Goat miR-92a-3p Targets APOL6 Gene to Regulate the Differentiation of Intramuscular Precursor Adipocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Isolation, Culture, and Induction of Differentiation
2.2. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.3. Vector Construction
2.4. Luciferase Reporter Assaying
2.5. Lipid Droplets Staining
2.6. Statistical Analysis
3. Results
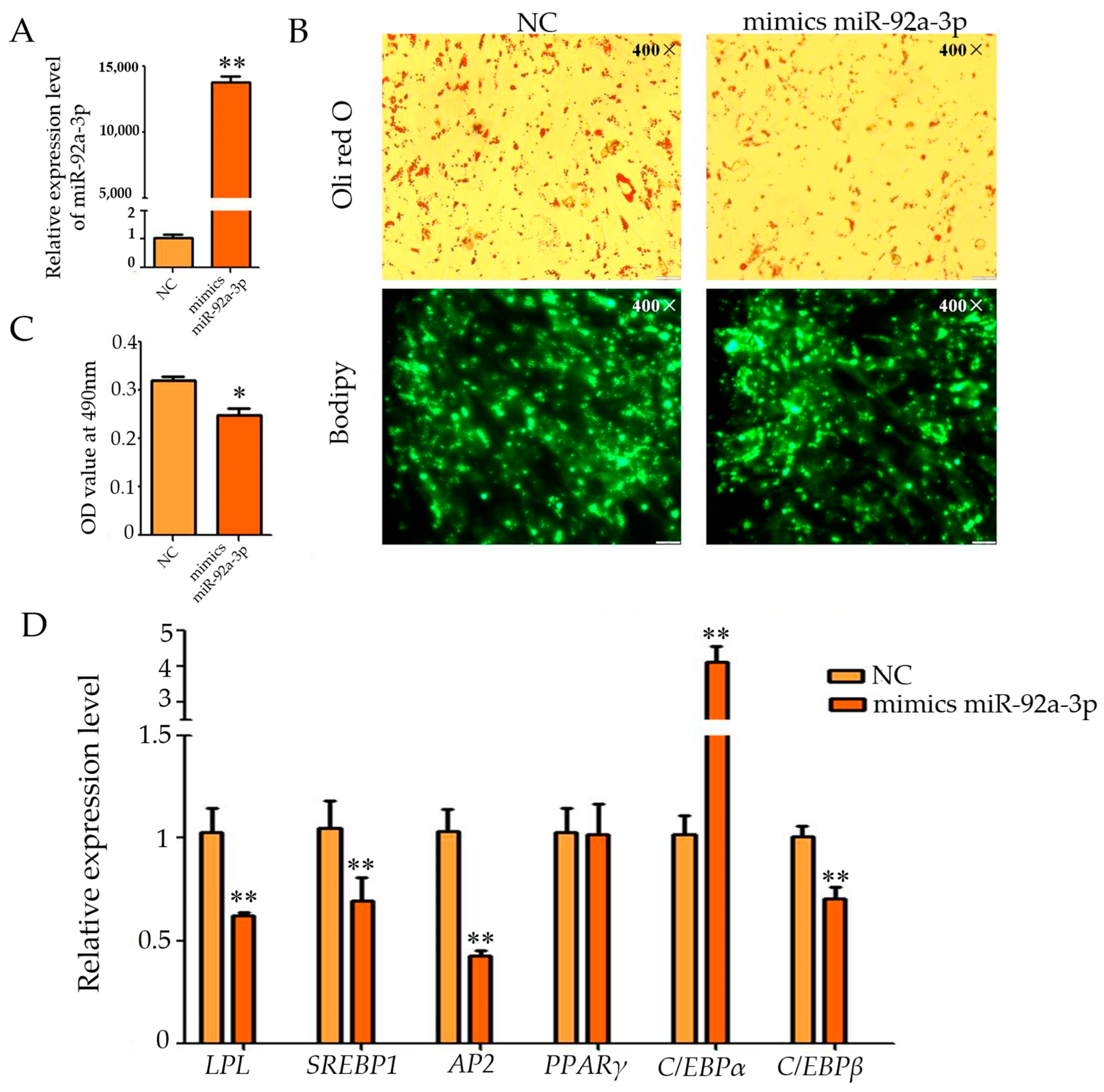
3.1. Effect of Overexpression of miR-92a-3p on the Differentiation of Intramuscular Adipocytes in Goats
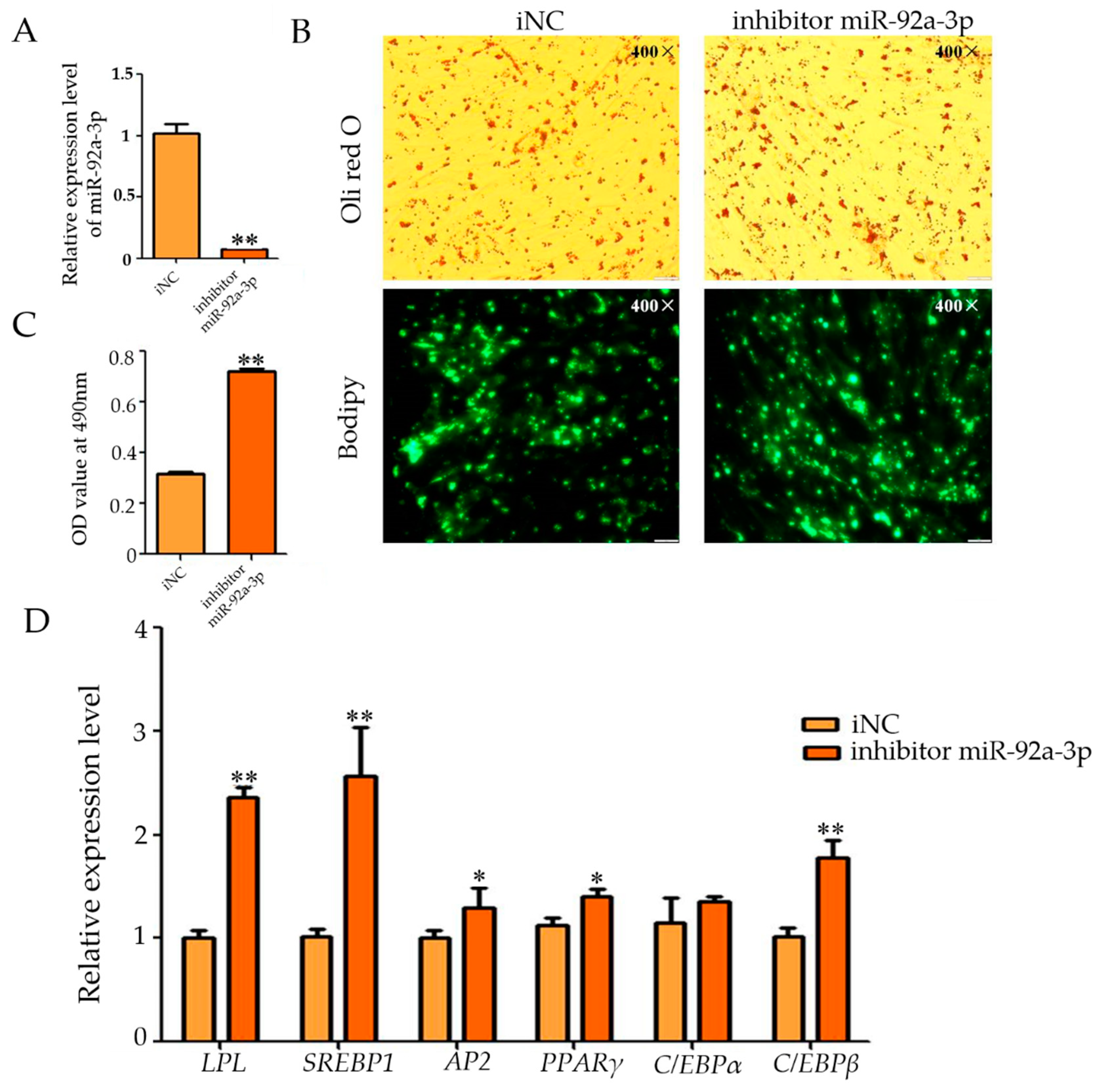
3.2. Effect of miR-92a-3p Inhibition on Intramuscular Adipocyte Differentiation in Goats
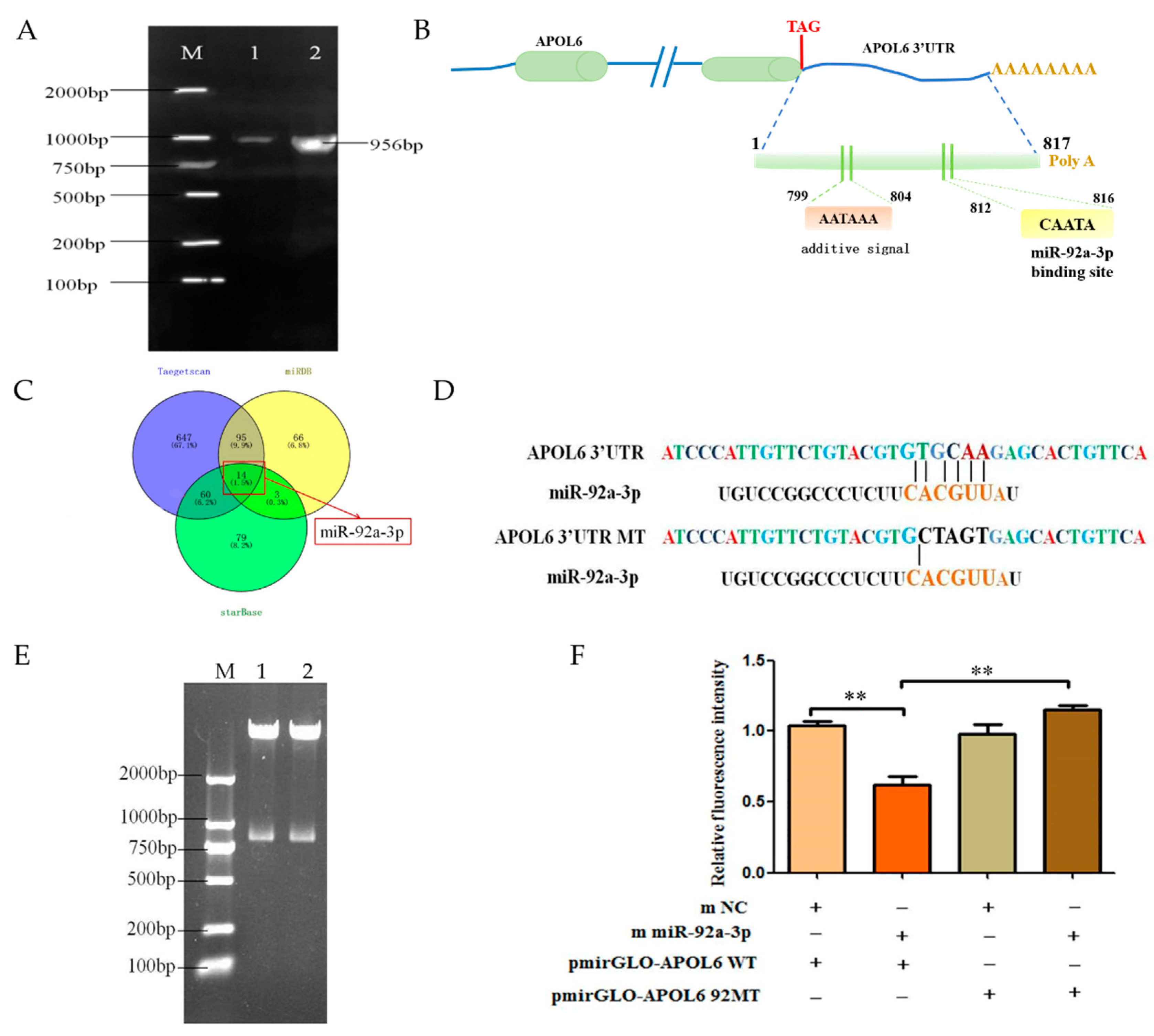
3.3. Effect of miR-92a-3p on the Expression of APOL6
3.4. miR-92a-3p Targets APOL6 3′UTR Binding
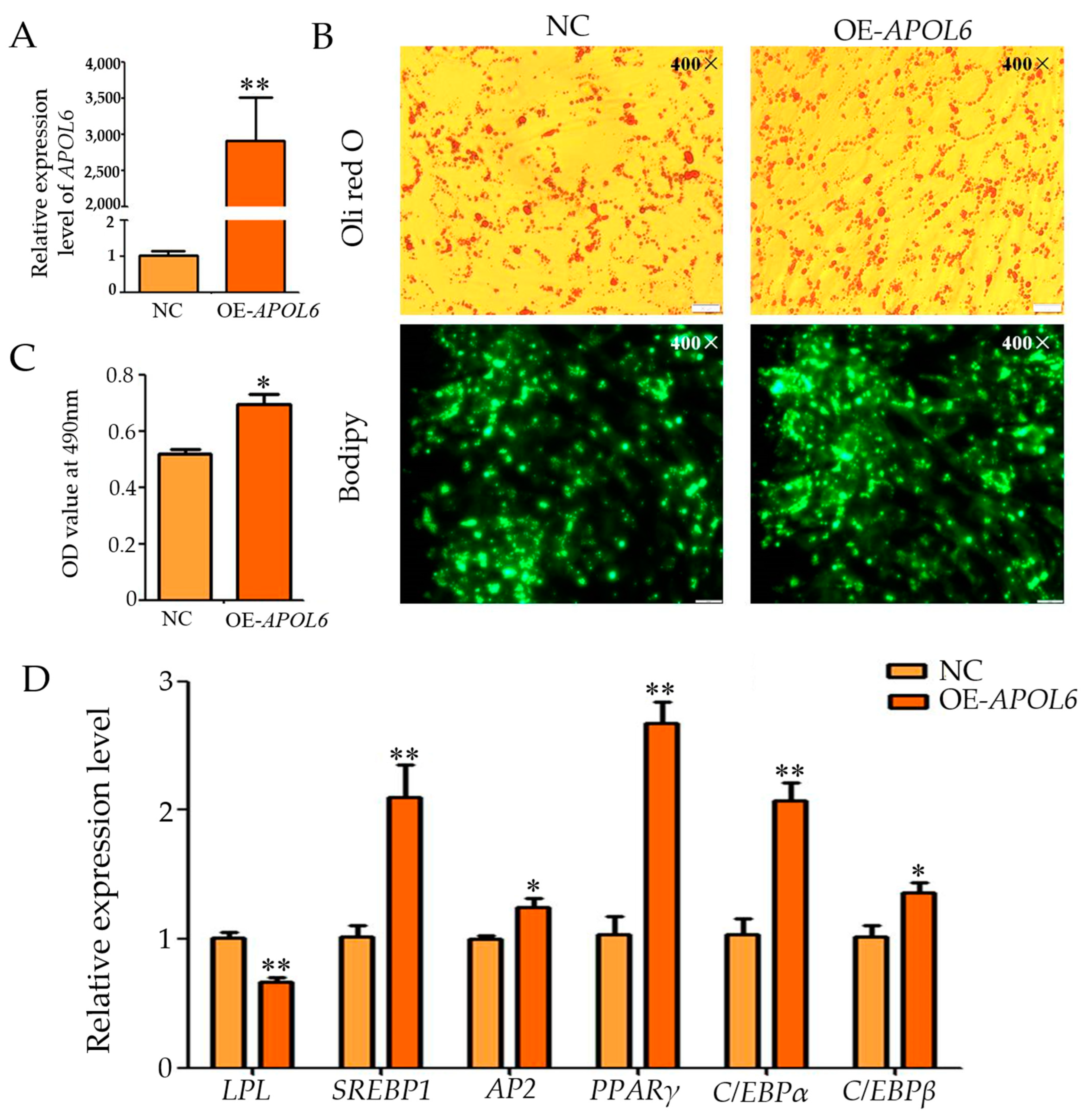
3.5. Effect of Overexpression of APOL6 on the Differentiation of Intramuscular Adipocytes in Goats
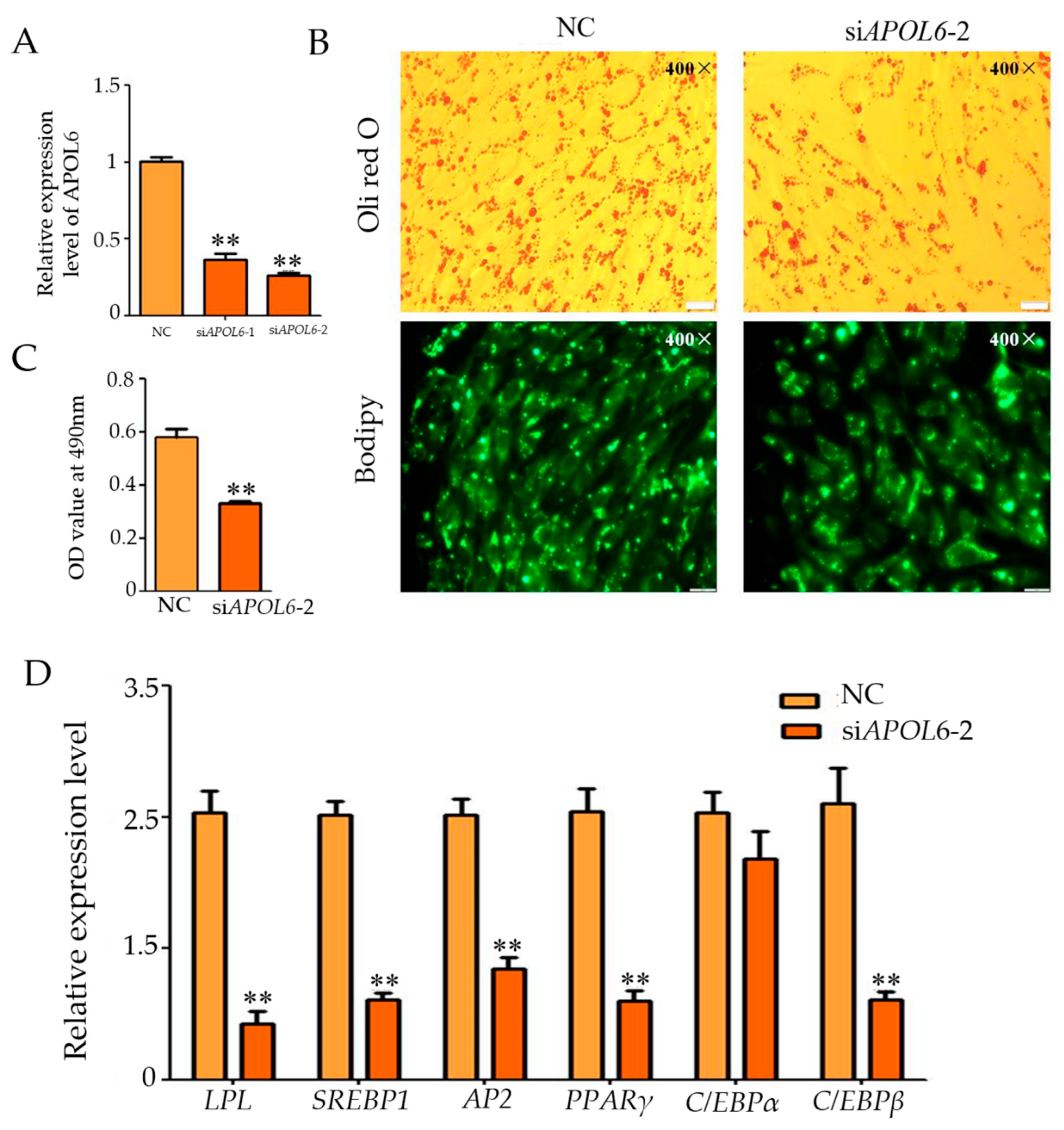
3.6. Effect of Inhibition of APOL6 on the Differentiation of Intramuscular Adipocytes in Goats
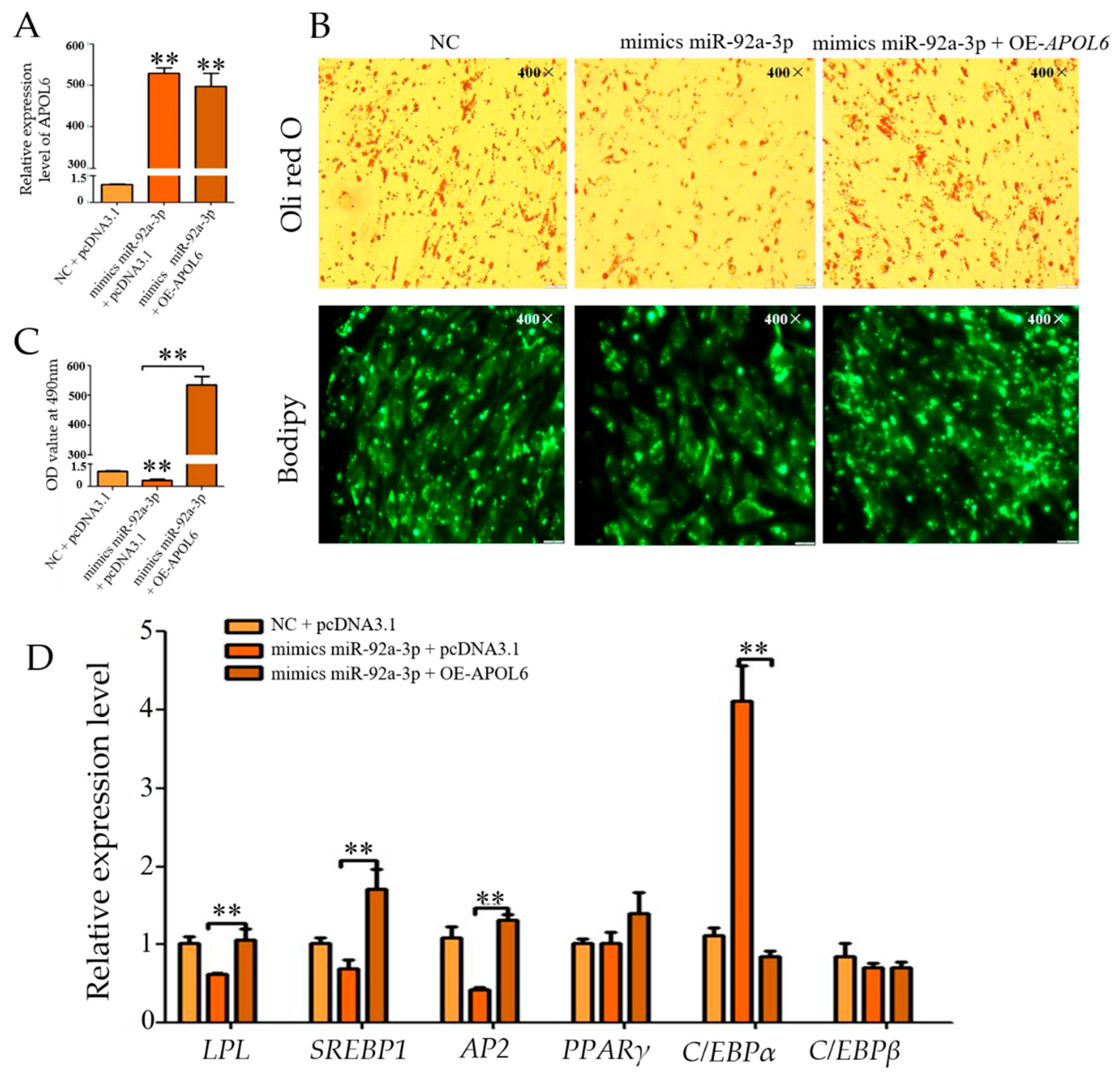
3.7. Effect of Overexpression of miR-92a-3p Followed by Overexpression of APOL6 on the Differentiation of Goat Intramuscular Adipocytes
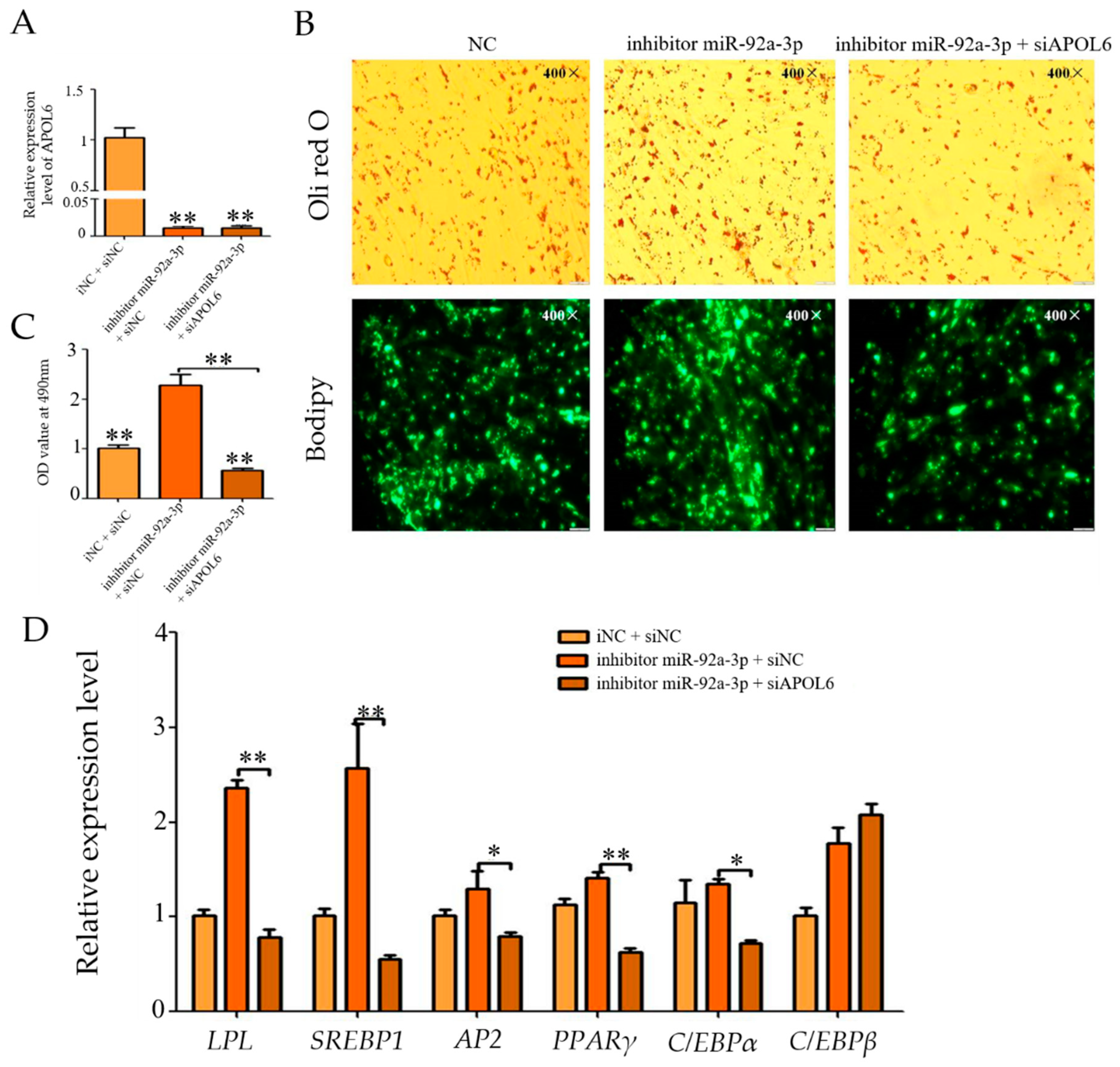
3.8. Effect of Inhibition of miR-92a-3p Followed by Interference with APOL6 on Goat Intramuscular Adipocyte Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stanišić, N.; Žujović, M.; Tomić, Z.; Maksimović, N.; Bijelić, Z.; Ivanović, S.; Memiši, N. The Effects of Crossing Balkan and Saanen Goat Breeds on Carcass Traits and Certain Quality Parameters of Kid Meat. Ann. Anim. Sci. 2012, 12, 53–62. [Google Scholar] [CrossRef]
- Xu, K.; Ji, M.; Huang, X.; Peng, Y.; Wu, W.; Zhang, J. Differential regulatory roles of MicroRNAs in porcine intramuscular and subcutaneous adipocytes. J. Agric. Food Chem. 2020, 68, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, Y.; Wu, J.; Qiao, M.; Xu, Z.; Peng, X.; Mei, S. Proteomic and lipidomic analyses reveal saturated fatty acids, phosphatidylinositol, phosphatidylserine, and associated proteins contributing to intramuscular fat deposition. J. Proteom. 2021, 241, 104235. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Ball, A.; Hughes, J.; Krishnamurthy, R.; Piyasiri, U.; Stark, J.; Watkins, P.; Warner, R. Sensory and flavor chemistry characteristics of Australian beef: Influence of intramuscular fat, feed, and breed. J. Agric. Food Chem. 2016, 64, 4299–4311. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Watkins, P.; Ball, A.; Krishnamurthy, R.; Piyasiri, U.; Sewell, J.; Ortuno, J.; Stark, J.; Warner, R. Impact of brassica and lucerne finishing feeds and intramuscular fat on lamb eating quality and flavor. A cross-cultural study using Chinese and Non-Chinese Australian consumers. J. Agric. Food Chem. 2016, 64, 6856–6868. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.S.; Raza, S.H.A.; Sun, Y.G.; Khan, R.; Ullah, I.; Han, Y.C. Detection of polymorphisms in the promoter of bovine SIRT1 gene and their effects on intramuscular fat content in Chinese indigenous cattle. Gene 2019, 700, 47–51. [Google Scholar] [CrossRef]
- Li, X.; Fu, X.; Yang, G.; Du, M. Review: Enhancing intramuscular fat development via targeting fibro-adipogenic progenitor cells in meat animals. Animal 2020, 14, 312–321. [Google Scholar] [CrossRef]
- Migdał, W.; Kawęcka, A.; Sikora, J.; Migdał, Ł. Meat quality of the native Carpathian goat breed in comparison with the Saanen breed. Animals 2021, 11, 2220. [Google Scholar] [CrossRef]
- Realini, C.E.; Pavan, E.; Johnson, P.L.; Font-I-Furnols, M.; Jacob, N.; Agnew, M.; Craigie, C.R.; Moon, C.D. Consumer liking of M. longissimus lumborum from New Zealand pasture-finished lamb is influenced by intramuscular fat. Meat Sci. 2021, 173, 108380. [Google Scholar] [CrossRef]
- Mota de Sá, P.; Richard, A.J.; Hang, H.; Stephens, J.M. Transcriptional Regulation of Adipogenesis. Compr. Physiol. 2017, 7, 635–674. [Google Scholar]
- Covas, D.T.; Panepucci, R.A.; Fontes, A.M.; Silva, W.A., Jr.; Orellana, M.D.; Freitas, M.C.; Neder, L.; Santos, A.R.; Peres, L.C.; Jamur, M.C.; et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp. Hematol. 2008, 36, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Chen, H.; Zhang, Y.; Zhu, C.; Gao, B.; Yin, Y.; Li, W.; Shi, Q.; Zheng, M.; Xu, Q.; et al. Cloning of the Xuhuai goat PPARγ gene and the preparation of Transgenic sheep. Biochem. Genet. 2013, 51, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Khan, R.; Raza, S.H.A.; Nurgulsim, K.; Suhail, S.M.; Rahman, A.; Ahmed, I.; Ijaz, A.; Ahmad, I.; Linsen, Z. Transcriptional regulation of adipogenic marker genes for the improvement of intramuscular fat in Qinchuan beef cattle. Anim. Biotechnol. 2022, 33, 776–795. [Google Scholar]
- Zhou, L.; Chen, S.Y.; Han, H.J.; Sun, J.Q. Lactate augments intramuscular triglyceride accumulation and mitochondrial biogenesis in rats. J. Biol. Regul. Homeost. Agents 2021, 35, 105–115. [Google Scholar] [PubMed]
- Deb, B.; Uddin, A.; Chakraborty, S. miRNAs and ovarian cancer: An overview. Cell Physiol. 2018, 233, 3846–3854. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.; Zhang, Z.; Liu, G.; Sun, S. Sun C miR-103 promotes 3T3-L1 cell adipogenesis through AKT/mTOR signal pathway with its target being MEF2D. Biol Chem. 2015, 396, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chen, F.F.; Ge, J.; Zhu, J.Y.; Shi, X.E.; Li, X.; Yu, T.Y.; Chu, G.Y.; Yang, G.S. miR-429 Inhibits Differentiation and Promotes Proliferation in Porcine Preadipocytes. Int. J. Mol. Sci. 2016, 17, 2047. [Google Scholar] [CrossRef]
- Li, B.; Huang, X.; Yang, C.; Ge, T.; Zhao, L.; Zhang, X.; Tian, L.; Zhang, E. miR-27a regulates sheep adipocyte differentiation by targeting CPT1B gene. Animals 2021, 12, 28. [Google Scholar] [CrossRef]
- Shan, B.; Yan, M.; Yang, K.; Lin, W.; Yan, J.; Wei, S.; Wei, W.; Chen, J.; Zhang, L. MiR-218-5p affects subcutaneous adipogenesis by targeting ACSL1, a novel candidate for pig fat deposition. Genes 2022, 13, 260. [Google Scholar] [CrossRef]
- Page, N.M.; Butlin, D.J.; Lomthaisong, K.; Lowry, P.J. The human apolipoprotein L gene cluster: Identification, classification, and sites of distribution. Genomics. 2001, 74, 71–78. [Google Scholar] [CrossRef]
- Schroeder, F.; Gallegos, A.M.; Atshaves, B.P.; Storey, S.M.; Mcintosh, A.L.; Petrescu, A.D.; Huang, H.; Starodub, O.; Chao, H.; Yang, H.; et al. Recent advances in membrane microdomains: Rafts, caveolae, and intracellular cholesterol trafficking. Exp. Biol. Med. 2001, 226, 873–890. [Google Scholar] [CrossRef]
- Xu, Q.; Lin, S.; Wang, Y.; Zhu, J.; Lin, Y. Fibroblast growth factor 10 (FGF10) promotes the adipogenesis of intramuscular preadipocytes in goat. Mol. Biol. Rep. 2018, 45, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.D.; Magnuson, A.M.; Fouts, J.; Wei, Y.; Wang, D.; Pagliassotti, M.J.; Foster, M.T. Subcutaneous adipose tissue accumulation protects systemic glucose tolerance and muscle metabolism. Adipocyte 2018, 7, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Esau, C.; Kang, X.; Peralta, E.; Hanson, E.; Marcusson, E.G.; Ravichandran, L.V.; Sun, Y.; Koo, S.; Perera, R.J.; Jain, R.; et al. MicroRNA-143 regulates adipocyte differentiation. J. Biol. Chem. 2004, 279, 52361–52365. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, H.; Cho, Y.M.; Kwon, O.J.; Kim, W.; Lee, E.K. TNFα-induced miR-130 resulted in adipocyte dysfunction during obesity-related inflammation. FEBS Lett. 2013, 587, 3853–3858. [Google Scholar] [CrossRef]
- Zhao, N.Q.; Li, X.Y.; Wang, L.; Feng, Z.L.; Li, X.F.; Wen, Y.F.; Han, J.X. Palmitate induces fat accumulation by activating C/EBPβ-mediated G0S2 expression in HepG2 cells. World J. Gastroenterol. 2017, 23, 7705–7715. [Google Scholar] [CrossRef]
- Stachecka, J.; Nowacka-Woszuk, J.; Kolodziejski, P.A.; Szczerbal, I. The importance of the nuclear positioning of the PPARG gene for its expression during porcine in vitro adipogenesis. Chromosome Res. 2019, 27, 271–284. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Haakonsson, A.K.; Lazar, M.A.; Mandrup, S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 2014, 25, 293–302. [Google Scholar] [CrossRef]
- Lee, J.E.; Ge, K. Transcriptional and epigenetic regulation of PPARγ expression during adipogenesis. Cell Biosci. 2014, 4, 29. [Google Scholar] [CrossRef]
- Fajas, L.; Schoonjans, K.; Gelman, L.; Kim, J.B.; Najib, J.; Martin, G.; Fruchart, J.C.; Briggs, M.; Spiegelman, B.M.; Auwerx, J. Regulation of peroxisome proliferator-activated receptor gamma expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: Implications for adipocyte differentiation and metabolism. Mol. Cell Biol. 1999, 19, 5495–5503. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, H.; Jiang, Z.; Pastuszyn, A.; Hu, C.A. Apolipoprotein l6, a novel proapoptotic Bcl-2 homology 3-only protein, induces mitochondria-mediated apoptosis in cancer cells. Mol. Cancer Res. 2005, 3, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Zhaorigetu, S.; Yang, Z.; Toma, I.; McCaffrey, T.A.; Hu, C.A. Apolipoprotein L6, induced in atherosclerotic lesions, promotes apoptosis and blocks Beclin 1-dependent autophagy in atherosclerotic cells. J. Biol. Chem. 2011, 286, 27389–27398. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Gu, S.; Chu, W.; Sun, W.; Wei, W.; Dang, X.; Tian, Y.; Liu, K.; Chen, J. miR-17-5p Regulates Differential Expression of NCOA3 in Pig Intramuscular and Subcutaneous Adipose Tissue. Lipids. 2017, 52, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Gan, M.; Fan, Y.; Li, L.; Zhong, Z.; Li, X.; Bai, L.; Zhao, Y.; Niu, L.; Shang, Y.; et al. miR-10b-5p regulates 3T3-L1 cells differentiation by targeting Apol6. Gene. 2019, 687, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Salmerón, C. Adipogenesis in fish. J. Exp. Biol. 2018, 221 (Suppl. S1), jeb161588. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Lane, M.D. Activation and centromeric localization of CCAAT/enhancer-binding proteins during the mitotic clonal expansion of adipocyte differentiation. Genes Dev. 1999, 13, 2231–2241. [Google Scholar] [CrossRef]
- Zhang, F.; Pan, T.; Nielsen, L.D.; Mason, R.J. Lipogenesis in fetal rat lung: Importance of C/EBPalpha, SREBP-1c, and stearoyl-CoA desaturase. Am. J. Respir. Cell Mol. Biol. 2004, 30, 174–183. [Google Scholar] [CrossRef]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1995, 79, 1147–1156. [Google Scholar] [CrossRef]
| Gene/miRNA | Accession Number | Sequence (5′~3′) | Tm/°C | Purpose | Product Length |
|---|---|---|---|---|---|
| U6 | S: TGGAACGCTTCACGAATTTGCG | NR_138,085.1 | 60 | qPCR | 68 |
| A: GGAACGATACAGAGAAGATTAGC | |||||
| UXT | S: GCAAGTGGATTTGGGCTGTAAC | XM_005700842.2 | 60 | qPCR | 180 |
| A: ATGGAGTCCTTGGTGAGGTTGT | |||||
| miR-92a-3p | S: CTGGAGTATTGCACTTGTCCCG | 60 | qPCR | ||
| A: GTGCAGGGTCCGAGGT | |||||
| LPL | S: TCCTGGAGTGACGGAATCTGT | NM_001285607.1 | 60 | qPCR | 156 |
| A: GACAGCCAGTCCACCACGAT | |||||
| C/EBPα | S: CCGTGGACAAGAACAGCAAC | XM_018062278 | 58 | qPCR | 142 |
| A: AGGCGGTCATTGTCACTGGT | |||||
| PPARγ | S: AAGCGTCAGGGTTCCACTATG | NM_001285658 | 60 | qPCR | 197 |
| A: GAACCTGATGGCGTTATGAGAC | |||||
| SREBP1 | S: AAGTGGTGGGCCTCTCTGA | NM_001285755 | 58 | qPCR | 127 |
| A: GCAGGGGTTTCTCGGACT | |||||
| C/EBPβ | S: CAAGAAGACGGTGGACAAGC | XM_018058020.1 | 65 | qPCR | 204 |
| A: AACAAGTTCCGCAGGGTG | |||||
| AP2 | S: TGAAGTCACTCCAGATGACAGG | NM_001285623.1 | 58 | qPCR | 143 |
| A: TGACACATTCCAGCACCAGC |
| miRNA | Sequence | Classification |
|---|---|---|
| NC | UUCUCCGAACGUGUCACGUTT | mimics |
| CAGUACUUUUGUGUAGUACAA | inhibitor | |
| miR-92a-3p | UAUUGCACUUGUCCCGGCCUGU | mimics |
| ACAGGCCGGGACAAGUGCAAUA | inhibitor |
| Gene | Primer Sequence (5′~3′) | Purpose |
|---|---|---|
| APOL6 | S: CGGCTAGCATGGACAAAATGAGCATG | RT-PCR |
| A: TTGCGGCCGCCTAAAATAGGAGCTGG | ||
| si-NC | S: UUCUCCGAACGUGUCACGUTT | Interference |
| A: ACGUGACACGUUCGGAGAATT | ||
| siAPOL6-1 | S: CAUGCCCUUGCAGACCACAUUGACA | Interference |
| A: UGUCAAUGUGGUCUGCAAGGGCAUG | ||
| siAPOL6-2 | S: UGGCCGCUGGAAAGGUGAUUCAGAA | Interference |
| A: UUCUGAAUCACCUUUCCAGCGGCCA |
| Group Name | Treatment |
|---|---|
| NC WT | pmirGLO-APOL6 WT + mimics NC |
| NC MT | pmirGLO-APOL6 28MT + mimics NC |
| 92a WT | pmirGLO-APOL6 WT + miR-92a-3p mimics |
| 92a MT | pmirGLO-APOL6 92aMT + miR-92a-3p mimics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qubi, W.; Zheng, J.; Wang, Y.; Xu, G.; Li, Y.; Xiong, Y.; Wang, Y.; Liu, W.; Lin, Y. Goat miR-92a-3p Targets APOL6 Gene to Regulate the Differentiation of Intramuscular Precursor Adipocytes. Genes 2024, 15, 57. https://doi.org/10.3390/genes15010057
Qubi W, Zheng J, Wang Y, Xu G, Li Y, Xiong Y, Wang Y, Liu W, Lin Y. Goat miR-92a-3p Targets APOL6 Gene to Regulate the Differentiation of Intramuscular Precursor Adipocytes. Genes. 2024; 15(1):57. https://doi.org/10.3390/genes15010057
Chicago/Turabian StyleQubi, Wuqie, Jianying Zheng, Youli Wang, Guishan Xu, Yanyan Li, Yan Xiong, Yong Wang, Wei Liu, and Yaqiu Lin. 2024. "Goat miR-92a-3p Targets APOL6 Gene to Regulate the Differentiation of Intramuscular Precursor Adipocytes" Genes 15, no. 1: 57. https://doi.org/10.3390/genes15010057
APA StyleQubi, W., Zheng, J., Wang, Y., Xu, G., Li, Y., Xiong, Y., Wang, Y., Liu, W., & Lin, Y. (2024). Goat miR-92a-3p Targets APOL6 Gene to Regulate the Differentiation of Intramuscular Precursor Adipocytes. Genes, 15(1), 57. https://doi.org/10.3390/genes15010057





