First Preliminary Molecular Assessment of Ants from Cabo Verde
Abstract
1. Introduction
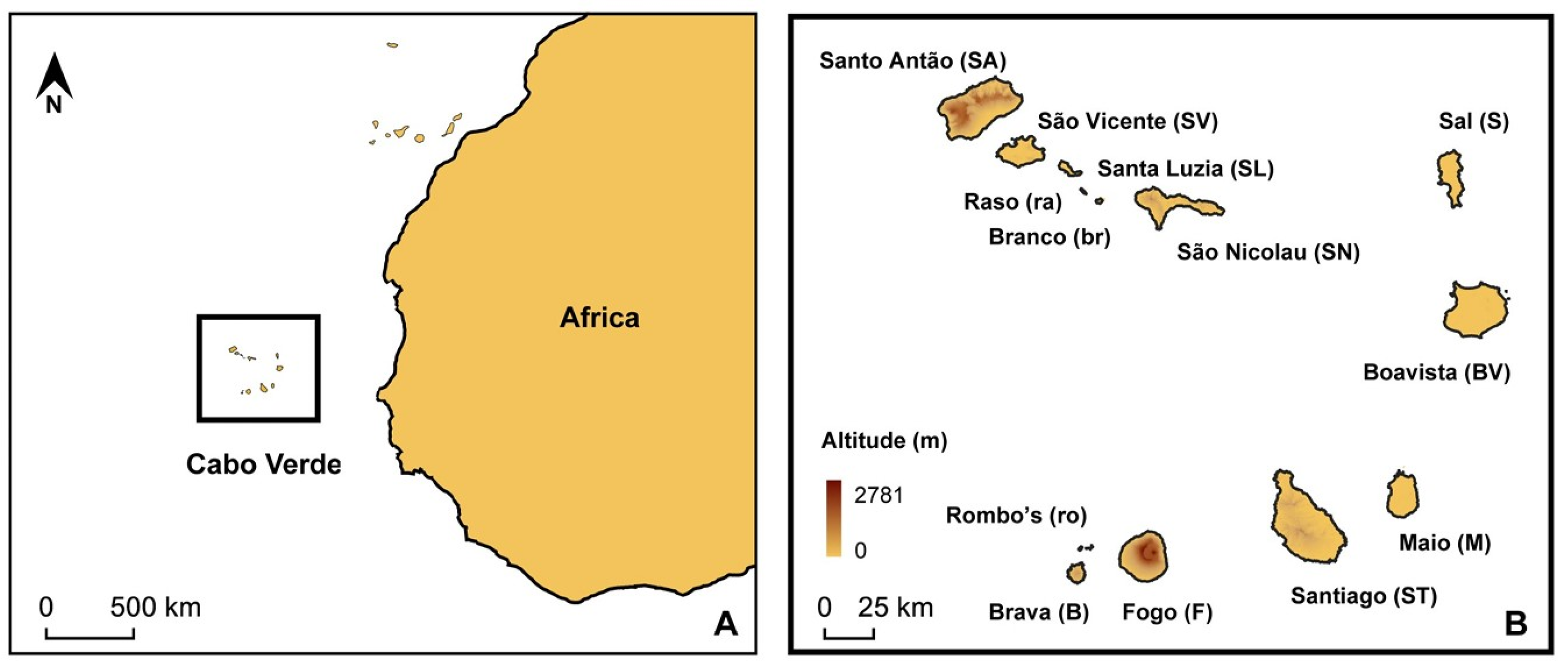
2. Materials and Methods
3. Results
3.1. The Samsum Ant Brachyponera sennaarensis (Mayr, 1862)
3.2. The Longhorn Crazy Ant Paratrechina longicornis (Latreille, 1802)
3.3. The African Big-Headed Ant Pheidole megacephala Fabricius, 1793
3.4. The Singapore Ant Trichomyrmex destructor (Jerdon, 1851)
3.5. The Carpenter Ant Camponotus sp. Mayr, 1861

3.6. The Plagiolepidine Ant Lepisiota sp. Santschi, 1926
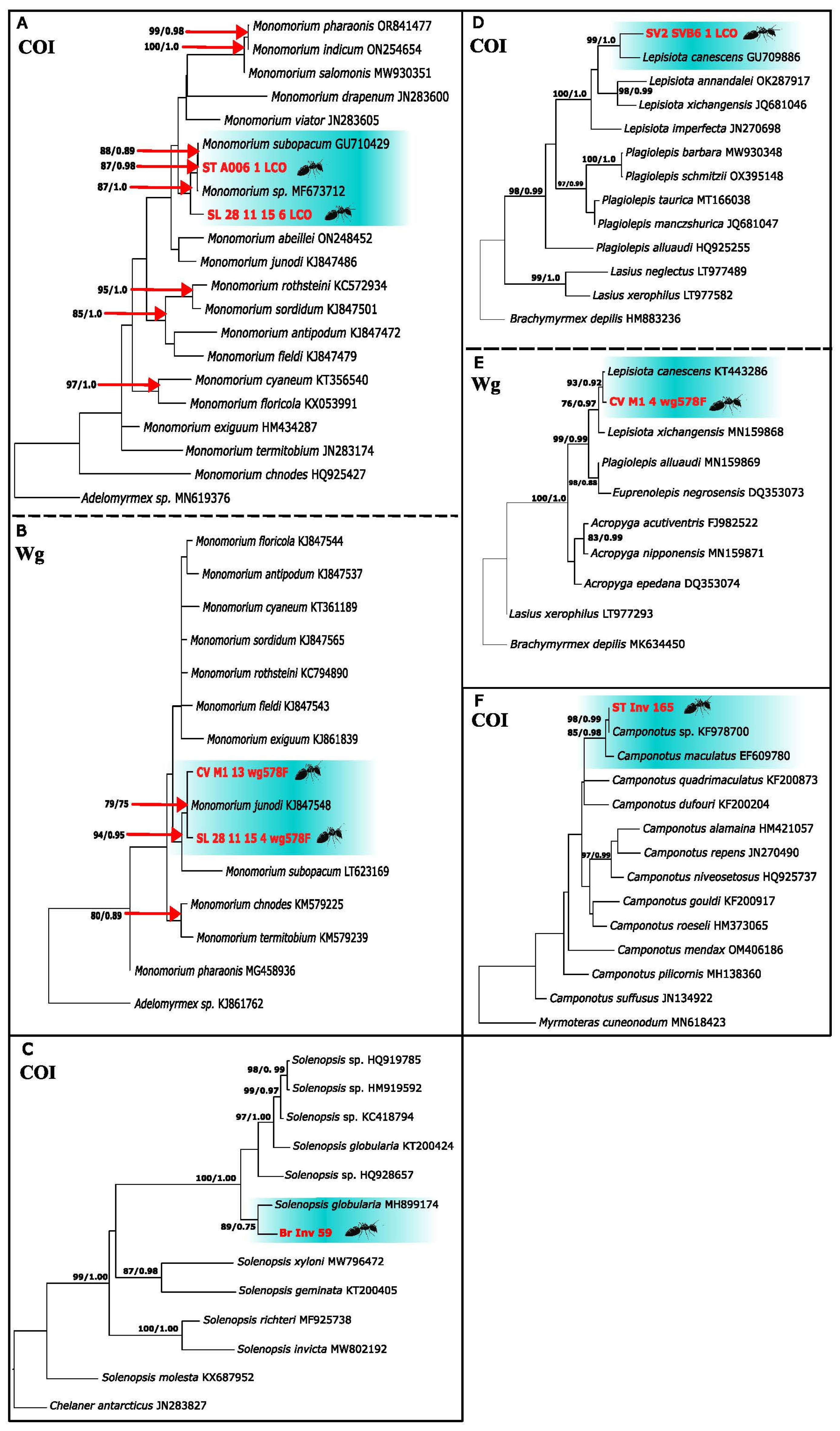
3.7. The Pharaoh Ants Monomorium sp. And Monomorium subopacum (F. Smith, 1858)
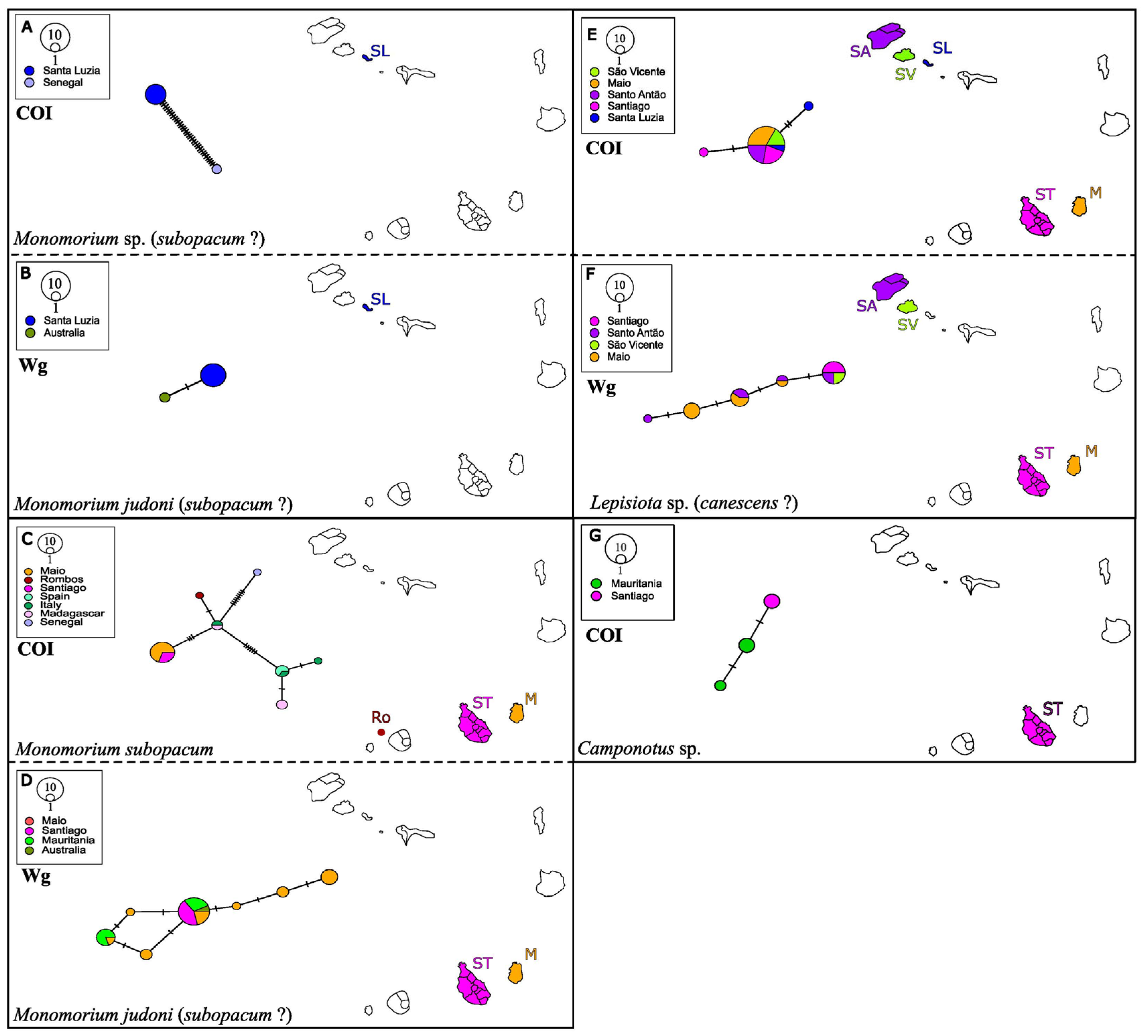
3.8. The Globular Thief Ant Solenopsis globularia Westwood, 1840
4. Discussion
4.1. Species Identification and Biodiversity
4.2. Cabo Verde Archipelago Colonization and Dispersal
4.3. Invasive and Exotic Ants in Cabo Verde
5. Conclusions
Future Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kass, J.M.; Guénard, B.; Dudley, K.L.; Jenkins, C.N.; Azuma, F.; Fisher, B.; Parr, C.L.; Gigg, H.; Longino, J.T.; Ward, P.S.; et al. The global distribution of known and undiscovered ant biodiversity. Sci. Adv. 2022, 8, abp9908. [Google Scholar] [CrossRef] [PubMed]
- Ellison, A.M.; Gotelli, N.J. Ants (Hymenoptera: Formicidae) and humans: From inspiration and metaphor to 21st-century symbiont. Myrmecol. News 2021, 31, 225–240. [Google Scholar] [CrossRef]
- Báez, M.; Oromí, P. Arthropoda. In Lista Preliminar de Especies Silvestres de Cabo Verde (Hongos, Plantas y Animales Terrestres); Arechavaleta, M., Zurita, N., Marrero, M.C., Martín, J.L., Eds.; Consejería de Medio Ambiente e Ordenación Territorial, Gobierno de Canarias: Santa Cruz de Tenerife, Spain, 2005; pp. 60–100. [Google Scholar]
- Wetterer, J.K.; Espadaler, X. Ants (Hymenoptera: Formicidae) of the Cabo Verde Islands. Trans. Am. Entomol. Soc. 2021, 147, 485–502. [Google Scholar] [CrossRef]
- Wojcik, D.P. Comparison of the Ecology of Red Imported Fire Ants in North and South America. Fla. Entomol. 1983, 66, 101–111. [Google Scholar] [CrossRef]
- Bruna, E.M.; Izzo, T.J.; Inouye, B.D.; Uriarte, M.; Vasconcelos, H.L. Asymmetric dispersal and colonization success of Amazonian plant-ants queens. PLoS ONE 2011, 6, e22937. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.A. The flight ecology of ants (Hymenoptera: Formicidae). Myrmecol. News 2017, 26, 19–30. [Google Scholar]
- Gama, C.; Tchepel, O.; Baldasano, J.M.; Basart, S.; Ferreira, J.; Pio, C.; Cardoso, J.; Borrego, C. Seasonal patterns of Saharan dust over Cape Verde—A combined approach using observations and modelling. Tellus B 2015, 67, 24410. [Google Scholar] [CrossRef]
- Morrison, L.W. The ecology of ants (Hymenoptera: Formicidae) on islands. Myrmecol. News 2016, 23, 1–14. [Google Scholar]
- Wittman, S.E. Impacts of invasive ants on native ant communities (Hymenoptera: Formicidae). Myrmecol. News 2014, 19, 111–123. [Google Scholar]
- Holway, D.A.; Lach, L.; Suarez, A.V.; Tsutsui, N.D.; Case, T.J. The causes and consequences of ants invasions. Annu. Rev. Ecol. Syst. 2002, 33, 181–233. [Google Scholar] [CrossRef]
- Westermann, F.L.; Suckling, D.M.; Lester, P.J. Disruption of Foraging by a Dominant Invasive Species to Decrease Its Competitive Ability. PLoS ONE 2014, 9, e90173. [Google Scholar] [CrossRef]
- Shimoji, H.; Suwabe, M.; Kikuchi, T.; Ohnishi, H.; Tanaka, H.; Kawara, K.; Hidaka, Y.; Enoki, T.; Tsuji, K. Resilience of native ant community against invasion of exotic ants after anthropogenic disturbances of forest habitats. Ecol. Evol. 2022, 12, e9073. [Google Scholar] [CrossRef]
- Zina, V.; Branco, M.; Franco, J.C. Impact of the Invasive Argentine Ant in Citrus Agroecosystems: Effects on the Diversity and Frequency of Native Ant Species Foraging on Tree Canopy. Insects 2020, 11, 785. [Google Scholar] [CrossRef] [PubMed]
- Archury, R.; Holway, D.A.; Suarez, A.V. Pervasive and persistent effects of ant invasion and fragmentation on native ant assemblages. Ecology 2021, 10, e03257. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Bamisile, B.S.; Khan, M.M.; Islam, W.; Hafeez, M.; Bodlah, I.; Xu, Y. Impact of invasive ant species on native fauna across similar habitats under global environmental changes. Environ. Sci. Pollut. Res. Int. 2021, 28, 54362–54382. [Google Scholar] [CrossRef]
- Lucky, A. Molecular phylogeny and biogeography of the spider ants, genus Leptomyrmex Mayr (Hymenoptera: Formicidae). Mol. Phylogenet. Evol. 2011, 59, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Schär, S.; Talavera, G.; Rana, J.D.; Espadaler, X.; Cover, S.P.; Shattuck, S.O.; Vila, R. Integrative taxonomy reveals cryptic diversity in North American Lasius ants, and an overlooked introduced species. Sci. Rep. 2022, 12, 5970. [Google Scholar] [CrossRef]
- Pava-Ripoll, M.; Miller, A.K.; Ziobro, G.C. Development of a Multiplex Polymerase Chain Reaction (PCR) Assay for the Potential Detection of Insect Contaminants in Food. J. Food Prot. 2023, 86, 100120. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Ward, P.S.; Brady, S.G.; Fisher, B.L.; Schultz, T.R. The evolution of myrmicine ants: Phylogeny and biogeography of a hyperdiverse ant clade (Hymenoptera: Formicidae). Syst. Entomol. 2015, 40, 61–81. [Google Scholar] [CrossRef]
- Abouheif, E.; Wray, G.A. Evolution of the gene network underlying wing polyphenism in ants. Science 2002, 297, 249–252. [Google Scholar] [CrossRef]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010. [Google Scholar] [CrossRef]
- Ward, P.S.; Blaimer, B.B.; Fisher, B.L. A revised phylogenetic classification of the ant subfamily Formicinae (Hymenoptera: Formicidae), with resurrection of the genera Colobopsis and Dinomyrmex. Zootaxa 2016, 4072, 343–357. [Google Scholar] [CrossRef]
- Ward, P.S.; Downie, D.A. The ant subfamily Pseudomyrmecinae (Hymenoptera: Formicidae): Phylogeny and evolution of big-eyed arboreal ants. Syst. Entomol. 2005, 30, 310–335. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MRBAYES 3.2: Efficient Bayesian phylogenetic inference and model selection across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Guindon, S.; Gascuel, O. A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef]
- Rambaut, A. Figtree, Version 1.4.4.; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2018. [Google Scholar]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Datasets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. PopART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Bandelt, H.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schmidt, C.A.; Shattuck, S.O. The higher classification of the ant subfamily Ponerinae (Hymenoptera: Formicidae), with a review of ponerine ecology and behavior. Zootaxa 2014, 3817, 1–242. [Google Scholar] [CrossRef] [PubMed]
- Coker, O.M. Importance of genetics in conservation of biodiversity. Nig. J. Wildl. Manag. 2017, 1, 11–18. [Google Scholar]
- Forel, A. Études myrmécologiques en 1879 (deuxième partie [1re partie en 1878]). Soc. Vaud. Sci. Nat. 1879, 16, 53–128. [Google Scholar]
- Forel, A. Ameisen aus der Kolonie Erythräa. Gesammelt von Prof. Dr. K. Escherich (nebst einigen in West-Abessinien von Herrn A. Ilg gesammelten Ameisen). Zool. Jahrb. Abt. Syst. Geog. Biol. Tiere 1910, 29, 243–274. [Google Scholar]
- Diame, L.; Taylor, B.; Blatrix, R.; Vayssières, J.F.; Rey, J.Y.; Grechi, I.; Diarra, K. A preliminary checklist of the ant (Hymenoptera, Formicidae) fauna of Senegal. J. Insect Biodivers. 2017, 5, 1–16. [Google Scholar] [CrossRef]
- Taheri, A.; Wetterer, J.K.; Najjari, A.; Reyes-López, J.L. Checklist of the Ants of Mauritania (Hymenoptera: Formicidae). Trans. Am. Entomol. Soc. 2024, 150, 231–238. [Google Scholar] [CrossRef]
- Wetterer, J.K. Worldwide spread of the longhorn crazy ant, Paratrechina longicornis (Hymenoptera: Formicidae). Myrmecol. News 2008, 11, 137–149. [Google Scholar]
- Harris, R.J.; Abbott, K.; Barton, K.; Berry, J.A.; Don, W.; Gunawardana, D.N.; Lester, P.J.; Rees, J.S.; Stanley, M.C.; Sutherland, A.; et al. Invasive ant pest risk assessment project for Biosecurity New Zealand. N. Z. J. Zool. 2007, 34, 161–178. [Google Scholar] [CrossRef]
- Wetterer, J.K.; Miller, S.E.; Wheeler, D.E.; Olson, C.A.; Polhemus, D.A.; Pitts, M.; Ashton, I.W.; Himler, A.G.; Yospin, M.M.; Helms, K.R.; et al. Ecological dominance by Paratrechina longicornis (Hymenoptera: Formicidae), an invasive tramp ant, in Biosphere 2. Fla. Entomol. 1999, 82, 381–388. [Google Scholar] [CrossRef]
- Wasmann, E. Zur Lebensweise einiger in- und ausländischer Ameisengäste (148. Beitrag zur Kenntnis der Myrmecophilen und Termitophilen). Z. Wiss. Insektenbiol. 1905, 10, 329–336. [Google Scholar]
- LaPolla, J.S.; Fisher, B.L. Then there were five: A reexamination of the ant genus Paratrechina (Hymenoptera, Formicidae). Zookeys 2014, 30, 35–48. [Google Scholar] [CrossRef]
- Dejean, A.; Moreau, C.S.; Kenne, M.; Leponce, M. The raiding success of Pheidole megacephala on other ants in both its native and introduced ranges. Comptes Rendus Biol. 2008, 331, 631–635. [Google Scholar] [CrossRef]
- Wetterer, J.K. Worlwide spread of the African big-headed ant, Pheidole megacephala (Hymeno-ptera: Formicidae). Myrmecol. News 2014, 17, 51–62. [Google Scholar]
- Dejean, A.; Moreau, C.S.; Uzac, P.; Le Breton, J.; Kenne, M. The predatory behavior of Pheidole megacephala. Comptes Rendus Biol. 2007, 330, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.D.; Andersen, A.N.; Hill, G. Impact of an introduced ant on native rainforest invertebrates: Pheidole megacephala in monsoonal Australia. Oecologia 1999, 120, 595–604. [Google Scholar] [CrossRef]
- Wetterer, J.K. Worldwide spread of the destroyer ant, Monomorium destructor (Hymenoptera: Formicidae). Myrmecol. News 2009, 12, 97–108. [Google Scholar]
- Espadaler, X.; Bernal, V. Exotic ants in the Canary Islands (Hymenoptera, Formicidae). Vieraea 2003, 31, 1–7. [Google Scholar]
- Wetterer, J.K. Geographic Spread of Solenopsis globularia (Hymenoptera, Formicidae). Sociobiology 2019, 66, 257. [Google Scholar] [CrossRef]
- Wetterer, J.K. Geographic spread of the samsum or sword ant, Pachycondyla (Brachyponera) sennaarensis (Hymenoptera: Formicidae). Myrmecol. News 2013, 18, 13–18. [Google Scholar]
- Dejean, A.; Lachaud, J.P. Ecology and behavior of the seed eating ponerine Brachyponera sennaarensis (Mayr). Insect Soc. 1994, 41, 191–210. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jowers, M.J.; Guouman Ferreyra, F.; Caut, S.; Brito, J.C.; Vasconcelos, R. First Preliminary Molecular Assessment of Ants from Cabo Verde. Genes 2025, 16, 725. https://doi.org/10.3390/genes16070725
Jowers MJ, Guouman Ferreyra F, Caut S, Brito JC, Vasconcelos R. First Preliminary Molecular Assessment of Ants from Cabo Verde. Genes. 2025; 16(7):725. https://doi.org/10.3390/genes16070725
Chicago/Turabian StyleJowers, Michael Joseph, Franco Guouman Ferreyra, Stephane Caut, José Carlos Brito, and Raquel Vasconcelos. 2025. "First Preliminary Molecular Assessment of Ants from Cabo Verde" Genes 16, no. 7: 725. https://doi.org/10.3390/genes16070725
APA StyleJowers, M. J., Guouman Ferreyra, F., Caut, S., Brito, J. C., & Vasconcelos, R. (2025). First Preliminary Molecular Assessment of Ants from Cabo Verde. Genes, 16(7), 725. https://doi.org/10.3390/genes16070725




