Molecular Genomics of Oral Submucous Fibrosis: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
Inclusion and Exclusion Criteria
- Language: Articles written in English;
- Time frame: Studies published between 2015–2025;
- Type of study: Original research articles.
- Language: Articles written in a language other than English;
- Time frame: Articles written before 2015;
- Type of study: Non-original research articles.
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, S.; Jawanda, M.K. Oral submucous fibrosis: An overview of a challenging entity. Indian. J. Dermatol. Venereol. Leprol. 2021, 87, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Oral submucous fibrosis: A demographic study. J. Indian Acad. Oral Med. Radiol. 2016, 28, 124–128. [Google Scholar] [CrossRef]
- Tilakaratne, W.M.; Klinikowski, M.F.; Saku, T.; Peters, T.J.; Warnakulasuriya, S. Oral submucous fibrosis: Review on aetiology and pathogenesis. Oral Oncol. 2006, 42, 561–568. [Google Scholar] [CrossRef]
- Van Schayck, C.P.; Loozen, J.M.C.; Wagena, E.; Akkermans, R.P.; Wesseling, G.J. Detecting patients at a high risk of developing chronic obstructive pulmonary disease in general practice: Cross sectional case finding study. BMJ 2002, 324, 1370. [Google Scholar] [CrossRef]
- Yang, Y.-H.C.; Warnakulasuriya, S. Lifestyle Factors; Springer: Berlin/Heidelberg, Germany, 2023; pp. 97–110. [Google Scholar] [CrossRef]
- Singh, A.G.; Roy, S.; Oza, S.; Singhavi, H.; Chatterjee, K.; Chaturvedi, P. A contemporary narrative review to guide molecular epidemiology of oral submucous fibrosis. Int. J. Mol. Epidemiol. Genet. 2021, 12, 61–70. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC8449189/ (accessed on 30 March 2025).
- Chole, R.H.; Gondivkar, S.M.; Gadbail, A.R.; Balsaraf, S.; Chaudhary, S.; Dhore, S.V.; Ghonmode, S.; Balwani, S.; Mankar, M.; Tiwari, M.; et al. Review of drug treatment of oral submucous fibrosis. Oral Oncol. 2012, 48, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, K.; Khatana, S.; Bali, R.; Kaur, A.; Dutt, N. Quality of Life in Oral Submucous Fibrosis: A Systematic Review. J. Oral Maxillofac. Surg. 2022, 21, 14–24. [Google Scholar] [CrossRef]
- Angadi, P.V.; Rao, S. Management of oral submucous fibrosis: An overview. Oral Maxillofac. Surg. 2010, 14, 133–142. [Google Scholar] [CrossRef]
- Angadi, P.V.; Rekha, P.K. Oral submucous fibrosis: A clinicopathologic review of 205 cases in Indians. J. Oral Maxillofac. Surg. 2011, 15, 15–19. [Google Scholar] [CrossRef]
- More, C.B.; Gavli, N.; Chen, Y.; Rao, N.R. A novel clinical protocol for therapeutic intervention in oral submucous fibrosis: An evidence based approach. J. Oral Maxillofac. Pathol. 2018, 22, 382–391. [Google Scholar] [CrossRef]
- Fedorowicz, Z.; Chan Shih-Yen, E.; Dorri, M.; Nasser, M.; Newton, T.; Shi, L. Interventions for the management of oral submucous fibrosis. Cochrane Database Syst. Rev. 2008, CD007156. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.M.; Patil, A.; Patil, K.; Prasant, M.C. Oral submucous fibrosis and its dermatological relation. Indian Dermatol. Online J. 2014, 5, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Pant, I.; Kumar, N.; Khan, I.; Rao, S.G.; Kondaiah, P. Role of Areca Nut Induced TGF-β and Epithelial-Mesenchymal Interaction in the Pathogenesis of Oral Submucous Fibrosis. PLoS ONE 2015, 10, e0129252. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Chen, L.; Mashrah, M.; Zhu, Y.; He, Z.; Hu, Y.; Xiang, T.; Yao, Z.; Guo, F.; Zhang, C. Expression and promoter methylation of Wnt inhibitory factor-1 in the development of oral submucous fibrosis. Oncol. Rep. 2015, 34, 2636–2642. [Google Scholar] [CrossRef]
- Yuan, Y.; Hou, X.; Feng, H.; Liu, R.; Xu, H.; Gong, W.; Deng, J.; Sun, C.; Gao, Y.; Peng, J.; et al. Proteomic identification of cyclophilin A as a potential biomarker and therapeutic target in oral submucous fibrosis. Oncotarget 2016, 7, 60348–60365. [Google Scholar] [CrossRef]
- Hou, X.; Liu, R.; Huang, C.; Jiang, L.; Zhou, Y.; Chen, Q. Cyclophilin A was revealed as a candidate marker for human oral submucous fibrosis by proteomic analysis. Cancer Biomark. 2017, 20, 345–356. [Google Scholar] [CrossRef]
- Das, T.; Prodhan, C.; Patsa, S.; Ray, J.G.; Chaudhuri, K. Identification of over expressed proteins in oral submucous fibrosis by proteomic analysis. J. Cell Biochem. 2018, 119, 4361–4371. [Google Scholar] [CrossRef]
- Chen, P.N.; Lin, C.W.; Yang, S.F.; Chang, Y.C. Oral submucous fibrosis stimulates invasion and epithelial-mesenchymal transition in oral squamous cell carcinoma by activating MMP-2 and IGF-IR. J. Cell. Mol. Med. 2021, 25, 9814–9825. [Google Scholar] [CrossRef]
- Anne, A.; Bindra, S.; Reddy, K.V.R.; Gummanur, M.R.; Mohan, K.N. Comparative Somatic Variant Analysis of a Rare Case with Concurrent Oral Leukoplakia and Oral Submucosal Fibrosis. Cytogenet. Genome Res. 2022, 162, 207–213. [Google Scholar] [CrossRef]
- Kundu, P.; Pant, I.; Jain, R.; Rao, S.G.; Kondaiah, P. Genome-wide DNA methylation changes in oral submucous fibrosis. Oral Dis. 2022, 28, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Jing, F.; Zhu, L.; Bai, J.; Cai, X.; Zhou, X.; Zhang, J.; Zhang, H.; Li, T. Molecular mechanisms underlying the epigallocatechin-3-gallate-mediated inhibition of oral squamous cell carcinogenesis. Arch. Oral Biol. 2023, 153, 105740. [Google Scholar] [CrossRef] [PubMed]
- Mannan, A.; Bhinder, M.A.; Sadia, H.; Yousaf, Z.; Hussain, Z.; Akram, M.; Shakoor, M.; Zahoor, M.Y.; Rehman, R.A. Association of Oral Submucous Fibrosis Risk with GSTM1 and GSTT1 Gene Polymorphisms. J. Coll. Physicians Surg. Pak. 2024, 34, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Yang, L.; Li, Z.; Wang, J.; Zheng, K.; Mei, J.; Sun, H.; Huang, Y.; Yang, C.; Luo, W. DNA methyltransferase 3A induces the occurrence of oral submucous fibrosis by promoting the methylation of the von Hippel-Lindau. Oral Dis. 2024, 30, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Sharma, K.; Singh, P.; Chandra, S.; Singh, G.; Gupta, S. Quantitative proteogenomic characterization in MUC1 and MUC4 in oral squamous cell carcinoma, oral potentially malignant disorders, and normal oral mucosa in carcinogenesis. Eur. Arch. Oto-Rhino-Laryngol. 2025, 282, 2033–2042. [Google Scholar] [CrossRef]
- Kamath, V.V.; Satelur, K.; Komali, Y. Biochemical markers in oral submucous fibrosis: A review and update. Dent. Res. J. 2013, 10, 576–584. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC3858729/ (accessed on 29 March 2025).
- Panda, A.; Mishra, P.; Mohanty, A.; Sundaragiri, K.S.; Singh, A.; Jha, K. Is Epithelial-Mesenchymal Transition a New Roadway in the Pathogenesis of Oral Submucous Fibrosis: A Comprehensive Review. Cureus 2022, 14, e29636. [Google Scholar] [CrossRef]
- Gupta, S.; Shetty, D.C.; Gulati, N.; Juneja, S.; Jain, A. Potentiated action on the progression of OSMF by hypoxia mediated signaling pathway by the epithelial mesenchymal transition and angiogenic apparatus. J. Cancer Res. Ther. 2023, 19 (Suppl. S1), S389–S396. [Google Scholar] [CrossRef]
- Khan, S.; Chatra, L.; Prashanth, S.K.; Veena, K.M.; Rao, P.K. Pathogenesis of oral submucous fibrosis. J. Cancer Res. Ther. 2012, 8, 199–203. [Google Scholar] [CrossRef]
- Salati, N.A.; Sharma, M.; Rao, N.N.; Shetty, S.S.; Radhakrishnan, R.A. Role of osteopontin in oral epithelial dysplasia, oral submucous fibrosis and oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2023, 27, 706–714. [Google Scholar] [CrossRef]
- Angadi, P.V.; Krishnapillai, R. Evaluation of PTEN Immunoexpression in Oral Submucous Fibrosis: Role in Pathogenesis and Malignant Transformation. Head Neck Pathol. 2012, 6, 314–321. [Google Scholar] [CrossRef]
- James, A.; Jayan, L.; Ramadoss, R.; Arunachalam, P. Leaving no stone unturned: Role of profibrotic genes in oral submucous fibrosis—A systematic review. J. Oral Maxillofac. Pathol. 2022, 26, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, J.; Zhou, Z.; Cui, X.; Tu, H.; Jia, J.; Chen, B.; Dai, X.; Liu, O. Oral submucous fibrosis: Pathogenesis and therapeutic approaches. Int. J. Oral Sci. 2025, 17, 8. [Google Scholar] [CrossRef]
- Mehta, C.H.; Paliwal, S.; Muttigi, M.S.; Seetharam, R.N.; Prasad, A.S.B.; Nayak, Y.; Acharya, S.; Nayak, U.Y. Polyphenol-based targeted therapy for oral submucous fibrosis. Inflammopharmacology 2023, 31, 2349–2368. [Google Scholar] [CrossRef]
- Mulk, B.S.; Deshpande, P.; Velpula, N.; Chappidi, V.; Chintamaneni, R.L.; Goyal, S. Spirulina and Pentoxyfilline—A Novel Approach for Treatment of Oral Submucous Fibrosis. J. Clin. Diagn. Res. 2013, 7, 3048–3050. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, H.; Li, R.; Chen, Y.; Xu, Z.; Shang, Z. Stromal thrombospondin 1 suppresses angiogenesis in oral submucous fibrosis. Int. J. Oral Sci. 2024, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, R.K.; Maheswari, S.U.; Appukuttan, D.; Victor, D.J.; Prakash, P.S.G.; Crena, J. Ultrasonographic Evaluation of Masseter Muscle in Patients with Oral Submucous Fibrosis. J. Interdiscip. Dent. 2024, 14, 200–206. [Google Scholar] [CrossRef]
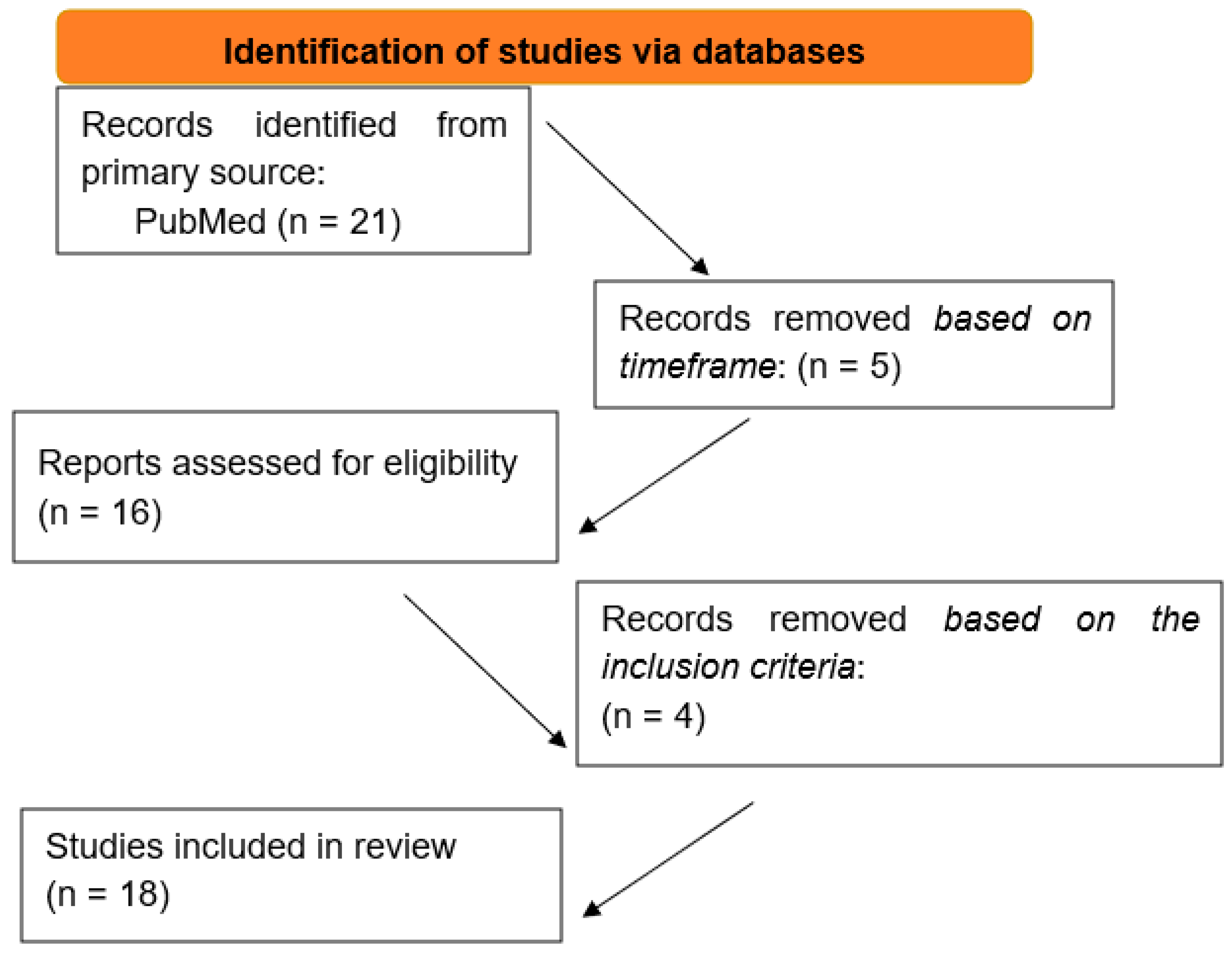
| 21 Articles Through Keywords | |
|---|---|
| Time frame 2015–2025 | |
| 16 articles remained | 5 articles excluded |
| The rest of the inclusion criteria | |
| 12 articles remained | 4 articles excluded |
| Study | Country | Year | Sample Size | Method |
|---|---|---|---|---|
| [14] | India | 2015 | - | RT-PCR, microarray, data analysis, and immunohistochemistry |
| [15] | China | 2015 | 45 (OSF) | RT-PCR, methylation-specific PCR, and immunohistochemistry |
| [16] | China | 2016 | 60 (OSF) | Two-dimensional electrophoresis-based proteomic approach, western blot, and immunohistochemistry |
| [17] | China | 2017 | - | Two-dimensional electrophoresis-based proteomic approach, western blot, and immunohistochemistry |
| [18] | India | 2018 | 63 (OSF) | Two-dimensional electrophoresis-based proteomic approach, immunohistochemistry, and RT-PCR |
| [19] | Taiwan | 2021 | 3 (OSF) | IHC, RT-PCR, Cell assay, and western blot |
| [20] | India | 2022 | 1 (OSF) | Exome sequencing of a rare case report |
| [21] | India | 2022 | 7 (OSF) | MS- PCR and RT- PCR |
| [22] | China | 2023 | 3 (OSF) | Functional annotation and pathway enrichment analysis. |
| [23] | Pakistan | 2024 | 108 (OSF) | DNA extraction and PCR amplification. |
| [24] | China | 2024 | 32 (OSF) | Cell culture, MS-PCR, RT-PCR, western blot, and immunohistochemistry. |
| [25] | India | 2025 | 63 (OSCC, OPMD, OSF, NOM) | RT-PCR and immunohistochemistry. |
| Markers | Role |
|---|---|
| TGF-β | Signaling pathways |
| WNt IF-1 | Signaling pathways |
| MMP-2 | Signaling pathways |
| IGF-IR | Signaling pathways |
| Insulin signaling | Signaling pathways |
| Ubiquitin proteolysis | Signaling pathways |
| RAS/MAPK | Signaling pathways |
| EGCG | Signaling pathways |
| GSTM1 | Gene polymorphism |
| GSTT1 | Gene polymorphism |
| VHL | Epigenetics |
| FGF13 | Epigenetics |
| RPS6KA3 | Epigenetics |
| ACSL4 | Epigenetics |
| XIST | Epigenetics |
| CypA | Proteomics |
| Hsp-70 | Proteomics |
| Calreticulin | Proteomics |
| Lumican | Proteomics |
| Enolase 1 | Proteomics |
| MUC1 | Proteomics |
| MUC4 | Proteomics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zisis, V.; Zisis, S.; Charisi, C.; Poulopoulos, K.; Sarkisian, A.; Poulopoulos, A. Molecular Genomics of Oral Submucous Fibrosis: A Narrative Review. Genes 2025, 16, 612. https://doi.org/10.3390/genes16060612
Zisis V, Zisis S, Charisi C, Poulopoulos K, Sarkisian A, Poulopoulos A. Molecular Genomics of Oral Submucous Fibrosis: A Narrative Review. Genes. 2025; 16(6):612. https://doi.org/10.3390/genes16060612
Chicago/Turabian StyleZisis, Vasileios, Stefanos Zisis, Christina Charisi, Konstantinos Poulopoulos, Aristeidis Sarkisian, and Athanasios Poulopoulos. 2025. "Molecular Genomics of Oral Submucous Fibrosis: A Narrative Review" Genes 16, no. 6: 612. https://doi.org/10.3390/genes16060612
APA StyleZisis, V., Zisis, S., Charisi, C., Poulopoulos, K., Sarkisian, A., & Poulopoulos, A. (2025). Molecular Genomics of Oral Submucous Fibrosis: A Narrative Review. Genes, 16(6), 612. https://doi.org/10.3390/genes16060612






