Genomic Regions Associated with Resistance to Gastrointestinal Nematode Parasites in Sheep—A Review
Abstract
1. Introduction
2. Association Studies for Resistance to Gastrointestinal Nematodes in Sheep
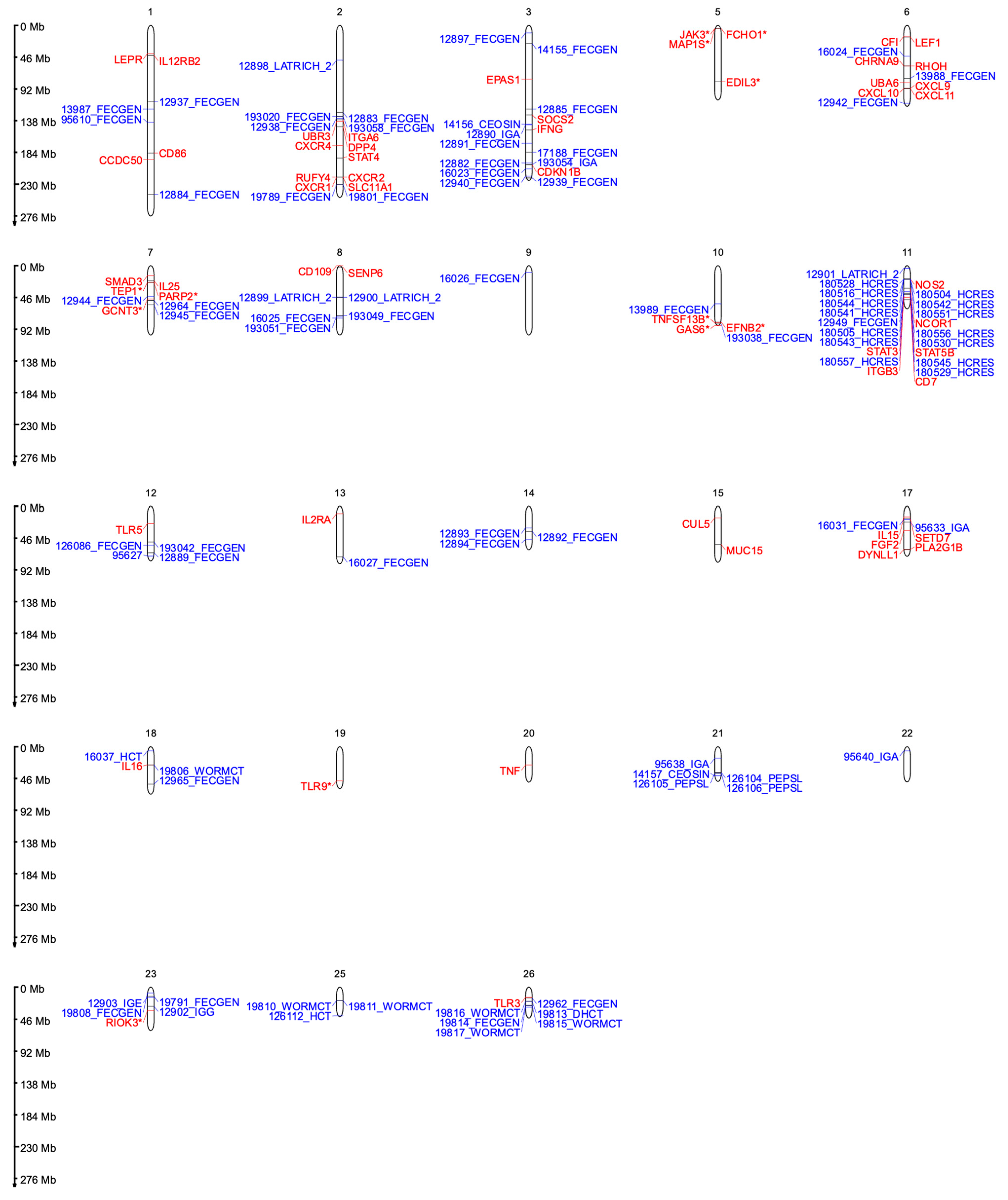
2.1. Chromosome 1
| Chromosome | QTL 1 | Associated Phenotype 2 | Breed 3 | Reference |
|---|---|---|---|---|
| 1 | 12937 | Average FEC | Merino | [57] |
| EBV of the average FEC | Multiple breeds | [19] | ||
| 13987 | FEC | Spanish Churra | [58] | |
| FEC | Tunisian | [24] | ||
| 95610 | FEC | Spanish Churra | [27] | |
| FEC | Florida Native | [59] | ||
| 12884 | T. colubriformis FEC | Merino | [60] | |
| Antigen-specific IgA activity | Spanish Churra | [27] | ||
| 2 | 12898 | Trichostrongylus spp. adults and late-stage larvae counts | Romney × Coopworth | [35] |
| FEC | Tunisan | [24] | ||
| FEC | Florida Native | [59] | ||
| PCV | Djallonké | [25] | ||
| FAMACHA© | Djallonké | [25] | ||
| Resistance to GINs 5 | Scottish Blackface | [46] | ||
| 193058 | Nematodirus spp. average FEC | Scottish Blackface | [61] | |
| EBV of the average FEC | Multiple breeds | [19] | ||
| 193020 | Nematodirus spp. FEC at 20 weeks of age | Scottish Blackface | [61] | |
| EBV of the average FEC | Multiple breeds | [19] | ||
| 12883 | EBV of the average FEC | Multiple breeds | [19] | |
| Nematodirus spp. FEC at 20 weeks of age | Scottish Blackface | [36] | ||
| 12938 | EBV of the average FEC | Multiple breeds | [19] | |
| Average FEC | Merino | [57] | ||
| 19801 | FEC | Red Massai × Dorper | [38] | |
| Resistance to GINs 5 | Scottish Blackface | [46] | ||
| 19789 | FEC | Red Massai × Dorper | [38] | |
| Resistance to GINs 5 | Scottish Blackface | [46] | ||
| 3 | 12882 | Nematodirus spp. FEC | Scottish Blackface | [36] |
| EBV of the dag at 8 months | Multiple breeds | [19] | ||
| FEC | Tunisian | [24] | ||
| 12885 | T. colubriformis FEC | Merino | [60] | |
| Resistance to GINs 5 | Scottish Blackface | [46] | ||
| 12890 | Antigen-specific IgA activity | Scottish Blackface | [36] | |
| EBV of the FEC | Multiple breeds | [19] | ||
| EBV of the FEC | Multiple breeds | [19] | ||
| 12891 | Strongyle FEC | Scottish Blackface | [36] | |
| Antigen-specific IgA activity | Spanish Churra | [27] | ||
| 12897 | FEC | Merino | [57] | |
| FEC | Tunisian | [24] | ||
| Resistance to GINs 5 | Scottish Blackface | [46] | ||
| 12939 | FEC | Merino | [57] | |
| FEC | Florida Native | [59] | ||
| 12940 | FEC | Merino | [57] | |
| FEC | Florida Native | [59] | ||
| FEC | Florida Native | [59] | ||
| 14155 | Average FEC | Merino × Romney | [37] | |
| FEC | Tunisian | [24] | ||
| 14156 | Eosinophil level | Merino × Romney | [37] | |
| EBV of the FEC | Multiple breeds | [19] | ||
| EBV of the FEC | Multiple breeds | [19] | ||
| 16023 | Average FEC [45] | Red Massai × Dorper | [39] | |
| FEC | Tunisian | [24] | ||
| 17188 | Nematodirus spp. FEC | Texel, Suffolk | [62] | |
| FEC | Tunisian | [24] | ||
| 193054 | Antigen-specific IgA activity | Scottish Blackface | [61] | |
| EBV of the FEC | Multiple breeds | [19] | ||
| EBV of the FEC | Multiple breeds | [19] | ||
| 6 | 16024 | Average FEC | Red Massai × Dorper | [39] |
| FEC | Tunisian | [24] | ||
| FEC | Spanish Churra | [27] | ||
| Resistance to GINs 5 | Scottish Blackface | [46] | ||
| 12942 | FEC | Merino | [57] | |
| Average FEC | Multiple breeds | [19] | ||
| 13988 | EBV of the FEC | Spanish Churra | [58] | |
| FEC | Spanish Churra | [27] | ||
| 7 | 12944 | FEC | Merino | [57] |
| EBV of the FEC | Multiple breeds | [19] | ||
| 12945 | FEC | Merino | [57] | |
| EBV of the FEC | Multiple breeds | [19] | ||
| 12964 | FEC | Merino | [57] | |
| FEC | Spanish Churra | [27] | ||
| EBV of the FEC | Multiple breeds | [19] | ||
| 8 | 16025 | Average FEC | Red Massai × Dorper | [39] |
| Antigen-specific IgA activity | Spanish Churra | [27] | ||
| FEC | Tunisan | [24] | ||
| 193049 | Strongyles FEC | Scottish Blackface | [61] | |
| EBV of the dag at 8 months | Multiple breeds | [19] | ||
| 193051 | Strongyles FEC | Scottish Blackface | [61] | |
| EBV of the dag at 8 months | Multiple breeds | [19] | ||
| 12899 | Total counts of adults and late-stage larvae of Trichostrongylus spp. found in the abomasum | Romney × Coopworth | [35] | |
| FEC | Tunisan | [24] | ||
| FEC | Djallonké | [25] | ||
| PCV | Djallonké | [25] | ||
| Antigen-specific IgA activity | Spanish Churra | [27] | ||
| FAMACHA© | Florida Native | [59] | ||
| Resistance to GINs 5 | Scottish Blackface | [46] | ||
| 12900 | Total counts of adults and late-stage larvae of Trichostrongylus spp. found in the small intestine | Romney × Coopworth | [35] | |
| FAMACHA© | Florida Native | [59] | ||
| Antigen-specific IgA activity | Spanish Churra | [27] | ||
| FEC | Tunisan | [24] | ||
| Resistance to GINs 5 | Scottish Blackface | [46] | ||
| FEC | Djallonké | [25] | ||
| PCV | Djallonké | [25] | ||
| 9 | 16026 | Average FEC | Red Massai × Dorper | [39] |
| FEC | Spanish Churra | [27] | ||
| 10 | 193038 | Strongyles FEC | Scottish Blackface | [61] |
| H. contortus FEC | Florida Native | [47] | ||
| PVC | Florida Native | [47] | ||
| 13989 | FEC | Spanish Churra | [58] | |
| FEC | Tunisan | [24] | ||
| Antigen-specific IgA activity | Spanish Churra | [27] | ||
| FEC | Florida Native | [59] | ||
| Resistance to GINs 5 | Scottish Blackface | [46] | ||
| 11 | 180504 | H. contortus resistance 4 | Dorper, Katahdin, St. Croix | [44] |
| FEC | Florida Native | [47] | ||
| 180505 | H. contortus resistance 4 | Dorper, Katahdin, St. Croix | [44] | |
| FEC | Florida Native | [47] | ||
| 180516 | H. contortus resistance 4 | Dorper, Katahdin, St. Croix | [44] | |
| FEC | Florida Native | [47] | ||
| 180528 | H. contortus resistance 4 | Dorper, Katahdin, St. Croix | [44] | |
| FEC | Florida Native | [47] | ||
| 180529 | H. contortus resistance 4 | Dorper, Katahdin, St. Croix | [44] | |
| FEC | Florida Native | [47] | ||
| 180530 | H. contortus resistance 4 | Dorper, Katahdin, St. Croix | [44] | |
| FEC | Florida Native | [47] | ||
| 180541 | H. contortus resistance 4 | Dorper, Katahdin, St. Croix | [44] | |
| FEC | Florida Native | [47] | ||
| 180542 | H. contortus resistance 4 | Dorper, Katahdin, St. Croix | [44] | |
| FEC | Florida Native | [47] | ||
| 180543 | H. contortus resistance 4 | Dorper, Katahdin, St. Croix | [44] | |
| FEC | Florida Native | [47] | ||
| 180544 | H. contortus resistance 4 | Dorper, Katahdin, St. Croix | [44] | |
| FEC | Florida Native | [47] | ||
| 180545 | H. contortus resistance 4 | Dorper, Katahdin, St. Croix | [44] | |
| FEC | Florida Native | [47] | ||
| 180551 | H. contortus resistance 4 | Dorper, Katahdin, St. Croix | [44] | |
| FEC | Florida Native | [47] | ||
| 180556 | H. contortus resistance 4 | Dorper, Katahdin, St. Croix | [44] | |
| FEC | Florida Native | [47] | ||
| 180557 | H. contortus resistance 4 | Dorper, Katahdin, St. Croix | [44] | |
| FEC | Florida Native | [47] | ||
| 12949 | H. contortus FEC | Merino | [57] | |
| EBV of the FEC | Multiple breeds | [19] | ||
| EBV of the FEC | Multiple breeds | [19] | ||
| 12901 | Total counts of adults and late-stage larvae of Trichostrongylus spp. found in the small intestine | Romney × Coopworth | [35] | |
| FEC | Tunisian | [24] | ||
| H. contortus FEC | Florida Native | [59] | ||
| Resistance to GINs 5 | Scottish Blackface | [46] | ||
| 12 | 193042 | Nematodirus spp. FEC | Scottish Blackface | [61] |
| EBV of the FEC | Multiple breeds | [19] | ||
| 126086 | EBV of the FEC | Multiple breeds | [19] | |
| H. contortus FEC | Martinik Black Belly × Romane | [63] | ||
| 95627 | Antigen-specific IgA activity | Spanish Churra | [27] | |
| FEC | Tunisan | [24] | ||
| Resistance to GINs 5 | Scottish Blackface | [46] | ||
| 12889 | T. colubriformis FEC | Merino | [60] | |
| Antigen-specific IgA activity | Spanish Churra | [27] | ||
| 13 | 16027 | Average FEC | Red Massai × Dorper | [39] |
| FAMACHA© | Djallonké | [25] | ||
| 14 | 12892 | Nematodirus spp. FEC | Scottish Blackface | [36] |
| FEC | Tunisan | [24] | ||
| RBC | Santa Inês | [29] | ||
| 12893 | Nematodirus spp. FEC | Scottish Blackface | [36] | |
| FEC | Tunisan | [24] | ||
| 12894 | Nematodirus spp. FEC | Scottish Blackface | [36] | |
| Resistance to GINs 5 | Scottish Blackface | [46] | ||
| 15 | 16029 | Average FEC | Red Masaai × Dorper | [39] |
| Antigen-specific IgA activity | Spanish Churra | [27] | ||
| EBV of the dag at 3 months | Multiple breeds | [19] | ||
| EBV of the dag at 8 months | Multiple breeds | [19] | ||
| 17 | 16031 | Average FEC | Red Masaai × Dorper | [39] |
| FEC | Spanish Churra | [27] | ||
| Antigen-specific IgA activity | Spanish Churra | [27] | ||
| Resistance to GINs 5 | Scottish Blackface | [46] | ||
| 95633 | Antigen-specific IgA activity | Spanish Churra | [27] | |
| FEC | Tunisan | [24] | ||
| Resistance to GINs 5 | Scottish Blackface | [46] | ||
| 18 | 16037 | PCV | Red Masaai × Dorper | [39] |
| FAMACHA© | Djallonké | [25] | ||
| 19806 | Number of adult worms found in the abomasum at necropsy | Red Masaai × Dorper | [38] | |
| FEC | Tunisan | [24] | ||
| 12965 | H. contortus FEC | Merino | [57] | |
| Resistance to GINs 5 | Scottish Blackface | [46] | ||
| 21 | 14157 | Eosinophil number | Merino × Romney | [37] |
| FEC | Tunisan | [24] | ||
| FEC | Spanish Churra | [27] | ||
| Antigen-specific IgA activity | Spanish Churra | [27] | ||
| 95638 | Antigen-specific IgA activity | Spanish Churra | [27] | |
| H. contortus FEC | Florida Native | [59] | ||
| 126104 | Serum pepsinogen level | Martinik Black Belly × Romane, | [63] | |
| FEC | Tunisan | [24] | ||
| 126105 | Serum pepsinogen level | Martinik Black Belly × Romane | [63] | |
| FEC | Tunisan | [24] | ||
| 126106 | Serum pepsinogen level | Martinik Black Belly × Romane | [63] | |
| FEC | Tunisan | [24] | ||
| 22 | 95640 | Antigen-specific IgA activity | Spanish Churra | [27] |
| FAMACHA© | Djallonké | [25] | ||
| 23 | 19808 | FEC | Red Masaai × Dorper | [38] |
| Resistance to GINs 5 | Scottish Blackface | [46] | ||
| FEC | Santa Inês | [30] | ||
| HCT | Santa Inês | [30] | ||
| HGB | Santa Inês | [30] | ||
| RBC | Santa Inês | [30] | ||
| 19791 | FEC | Red Masaai × Dorper | [38] | |
| Resistance to GINs 5 | Scottish Blackface | [46] | ||
| 12902 | Total IgE level | Romney × Coopworth | [35] | |
| Antigen-specific IgA activity | Spanish Churra | [27] | ||
| 12903 | Antigen-specific IgG level | Romney × Coopworth | [35] | |
| Antigen-specific IgA activity | Spanish Churra | [27] | ||
| Resistance to GINs 5 | Scottish Blackface | [46] | ||
| 25 | 19810 | Total counts of adult and immature worms at necropsy | Red Masaai × Dorper | [38] |
| EBV of the FEC | Multiple breeds | [19] | ||
| 19811 | Total count of adult worms at necropsy | Red Masaai × Dorper | [38] | |
| EBV of the FEC | Multiple breeds | [19] | ||
| 126112 | PCV | Martinik Black Belly × Romane | [63] | |
| EBV of the FEC | Multiple breeds | [19] | ||
| 26 | 19813 | PCV | Merino | [57] |
| EBV of the FEC | Multiple breeds | [19] | ||
| 19814 | FEC | Merino | [57] | |
| EBV of the FEC | Multiple breeds | [19] | ||
| 19815 | Total counts of adult and immature worms at necropsy | Merino | [57] | |
| EBV of the FEC | Multiple breeds | [19] | ||
| 19816 | Total count of adult worms at necropsy | Merino | [57] | |
| EBV of the FEC | Multiple breeds | [19] | ||
| 19817 | Total count of immature worms at necropsy | Merino | [57] | |
| EBV of the FEC | Multiple breeds | [19] | ||
| EBV of the FEC | Multiple breeds | [19] | ||
| EBV of the FEC | Multiple breeds | [19] | ||
| 12962 | H. contortus FEC | Merino | [57] | |
| EBV of the FEC | Multiple breeds | [19] |
2.2. Chromosome 2
2.3. Chromosome 3
2.4. Chromosome 6
2.5. Chromosome 7
2.6. Chromosome 8
2.7. Chromosome 9
2.8. Chromosome 10
2.9. Chromosome 11
2.10. Chromosome 12
2.11. Chromosome 13
2.12. Chromosome 14
2.13. Chromosome 15
2.14. Chromosome 17
2.15. Chromosome 18
2.16. Chromosome 20
2.17. Chromosome 21
2.18. Chromosome 22
2.19. Chromosome 23
2.20. Chromosome 24
2.21. Chromosome 25
2.22. Chromosome 26
2.23. Chromosomes 4, 5, 16, 19
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riggio, V.; Pong-Wong, R.; Sallé, G.; Usai, M.G.; Casu, S.; Moreno, C.R.; Matika, O.; Bishop, S.C. A Joint Analysis to Identify Loci Underlying Variation in Nematode Resistance in Three E Uropean Sheep Populations. J. Anim. Breed. Genet. 2014, 131, 426–436. [Google Scholar] [CrossRef]
- Karrow, N.A.; Goliboski, K.; Stonos, N.; Schenkel, F.; Peregrine, A. Review: Genetics of Helminth Resistance in Sheep. Can. J. Anim. Sci. 2014, 94, 1–9. [Google Scholar] [CrossRef]
- Miller, J.E.; Kaplan, R.M.; Pugh, D.G. Internal Parasites. In Sheep and Goat Medicine; Elsevier: Amsterdam, The Netherlands, 2012; pp. 106–125. ISBN 978-1-4377-2353-3. [Google Scholar]
- Galyon, H.R.; Zajac, A.M.; Wright, D.L.; Greiner, S.P.; Bradford, H.L. Evaluating the Relationship between Fecal Egg Count, FAMACHA Score, and Weight in Dewormed and Non-Dewormed Katahdin Rams during a Parasite Challenge. Transl. Anim. Sci. 2020, 4, txaa178. [Google Scholar] [CrossRef]
- Kaplan, R.M.; Burke, J.M.; Terrill, T.H.; Miller, J.E.; Getz, W.R.; Mobini, S.; Valencia, E.; Williams, M.J.; Williamson, L.H.; Larsen, M.; et al. Validation of the FAMACHA Eye Color Chart for Detecting Clinical Anemia in Sheep and Goats on Farms in the Southern United States. Vet. Parasitol. 2004, 123, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Benavides, M.V.; Sonstegard, T.S.; Kemp, S.; Mugambi, J.M.; Gibson, J.P.; Baker, R.L.; Hanotte, O.; Marshall, K.; Van Tassell, C. Identification of Novel Loci Associated with Gastrointestinal Parasite Resistance in a Red Maasai x Dorper Backcross Population. PLoS ONE 2015, 10, e0122797. [Google Scholar] [CrossRef] [PubMed]
- McLeod, R.S. Costs of Major Parasites to the Australian Livestock Industries. Int. J. Parasitol. 1995, 25, 1363–1367. [Google Scholar] [CrossRef]
- Shaw, R.J.; Morris, C.A.; Wheeler, M.; Tate, M.; Sutherland, I.A. Salivary IgA: A Suitable Measure of Immunity to Gastrointestinal Nematodes in Sheep. Vet. Parasitol. 2012, 186, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Saddiqi, H.A.; Sarwar, M.; Iqbal, Z.; Nisa, M.; Shahzad, M.A. Markers/Parameters for the Evaluation of Natural Resistance Status of Small Ruminants against Gastrointestinal Nematodes. Animal 2012, 6, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Khusro, M.; Van Der Werf, J.H.J.; Brown, D.J.; Ball, A. Across Flock (Co)Variance Components for Faecal Worm Egg Count, Live Weight, and Fleece Traits for Australian Merinos. Livest. Prod. Sci. 2004, 91, 35–43. [Google Scholar] [CrossRef]
- Sallé, G.; Deiss, V.; Marquis, C.; Tosser-Klopp, G.; Cortet, J.; Serreau, D.; Koch, C.; Marcon, D.; Bouvier, F.; Jacquiet, P.; et al. Genetic × Environment Variation in Sheep Lines Bred for Divergent Resistance to Strongyle Infection. Evol. Appl. 2021, 14, 2591–2602. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.C. Possibilities to Breed for Resistance to Nematode Parasite Infections in Small Ruminants in Tropical Production Systems. Animal 2012, 6, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.; McNally, J.; Smith, D.V.; Rahman, A.; Hunt, P.; Kotze, A.C.; Dominik, S.; Ingham, A. Quantification of Differences in Resistance to Gastrointestinal Nematode Infections in Sheep Using a Multivariate Blood Parameter. Vet. Parasitol. 2019, 270, 31–39. [Google Scholar] [CrossRef]
- Bishop, S.C. A Consideration of Resistance and Tolerance for Ruminant Nematode Infections. Front. Genet. 2012, 3, 168. [Google Scholar] [CrossRef] [PubMed]
- Boareki, M.N.; Schenkel, F.S.; Willoughby, O.; Suarez-Vega, A.; Kennedy, D.; Cánovas, A. Comparison between Methods for Measuring Fecal Egg Count and Estimating Genetic Parameters for Gastrointestinal Parasite Resistance Traits in Sheep. J. Anim. Sci. 2021, 99, skab341. [Google Scholar] [CrossRef] [PubMed]
- Aguerre, S.; Jacquiet, P.; Brodier, H.; Bournazel, J.P.; Grisez, C.; Prévot, F.; Michot, L.; Fidelle, F.; Astruc, J.M.; Moreno, C.R. Resistance to Gastrointestinal Nematodes in Dairy Sheep: Genetic Variability and Relevance of Artificial Infection of Nucleus Rams to Select for Resistant Ewes on Farms. Vet. Parasitol. 2018, 256, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Hollema, B.L.; Bijma, P.; van der Werf, J.H.J. Sensitivity of the Breeding Values for Growth Rate and Worm Egg Count to Environmental Worm Burden in Australian Merino Sheep. J. Anim. Breed. Genet. 2018, 135, 357–365. [Google Scholar] [CrossRef]
- Hayward, A.D. Genetic Parameters for Resistance to Gastrointestinal Nematodes in Sheep: A Meta-Analysis. Int. J. Parasitol. 2022, 52, 843–853. [Google Scholar] [CrossRef]
- Pickering, N.K.; Auvray, B.; Dodds, K.G.; McEwan, J.C. Genomic Prediction and Genome-Wide Association Study for Dagginess and Host Internal Parasite Resistance in New Zealand Sheep. BMC Genom. 2015, 16, 958. [Google Scholar] [CrossRef]
- Kemper, K.E.; Palmer, D.G.; Liu, S.M.; Greeff, J.C.; Bishop, S.C.; Karlsson, L.J.E. Reduction of Faecal Worm Egg Count, Worm Numbers and Worm Fecundity in Sheep Selected for Worm Resistance Following Artificial Infection with Teladorsagia Circumcincta and Trichostrongylus Colubriformis. Vet. Parasitol. 2010, 171, 238–246. [Google Scholar] [CrossRef]
- Boareki, M.; Schenkel, F.; Kennedy, D.; Cánovas, A. Prediction of Genetic Resistance for Scrapie in Ungenotyped Sheep Using a Linear Animal Model. Genes 2021, 12, 1432. [Google Scholar] [CrossRef]
- Dixon, S.; Karrow, N.A.; Borkowski, E.; Suarez-Vega, A.; Menzies, P.I.; Kennedy, D.; Peregrine, A.S.; Mallard, B.A.; Cánovas, Á. Identifying Hepatic Genes Regulating the Ovine Response to Gastrointestinal Nematodes Using RNA-Sequencing. Front. Genet. 2023, 14, 1111426. [Google Scholar] [CrossRef]
- Becker, G.M.; Burke, J.M.; Lewis, R.M.; Miller, J.E.; Morgan, J.L.M.; Rosen, B.D.; Van Tassell, C.P.; Notter, D.R.; Murdoch, B.M. Variants Within Genes EDIL3 and ADGRB3 Are Associated With Divergent Fecal Egg Counts in Katahdin Sheep at Weaning. Front. Genet. 2022, 13, 817319. [Google Scholar] [CrossRef]
- Ahbara, A.M.; Rouatbi, M.; Gharbi, M.; Rekik, M.; Haile, A.; Rischkowsky, B.; Mwacharo, J.M. Genome-Wide Insights on Gastrointestinal Nematode Resistance in Autochthonous Tunisian Sheep. Sci. Rep. 2021, 11, 9250. [Google Scholar] [CrossRef]
- Álvarez, I.; Fernández, I.; Soudré, A.; Traoré, A.; Pérez-Pardal, L.; Sanou, M.; Tapsoba, S.A.R.; Menéndez-Arias, N.A.; Goyache, F. Identification of Genomic Regions and Candidate Genes of Functional Importance for Gastrointestinal Parasite Resistance Traits in Djallonké Sheep of Burkina Faso. Arch. Anim. Breed. 2019, 62, 313–323. [Google Scholar] [CrossRef]
- Arzik, Y.; Kizilaslan, M.; White, S.N.; Piel, L.M.W.; Çınar, M.U. Genomic Analysis of Gastrointestinal Parasite Resistance in Akkaraman Sheep. Genes 2022, 13, 2177. [Google Scholar] [CrossRef]
- Atlija, M.; Arranz, J.-J.; Martinez-Valladares, M.; Gutiérrez-Gil, B. Detection and Replication of QTL Underlying Resistance to Gastrointestinal Nematodes in Adult Sheep Using the Ovine 50K SNP Array. Genet. Sel. Evol. 2016, 48, 4. [Google Scholar] [CrossRef]
- Becker, G.M.; Davenport, K.M.; Burke, J.M.; Lewis, R.M.; Miller, J.E.; Morgan, J.L.M.; Notter, D.R.; Murdoch, B.M. Genome-wide Association Study to Identify Genetic Loci Associated with Gastrointestinal Nematode Resistance in Katahdin Sheep. Anim. Genet. 2020, 51, 330–335. [Google Scholar] [CrossRef]
- Berton, M.P.; De Oliveira Silva, R.M.; Peripolli, E.; Stafuzza, N.B.; Martin, J.F.; Álvarez, M.S.; Gavinã, B.V.; Toro, M.A.; Banchero, G.; Oliveira, P.S.; et al. Genomic Regions and Pathways Associated with Gastrointestinal Parasites Resistance in Santa Inês Breed Adapted to Tropical Climate. J. Anim. Sci. Biotechnol. 2017, 8, 73. [Google Scholar] [CrossRef]
- Berton, M.P.; Da Silva, R.P.; Banchero, G.; Mourão, G.B.; Ferraz, J.B.S.; Schenkel, F.S.; Baldi, F. Genomic Integration to Identify Molecular Biomarkers Associated with Indicator Traits of Gastrointestinal Nematode Resistance in Sheep. J. Anim. Breed. Genet. 2022, 139, 502–516. [Google Scholar] [CrossRef]
- Carracelas, B.; Navajas, E.A.; Vera, B.; Ciappesoni, G. Genome-Wide Association Study of Parasite Resistance to Gastrointestinal Nematodes in Corriedale Sheep. Genes. 2022, 13, 1548. [Google Scholar] [CrossRef]
- Casu, S.; Usai, M.G.; Sechi, T.; Salaris, S.L.; Miari, S.; Mulas, G.; Tamponi, C.; Varcasia, A.; Scala, A.; Carta, A. Association Analysis and Functional Annotation of Imputed Sequence Data within Genomic Regions Influencing Resistance to Gastro-Intestinal Parasites Detected by an LDLA Approach in a Nucleus Flock of Sarda Dairy Sheep. Genet. Sel. Evol. 2022, 54, 2. [Google Scholar] [CrossRef]
- Beraldi, D.; McRae, A.F.; Gratten, J.; Pilkington, J.G.; Slate, J.; Visscher, P.M.; Pemberton, J.M. Quantitative Trait Loci (QTL) Mapping of Resistance to Strongyles and Coccidia in the Free-Living Soay Sheep (Ovis Aries). Int. J. Parasitol. 2007, 37, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Coltman, D.W.; Wilson, K.; Pilkington, J.G.; Stear, M.J.; Pemberton, J.M. A Microsatellite Polymorphism in the γ Interferon Gene Is Associated with Resistance to Gastrointestinal Nematodes in a Naturally-Parasitized Population of Soay Sheep. Parasitology 2001, 122, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.M.; Paterson, K.A.; Dodds, K.G.; Diez Tascon, C.; Williamson, P.A.; Roberts Thomson, M.; Bisset, S.A.; Beattie, A.E.; Greer, G.J.; Green, R.S.; et al. Discovery of Quantitative Trait Loci for Resistance to Parasitic Nematode Infection in Sheep: I. Analysis of Outcross Pedigrees. BMC Genom. 2006, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.; Stear, M.J.; Benothman, M.; Abuagob, O.; Kerr, A.; Mitchell, S.; Bishop, S.C. Quantitative Trait Loci Associated with Parasitic Infection in Scottish Blackface Sheep. Heredity 2006, 96, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Dominik, S.; Hunt, P.W.; McNALLY, J.; Murrell, A.; Hall, A.; Purvis, I.W. Detection of quantitative trait loci for internal parasite resistance in sheep. I. Linkage analysis in a Romney × Merino sheep backcross population. Parasitology 2010, 137, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.; Mugambi, J.M.; Nagda, S.; Sonstegard, T.S.; Van Tassell, C.P.; Baker, R.L.; Gibson, J.P. Quantitative Trait Loci for Resistance to Haemonchus Contortus Artificial Challenge in Red Maasai and Dorper Sheep of East Africa. Anim. Genet. 2013, 44, 285–295. [Google Scholar] [CrossRef]
- Silva, M.V.B.; Sonstegard, T.S.; Hanotte, O.; Mugambi, J.M.; Garcia, J.F.; Nagda, S.; Gibson, J.P.; Iraqi, F.A.; McClintock, A.E.; Kemp, S.J.; et al. Identification of Quantitative Trait Loci Affecting Resistance to Gastrointestinal Parasites in a Double Backcross Population of Red Maasai and Dorper Sheep: Parasite Indicator QTL of Red Maasai Sheep. Anim. Genet. 2012, 43, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Niciura, S.C.M.; Benavides, M.V.; Okino, C.H.; Ibelli, A.M.G.; Minho, A.P.; Esteves, S.N.; Chagas, A.C.D.S. Genome-Wide Association Study for Haemonchus Contortus Resistance in Morada Nova Sheep. Pathogens 2022, 11, 939. [Google Scholar] [CrossRef]
- Estrada-Reyes, Z.M.; Rae, O.; Postley, C.; Jiménez Medrano, M.B.; Leal Gutiérrez, J.D.; Mateescu, R.G. Association Study Reveals Th17, Treg, and Th2 Loci Related to Resistance to Haemonchus Contortus in Florida Native Sheep1. J. Anim. Sci. 2019, 97, 4428–4444. [Google Scholar] [CrossRef]
- Hu, Z.-L.; Park, C.A.; Reecy, J.M. Bringing the Animal QTLdb and CorrDB into the Future: Meeting New Challenges and Providing Updated Services. Nucleic Acids Res. 2022, 50, D956–D961. [Google Scholar] [CrossRef]
- Chao, J.; Li, Z.; Sun, Y.; Aluko, O.O.; Wu, X.; Wang, Q.; Liu, G. MG2C: A User-Friendly Online Tool for Drawing Genetic Maps. Mol. Hortic. 2021, 1, 16. [Google Scholar] [CrossRef]
- Estrada-Reyes, Z.M.; Tsukahara, Y.; Amadeu, R.R.; Goetsch, A.L.; Gipson, T.A.; Sahlu, T.; Puchala, R.; Wang, Z.; Hart, S.P.; Mateescu, R.G. Signatures of Selection for Resistance to Haemonchus Contortus in Sheep and Goats. BMC Genom. 2019, 20, 735. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Reyes, Z.M.; Tsukahara, Y.; Goetsch, A.L.; Gipson, T.A.; Sahlu, T.; Puchala, R.; Mateescu, R.G. Association Analysis of Immune Response Loci Related to Haemonchus Contortus Exposure in Sheep and Goats Using a Targeted Approach. Livest. Sci. 2019, 228, 109–119. [Google Scholar] [CrossRef]
- Farahani, A.H.K.; Mohammadi, H.; Moradi, M.H. Genome-Wide Association Study Using Fix-Length Haplotypes and Network Analysis Revealed New Candidate Genes for Nematode Resistance and Body Weight in Blackface Lambs. Ann. Anim. Sci. 2020, 20, 445–464. [Google Scholar] [CrossRef]
- Estrada-Reyes, Z.M.; Ogunade, I.M.; Pech-Cervantes, A.A.; Terrill, T.H. Copy Number Variant-based Genome Wide Association Study Reveals Immune-related Genes Associated with Parasite Resistance in a Heritage Sheep Breed from the United States. Parasite Immunol. 2022, 44, e12943. [Google Scholar] [CrossRef] [PubMed]
- Tsukiyama, T.; Matsuda-Tsukiyama, M.; Bohgaki, M.; Terai, S.; Tanaka, S.; Hatakeyama, S. Ymer Acts as a Multifunctional Regulator in Nuclear Factor-κB and Fas Signaling Pathways. Mol. Med. 2012, 18, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Bohgaki, M.; Tsukiyama, T.; Nakajima, A.; Maruyama, S.; Watanabe, M.; Koike, T.; Hatakeyama, S. Involvement of Ymer in Suppression of NF-κB Activation by Regulated Interaction with Lysine-63-Linked Polyubiquitin Chain. Biochim. Et. Biophys. Acta (BBA)—Mol. Cell Res. 2008, 1783, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Friedman, T.B. Growth Factor and Receptor Malfunctions Associated with Human Genetic Deafness. Clin. Genet. 2020, 97, 138–155. [Google Scholar] [CrossRef]
- Kennedy, A.; Waters, E.; Rowshanravan, B.; Hinze, C.; Williams, C.; Janman, D.; Fox, T.A.; Booth, C.; Pesenacker, A.M.; Halliday, N.; et al. Differences in CD80 and CD86 Transendocytosis Reveal CD86 as a Key Target for CTLA-4 Immune Regulation. Nat. Immunol. 2022, 23, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Airoldi, I. Lack of Il12rb2 Signaling Predisposes to Spontaneous Autoimmunity and Malignancy. Blood 2005, 106, 3846–3853. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-12 and the Regulation of Innate Resistance and Adaptive Immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, W.; Zhu, Y. Immunoregulatory Functions of the IL-12 Family of Cytokines in Antiviral Systems. Viruses 2019, 11, 772. [Google Scholar] [CrossRef]
- Gómez, I.; Thomas, M.C.; Palacios, G.; Egui, A.; Carrilero, B.; Simón, M.; Valladares, B.; Segovia, M.; Carmelo, E.; López, M.C. Differential Expression of Immune Response Genes in Asymptomatic Chronic Chagas Disease Patients Versus Healthy Subjects. Front. Cell. Infect. Microbiol. 2021, 11, 722984. [Google Scholar] [CrossRef] [PubMed]
- Alti, D.; Sambamurthy, C.; Kalangi, S.K. Emergence of Leptin in Infection and Immunity: Scope and Challenges in Vaccines Formulation. Front. Cell. Infect. Microbiol. 2018, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.; Maddox, J.F.; Lee, S.H.; Zhang, Y.; Kahn, L.; Graser, H.-U.; Gondro, C.; Walkden-Brown, S.W.; van der Werf, J.H.J. Genetic Mapping of Quantitative Trait Loci for Resistance to Haemonchus Contortus in Sheep. Anim. Genet. 2009, 40, 262–272. [Google Scholar] [CrossRef]
- Gutiérrez-Gil, B.; Pérez, J.; Álvarez, L.; Martínez-Valladares, M.; De La Fuente, L.-F.; Bayón, Y.; Meana, A.; Primitivo, F.S.; Rojo-Vázquez, F.-A.; Arranz, J.-J. Quantitative Trait Loci for Resistance to Trichostrongylid Infection in Spanish Churra Sheep. Genet. Sel. Evol. 2009, 41, 46. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Reyes, Z.M.; Rae, D.O.; Mateescu, R.G. Genome-Wide Scan Reveals Important Additive and Non-Additive Genetic Effects Associated with Resistance to Haemonchus Contortus in Florida Native Sheep. Int. J. Parasitol. 2021, 51, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Beh, K.J.; Hulme, D.J.; Callaghan, M.J.; Leish, Z.; Lenane, I.; Windon, R.G.; Maddox, J.F. A Genome Scan for Quantitative Trait Loci Affecting Resistance to Trichostrongylus Colubriformis in Sheep: A Genome Scan for Quantitative Trait Loci. Anim. Genet. 2002, 33, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Riggio, V.; Matika, O.; Pong-Wong, R.; Stear, M.J.; Bishop, S.C. Genome-Wide Association and Regional Heritability Mapping to Identify Loci Underlying Variation in Nematode Resistance and Body Weight in Scottish Blackface Lambs. Heredity 2013, 110, 420–429. [Google Scholar] [CrossRef]
- Matika, O.; Pong-Wong, R.; Woolliams, J.A.; Bishop, S.C. Confirmation of Two Quantitative Trait Loci Regions for Nematode Resistance in Commercial British Terminal Sire Breeds. Animal 2011, 5, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Sallé, G.; Jacquiet, P.; Gruner, L.; Cortet, J.; Sauvé, C.; Prévot, F.; Grisez, C.; Bergeaud, J.P.; Schibler, L.; Tircazes, A.; et al. A Genome Scan for QTL Affecting Resistance to Haemonchus Contortus in Sheep. J. Anim. Sci. 2012, 90, 4690–4705. [Google Scholar] [CrossRef] [PubMed]
- Golbert, D.C.F.; Santana-Van-Vliet, E.; Ribeiro-Alves, M.; Fonsêca, M.M.B.d.; Lepletier, A.; Mendes-da-Cruz, D.A.; Loss, G.; Cotta-de-Almeida, V.; Vasconcelos, A.T.R.; Savino, W. Small Interference ITGA6 Gene Targeting in the Human Thymic Epithelium Differentially Regulates the Expression of Immunological Synapse-Related Genes. Cell Adh. Migr. 2018, 12, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Pilling, J.E.; Galvin, A.; Robins, A.M.; Sewell, H.F.; Mahida, Y.R. Expression of Alpha5 (CD49e) and Alpha6 (CD49f) Integrin Subunits on T Cells in the Circulation and the Lamina Propria of Normal and Inflammatory Bowel Disease Colonic Mucosa. Scand. J. Immunol. 1998, 48, 425–428. [Google Scholar] [CrossRef]
- Richardson, R.M.; Marjoram, R.J.; Barak, L.S.; Snyderman, R. Role of the Cytoplasmic Tails of CXCR1 and CXCR2 in Mediating Leukocyte Migration, Activation, and Regulation. J. Immunol. 2003, 170, 2904–2911. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.J.; Haque, S.; Banks, C.; Johnson, P.; Sarsfield, P.; Sheron, N. Distribution of the Interleukin-8 Receptors, CXCR1 and CXCR2, in Inflamed Gut Tissue. J. Pathol. 2000, 192, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, C.; Sadaria, M.; Bhat-Nakshatri, P.; Goulet, R.; Edenberg, H.J.; McCarthy, B.P.; Chang, C.-H.; Srour, E.F.; Nakshatri, H. Negative Regulation of MHC Class II Gene Expression by CXCR4. Exp. Hematol. 2006, 34, 1085–1092. [Google Scholar] [CrossRef]
- Contento, R.L.; Molon, B.; Boularan, C.; Pozzan, T.; Manes, S.; Marullo, S.; Viola, A. CXCR4–CCR5: A Couple Modulating T Cell Functions. Proc. Natl. Acad. Sci. USA 2008, 105, 10101–10106. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, Q.; Chen, L.; Luo, Y.; Shen, L.; Cao, Z.; Wang, Q. UBR3 Promotes Inflammation and Apoptosis via DUSP1/P38 Pathway in the Nucleus Pulposus Cells of Patients with Intervertebral Disc Degeneration. Hum. Cell 2022, 35, 792–802. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, Z.J. The Role of Ubiquitylation in Immune Defence and Pathogen Evasion. Nat. Rev. Immunol. 2012, 12, 35–48. [Google Scholar] [CrossRef]
- Germic, N.; Frangez, Z.; Yousefi, S.; Simon, H.-U. Regulation of the Innate Immune System by Autophagy: Monocytes, Macrophages, Dendritic Cells and Antigen Presentation. Cell Death Differ. 2019, 26, 715–727. [Google Scholar] [CrossRef]
- Terawaki, S.; Camosseto, V.; Pierre, P.; Gatti, E. RUFY4: Immunity Piggybacking on Autophagy? Autophagy 2016, 12, 598–600. [Google Scholar] [CrossRef]
- Klemann, C.; Wagner, L.; Stephan, M.; Von Hörsten, S. Cut to the Chase: A Review of CD26/Dipeptidyl Peptidase-4′s (DPP4) Entanglement in the Immune System. Clin. Exp. Immunol. 2016, 185, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Joseph, S. Role of SLC11A1 Gene in Disease Resistance. Bio Anim. Husb. 2012, 28, 99–106. [Google Scholar] [CrossRef][Green Version]
- Pires, V.S.; Ganzella, F.A.D.O.; Minozzo, G.A.; Dias De Castro, L.L.; Moncada, A.D.B.; Klassen, G.; Ramos, E.A.S.; Molento, M.B. Epigenetic Regulation of SLC11a1 Gene in Horses Infected with Cyathostomins. Gene Rep. 2021, 25, 101410. [Google Scholar] [CrossRef]
- Awomoyi, A.A. The Human Solute Carrier Family 11 Member 1 Protein (SLC11A1): Linking Infections, Autoimmunity and Cancer? FEMS Immunol. Med. Microbiol. 2007, 49, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Varadé, J.; Márquez, A.; Cénit, M.C.; Espino, L.; Perdigones, N.; Santiago, J.L.; Fernández-Arquero, M.; De La Calle, H.; Arroyo, R.; et al. Association of the STAT4 Gene with Increased Susceptibility for Some Immune-Mediated Diseases. Arthritis Rheum. 2008, 58, 2598–2602. [Google Scholar] [CrossRef]
- Kaplan, M.H. STAT4: A Critical Regulator of Inflammation In Vivo. Immunol. Res. 2005, 31, 231–242. [Google Scholar] [CrossRef]
- Otani, N.; Nakajima, K.; Ishikawa, K.; Ichiki, K.; Ueda, T.; Takesue, Y.; Yamamoto, T.; Tanimura, S.; Shima, M.; Okuno, T. Changes in Cell-Mediated Immunity (IFN-γ and Granzyme B) Following Influenza Vaccination. Viruses 2021, 13, 1137. [Google Scholar] [CrossRef]
- Jia, W.; He, M.-X.; McLeod, I.X.; Guo, J.; Ji, D.; He, Y.-W. Autophagy Regulates T Lymphocyte Proliferation through Selective Degradation of the Cell-Cycle Inhibitor CDKN1B/p27Kip1. Autophagy 2015, 11, 2335–2345. [Google Scholar] [CrossRef]
- Kunimura, K.; Uruno, T.; Fukui, Y. DOCK Family Proteins: Key Players in Immune Surveillance Mechanisms. Int. Immunol. 2020, 32, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Perrigoue, J.G.; Zaph, C.; Guild, K.; Du, Y.; Artis, D. IL-31-IL-31R Interactions Limit the Magnitude of Th2 Cytokine-Dependent Immunity and Inflammation Following Intestinal Helminth Infection. J. Immunol. 2009, 182, 6088–6094. [Google Scholar] [CrossRef] [PubMed]
- Cramer, A.; De Lima Oliveira, B.C.; Leite, P.G.; Rodrigues, D.H.; Brant, F.; Esper, L.; Pimentel, P.M.O.; Rezende, R.M.; Rachid, M.A.; Teixeira, A.L.; et al. Role of SOCS2 in the Regulation of Immune Response and Development of the Experimental Autoimmune Encephalomyelitis. Mediat. Inflamm. 2019, 2019, 1872593. [Google Scholar] [CrossRef]
- Rico-Bautista, E.; Flores-Morales, A.; Fernández-Pérez, L. Suppressor of Cytokine Signaling (SOCS) 2, a Protein with Multiple Functions. Cytokine Growth Factor. Rev. 2006, 17, 431–439. [Google Scholar] [CrossRef]
- Bouzroud, W.; Tazzite, A.; Yousra, I.; Gazzaz, B.; Dehbi, H. Complement Factor I Deficiency: A Novel Homozygous CFI Gene Mutation. SAGE Open Med. Case Rep. 2022, 10, 2050313X2211059. [Google Scholar] [CrossRef]
- Kerepesi, L.A.; Hess, J.A.; Nolan, T.J.; Schad, G.A.; Abraham, D. Complement Component C3 Is Required for Protective Innate and Adaptive Immunity to Larval Strongyloides Stercoralis in Mice. J. Immunol. 2006, 176, 4315–4322. [Google Scholar] [CrossRef] [PubMed]
- Toscano, J.H.B.; Okino, C.H.; Dos Santos, I.B.; Giraldelo, L.A.; Von Haehling, M.B.; Esteves, S.N.; De Souza Chagas, A.C. Innate Immune Responses Associated with Resistance against Haemonchus Contortus in Morada Nova Sheep. J. Immunol. Res. 2019, 2019, 3562672. [Google Scholar] [CrossRef]
- Hecker, A.; Küllmar, M.; Wilker, S.; Richter, K.; Zakrzewicz, A.; Atanasova, S.; Mathes, V.; Timm, T.; Lerner, S.; Klein, J.; et al. Phosphocholine-Modified Macromolecules and Canonical Nicotinic Agonists Inhibit ATP-Induced IL-1β Release. J. Immunol. 2015, 195, 2325–2334. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Ferris, R.L.; Matthews, T.; Hiel, H.; Lopez-Albaitero, A.; Lustig, L.R. Characterization of the Human Nicotinic Acetylcholine Receptor Subunit α (α) 9 (CHRNA9) and α (α) 10 (CHRNA10) in Lymphocytes. Life Sci. 2004, 76, 263–280. [Google Scholar] [CrossRef]
- Nakata, Y.; Miura, K.; Yamasaki, N.; Ogata, S.; Miura, S.; Hosomi, N.; Kaminuma, O. Expression and Function of Nicotinic Acetylcholine Receptors in Induced Regulatory T Cells. Int. J. Mol. Sci. 2022, 23, 1779. [Google Scholar] [CrossRef]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.-J. CXCL9, CXCL10, CXCL11/CXCR3 Axis for Immune Activation—A Target for Novel Cancer Therapy. Cancer Treat. Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef]
- Romagnani, P.; Crescioli, C. CXCL10: A Candidate Biomarker in Transplantation. Clin. Chim. Acta 2012, 413, 1364–1373. [Google Scholar] [CrossRef]
- Zhai, Y.; Shen, X.-D.; Gao, F.; Zhao, A.; Freitas, M.C.; Lassman, C.; Luster, A.D.; Busuttil, R.W.; Kupiec-Weglinski, J.W. CXCL10 Regulates Liver Innate Immune Response against Ischemia and Reperfusion Injury. Hepatology 2007, 47, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Kouroumalis, A.; Nibbs, R.J.; Aptel, H.; Wright, K.L.; Kolios, G.; Ward, S.G. The Chemokines CXCL9, CXCL10, and CXCL11 Differentially Stimulate Gαi-Independent Signaling and Actin Responses in Human Intestinal Myofibroblasts. J. Immunol. 2005, 175, 5403–5411. [Google Scholar] [CrossRef]
- Datta, R.; deSchoolmeester, M.L.; Hedeler, C.; Paton, N.W.; Brass, A.M.; Else, K.J. Identification of Novel Genes in Intestinal Tissue That Are Regulated after Infection with an Intestinal Nematode Parasite. Infect. Immun. 2005, 73, 4025–4033. [Google Scholar] [CrossRef]
- Hui, W.; Jiang, S.; Tang, J.; Hou, H.; Chen, S.; Jia, B.; Ban, Q. An Immediate Innate Immune Response Occurred In the Early Stage of E. granulosus Eggs Infection in Sheep: Evidence from Microarray Analysis. PLoS ONE 2015, 10, e0135096. [Google Scholar] [CrossRef]
- Cliffe, L.J.; Humphreys, N.E.; Lane, T.E.; Potten, C.S.; Booth, C.; Grencis, R.K. Accelerated Intestinal Epithelial Cell Turnover: A New Mechanism of Parasite Expulsion. Science 2005, 308, 1463–1465. [Google Scholar] [CrossRef] [PubMed]
- Steinke, F.C.; Xue, H.-H. From Inception to Output, Tcf1 and Lef1 Safeguard Development of T Cells and Innate Immune Cells. Immunol. Res. 2014, 59, 45–55. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Chen, S.-H.; Hou, J.; Ke, Z.-B.; Wu, Y.-P.; Lin, T.-T.; Wei, Y.; Xue, X.-Y.; Zheng, Q.-S.; Huang, J.-B.; et al. Identifying Hub Genes of Clear Cell Renal Cell Carcinoma Associated with the Proportion of Regulatory T Cells by Weighted Gene Co-Expression Network Analysis. Aging 2019, 11, 9478–9491. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zeng, X.; Fan, Z.; Lim, B. RhoH Modulates Pre-TCR and TCR Signalling by Regulating LCK. Cell. Signal. 2011, 23, 249–258. [Google Scholar] [CrossRef]
- Shah, K.; Al-Haidari, A.; Sun, J.; Kazi, J.U. T Cell Receptor (TCR) Signaling in Health and Disease. Sig. Transduct. Target. Ther. 2021, 6, 412. [Google Scholar] [CrossRef]
- Gu, Y.; Chae, H.-D.; Siefring, J.E.; Jasti, A.C.; Hildeman, D.A.; Williams, D.A. RhoH GTPase Recruits and Activates Zap70 Required for T Cell Receptor Signaling and Thymocyte Development. Nat. Immunol. 2006, 7, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhao, B. UBA6 and Its Bispecific Pathways for Ubiquitin and FAT10. Int. J. Mol. Sci. 2019, 20, 2250. [Google Scholar] [CrossRef]
- Lee, J.Y.; An, E.-K.; Hwang, J.; Jin, J.-O.; Lee, P.C.W. Ubiquitin Activating Enzyme UBA6 Regulates Th1 and Tc1 Cell Differentiation. Cells 2021, 11, 105. [Google Scholar] [CrossRef]
- Łyszkiewicz, M.; Ziętara, N.; Frey, L.; Pannicke, U.; Stern, M.; Liu, Y.; Fan, Y.; Puchałka, J.; Hollizeck, S.; Somekh, I.; et al. Human FCHO1 Deficiency Reveals Role for Clathrin-Mediated Endocytosis in Development and Function of T Cells. Nat. Commun. 2020, 11, 1031. [Google Scholar] [CrossRef] [PubMed]
- Thomis, D.C.; Berg, L.J. Peripheral Expression of Jak3 Is Required to Maintain T Lymphocyte Function. J. Exp. Med. 1997, 185, 197–206. [Google Scholar] [CrossRef]
- Macchi, P.; Villa, A.; Giliani, S.; Sacco, M.G.; Frattini, A.; Porta, F.; Ugazio, A.G.; Johnston, J.A.; Candotti, F.; O’Shea, J.J. Mutations of Jak-3 Gene in Patients with Autosomal Severe Combined Immune Deficiency (SCID). Nature 1995, 377, 65–68. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, Y.; Liu, L.; Zhang, T.; Han, F.; Cleveland, J.; Wang, F.; McKeehan, W.L.; Li, Y.; Zhang, D. MAP1S Protein Regulates the Phagocytosis of Bacteria and Toll-like Receptor (TLR) Signaling. J. Biol. Chem. 2016, 291, 1243–1250. [Google Scholar] [CrossRef]
- Haimovici, A.; Brigger, D.; Torbett, B.E.; Fey, M.F.; Tschan, M.P. Induction of the Autophagy-Associated Gene MAP1S via PU.1 Supports APL Differentiation. Leuk. Res. 2014, 38, 1041–1047. [Google Scholar] [CrossRef]
- Malhotra, N.; Kang, J. SMAD Regulatory Networks Construct a Balanced Immune System. Immunology 2013, 139, 1–10. [Google Scholar] [CrossRef]
- Weinstein, M.; Yang, X.; Deng, C.-X. Functions of Mammalian Smad Genes as Revealed by Targeted Gene Disruption in Mice. Cytokine Growth Factor. Rev. 2000, 11, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Urban, J.F.; Sun, R.; Stiltz, J.; Morimoto, M.; Notari, L.; Madden, K.B.; Yang, Z.; Grinchuk, V.; Ramalingam, T.R.; et al. Critical Role of IL-25 in Nematode Infection-Induced Alterations in Intestinal Function. J. Immunol. 2010, 185, 6921–6929. [Google Scholar] [CrossRef] [PubMed]
- Aono, Y.; Suzuki, Y.; Horiguchi, R.; Inoue, Y.; Karayama, M.; Hozumi, H.; Furuhashi, K.; Enomoto, N.; Fujisawa, T.; Nakamura, Y.; et al. CD109 on Dendritic Cells Regulates Airway Hyperreactivity and Eosinophilic Airway Inflammation. Am. J. Respir. Cell Mol. Biol. 2023, 68, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Carnevale, G.; Polese, B.; Simard, M.; Thurairajah, B.; Khan, N.; Gentile, M.E.; Fontes, G.; Vinh, D.C.; Pouliot, R.; et al. CD109 Restrains Activation of Cutaneous IL-17-Producing Γδ T Cells by Commensal Microbiota. Cell Rep. 2019, 29, 391–405.e5. [Google Scholar] [CrossRef] [PubMed]
- Taki, T.; Shiraki, Y.; Enomoto, A.; Weng, L.; Chen, C.; Asai, N.; Murakumo, Y.; Yokoi, K.; Takahashi, M.; Mii, S. CD109 Regulates in Vivo Tumor Invasion in Lung Adenocarcinoma through TGF-β Signaling. Cancer Sci. 2020, 111, 4616–4628. [Google Scholar] [CrossRef]
- Liu, X.; Chen, W.; Wang, Q.; Li, L.; Wang, C. Negative Regulation of TLR Inflammatory Signaling by the SUMO-Deconjugating Enzyme SENP6. PLoS Pathog. 2013, 9, e1003480. [Google Scholar] [CrossRef]
- Mukherjee, S.; Huda, S.; Sinha Babu, S.P. Toll-like Receptor Polymorphism in Host Immune Response to Infectious Diseases: A Review. Scand. J. Immunol. 2019, 90, e12771. [Google Scholar] [CrossRef]
- Ekman, C.; Linder, A.; Åkesson, P.; Dahlbäck, B. Plasma Concentrations of Gas6 (Growth Arrest Specific Protein 6) and Its Soluble Tyrosine Kinase Receptor sAxl in Sepsis and Systemic Inflammatory Response Syndromes. Crit. Care 2010, 14, R158. [Google Scholar] [CrossRef]
- Li, R.W.; Li, C.; Elsasser, T.H.; Liu, G.; Garrett, W.M.; Gasbarre, L.C. Mucin Biosynthesis in the Bovine Goblet Cell Induced by Cooperia Oncophora Infection. Vet. Parasitol. 2009, 165, 281–289. [Google Scholar] [CrossRef]
- Navarro, J.; Gozalbo-López, B.; Méndez, A.C.; Dantzer, F.; Schreiber, V.; Martínez, C.; Arana, D.M.; Farrés, J.; Revilla-Nuin, B.; Bueno, M.F.; et al. PARP-1/PARP-2 Double Deficiency in Mouse T Cells Results in Faulty Immune Responses and T Lymphomas. Sci. Rep. 2017, 7, 41962. [Google Scholar] [CrossRef]
- Saraiva, R.G.; Kang, S.; Simões, M.L.; Angleró-Rodríguez, Y.I.; Dimopoulos, G. Mosquito Gut Antiparasitic and Antiviral Immunity. Dev. Comp. Immunol. 2016, 64, 53–64. [Google Scholar] [CrossRef]
- Reinhold, U.; Abken, H. CD4+ CD7− T Cells: A Separate Subpopulation of Memory T Cells? J. Clin. Immunol. 1997, 17, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Borowiec, B.; Bryl, R.; Stefańska, K.; Dyszkiewicz-Konwińska, M. Potential Role of LYN, CCL2, ITGB3 and IL6 Genes in the Immune Response of Porcine Buccal Mucosa Cells. Med. J. Cell Biol. 2022, 10, 49–55. [Google Scholar] [CrossRef]
- Hainberger, D.; Stolz, V.; Zhu, C.; Schuster, M.; Müller, L.; Hamminger, P.; Rica, R.; Waltenberger, D.; Alteneder, M.; Krausgruber, T.; et al. NCOR1 Orchestrates Transcriptional Landscapes and Effector Functions of CD4+ T Cells. Front. Immunol. 2020, 11, 579. [Google Scholar] [CrossRef] [PubMed]
- Brunet, L.R. Nitric Oxide in Parasitic Infections. Int. Immunopharmacol. 2001, 1, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Lai, D.-H.; Wilson, R.A.; Chen, Y.-F.; Wang, L.-F.; Yu, Z.-L.; Li, M.-Y.; He, P.; Hide, G.; Sun, X.; et al. Nitric Oxide Blocks the Development of the Human Parasite Schistosoma Japonicum. Proc. Natl. Acad. Sci. USA 2017, 114, 10214–10219. [Google Scholar] [CrossRef] [PubMed]
- Li, R.W.; Li, C.; Gasbarre, L.C. The Vitamin D Receptor and Inducible Nitric Oxide Synthase Associated Pathways in Acquired Resistance to Cooperia Oncophora Infection in Cattle. Vet. Res. 2011, 42, 48. [Google Scholar] [CrossRef] [PubMed]
- Villarino, A.V.; Kanno, Y.; O’Shea, J.J. Mechanisms and Consequences of Jak–STAT Signaling in the Immune System. Nat. Immunol. 2017, 18, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.Y. STAT3 in Immune Responses and Inflammatory Bowel Diseases. Cell Res. 2006, 16, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Wittkopf, N.; Pickert, G.; Billmeier, U.; Mahapatro, M.; Wirtz, S.; Martini, E.; Leppkes, M.; Neurath, M.F.; Becker, C. Activation of Intestinal Epithelial Stat3 Orchestrates Tissue Defense during Gastrointestinal Infection. PLoS ONE 2015, 10, e0118401. [Google Scholar] [CrossRef] [PubMed]
- Kanai, T.; Jenks, J.; Nadeau, K.C. The STAT5b Pathway Defect and Autoimmunity. Front. Immun. 2012, 3, 234. [Google Scholar] [CrossRef]
- Jin, W.; Qi, S.; Luo, H. T Cell-Specific Deletion of EFNB2 Minimally Affects T Cell Development and Function. Mol. Immunol. 2012, 52, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zhang, C.; Chen, S.; He, R.; Chao, G.; Zhang, S. TLR5 Signaling in the Regulation of Intestinal Mucosal Immunity. J. Inflamm. Res. 2023, 16, 2491–2501. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; Nemati, M.; Salarkia, E.; Yadav, S.; Aminizadeh, N.; Jafarzadeh, S.; Yadav, M. Inflammatory Responses during Trichomoniasis: The Role of Toll-like Receptors and Inflammasomes. Parasite Immunol. 2023, 45, e13000. [Google Scholar] [CrossRef] [PubMed]
- Novak, A.J.; Slager, S.L.; Fredericksen, Z.S.; Wang, A.H.; Manske, M.M.; Ziesmer, S.; Liebow, M.; Macon, W.R.; Dillon, S.R.; Witzig, T.E.; et al. Genetic Variation in B-Cell–Activating Factor Is Associated with an Increased Risk of Developing B-Cell Non–Hodgkin Lymphoma. Cancer Res. 2009, 69, 4217–4224. [Google Scholar] [CrossRef] [PubMed]
- Schweighoffer, E.; Tybulewicz, V.L. BAFF Signaling in Health and Disease. Curr. Opin. Immunol. 2021, 71, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, N.; Mercado, D.; Vergara, C.; Sánchez, J.; Kennedy, M.W.; Jiménez, S.; Fernández, A.M.; Gutiérrez, M.; Puerta, L.; Caraballo, L. Association between Total Immunoglobulin E and Antibody Responses to Naturally Acquired Ascaris Lumbricoides Infection and Polymorphisms of Immune System-Related LIG4, TNFSF13B and IRS2 Genes. Clin. Exp. Immunol. 2009, 157, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Chinen, T.; Kannan, A.K.; Levine, A.G.; Fan, X.; Klein, U.; Zheng, Y.; Gasteiger, G.; Feng, Y.; Fontenot, J.D.; Rudensky, A.Y. An Essential Role for the IL-2 Receptor in Treg Cell Function. Nat. Immunol. 2016, 17, 1322–1333. [Google Scholar] [CrossRef]
- Ingham, A.; Reverter, A.; Windon, R.; Hunt, P.; Menzies, M. Gastrointestinal Nematode Challenge Induces Some Conserved Gene Expression Changes in the Gut Mucosa of Genetically Resistant Sheep. Int. J. Parasitol. 2008, 38, 431–442. [Google Scholar] [CrossRef]
- Kumar, B.; Field, N.S.; Kim, D.D.; Dar, A.A.; Chen, Y.; Suresh, A.; Pastore, C.F.; Hung, L.-Y.; Porter, N.; Sawada, K.; et al. The Ubiquitin Ligase Cul5 Regulates CD4+ T Cell Fate Choice and Allergic Inflammation. Nat. Commun. 2022, 13, 2786. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Lindén, S.K.; Sutton, P.; Florin, T.H. Mucin Dynamics and Enteric Pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar] [CrossRef]
- King, A.; Li, L.; Wong, D.M.; Liu, R.; Bamford, R.; Strasser, A.; Tarlinton, D.M.; Heierhorst, J. Dynein Light Chain Regulates Adaptive and Innate B Cell Development by Distinctive Genetic Mechanisms. PLoS Genet. 2017, 13, e1007010. [Google Scholar] [CrossRef]
- Liu, R.; King, A.; Tarlinton, D.; Heierhorst, J. The ASCIZ-DYNLL1 Axis Is Essential for TLR4-Mediated Antibody Responses and NF-κ B Pathway Activation. Mol. Cell. Biol. 2021, 41, e00251-21. [Google Scholar] [CrossRef]
- Song, X.; Dai, D.; He, X.; Zhu, S.; Yao, Y.; Gao, H.; Wang, J.; Qu, F.; Qiu, J.; Wang, H.; et al. Growth Factor FGF2 Cooperates with Interleukin-17 to Repair Intestinal Epithelial Damage. Immunity 2015, 43, 488–501. [Google Scholar] [CrossRef] [PubMed]
- Ohteki, T.; Suzue, K.; Maki, C.; Ota, T.; Koyasu, S. Critical Role of IL-15-IL-15R for Antigen-Presenting Cell Functions in the Innate Immune Response. Nat. Immunol. 2001, 2, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Reinecker, H.C.; MacDermott, R.P.; Mirau, S.; Dignass, A.; Podolsky, D.K. Intestinal Epithelial Cells Both Express and Respond to Interleukin 15. Gastroenterology 1996, 111, 1706–1713. [Google Scholar] [CrossRef]
- Raeber, M.E.; Zurbuchen, Y.; Impellizzieri, D.; Boyman, O. The Role of Cytokines in T-Cell Memory in Health and Disease. Immunol. Rev. 2018, 283, 176–193. [Google Scholar] [CrossRef]
- Entwistle, L.J.; Pelly, V.S.; Coomes, S.M.; Kannan, Y.; Perez-Lloret, J.; Czieso, S.; Silva Dos Santos, M.; MacRae, J.I.; Collinson, L.; Sesay, A.; et al. Epithelial-Cell-Derived Phospholipase A 2 Group 1B Is an Endogenous Anthelmintic. Cell Host Microbe 2017, 22, 484–493.e5. [Google Scholar] [CrossRef]
- Palma, M.; El-Naccache, D.W.; Gause, W.C. Pla2g1b Places Worms in Peril. Cell Host Microbe 2017, 22, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Oudhoff, M.J.; Antignano, F.; Chenery, A.L.; Burrows, K.; Redpath, S.A.; Braam, M.J.; Perona-Wright, G.; Zaph, C. Intestinal Epithelial Cell-Intrinsic Deletion of Setd7 Identifies Role for Developmental Pathways in Immunity to Helminth Infection. PLoS Pathog. 2016, 12, e1005876. [Google Scholar] [CrossRef] [PubMed]
- Cruikshank, W.W.; Kornfeld, H.; Center, D.M. Interleukin-16. J. Leukoc. Biol. 2000, 67, 757–766. [Google Scholar] [CrossRef]
- Grencis, R.K. Cytokine Regulation of Resistance and Susceptibility to Intestinal Nematode Infection—From Host to Parasite. Vet. Parasitol. 2001, 100, 45–50. [Google Scholar] [CrossRef]
- Pernthaner, A.; Cole, S.-A.; Morrison, L.; Hein, W.R. Increased Expression of Interleukin-5 (IL-5), IL-13, and Tumor Necrosis Factor α Genes in Intestinal Lymph Cells of Sheep Selected for Enhanced Resistance to Nematodes during Infection with Trichostrongylus Colubriformis. Infect. Immun. 2005, 73, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Kosik-Bogacka, D.I.; Wojtkowiak-Giera, A.; Kolasa, A.; Baranowska-Bosiacka, I.; Lanocha, N.; Wandurska-Nowak, E.; Izabela, G.; Salamatin, R.; Jagodzinski, P.P. Hymenolepis Diminuta: Analysis of the Expression of Toll-like Receptor Genes and Protein (TLR3 and TLR9) in the Small and Large Intestines of Rats. Exp. Parasitol. 2014, 145, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Bisom, T.C.; White, L.A.; Lanchy, J.-M.; Lodmell, J.S. RIOK3 and Its Alternatively Spliced Isoform Have Disparate Roles in the Innate Immune Response to Rift Valley Fever Virus (MP12) Infection. Viruses 2022, 14, 2064. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Tang, K.; Chen, D.; Hong, M.; Sun, F.; Wang, S.; Ke, Y.; Wu, T.; Sun, R.; Qian, J.; et al. Riok3 Inhibits the Antiviral Immune Response by Facilitating TRIM40-Mediated RIG-I and MDA5 Degradation. Cell Rep. 2021, 35, 109272. [Google Scholar] [CrossRef]
- Feng, J.; De Jesus, P.D.; Su, V.; Han, S.; Gong, D.; Wu, N.C.; Tian, Y.; Li, X.; Wu, T.-T.; Chanda, S.K.; et al. RIOK3 Is an Adaptor Protein Required for IRF3-Mediated Antiviral Type I Interferon Production. J. Virol. 2014, 88, 7987–7997. [Google Scholar] [CrossRef]
- Vanhoutte, F.; Breuilh, L.; Fontaine, J.; Zouain, C.S.; Mallevaey, T.; Vasseur, V.; Capron, M.; Goriely, S.; Faveeuw, C.; Ryffel, B.; et al. Toll-like Receptor (TLR)2 and TLR3 Sensing Is Required for Dendritic Cell Activation, but Dispensable to Control Schistosoma Mansoni Infection and Pathology. Microbes Infect. 2007, 9, 1606–1613. [Google Scholar] [CrossRef]
- Sawada, Y.; Nakatsuji, T.; Dokoshi, T.; Kulkarni, N.N.; Liggins, M.C.; Sen, G.; Gallo, R.L. Cutaneous Innate Immune Tolerance Is Mediated by Epigenetic Control of MAP2K3 by HDAC8/9. Sci. Immunol. 2021, 6, eabe1935. [Google Scholar] [CrossRef]
| Chromosome | Associated Gene | Associated Phenotype 1 | Breed 2 | Reference |
|---|---|---|---|---|
| 1 | CCDC50 | Average FEC | Red Masaai × Dorper | [6] |
| FAMACHA© | Santa Inês | [30] | ||
| HCT | Santa Inês | [30] | ||
| RBC | Santa Inês | [30] | ||
| CD86 | H. contortus resistance 3 | Dorper, Katahdin, St. Croix | [44] | |
| RBC | Santa Inês | [29] | ||
| PLT | Santa Inês | [29] | ||
| HCT | Santa Inês | [29] | ||
| IL12RB2 | PLT | Santa Inês | [29] | |
| HCT | Santa Inês | [29] | ||
| FEC | Florida Native | [41] | ||
| FAMACHA© | Florida Native | [41] | ||
| Total IgM level | Dorper, Katahdin, St. Croix | [45] | ||
| LEPR | FEC | Corriedale | [31] | |
| RBC | Santa Inês | [29] | ||
| PLT | Santa Inês | [29] | ||
| HCT | Santa Inês | [29] | ||
| 2 | ITGA6 | H. contortus resistance 4 | Morada Nova | [40] |
| PLT | Santa Inês | [30] | ||
| CXCR1 | FEC | Tunisan | [24] | |
| HGB | Tunisan | [24] | ||
| CXCR2 | FEC | Tunisan | [24] | |
| HGB | Tunisan | [24] | ||
| CXCR4 | RBC | Santa Inês | [29] | |
| FEC | Santa Inês | [30] | ||
| PLT | Santa Inês | [30] | ||
| Resistance to GINs 5 | Scottish Blackface | [46] | ||
| UBR3 | H. contortus resistance 4 | Morada Nova | [40] | |
| PLT | Santa Inês | [30] | ||
| RUFY4 | FEC | Tunisan | [24] | |
| HGB | Santa Inês | [30] | ||
| DPP4 | RBC | Santa Inês | [29] | |
| PLT | Santa Inês | [29] | ||
| H. contortus resistance 4 | Morada Nova | [40] | ||
| SLC11A1 | FEC | Tunisian | [24] | |
| HGB | Santa Inês | [30] | ||
| STAT4 | Resistance to GINs 5 | Scottish Blackface | [46] | |
| HGB | Santa Inês | [30] | ||
| RBC | Santa Inês | [30] | ||
| 3 | IFNG | FEC | Soay | [34] |
| CDKN1B | FEC | Tunisian | [24] | |
| HCT | Santa Inês | [29] | ||
| PLT | Santa Inês | [30] | ||
| EPAS1 | FAMACHA© | Djallonqe | [25] | |
| HCT | Santa Inês | [29] | ||
| SOCS2 | Average FEC | Red Masaai × Dorper | [6] | |
| H. contortus resistance 3 | Dorper, Katahdin, St. Croix | [44] | ||
| 6 | CFI | FEC | Florida Native | [41] |
| H. contortus resistance 4 | Morada Nova | [40] | ||
| CHRNA9 | FEC | Tunisan | [24] | |
| RBC | Santa Inês | [30] | ||
| WBC | Santa Inês | [30] | ||
| CXCL9 | FEC | Spanish Churra | [27] | |
| WBC | Santa Inês | [30] | ||
| FAMACHA© | Santa Inês | [30] | ||
| CXCL10 | FEC | Spanish Churra | [27] | |
| PLT | Santa Inês | [29] | ||
| Neutrophil count | Florida Native | [41] | ||
| WBC | Santa Inês | [30] | ||
| FAMACHA© | Santa Inês | [30] | ||
| CXCL11 | FEC | Spanish Churra | [27] | |
| WBC | Santa Inês | [30] | ||
| FAMACHA© | Santa Inês | [30] | ||
| LEF1 | HGB | Santa Inês | [29] | |
| H. contortus resistance 4 | Morada Nova | [40] | ||
| RHOH | FEC | Tunisan | [24] | |
| WBC | Santa Inês | [30] | ||
| RBC | Santa Inês | [30] | ||
| UBA6 | FEC | Spanish Churra | [27] | |
| PLT | Santa Inês | [29] | ||
| PLT | Santa Inês | [30] | ||
| 7 | FCHO1 | FEC | Spanish Churra | [27] |
| FAMACHA© | Santa Inês | [30] | ||
| JAK3 | FEC | Spanish Churra | [27] | |
| FAMACHA© | Santa Inês | [30] | ||
| MAP1S | FEC | Spanish Churra | [27] | |
| FAMACHA© | Santa Inês | [30] | ||
| SMAD3 | FEC | Spanish Churra | [27] | |
| FEC | Santa Inês | [30] | ||
| IL25 | FEC | Spanish Churra | [27] | |
| FEC | Santa Inês | [30] | ||
| EDIL3 | FEC | Katahdin | [23] | |
| WBC | Santa Inês | [30] | ||
| 8 | CD109 | FEC | Spanish Churra | [27] |
| RBC | Santa Inês | [29] | ||
| HGB | Santa Inês | [29] | ||
| HCT | Santa Inês | [29] | ||
| H. contortus resistance 4 | Morada Nova | [40] | ||
| SENP6 | FEC | Spanish Churra | [27] | |
| RBC | Santa Inês | [29] | ||
| HCT | Santa Inês | [29] | ||
| HGB | Santa Inês | [29] | ||
| 10 | GAS6 | FEC | Florida Native | [47] |
| PCV | Florida Native | [47] | ||
| PCV | Florida Native | [47] | ||
| RBC | Santa Inês | [29] | ||
| GCNT3 | H. contortus resistance 4 | Morada Nova | [40] | |
| WBC | Santa Inês | [30] | ||
| PARP2 | FEC | Spanish Churra | [27] | |
| FEC | Santa Inês | [30] | ||
| TEP1 | FEC | Spanish Churra | [27] | |
| FEC | Santa Inês | [30] | ||
| 11 | CD7 | Antigen-specific IgA activity | Spanish Churra | [27] |
| FEC | Florida Native | [47] | ||
| ITGB3 | Antigen-specific IgA activity | Spanish Churra | [27] | |
| WBC | Santa Inês | [29] | ||
| FEC | Florida Native | [47] | ||
| NCOR1 | H. contortus resistance 4 | Morada Nova | [40] | |
| PLT | Santa Inês | [30] | ||
| NOS2 | H. contortus FEC | Florida Native | [47] | |
| H. contortus resistance 3 | Dorper, Katahdin, St. Croix | [44] | ||
| STAT3 | H. contortus FEC | Florida Native | [41] | |
| H. contortus FEC | Florida Native | [47] | ||
| STAT5B | H. contortus FEC | Florida Native | [47] | |
| H. contortus resistance 3 | Dorper, Katahdin, St. Croix | [44] | ||
| 12 | EFNB2 | H. contortus FEC | Florida Native | [47] |
| PCV | Florida Native | [47] | ||
| PCV | Florida Native | [47] | ||
| HCT | Santa Inês | [30] | ||
| HGB | Santa Inês | [30] | ||
| RBC | Santa Inês | [30] | ||
| TLR5 | PLT | Santa Inês | [29] | |
| FEC | Corriedale | [31] | ||
| TNFSF13B | H. contortus FEC | Florida Native | [47] | |
| PCV | Florida Native | [47] | ||
| PCV | Florida Native | [47] | ||
| HCT | Santa Inês | [30] | ||
| HGB | Santa Inês | [30] | ||
| RBC | Santa Inês | [30] | ||
| 13 | IL2RA | WBC | Santa Inês | [29] |
| PLT | Santa Inês | [29] | ||
| RBC | Santa Inês | [30] | ||
| H. contortus resistance 3 | Dorper, Katahdin, St. Croix | [44] | ||
| 15 | CUL5 | H. contortus resistance 4 | Morada Nova | [40] |
| FAMACHA© | Santa Inês | [30] | ||
| MUC15 | Traits measuring resistance to GINs 6 | Red Masaai × Dorper | [6] | |
| FEC | Florida Native | [41] | ||
| WBC | Florida Native | [41] | ||
| 17 | DYNLL1 | Antigen-specific IgA activity | Spanish Churra | [27] |
| H. contortus resistance 4 | Morada Nova | [40] | ||
| FGF2 | FEC | Tunisan | [24] | |
| FEC | Santa Inês | [29] | ||
| FEC | Santa Inês | [30] | ||
| PLT | Santa Inês | [30] | ||
| HCT | Santa Inês | [30] | ||
| HGB | Santa Inês | [30] | ||
| RBC | Santa Inês | [30] | ||
| IL15 | Antigen-specific IgA activity | Spanish Churra | [27] | |
| FEC | Santa Inês | [29] | ||
| WBC | Santa Inês | [29] | ||
| FEC | Santa Inês | [30] | ||
| PLA2G1B | Antigen-specific IgA activity | Spanish Churra | [27] | |
| H. contortus resistance 4 | Morada Nova | [40] | ||
| SETD7 | Antigen-specific IgA activity | Spanish Churra | [27] | |
| FEC | Santa Inês | [30] | ||
| 18 | IL16 | PLT | Santa Inês | [29] |
| RBC | Florida Native | [41] | ||
| HGB | Florida Native | [41] | ||
| 20 | TNF | FEC | Sarda Dairy × Lacaune | [32] |
| Neutrophil count | Florida Native | [41] | ||
| 22 | TLR9 | FEC | Corriedale | [31] |
| WBC | Santa Inês | [30] | ||
| PLT | Santa Inês | [30] | ||
| 24 | RIOK3 | Antigen-specific IgA activity | Spanish Churra | [27] |
| FEC | Santa Inês | [30] | ||
| 26 | TLR3 | WBC | Santa Inês | [29] |
| FEC | Florida Native | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha, S.M.F.; Lam, S.; Mallard, B.; Karrow, N.A.; Cánovas, Á. Genomic Regions Associated with Resistance to Gastrointestinal Nematode Parasites in Sheep—A Review. Genes 2024, 15, 187. https://doi.org/10.3390/genes15020187
Cunha SMF, Lam S, Mallard B, Karrow NA, Cánovas Á. Genomic Regions Associated with Resistance to Gastrointestinal Nematode Parasites in Sheep—A Review. Genes. 2024; 15(2):187. https://doi.org/10.3390/genes15020187
Chicago/Turabian StyleCunha, Samla Marques Freire, Stephanie Lam, Bonnie Mallard, Niel A. Karrow, and Ángela Cánovas. 2024. "Genomic Regions Associated with Resistance to Gastrointestinal Nematode Parasites in Sheep—A Review" Genes 15, no. 2: 187. https://doi.org/10.3390/genes15020187
APA StyleCunha, S. M. F., Lam, S., Mallard, B., Karrow, N. A., & Cánovas, Á. (2024). Genomic Regions Associated with Resistance to Gastrointestinal Nematode Parasites in Sheep—A Review. Genes, 15(2), 187. https://doi.org/10.3390/genes15020187





