] [PubMed]



| Type | Number | References |
|---|---|---|
| diagnostic | 16 | [23,26,27,29,34,35,36,38,39,41,45,48,51,52] |
| predictive | 14 | [22,23,24,25,31,32,37,40,41,42,45,46,47,49,50,53] |
| carrier screening | 5 | [24,26,30,33,34] |
| hypothetical testing scenarios | 2 | [39,44] |
| Preconception (n = 4) | Prenatal (n = 9) | Pediatric (n = 11) | Adult (n = 13) | |
|---|---|---|---|---|
| Promoters | ||||
| actionable results | [22,43,53] | [36,43,48,53] | [23,26,31,35,37,40,41,42,49,50] | [25,31,34,38,39,45,47,50,52] |
| personal/family history | [22,30,43,53] | [24,27,30,43,53] | [37,40,41] | [29,33,47] |
| emotional relief | [22,43] | [53] | [23,31,35,37,41,42,49] | [25,31,38,45,47] |
| altruism | [22] | [24] | [23,31,37,42,46,49] | [25,31,38,44,45,46,47,52] |
| understanding of GT | [30] | [24,27,28,30,36] | [31,37,42,50] | [29,31,33,34,47,50,52] |
| patient empowerment | - | [24,28,48] | [23,31,37,40,41,42,49] | [25,31,34,39,52] |
| woman’s age | - | [24,32] | - | - |
| Barriers | ||||
| stigmatization/ discrimination | [22,30] | [28,30,51] | [23,35,46,49] | [38,44,46,52] |
| psychological distress | [30] | [24,28,30,51] | [23,26,31,35,42,46,49] | [31,34,38,39,46,47,52] |
| access and financial barriers | - | [24,28] | [26,37] | [52] |
| limited under- standing of results | - | [24,28,51] | [26,37,41] | [38,39,46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strasser, S.; McDonald, I.R.; Uveges, M.K.; Hesse-Biber, S.; Keels, J.; Smith, N.; Dwyer, A.A. Psychosocial Factors Involved in Genetic Testing for Rare Diseases: A Scoping Review. Genes 2025, 16, 614. https://doi.org/10.3390/genes16060614
Strasser S, McDonald IR, Uveges MK, Hesse-Biber S, Keels J, Smith N, Dwyer AA. Psychosocial Factors Involved in Genetic Testing for Rare Diseases: A Scoping Review. Genes. 2025; 16(6):614. https://doi.org/10.3390/genes16060614
Chicago/Turabian StyleStrasser, Samantha, Isabella R. McDonald, Melissa K. Uveges, Sharlene Hesse-Biber, Jordan Keels, Neil Smith, and Andrew A. Dwyer. 2025. "Psychosocial Factors Involved in Genetic Testing for Rare Diseases: A Scoping Review" Genes 16, no. 6: 614. https://doi.org/10.3390/genes16060614
APA StyleStrasser, S., McDonald, I. R., Uveges, M. K., Hesse-Biber, S., Keels, J., Smith, N., & Dwyer, A. A. (2025). Psychosocial Factors Involved in Genetic Testing for Rare Diseases: A Scoping Review. Genes, 16(6), 614. https://doi.org/10.3390/genes16060614







