Hepatic Transcriptomics Reveals Reduced Lipogenesis in High-Salt Diet Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Animals
2.2. Immunohistochemistry
2.3. Biochemical Tests
2.4. RNA Isolation
2.5. Library Construction and Sequencing
2.6. Data Processing
2.7. RT-qPCR Validation
2.8. Statistical Analysis
3. Results
3.1. Metabolic Phenotypes of HSD Mice
3.2. Analysis of Differentially Expressed Genes
3.3. GO and KEGG Pathway Analysis of DEGs
3.4. Transcriptome Data Validation by qPCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, I.J.; Tzoulaki, I.; Candeias, V.; Elliott, P. Salt intakes around the world: Implications for public health. Int. J. Epidemiol. 2009, 38, 791–813. [Google Scholar] [CrossRef] [PubMed]
- Hipgrave, D.B.; Chang, S.; Li, X.; Wu, Y. Salt and Sodium Intake in China. JAMA 2016, 315, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Powles, J.; Fahimi, S.; Micha, R.; Khatibzadeh, S.; Shi, P.; Ezzati, M.; Engell, R.E.; Lim, S.S.; Danaei, G.; Mozaffarian, D.; et al. Global, regional, and national sodium intakes in 1990 and 2010: A systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013, 3, e003733. [Google Scholar] [CrossRef] [PubMed]
- Aburto, N.J.; Ziolkovska, A.; Hooper, L.; Elliott, P.; Cappuccio, F.P.; Meerpohl, J.J. Effect of lower sodium intake on health: Systematic review and meta-analyses. BMJ 2013, 346, f1326. [Google Scholar] [CrossRef] [PubMed]
- Graudal, N.A.; Hubeck-Graudal, T.; Jurgens, G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst. Rev. 2020, 12, CD004022. [Google Scholar]
- Yoon, Y.S.; Oh, S.W. Sodium density and obesity; the Korea National Health and Nutrition Examination Survey 2007–2010. Eur. J. Clin. Nutr. 2013, 67, 141–146. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, F.; Liu, F.Q.; Chu, C.; Wang, Y.; Wang, D.; Guo, T.S.; Wang, J.K.; Guan, G.C.; Ren, K.Y.; et al. Elevation of Fasting Ghrelin in Healthy Human Subjects Consuming a High-Salt Diet: A Novel Mechanism of Obesity? Nutrients 2016, 8, 323. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Kuwabara, M.; Andres-Hernando, A.; Li, N.; Cicerchi, C.; Jensen, T.; Orlicky, D.J.; Roncal-Jimenez, C.A.; Ishimoto, T.; Nakagawa, T.; et al. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 3138–3143. [Google Scholar] [CrossRef]
- Zhou, L.; Stamler, J.; Chan, Q.; Van Horn, L.; Daviglus, M.L.; Dyer, A.R.; Miura, K.; Okuda, N.; Wu, Y.; Ueshima, H.; et al. Salt intake and prevalence of overweight/obesity in Japan, China, the United Kingdom, and the United States: The INTERMAP Study. Am. J. Clin. Nutr. 2019, 110, 34–40. [Google Scholar] [CrossRef]
- Ogihara, T.; Asano, T.; Ando, K.; Chiba, Y.; Sekine, N.; Sakoda, H.; Anai, M.; Onishi, Y.; Fujishiro, M.; Ono, H.; et al. Insulin resistance with enhanced insulin signaling in high-salt diet-fed rats. Diabetes 2001, 50, 573–583. [Google Scholar] [CrossRef]
- Horikawa, C.; Yoshimura, Y.; Kamada, C.; Tanaka, S.; Tanaka, S.; Hanyu, O.; Araki, A.; Ito, H.; Tanaka, A.; Ohashi, Y.; et al. Dietary sodium intake and incidence of diabetes complications in Japanese patients with type 2 diabetes: Analysis of the Japan Diabetes Complications Study (JDCS). J. Clin. Endocrinol. Metab. 2014, 99, 3635–3643. [Google Scholar] [CrossRef]
- Abdulai, T.; Runqi, T.; Mao, Z.; Oppong, T.B.; Amponsem-Boateng, C.; Wang, Y.; Liu, X.; Zhang, H.; Wang, C. Preference for High Dietary Salt Intake Is Associated with Undiagnosed Type 2 Diabetes: The Henan Rural Cohort. Front. Nutr. 2020, 7, 537049. [Google Scholar] [CrossRef]
- Zivkovic, A.M.; German, J.B.; Sanyal, A.J. Comparative review of diets for the metabolic syndrome: Implications for nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2007, 86, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Guo, X.; Chen, X.; Tang, J.; Yan, L.; Ren, J.; Zhang, J.; Lu, Z.; Dong, J.; Xu, J.; et al. Association between 24 h urinary sodium and potassium excretion and the metabolic syndrome in Chinese adults: The Shandong and Ministry of Health Action on Salt and Hypertension (SMASH) study. Br. J. Nutr. 2015, 113, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Kosaki, K.; Sugasawa, T.; Matsui, M.; Yoshioka, M.; Aoki, K.; Kuji, T.; Mizuno, R.; KuroO, M.; Yamagata, K.; et al. High Salt Diet Impacts the Risk of Sarcopenia Associated with Reduction of Skeletal Muscle Performance in the Japanese Population. Nutrients 2020, 12, 3474. [Google Scholar] [CrossRef] [PubMed]
- Kleinewietfeld, M.; Manzel, A.; Titze, J.; Kvakan, H.; Yosef, N.; Linker, R.A.; Muller, D.N.; Hafler, D.A. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013, 496, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yosef, N.; Thalhamer, T.; Zhu, C.; Xiao, S.; Kishi, Y.; Regev, A.; Kuchroo, V.K. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 2013, 496, 513–517. [Google Scholar] [CrossRef]
- Gao, B.; Jeong, W.I.; Tian, Z. Liver: An organ with predominant innate immunity. Hepatology 2008, 47, 729–736. [Google Scholar] [CrossRef]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef]
- Huh, J.H.; Lee, K.J.; Lim, J.S.; Lee, M.Y.; Park, H.J.; Kim, M.Y.; Kim, J.W.; Chung, C.H.; Shin, J.Y.; Kim, H.S.; et al. High Dietary Sodium Intake Assessed by Estimated 24-h Urinary Sodium Excretion Is Associated with NAFLD and Hepatic Fibrosis. PLoS ONE 2015, 10, e0143222. [Google Scholar] [CrossRef]
- Takahashi, F.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Kawate, Y.; Okamura, T.; Kitagawa, N.; Okada, H.; Nakanishi, N.; Majima, S.; et al. The Association of Salt Intake and Non-alcoholic Fatty Liver Disease in People with Type 2 Diabetes: A Cross-Sectional Study. Front. Nutr. 2022, 9, 943790. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, J.E.; Chang, Y.; Kim, M.K.; Sung, E.; Shin, H.; Ryu, S. Dietary sodium and potassium intake in relation to non-alcoholic fatty liver disease. Br. J. Nutr. 2016, 116, 1447–1456. [Google Scholar] [CrossRef]
- Gao, P.; You, M.; Li, L.; Zhang, Q.; Fang, X.; Wei, X.; Zhou, Q.; Zhang, H.; Wang, M.; Lu, Z.; et al. Salt-Induced Hepatic Inflammatory Memory Contributes to Cardiovascular Damage through Epigenetic Modulation of SIRT3. Circulation 2022, 145, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Wang, E.; Xu, J.; Lu, J.; Yan, G.; Fu, L.; Jiao, Y.; Wu, L.; Liu, T.; Li, Y. Transcriptome analysis of multiple metabolic tissues in high-salt diet-fed mice. Front. Endocrinol. 2022, 13, 887843. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, H.; Yu, J.; Zhao, Z.; Xiao, F.; Xia, T.; Wang, C.; Li, K.; Deng, J.; Guo, Y.; et al. Activation of ERK1/2 Ameliorates Liver Steatosis in Leptin Receptor-Deficient (db/db) Mice via Stimulating ATG7-Dependent Autophagy. Diabetes 2016, 65, 393–405. [Google Scholar] [CrossRef]
- Jena, P.K.; Sheng, L.; Nagar, N.; Wu, C.; Barile, D.; Mills, D.A.; Wan, Y.Y. Synbiotics Bifidobacterium infantis and milk oligosaccharides are effective in reversing cancer-prone nonalcoholic steatohepatitis using western diet-fed FXR knockout mouse models. J. Nutr. Biochem. 2018, 57, 246–254. [Google Scholar] [CrossRef]
- Young, J.M.; Shykind, B.M.; Lane, R.P.; Tonnes-Priddy, L.; Ross, J.A.; Walker, M.; Williams, E.M.; Trask, B.J. Odorant receptor expressed sequence tags demonstrate olfactory expression of over 400 genes, extensive alternate splicing and unequal expression levels. Genome Biol. 2003, 4, R71. [Google Scholar] [CrossRef]
- Gaudet, P.; Livstone, M.S.; Lewis, S.E.; Thomas, P.D. Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief. Bioinform. 2011, 12, 449–462. [Google Scholar] [CrossRef]
- Murray, P.; Edgar, D. Regulation of programmed cell death by basement membranes in embryonic development. J. Cell Biol. 2000, 150, 1215–1221. [Google Scholar] [CrossRef]
- Gadadhar, S.; Alvarez Viar, G.; Hansen, J.N.; Gong, A.; Kostarev, A.; Ialy-Radio, C.; Leboucher, S.; Whitfield, M.; Ziyyat, A.; Touré, A.; et al. Tubulin glycylation controls axonemal dynein activity, flagellar beat, and male fertility. Science 2021, 371, eabd4914. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Paragas, N.; Ned, R.M.; Qiu, A.; Viltard, M.; Leete, T.; Drexler, I.R.; Chen, X.; Sanna Cherchi, S.; Mohammed, F.; et al. Scara5 is a ferritin receptor mediating non-transferrin iron delivery. Dev. Cell 2009, 16, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Hagenbuch, B.; Adler, I.D.; Schmid, T.E. Molecular cloning and functional characterization of the mouse organic-anion-transporting polypeptide 1 (Oatp1) and mapping of the gene to chromosome X. Biochem. J. 2000, 345 Pt 1, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Tamási, V.; Fülöp, A.K.; Hegyi, K.; Monostory, K.; Falus, A. Upregulation of CYP2e1 and CYP3a activities in histamine-deficient histidine decarboxylase gene targeted mice. Cell Biol. Int. 2003, 27, 1011–1015. [Google Scholar] [CrossRef]
- Archambaud, C.; Nahori, M.A.; Soubigou, G.; Bécavin, C.; Laval, L.; Lechat, P.; Smokvina, T.; Langella, P.; Lecuit, M.; Cossart, P. Impact of lactobacilli on orally acquired listeriosis. Proc. Natl. Acad. Sci. USA 2012, 109, 16684–16689. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Hoersch, S.; Liu, H.; Carr, S.A.; Hynes, R.O. The matrisome: In silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell Proteom. 2012, 11, M111.014647. [Google Scholar] [CrossRef]
- Gao, F.J.; Shi, L.; Hines, T.; Hebbar, S.; Neufeld, K.L.; Smith, D.S. Insulin signaling regulates functional interaction between adenomatous polyposis coli and cytoplasmic dynein. Mol. Biol. Cell 2017, 28, 587–599. [Google Scholar] [CrossRef]
- Barnes, B.R.; Glund, S.; Long, Y.C.; Hjälm, G.; Andersson, L.; Zierath, J.R. 5′-AMP activated protein kinase regulates skeletal muscle glycogen content and ergogenics. FASEB J. 2005, 19, 773–779. [Google Scholar] [CrossRef]
- Lazaro, J.B.; Bailey, P.J.; Lassar, A.B. Cyclin D-cdk4 activity modulates the subnuclear localization and interaction of MEF2 with SRC-family coactivators during skeletal muscle differentiation. Genes Dev. 2002, 16, 1792–1805. [Google Scholar] [CrossRef]
- Vairapandi, M.; Balliet, A.G.; Hoffman, B.; Liebermann, D.A. GADD45b and GADD45g are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotoxic stress. J. Cell Physiol. 2002, 192, 327–338. [Google Scholar] [CrossRef]
- Ozurumba, E.; Mathew, O.; Ranganna, K.; Choi, M.; Oyekan, A. Regulation of hypoxia inducible factor/prolyl hydroxylase binding domain proteins 1 by PPARα and high salt diet. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 165–173. [Google Scholar] [CrossRef]
- Ma, Y.; He, F.J.; MacGregor, G.A. High salt intake: Independent risk factor for obesity? Hypertension 2015, 66, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.E.; Kim, S.M.; Choi, M.K.; Heo, Y.R.; Hyun, T.S.; Lyu, E.S.; Oh, S.Y.; Park, H.R.; Ro, H.K.; Han, K.; et al. Association between 24-h urinary sodium excretion and obesity in Korean adults: A multicenter study. Nutrition 2017, 41, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Alaniz, M.H.; Brito, L.C.; Borges-Silva, C.N.; Takada, J.; Andreotti, S.; Lima, F.B. High dietary sodium intake increases white adipose tissue mass and plasma leptin in rats. Obesity 2007, 15, 2200–2208. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Alaniz, M.H.; Takada, J.; Andreotti, S.; de Campos, T.B.; Campaña, A.B.; Borges-Silva, C.N.; Lima, F.B. High sodium intake enhances insulin-stimulated glucose uptake in rat epididymal adipose tissue. Obesity 2008, 16, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Sorn, S.R.; Lee, Y.; Kang, I. Salt Induces Adipogenesis/Lipogenesis and Inflammatory Adipocytokines Secretion in Adipocytes. Int. J. Mol. Sci. 2019, 20, 160. [Google Scholar] [CrossRef]
- Van den Berg, E.H.; Gruppen, E.G.; Blokzijl, H.; Bakker, S.; Dullaart, R. Higher Sodium Intake Assessed by 24 Hour Urinary Sodium Excretion Is Associated with Non-Alcoholic Fatty Liver Disease: The PREVEND Cohort Study. J. Clin. Med. 2019, 8, 2157. [Google Scholar] [CrossRef]
- Shen, X.; Jin, C.; Wu, Y.; Zhang, Y.; Wang, X.; Huang, W.; Li, J.; Wu, S.; Gao, X. Prospective study of perceived dietary salt intake and the risk of non-alcoholic fatty liver disease. J. Hum. Nutr. Diet. 2019, 32, 802–809. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, Y.; Feng, Y.; Zhao, X.; Fan, Y.; Rong, J.; Zhao, L.; Yu, Y. Association between dietary sodium intake and non-alcoholic fatty liver disease in the US population. Public Health Nutr. 2021, 24, 993–1000. [Google Scholar] [CrossRef]
- Luo, X.; Li, Y.; Zhou, Y.; Zhang, C.; Li, L.; Luo, Y.; Wang, J.; Duan, Y.; Xie, J. Association of Non-alcoholic Fatty Liver Disease with Salt Intake and Dietary Diversity in Chinese Medical Examination Adults Aged 18–59 Years: A Cross-Sectional Study. Front. Nutr. 2022, 9, 930316. [Google Scholar] [CrossRef] [PubMed]
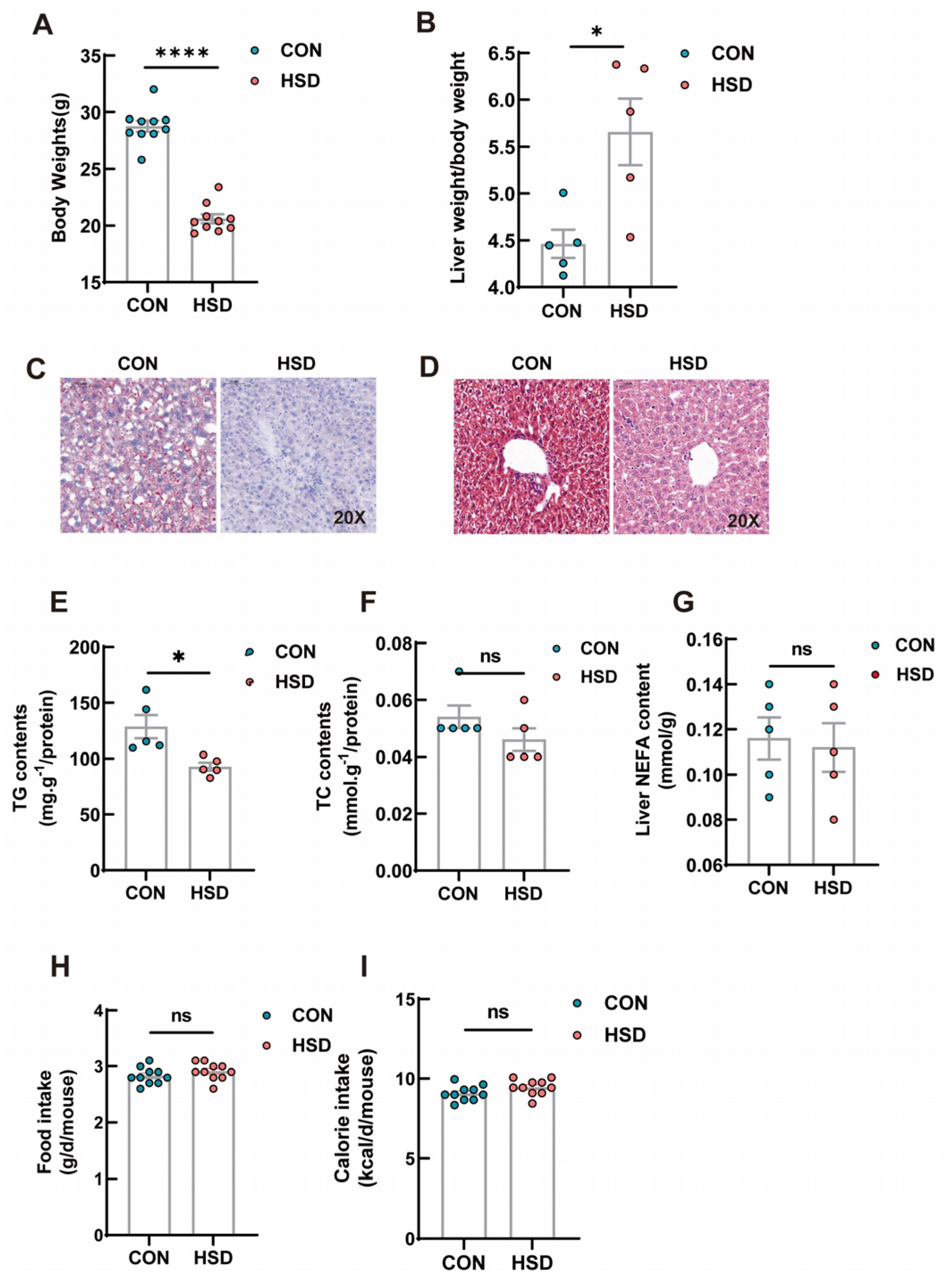
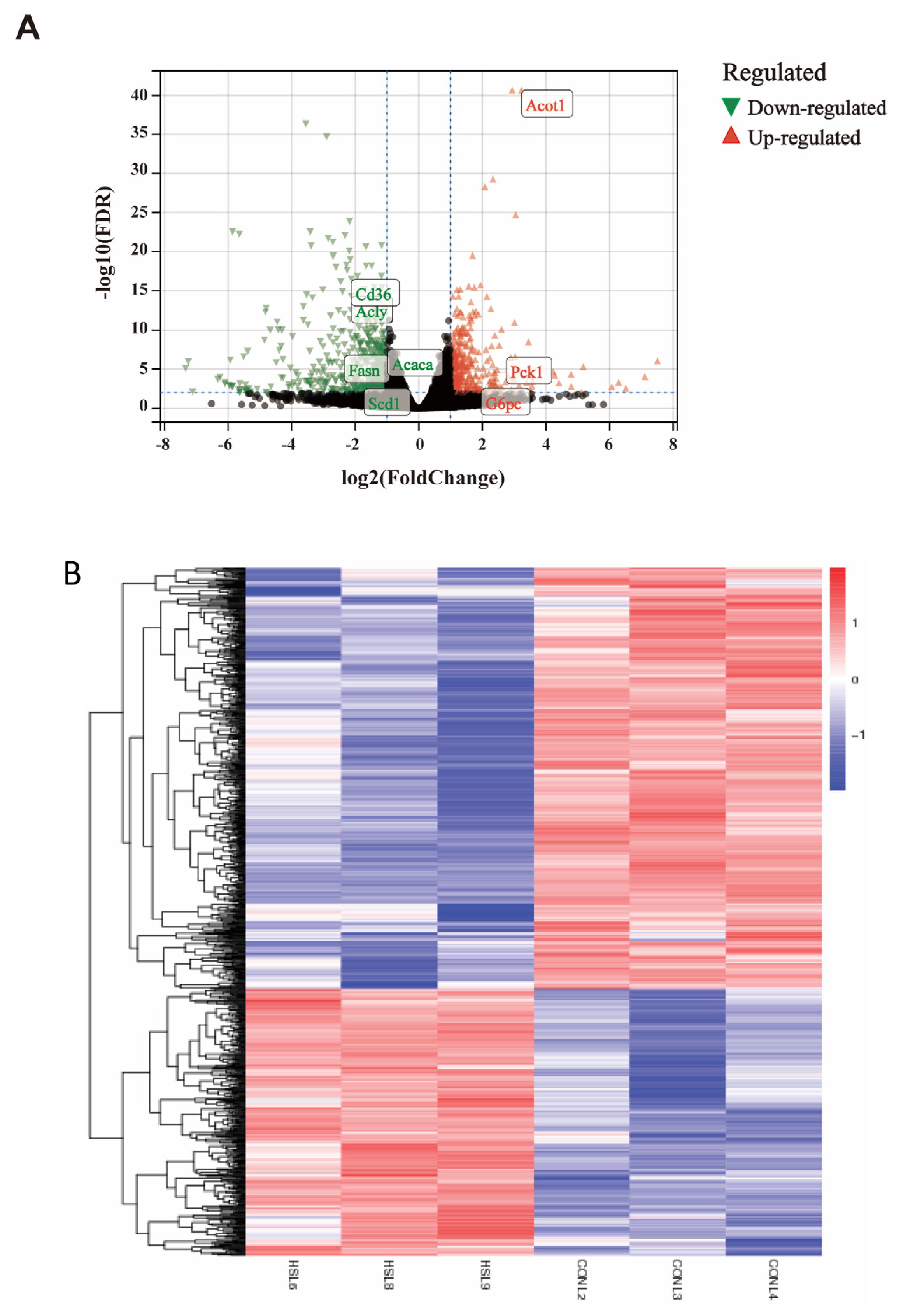
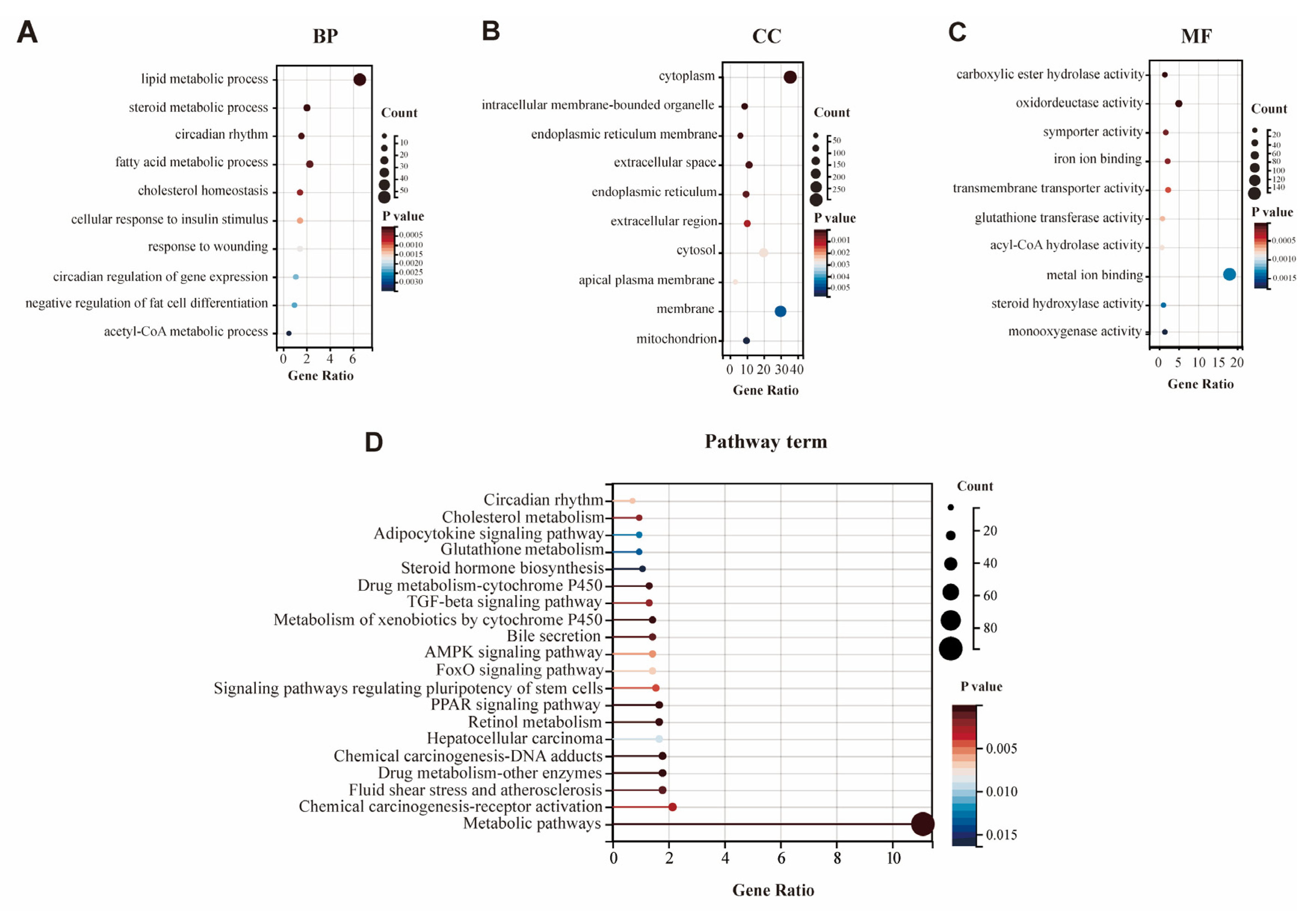

| Gene Symbol | Gene Description | Log2fold-Change | Padj | Known Function |
|---|---|---|---|---|
| Down-Regulated | ||||
| Gm28548 | Predicted gene 28548 | −7.3357 | 7.94 × 10−6 | LncRNA gene; unknown function |
| Gm40457 | Predicted gene 40457 | −7.25067 | 1.10 × 10−6 | LncRNA gene; unknown function |
| Sult2a3 | Sulfotransferase family 2A | −7.11578 | 0.008238 | Hepatic sulfonation of bile acid [27] |
| Gm32468 | Predicted gene 32468 | −6.67475 | 5.61 × 10−44 | LncRNA gene; unknown function |
| Olfr702 | Olfactory receptor family 13 subfamily N member 4 | −6.3241 | 0.000196 | Upstream of or within G protein-coupled receptor signaling pathway and sensory perception of smell [28] |
| Igkv3-2 | Immunoglobulin kappa variable 3-2 | −6.2531 | 0.000105 | Immune response [29] |
| Gm43626 | Predicted gene 43626 | −6.09587 | 0.000763 | Pseudogene; unknown function |
| AC166052.1 | Hypothetical protein I79_021887 | −6.09463 | 0.000458 | Unknown function |
| Olfr703 | Olfactory receptor family 2 subfamily AG member 19 | −5.96724 | 0.001053 | G protein-coupled receptor signaling pathway [28] |
| D830013O20Rik | RIKEN cDNA D830013O20 gene | −5.94681 | 0.001425 | LncRNA gene; unknown function |
| Pla2g4f | Phospholipase A2, group IVF | −5.93077 | 6.99 × 10−7 | Protein coding gene; glycerophospholipid catabolic process [29] |
| 5330426L24Rik | RIKEN cDNA 5330426L24 gene | −5.89239 | 0.001175 | LncRNA gene; unknown function |
| Igkv15-103 | Immunoglobulin kappa chain variable 15-103 | −5.86438 | 0.001724 | Immune response [29] |
| Cdh19 | Cadherin 19, type 2 | −5.86188 | 2.76 × 10−23 | Adherens junction organization [29] |
| Psg18 | Pregnancy-specific beta-1-glycoprotein 18 | −5.85541 | 0.001422 | Regulation of immune system process [29]; regulation of interleukin-10 production [30] |
| Mup-ps10 | Major urinary protein, pseudogene 10 | −5.65876 | 0.004965 | Pseudogene; unknown function |
| Cap2 | CAP, adenylate cyclase-associated protein, 2 (yeast) | −5.64668 | 5.44 × 10−23 | cAMP-mediated signaling [29] |
| Selenok-ps7 | Selenoprotein K, pseudogene 7 | −5.63814 | 0.009325 | Pseudogene; unknown function |
| 6430710C18Rik | RIKEN cDNA 6430710C18 gene | −5.63211 | 0.00067 | LncRNA gene; unknown function |
| Zfp385c | Zinc finger protein 385C | −5.62978 | 0.003875 | Enables nucleic acid binding activity and zinc ion binding activity and is predicted to be active in the nucleus [29] |
| Gene Symbol | Gene Description | Log2fold-Change | Padj | Known Function |
|---|---|---|---|---|
| Up-Regulated | ||||
| Ttll8 | Tubulin tyrosine ligase-like family, member 8 | 7.505617986 | 8.68 × 10−7 | Flagellated sperm motility and protein polyglycylation [31] |
| Serpina4-ps1 | Serine (or cysteine) peptidase inhibitor, clade A, member 4, pseudogene 1 | 7.110133615 | 8.81 × 10−5 | Pseudogene; unknown function |
| Gm45301 | Predicted gene 45301 | 6.512918923 | 0.0027869 | LncRNA gene; unknown function |
| Ubap1l | Ubiquitin-associated protein 1-like | 6.285266885 | 0.0004596 | Ubiquitin-dependent protein catabolic process via the multivesicular body sorting pathway [29] |
| Scara5 | Scavenger receptor class A, member 5 | 6.070547377 | 0.0017622 | Enables ferritin receptor activity [32] |
| Plin4 | Perilipin 4 | 5.174215259 | 4.81 × 10−6 | Located in plasma membranes; unknown function |
| Gm8251 | Predicted gene 8251 | 5.118764438 | 0.0088949 | Protein coding gene; unknown function |
| Gm43305 | Predicted gene 43305 | 4.796490714 | 0.0015396 | LncRNA gene; unknown function |
| Slco1a1 | Solute carrier organic anion transporter family, member 1a1 | 4.791203008 | 4.28 × 10−5 | Enables organic anion transmembrane transporter activity; response to stilbenoid [33,34] |
| Cyp3a11 | Cytochrome P450, family 3, subfamily a, polypeptide 11 | 4.71643503 | 4.06 × 10−76 | Enables monooxygenase activity; upstream of or within the response to bacterium [35,36] |
| 1700045H11Rik | RIKEN cDNA 1700045H11 gene | 4.407589228 | 0.0005687 | LncRNA gene; unknown function |
| Tmc7 | Transmembrane channel-like gene family 7 | 4.275886302 | 5.53 × 10−5 | Enable mechanosensitive ion channel activity [29] |
| Sftpa1 | Surfactant-associated protein A1 | 4.228839366 | 2.17 × 10−5 | Positive regulation of phagocytosis [29,37] |
| Dnaic1 | Dynein axonemal intermediate chain 1 | 3.580195937 | 0.0003139 | Enables both dynein heavy and light chain binding activity; insulin receptor signaling pathway [38] |
| Prkag3 | Protein kinase, AMP-activated, gamma 3 non-catalytic subunit | 3.553918438 | 0.0013324 | Contributes to AMP-activated protein kinase activity; upstream of or within glycogen biosynthetic process; glycolytic process [29,39] |
| Cdkn1a | Cyclin-dependent kinase inhibitor 1A (P21) | 3.525502285 | 0.0095903 | Enables cyclin binding activity [29,40,41] |
| Omd | Osteomodulin | 3.523000255 | 0.0063899 | Regulation of bone mineralization; upstream of or within cell adhesion [29] |
| CT010575.2 | Mus musculus chromosome 13 clone RP23-217J21 | 3.501322391 | 3.69 × 10−5 | Long intervening noncoding RNAs (lincRNAs) |
| Fam222a | Family with sequence similarity 222, member A | 3.450260655 | 3.20 × 10−9 | Protein coding gene; unknown function |
| Serpina9 | Serine (or cysteine) peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 9 | 3.392001189 | 0.0004838 | Enable serine-type endopeptidase inhibitor activity [29] |
| Genes | Annotation | Function | HSD vs. CON Log2fold-Change * |
|---|---|---|---|
| Pck1 | Phosphoenolpyruvate carboxykinase 1 | Gluconeogenesis | 2.37 |
| G6Pase | Glucose-6-phosphatase | Gluconeogenesis | 1.97 |
| Acss2 | Acyl-CoA synthetase short-chain family member 2 | Fatty acid synthesis | −2.70 |
| Fasn | Fatty acid synthase | Fatty acid synthesis | −1.17 |
| Acaca | Acetyl-CoA carboxylase alpha | Fatty acid synthesis | −1.02 |
| Me1 | Malic Enzyme 1 | Fatty acid synthesis | −1.84 |
| Scd1 | Stearoyl-Coenzyme A desaturase 1 | Fatty acid synthesis | −1.72 |
| Elovl6 | ELOVL fatty acid elongase 6 | Fatty acid synthesis | −1.64 |
| Acly | ATP citrate lyase | Fatty acid synthesis | −2.12 |
| Acc | Acetyl-CoA carboxylase | Fatty acid synthesis | −1.26 |
| Cd36 | CD36 molecule | Fatty acid transporter | −2.12 |
| Fabp5 | Fatty acid binding protein 5 | Fatty acid transporter | −2.27 |
| Acsl3 | Acyl-CoA synthetase long-chain family member 3 | Fatty acid transporter | −0.92 |
| Cyp4a10 | Cytochrome P450, family 4, subfamily a, polypeptide 10 | Fatty acid oxidation | 1.65 |
| Cyp4a14 | Cytochrome P450, family 4, subfamily a, polypeptide 14 | Fatty acid oxidation | 1.44 |
| Cyp4a31 | Cytochrome P450, family 4, subfamily a, polypeptide 31 | Fatty acid oxidation | 2.53 |
| Sc5d | Sterol-C5-desaturase | Cholesterol synthesis | −1.66 |
| Gpam | Glycerol-3-phosphate acyltransferase | Triglyceride synthesis | −1.36 |
| Cyp7a1 | Cholesterol 7α hydroxylase | Bile acids synthesis | 1.30 |
| Cyp17a1 | Cytochrome P450 family 17 subfamily A member 1 | Steroidogenesis | −1.05 |
| Canonical Pathways | Map Number | −log(p-Value) | Gene |
|---|---|---|---|
| Metabolic pathways | map01100 | 1.06 × 10−6 | Colgalt2, Alas1, Rdh9, Cyp3a11, Hnmt, Lipg, Pde4b, Me1, Scd1, Kmt5a, Nat8f7, Glul, Pla2g4f, G6pc, Cyp4a10, Cad, Cyp3a44, Acsl5, Sirt5, Cyp4a14, Ctps, Gcat, Inmt, Acly, Dct, Cyp2a4, Ehhadh, Cyp3a41b, Pgp, Adssl1, Acot2, Acot1, Adprm, Aldh1a7, Mgll, Dgkh, Ndufb9, Pfkfb3, Nnmt, Galt, Acss2, Pde1a, Mgst3, Gstp1, Hdc, Haao, Atp5k, Gstt2, Adcy1, Acnat2, Ak6, Cyp7a1, Acacb, Papss2, Acaca, Tymp, Cyp17a1, Neu2, Rdh11, Ugt1a5, Rdh16, Atp6v0a2, Sc5d, Hyi, St3gal6, 9130409i23rik, Ugt1a9, Pck1, Hao2, Gstm4, Car3, Nqo1, Gstm2, Car1, Cyp4a31, Sqor, Ugt2b37, Setd1a, Dhcr24, Nmnat3, Mthfs, Fmo5, Qdpr, Rpia, Gale, Gpam, Gsta4, Gnpda2, P4ha2, Gsta2, Fasn, Echdc1, Cryl1 |
| Chemical carcinogenesis-DNA adducts | map05204 | 1.30 × 10−6 | Gstm4, Gstm2, Mgst3, Gstp1, Ugt2b37, Cyp3a44, Cyp3a11, Gstt2, Gsta4, Gsta2, Cyp3a41b, Ugt1a5, Sult2a7, Ugt1a9, Sult2a3 |
| Drug metabolism-other enzymes | map00983 | 4.00 × 10−6 | Gstm4, Gstm2, Mgst3, Gstp1, Ugt2b37, Gstt2, Tymp, Ces2c, Gsta4, Ces2e, Ces1e, Gsta2, Ces2h, Ugt1a5, Ugt1a9 |
| PPAR signaling pathway | map03320 | 1.40 × 10−5 | Cyp4a31, Cyp4a10, Acsl5, Cyp4a14, Cyp7a1, Fabp5, Ehhadh, Plin4, Me1, Plin2, Cd36, Scd1, Pck1, Plin5 |
| Retinol metabolism | map00830 | 3.60 × 10−5 | Rdh9, Cyp4a31, Cyp4a10, Ugt2b37, Cyp3a44, Cyp3a11, Cyp4a14, Cyp2a4, Rdh11, Cyp3a41b, Rdh16, Ugt1a5, Ugt1a9, Aldh1a7 |
| Metabolism of xenobiotics by cytochrome P450 | map00980 | 4.80 × 10−5 | Gstm4, Gstm2, Gsta4, Gstp1, Gsta2, Mgst3, Ugt2b37, Ugt1a5, Sult2a7, Gstt2, Ugt1a9, Sult2a3 |
| Drug metabolism-cytochrome P450 | map00982 | 1.89 × 10−4 | Gstm4, Gstm2, Gsta4, Gstp1, Gsta2, Mgst3, Ugt2b37, Ugt1a5, Gstt2, Ugt1a9, Fmo5 |
| Fluid shear stress and atherosclerosis | map05418 | 7.26 × 10−4 | Gstm4, Nqo1, Gstm2, Hsp90aa1, Il1r1, Dusp1, Itgb3, Mgst3, Gstp1, Gstt2, Acvr2b, Thbd, Gsta4, Gsta2, Rac3 |
| Bile secretion | map04976 | 8.14 × 10−4 | Abcg8, Slco1a1, Aqp8, Ugt2b37, Ugt1a5, Sult2a7, Adcy1, Acnat2, Ugt1a9, Abcb1a, Cyp7a1, Sult2a3 |
| Cholesterol metabolism | map04979 | 0.001805528 | Mylip, Abcg8, Sort1, Angptl8, Lipg, Apoa4, Cd36, Cyp7a1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Mao, F.; Lu, Y.; Liu, T.; Li, X.; Li, Y. Hepatic Transcriptomics Reveals Reduced Lipogenesis in High-Salt Diet Mice. Genes 2023, 14, 966. https://doi.org/10.3390/genes14050966
Xu J, Mao F, Lu Y, Liu T, Li X, Li Y. Hepatic Transcriptomics Reveals Reduced Lipogenesis in High-Salt Diet Mice. Genes. 2023; 14(5):966. https://doi.org/10.3390/genes14050966
Chicago/Turabian StyleXu, Jing, Fei Mao, Yan Lu, Tiemin Liu, Xiaoying Li, and Yao Li. 2023. "Hepatic Transcriptomics Reveals Reduced Lipogenesis in High-Salt Diet Mice" Genes 14, no. 5: 966. https://doi.org/10.3390/genes14050966
APA StyleXu, J., Mao, F., Lu, Y., Liu, T., Li, X., & Li, Y. (2023). Hepatic Transcriptomics Reveals Reduced Lipogenesis in High-Salt Diet Mice. Genes, 14(5), 966. https://doi.org/10.3390/genes14050966







