Insights into the Deep Phylogeny and Novel Divergence Time Estimation of Patellogastropoda from Complete Mitogenomes
Abstract
:1. Introduction
2. Materials and Method
2.1. Sample Collection, Identification, and DNA Extraction
2.2. Mitogenomes Sequencing, Assembly, and Annotation
2.3. Sequence Analyses of Mitogenomes
2.4. Phylogenetic Inference
2.5. Divergence Time Estimation
3. Results and Discussion
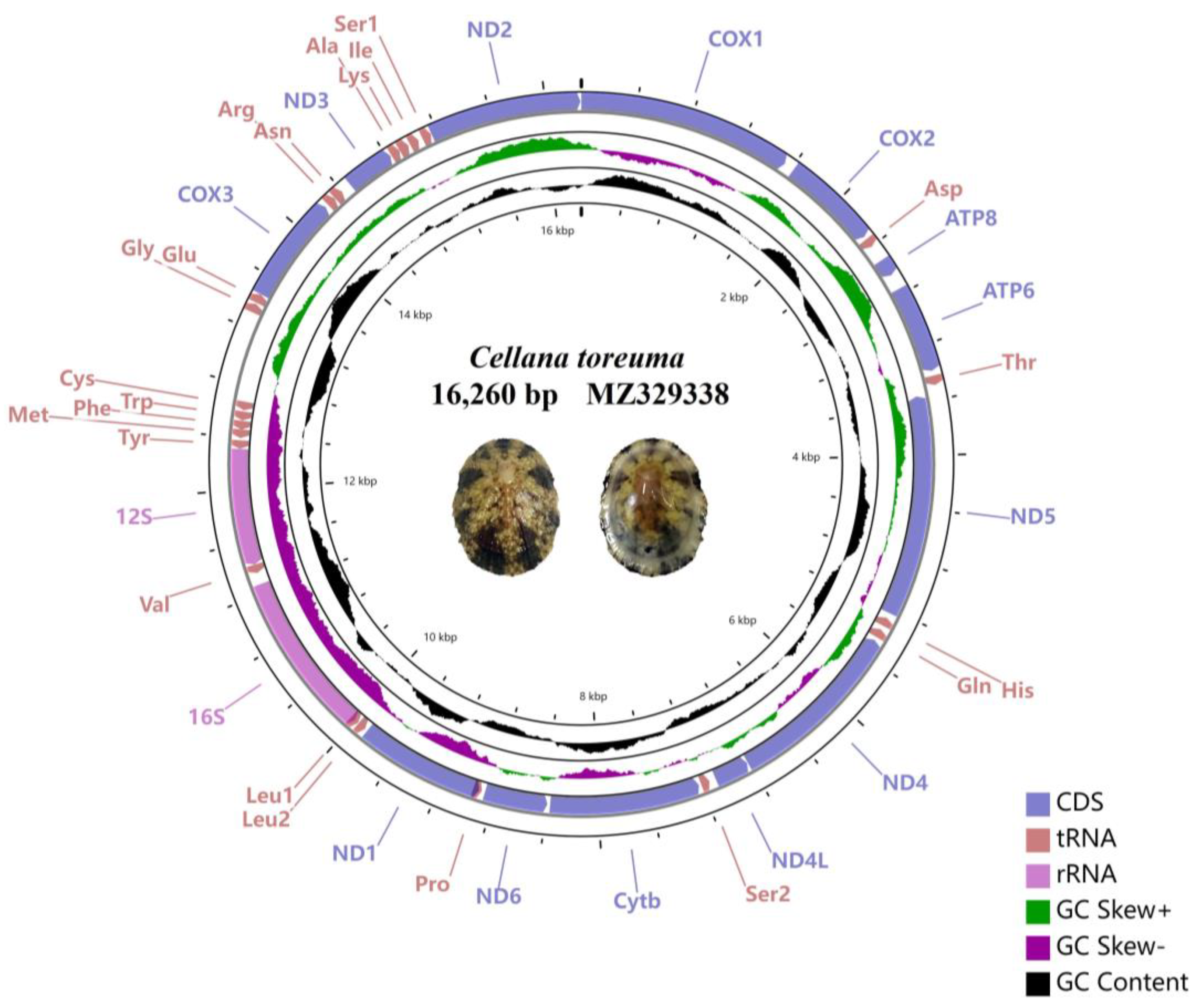
3.1. General Features of Entire Mitogenome
3.2. tRNA, rRNA, PCGs Genes, and Control Region
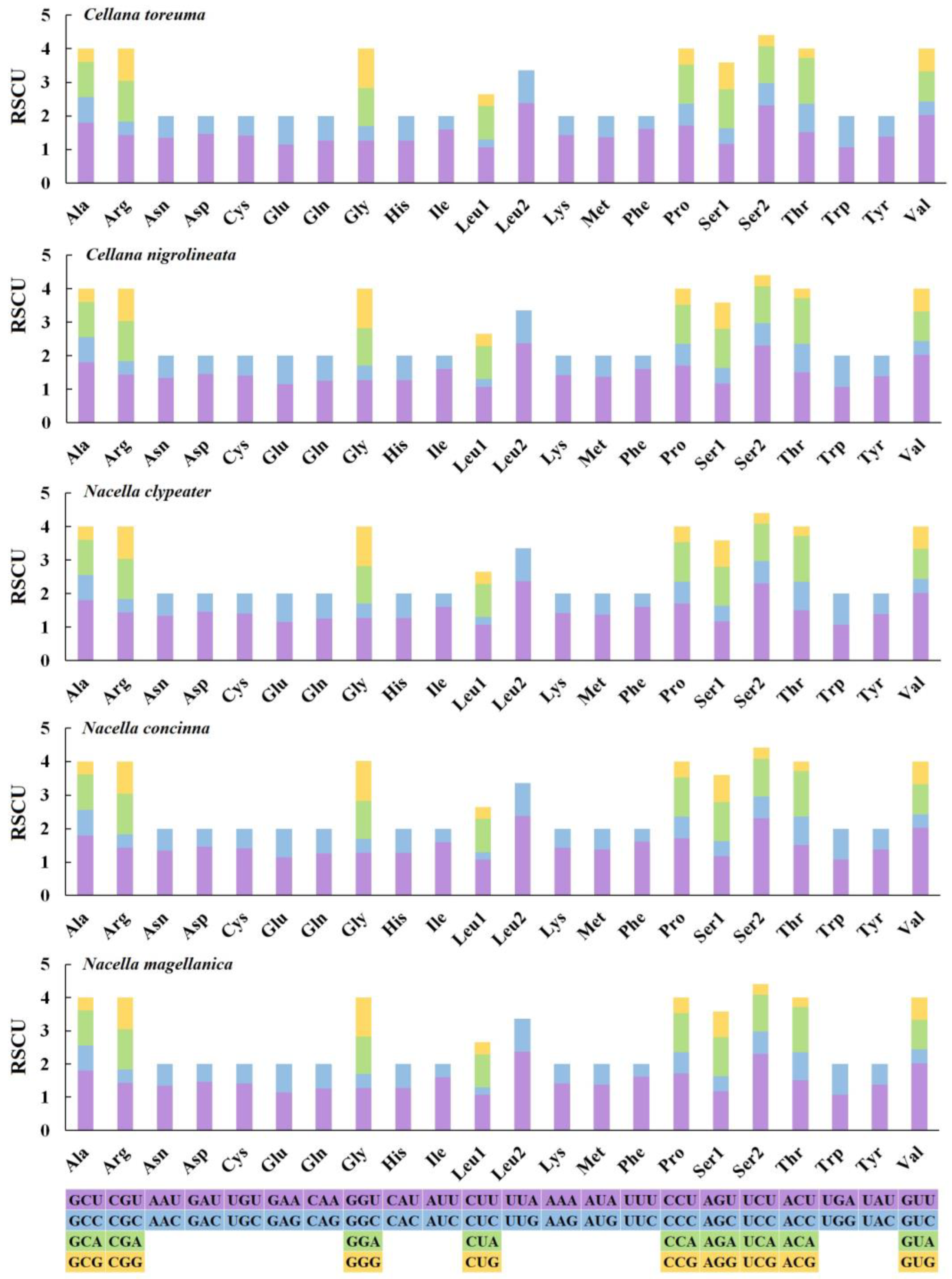
3.3. Mitochondrial Gene Codon Usage
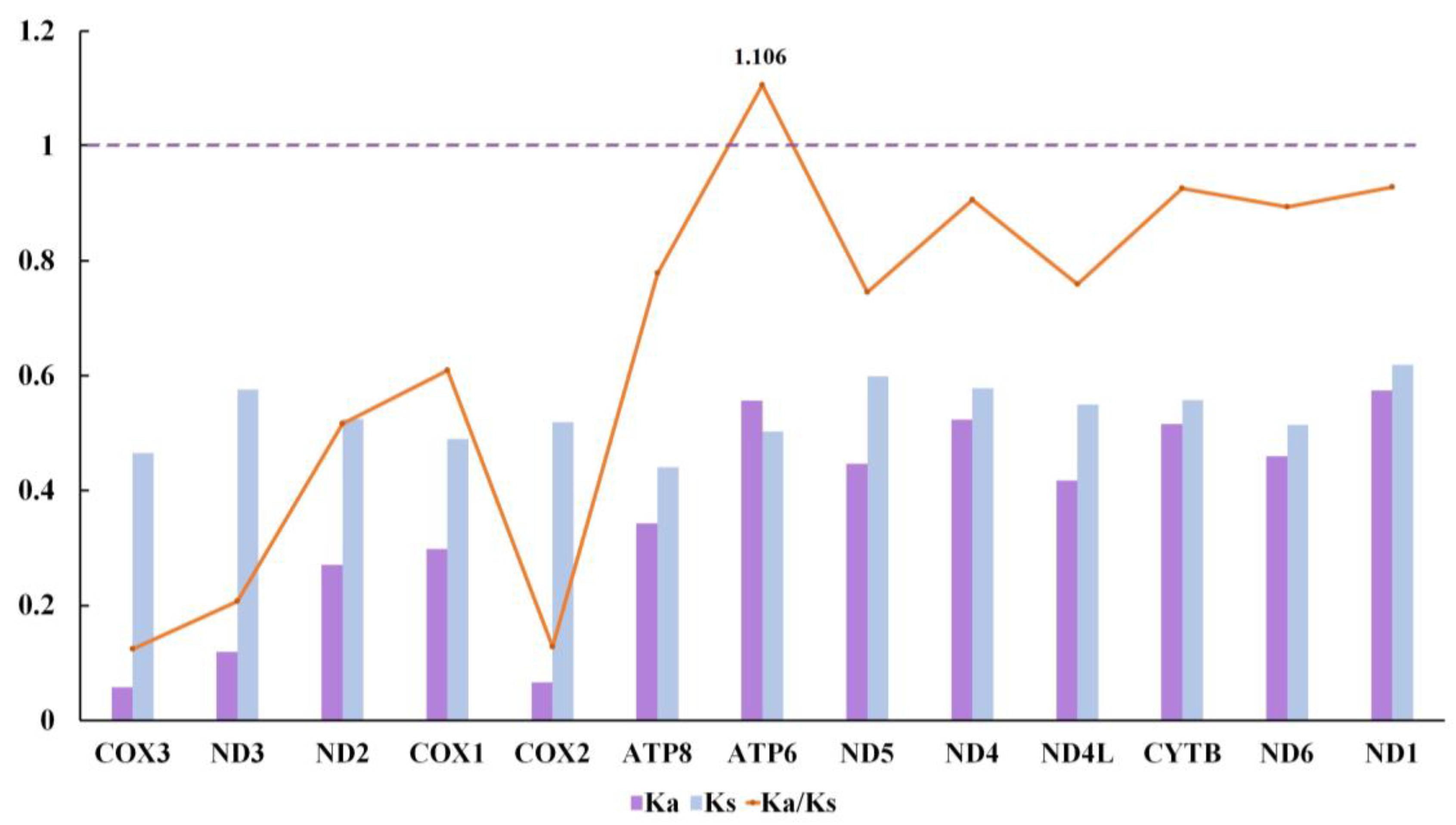
3.4. Selective Pressure Analysis
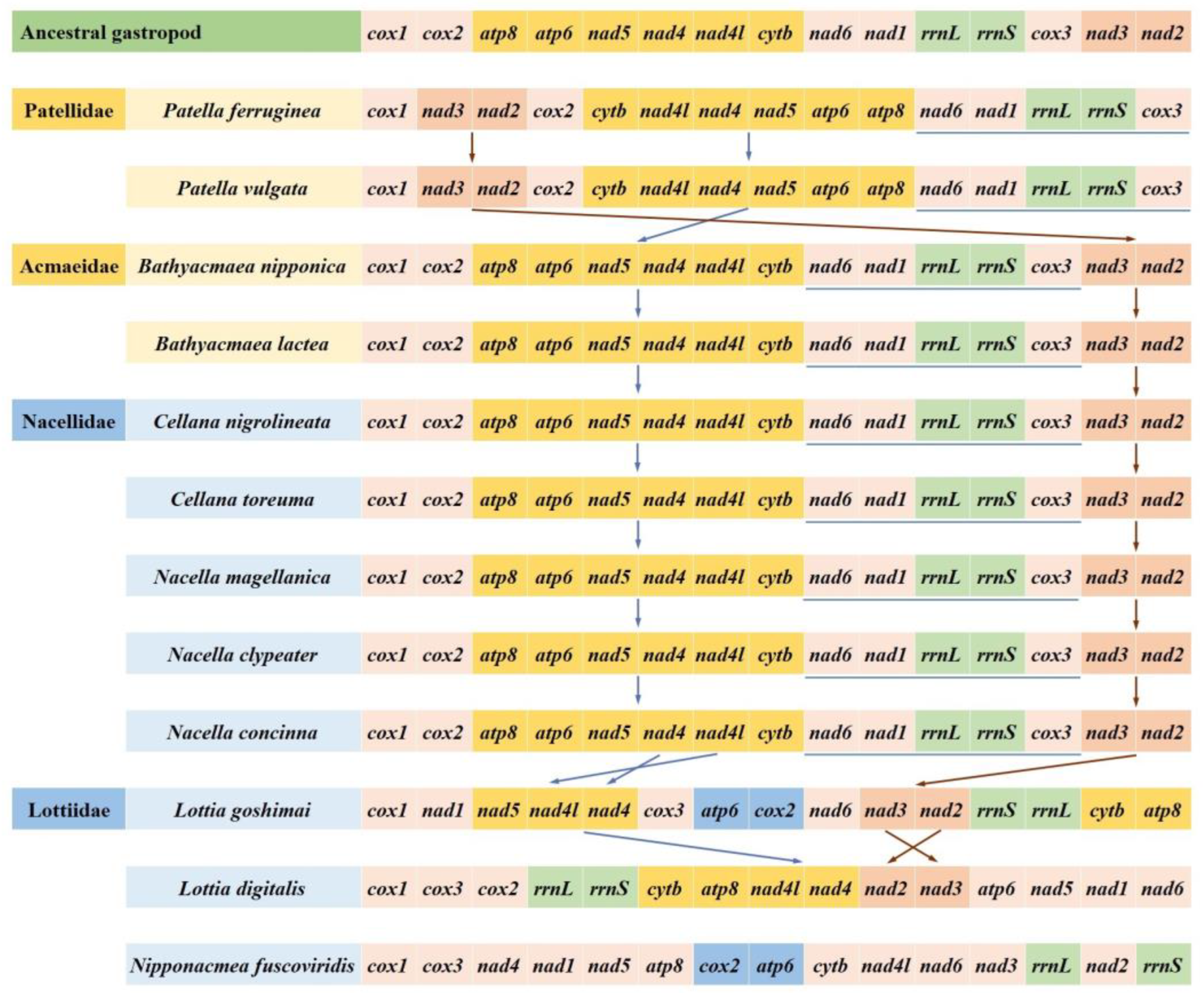
3.5. Gene Arrangement
3.6. Phylogenetic Relationship
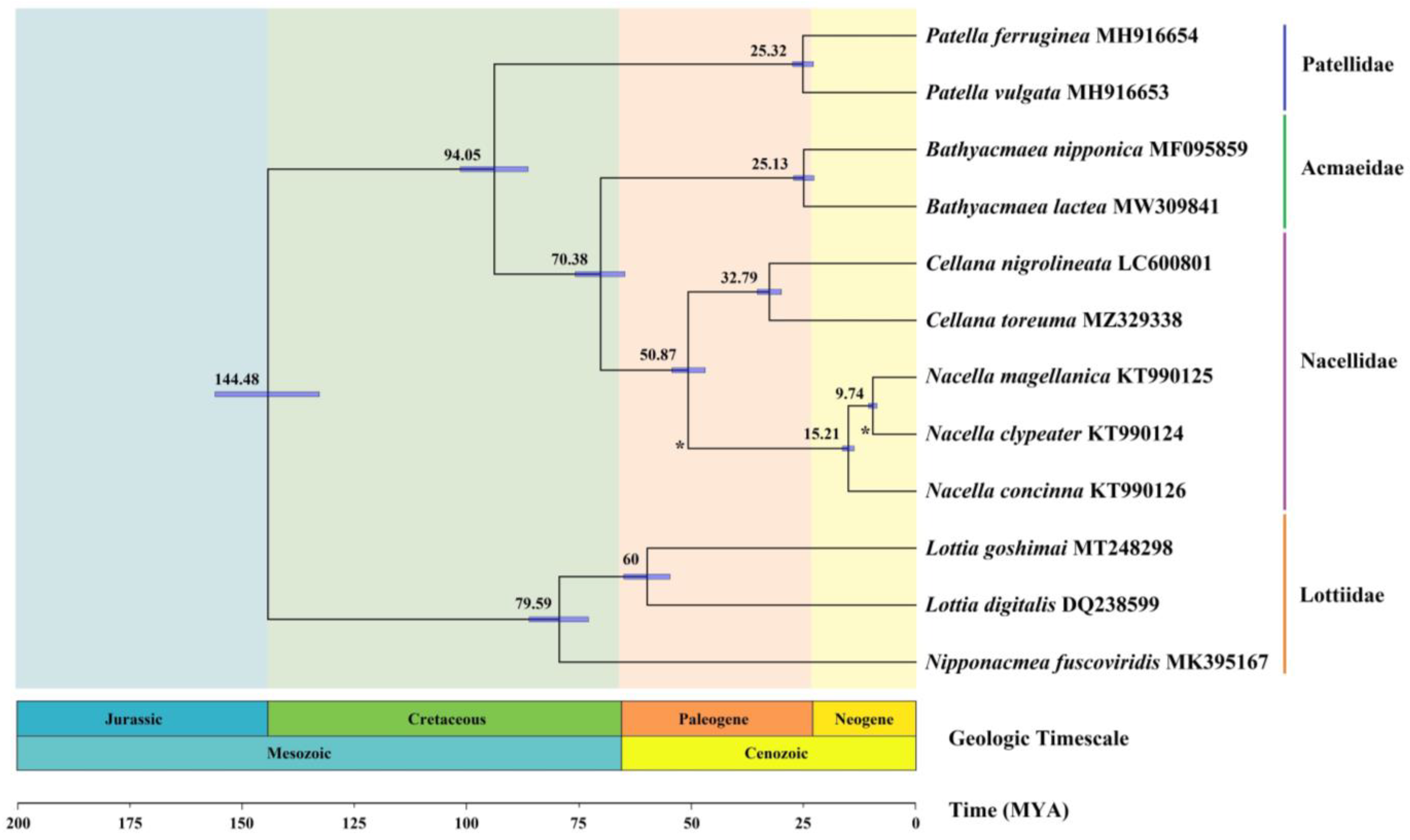
3.7. Divergence Times
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roger, A.J.; Muñoz-Gómez, S.A.; Kamikawa, R. The origin and diversification of mitochondria. Curr. Biol. 2017, 27, 1177–1192. [Google Scholar] [CrossRef] [Green Version]
- Hampl, V.; Čepička, I.; Eliáš, M. Was the mitochondrion necessary to start eukaryogenesis? Trends Microbiol. 2019, 27, 96–104. [Google Scholar] [CrossRef]
- Gray, M.W. Rickettsia, typhus and the mitochondrial connection. Nature 1998, 396, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, Z.; Yin, J.; Zhang, T.; Zhang, X.; Yuan, D.; Li, T.; Zhong, Y.; Ma, E.; Ren, Z. Complete mitochondrial genome of two Thitarodes species (Lepidoptera, Hepialidae), the host moths of Ophiocordyceps sinensis and phylogenetic implications. Int. J. Biol. Macromol. 2019, 140, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Mottis, A.; Herzig, S.; Auwerx, J. Mitocellular communication: Shaping health and disease. Science 2019, 366, 827–832. [Google Scholar] [CrossRef]
- Hämäläinen, R.H.; Landoni, J.C.; Ahlqvist, K.J.; Goffart, S.; Ryytty, S.; Rahman, M.O.; Brilhante, V.; Icay, K.; Hautaniemi, S.; Wang, L. Defects in mtDNA replication challenge nuclear genome stability through nucleotide depletion and provide a unifying mechanism for mouse progerias. Nat. Metab. 2019, 1, 958–965. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Zhu, K.; Tang, D.; Wang, Y.; Wang, Y.; Zhang, G.; Geng, Y.; Yu, H. Comparative analysis of mitochondrial genome features among four Clonostachys species and insight into their systematic positions in the order hypocreales. Int. J. Mol. Sci. 2021, 22, 5530. [Google Scholar] [CrossRef]
- Williams, S.; Foster, P.; Littlewood, D. The complete mitochondrial genome of a turbinid Vetigastropod from MiSeq Illumina sequencing of genomic DNA and steps towards a resolved gastropod phylogeny. Gene 2014, 533, 38–47. [Google Scholar] [CrossRef]
- Cole, L.W.; Guo, W.; Mower, J.P.; Palmer, J.D. High and variable rates of repeat-mediated mitochondrial genome rearrangement in a genus of plants. Mol. Biol. Evol. 2018, 35, 2773–2785. [Google Scholar] [CrossRef]
- Fritsch, E.S.; Chabbert, C.D.; Klaus, B.; Steinmetz, L.M. A genome-wide map of mitochondrial DNA recombination in yeast. Genetics 2014, 198, 755–771. [Google Scholar] [CrossRef] [Green Version]
- Crous, P.W.; Groenewald, J.Z. A phylogenetic re-evaluation of Arthrinium. IMA Fungus 2013, 4, 133–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowman, R.S. The morphology of Patella spp. juveniles in Britain, and some phylogenetic inferences. J. Mar. Biol. Assoc. UK 1981, 61, 647–666. [Google Scholar] [CrossRef]
- Branch, G.M.; Branch, M.L. Competition between Cellana tramoserica (Sowerby) (Gastropoda) and Patiriella exigua (Lamarck) (Asteroidea), and their influence on algal standing stocks. J. Exp. Mar. Biol. Ecol. 1980, 48, 35–49. [Google Scholar] [CrossRef]
- Fuchigami, T.; Sasaki, T. The shell structure of the recent Patellogastropoda (Mollusca: Gastropoda). Paleontolog. Res. 2005, 9, 143–168. [Google Scholar] [CrossRef]
- Hodgson, A.N. Spermatozoal morphology of Patellogastropoda and Vetigastropoda (Mollusca: Prosobranchia). Mem. Mus. Nat. D’histoire Nat. 1995, 166, 167–178. [Google Scholar]
- Hodgson, A.N.; Bernard, R.T.F. A comparison of the structure of the spermatozoa and spermatogenesis of 16 species of Patellid limpet (Mollusca: Gastropoda: Archaeogastropoda). J. Morphol. 1988, 195, 205–223. [Google Scholar] [CrossRef]
- Hodgson, A.N.; Ridgway, S.; Branch, G.M.; Hawkins, S.J. Spermatozoan morphology of 19 species of prosobranch limpets (Patellogastropoda) with a discussion of patellid relationships. Philos. Trans. R. Soc. Lond. Ser. B 1996, 351, 339–347. [Google Scholar] [CrossRef]
- Vakani, B.; Nakano, T.; Kundu, R. Diversity and taxonomy of the intertidal patellogastropod limpets of the mainland Indian coastline. Zootaxa 2020, 4728, 211–226. [Google Scholar] [CrossRef]
- Bouchet, P.; Rocroi, J.P. Classification and nomenclator of gastropod families. Malacologia 2005, 47, 85–397. [Google Scholar]
- Reeve, L.A. Monograph of the genus Patella. In Conchologia Iconica, or, Illustrations of the Shells of Molluscous Animals; Lovell Reeve: London, UK, 1854; Volume 8, pp. 1–42. [Google Scholar]
- Wang, Z.X.; Wu, C.W.; Liu, Z.Y. Preliminary study on the ecologicals, habits and characteristics of Cellana toreuma (Reeve) in the North of Zhejiang coastal area. J. Zhejiang Ocean Univ. 2001, 20, 7–13. [Google Scholar]
- Huang, X.; Wang, T.; Ye, Z.; Han, G.; Dong, Y. Temperature relations of aerial and aquatic physiological performance in a mid-intertidal limpet Cellana toreuma: Adaptation to rapid changes in thermal stress during emersion. Integr. Zool. 2015, 10, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.W.; Wang, H.S.; Han, G.D.; Ke, C.H.; Zhan, X.; Nakano, T.; Williams, G.A. The impact of Yangtze River discharge, ocean currents and historical events on the biogeographic pattern of Cellana toreuma along the China coast. PLoS ONE 2012, 7, e36178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Ganmanee, M.; Shau-Hwai, A.T.; Mujahid, A.; Dong, Y.W. Pleistocene events and present environmental factors have shaped the phylogeography of the intertidal limpet Cellana toreuma (Reeve, 1855) (Gastropoda: Nacellidae) in Southeast Asia and China. J. Molluscan Stud. 2016, 82, 378–390. [Google Scholar] [CrossRef] [Green Version]
- Powell, A.W.B. The patellid limpets of the world (Patellidae). Indo-Pac. Mollusca 1973, 3, 75–206. [Google Scholar]
- Nakano, T.; Ozawa, T. Worldwide phylogeography of limpets ofthe order Patellogastropoda: Molecular, morphological and palaeontological evidence. J. Molluscan Stud. 2007, 73, 79–99. [Google Scholar] [CrossRef]
- Nakano, T.; Espinosa, F. New alien species in the Atlantic Ocean? Mar. Biodivers. Rec. 2010, 3, e39. [Google Scholar] [CrossRef]
- Connell, J.H. Community interactions on marine rocky intertidal shores. Annu. Rev. Ecol. Syst. 1972, 3, 169. [Google Scholar] [CrossRef]
- Branch, G.M. The biology of limpets: Physical factors, energy flow, and ecological interactions. Oceanogr. Mar. Biol. Annu. Rev. 1981, 19, 235–380. [Google Scholar]
- Underwood, A.J.; Benedetti-Cecchi, L.; Åberg, P.; Arenas, F.; Arrontes, J.; Castro, J.O.; Hartnoll, R.G.; Jenkins, S.R.; Paula, J. A continental scale evaluation of the role of limpet grazing on rocky shores. Oecologia 2006, 147, 556–564. [Google Scholar] [CrossRef]
- Goldstien, S.J.; Schiel, D.R.; Gemmell, N.J. Comparative phylogeography of coastal limpets across a marine disjunction in New Zealand. Mol. Ecol. 2006, 15, 3259–3268. [Google Scholar] [CrossRef]
- Goldstien, S.J.; Gemmell, N.J.; Schiel, D.R. Molecular phylogenetics and biogeography of the nacellid limpets of New Zealand (Mollusca: Patellogastropoda). Mol. Phylogenet. Evol. 2006, 38, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Goldstien, S.J.; Gemmell, N.J.; Schiel, D.R. Colonisation and connectivity by intertidal limpets among New Zealand, Chatham and Sub-Antarctic Islands. I. Genetic connections. Mar. Ecol. Prog. Ser. 2009, 388, 111–119. [Google Scholar] [CrossRef]
- Ribeiro, P.A.; Branco, M.; Hawkins, S.J.; Santos, A.M. Recent changes in the distribution of a marine gastropod, Patella rustica, across the Iberian Atlantic coast did not result in diminished genetic diversity or increased connectivity. J. Biogeogr. 2010, 37, 1782–1796. [Google Scholar] [CrossRef]
- Editorial Board of Chinese Materia Medica, State Administration of Traditional Chinese Medicine. Chinese Materia Medica; Shanghai Science and Technology Publishers: Shanghai, China, 1999. [Google Scholar]
- Chen, D. Zoology of China: Mollusca; Science Press: Beijing, China, 1999. [Google Scholar]
- Firth, L.B.; Williams, G.A. The influence of multiple environmental stressors on the limpet Cellana toreuma during the summer monsoon season in Hong Kong. J. Exp. Mar. Biol. Ecol. 2009, 375, 70–75. [Google Scholar] [CrossRef]
- Bird, C.E.; Holland, B.S.; Bowen, B.W.; Toonen, R.J. Contrasting phylogeography in three endemic Hawaiian limpets (Cellana spp.) with similar life histories. Mol. Ecol. 2007, 16, 3173–3186. [Google Scholar] [CrossRef]
- Williams, G.A.; Morritt, D. Habitat partitioning and thermal tolerance in a tropical limpet, Cellana grata. Mar. Ecol. Prog. Ser. 1995, 124, 89–103. [Google Scholar] [CrossRef] [Green Version]
- Chelazzi, G.; De Pirro, M.; Williams, G.A. Cardiac responses to abiotic factors in two tropical limpets, occurring at different levels of the shore. Mar. Biol. 2001, 139, 79–85. [Google Scholar] [CrossRef]
- Dong, Y.W.; Williams, G.A. Variations in cardiac performance and heat shock protein expression to thermal stress in two differently zoned limpets on a tropical rocky shore. Mar. Biol. 2011, 158, 23–31. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Wu, C.W. Study on the age and growth of Cellauma toreuma (Reeve) in North Zhejiang coastal area. J. Zhejiang Ocean Univ. 2000, 19, 316–323. [Google Scholar]
- Qian, W.; Wang, Y.N.; Lu, K.H.; Wang, J.H.; Liu, L.G. Morphological discrimination of radula between two species of Cellana. Chinese J. Zool. 2011, 46, 76–85. [Google Scholar] [CrossRef]
- Hirano, Y. Studies on activity pattern of the patellid limpet Cellana toreuma (Reeve). J. Exp. Mar. Biol. Ecol. 1979, 40, 137–148. [Google Scholar] [CrossRef]
- Iwasaki, K. Interindividual trail following by the intertidal patellid limpet Cellana toreuma. J. Mar. Biol. Assoc. UK 1998, 78, 1019–1022. [Google Scholar] [CrossRef]
- Zhang, S.P. Seashells of China; China Ocean Press: Beijing, China, 2008; pp. 46–50. [Google Scholar]
- Aljanabi, S.M.; Martinez, I. Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic. Acids. Res. 1997, 25, 4692–4693. [Google Scholar] [CrossRef]
- Dierckxsens, N.; Mardulyn, P.; Smits, G. NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic. Acids. Res. 2017, 45, e18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.R.; Stothard, P. The CGView Server: A comparative genomics tool for circular genomes. Nucleic Acids Res. 2008, 36, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Hassanin, A.; Léger, N.; Deutsch, J. Evidence for multiple reversals of asymmetric mutational constraints during the evolution of the mitochondrial genome of Metazoa, and consequences for phylogenetic inferences. Syst. Biol. 2005, 54, 277–298. [Google Scholar] [CrossRef]
- Rozas, J.; Rozas, R. DnaSP, DNA sequence polymorphism: An interactive program for estimating population genetics parameters from DNA sequence data. Comput. Appl. Biosci. 1995, 11, 621–625. [Google Scholar] [CrossRef]
- Fernández-Pérez, J.; Nantón, A.; Ruiz-Ruano, F.J.; Camacho, J.P.M.; Méndez, J. First complete female mitochondrial genome in four bivalve species genus Donax and their phylogenetic relationships within the Veneroida order. PLoS ONE 2017, 12, e0184464. [Google Scholar] [CrossRef]
- Xia, X. DAMBE5: A comprehensive software package for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2013, 30, 1720–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587. [Google Scholar] [CrossRef] [Green Version]
- Nylander, J.A.; Ronquist, F.; Huelsenbeck, J.P.; Nieves-Aldrey, J. Bayesian phylogenetic analysis of combined data. Syst. Biol. 2004, 53, 47–67. [Google Scholar] [CrossRef] [Green Version]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v6: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, 242–245. [Google Scholar] [CrossRef]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef] [Green Version]
- González-Wevar, C.A.; Nakano, T.; Cañete, J.I.; Poulin, E. Molecular phylogeny and historical biogeography of Nacella (Patellogastropoda: Nacellidae) in the Southern Ocean. Mol. Phylogenet. Evol. 2010, 56, 115–124. [Google Scholar] [CrossRef]
- Rambaut, A.; Suchard, M.A.; Xie, D.; Drummond, A.J. Tracer v1.6. Available online: http://tree.bio.ed.ac.uk/software/tracer/2014 (accessed on 22 March 2022).
- Rambaut, A. FigTree, Version 1.4.3. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 1 July 2016).
- Nakashima, S.; Shimizu, M.; Hirota, K.; Hiruta, S.F.; Setiamarga, D.H.E. Complete mitochondrial genome of the pacific limpet Cellana nigrolineata (Gastropoda: Patellogastropoda) determined by shotgun sequencing using the Illumina ngs platform. Mitochondrial DNA Part B 2021, 6, 1857–1859. [Google Scholar] [CrossRef]
- Gaitan-Espitia, J.D.; Gonzalez-Wevar, C.A.; Poulin, E.; Cardenas, L. Antarctic and sub-Antarctic Nacella limpets reveal novel evolutionary characteristics of mitochondrial genomes in Patellogastropoda. Mol. Phylogenet. Evol. 2019, 131, 1–7. [Google Scholar] [CrossRef]
- Uribe, J.E.; Irisarri, I.; Templado, J.; Zardoya, R. New patellogastropod mitogenomes help counteracting long-branch attraction in the deep phylogeny of gastropod mollusks. Mol. Phylogenet. Evol. 2019, 133, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.T.; Guo, Y.H.; Yan, C.R.; Ye, Y.Y.; Li, J.J.; Guo, B.Y.; Lü, Z.M. Comparative analysis of the complete mitochondrial genomes in two limpets from Lottiidae (Gastropoda: Patellogastropoda): Rare irregular gene rearrangement within Gastropoda. Sci. Rep. 2020, 10, 19277. [Google Scholar] [CrossRef]
- Uwai, S.; Yotsukura, N.; Serisawa, Y.; Muraoka, D.; Hiraoka, M.; Kogame, K. Intraspecific genetic diversity of Undaria pinnatifida in Japan, based on the mitochondrial cox3 gene and the ITS1 of nrDNA. Hydrobiologia 2006, 553, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.M.; Diem, D.; Boo, S.M. Phylogenetic relationships of Rosenvingea (Scytosiphonaceae, Phaeophyceae) from Vietnam based on cox3 and psaA sequences. Algae 2014, 29, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Felsenstein, J. Cases in which parsimony or compatibility methods will be positively misleading. Syst. Zool. 1978, 27, 401–410. [Google Scholar] [CrossRef]
- Arquez, M.; Colgan, D.; Castro, L.R. Sequence and comparison of mitochondrial genomes in the genus Nerita (Gastropoda: Neritimorpha: Neritidae) and phylogenetic considerations among gastropods. Mar. Genom. 2014, 15, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Osca, D.; Templado, J.; Zardoya, R. The mitochondrial genome of Ifremeria nautilei and the phylogenetic position of the enigmatic deep-sea Abyssochrysoidea (Mollusca: Gastropoda). Gene 2014, 547, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Uribe, J.E.; Kano, Y.; Templado, J.; Zardoya, R. Mitogenomics of Vetigastropoda: Insights into the evolution of pallial symmetry. Zool. Scr. 2016, 45, 145–159. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Liu, Y.; Xu, T.; Zhang, Y.; Chen, C.; Qiu, J.W.; Qian, P.Y. The mitochondrial genome of the deep-sea limpet Bathyacmaea nipponica (Patellogastropoda: Pectinodontidae). Mitochondrial DNA Part B 2019, 4, 3175–3176. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.T.; Xia, L.P.; Yan, C.R.; Miao, J.; Ye, Y.Y.; Li, J.J.; Guo, B.Y.; Lü, Z.M. Characterization of four mitochondrial genomes of family Neritidae (Gastropoda: Neritimorpha) and insight into its phylogenetic relationships. Sci. Rep. 2021, 11, 11748. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Wang, M.; Bao, Z.; Wang, S. The complete mitochondrial genome and phylogenetic analysis of the deep-sea limpet Bathyacmaea lactea. Mitochondrial DNA Part B 2021, 6, 2090–2091. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Zeng, C.; Yan, G.Y.; He, L.S. Characterization of the mitochondrial genome of an ancient amphipod Halice sp. MT-2017 (Pardaliscidae) from 10,908 m in the Mariana Trench. Sci. Rep. 2019, 9, 2610. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Subclass | Family | Species | Size (bp) | Accession No. |
|---|---|---|---|---|
| Patellogastropoda | Lottiidae | Nipponacmea fuscoviridis | 18,720 | MK395167 |
| Lottia goshimai | 18,192 | MT248298 | ||
| Lottia digitalis | 26,835 | DQ238599 | ||
| Patellidae | Patella vulgata | 14,808 | MH916653 | |
| Patella ferruginea | 14,400 | MH916654 | ||
| Acmaeidae | Bathyacmaea nipponica | 16,792 | MF095859 | |
| Bathyacmaea lactea | 18,446 | MW309841 | ||
| Nacellidae | Cellana toreuma | 16,260 | MZ329338 | |
| Cellana nigrolineata | 16,153 | LC600801 | ||
| Nacella concinna | 16,761 | KT990126 | ||
| Nacella magellanica | 16,663 | KT990125 | ||
| Nacella clypeater | 16,742 | KT990124 | ||
| Heterobranchia | Polyceridae | Notodoris gardineri | 14,424 | DQ991934 |
| Roboastra europaea | 14,472 | NC_004321 | ||
| Nembrotha kubaryana | 14,395 | NC_034920 | ||
| Aplysiidae | Aplysia dactylomela | 14,128 | DQ991927 | |
| Aplysia vaccaria | 14,130 | DQ991928 | ||
| Aplysia kurodai | 14,131 | KF148053 | ||
| Siphonariidae | Siphonaria pectinate | 14,065 | AY345049 | |
| Siphonaria gigas | 14,518 | JN627205 | ||
| Volvatellidae | Ascobulla fragilis | 14,745 | AY345022 | |
| Placobranchidae | Thuridilla gracilis | 14,259 | DQ991939 | |
| Plakobranchus ocellatus | 14,173 | AP014544 | ||
| Elysia chlorotica | 14,132 | EU599581 | ||
| Elysia timida | 14,088 | NC_035490 | ||
| Elysia ornata | 14,188 | NC_030537 | ||
| Onchidiidae | Onchidella celtica | 14,150 | AY345048 | |
| Onchidella borealis | 14,510 | DQ991936 | ||
| Platevindex mortoni | 13,991 | NC_013934 | ||
| Peronia peronii | 13,968 | JN619346 | ||
| Ellobiidae | Carychium tridentatum | 13,908 | KT696545 | |
| Ovatella vulcani | 14,274 | JN615139 | ||
| Ellobium chinense | 13,979 | NC_034292 | ||
| Auriculinella bidentata | 14,135 | JN606066 | ||
| Auriculastra duplicata | 13,920 | NC_036959 | ||
| Caenogastropoda | Turritellidae | Turritella bacillum | 15,868 | NC_029717 |
| Pomatiopsidae | Oncomelania quadrasi | 15,184 | LC276227 | |
| Oncomelania hupensis nosophora | 15,182 | LC276226 | ||
| Xenophoridae | Onustus exutus | 16,043 | MK327366 | |
| Naticidae | Euspira pila | 15,244 | NC_046703 | |
| Euspira gilva | 15,315 | NC_046593 | ||
| Mammilla mammata | 15,319 | NC_046597 | ||
| Mammilla kurodai | 15,309 | NC_046596 | ||
| Turridae | Turricula nelliae spuria | 16,453 | MK251986 | |
| Conidae | Conus borgesi | 15,536 | EU827198 | |
| Conus tulipa | 15,756 | KR006970 | ||
| Conus betulinus | 16,240 | NC_039922 | ||
| Muricidae | Menathais tuberosa | 15,294 | NC_031405 | |
| Indothais lacera | 15,272 | NC_037221 | ||
| Concholepas concholepas | 15,495 | NC_017886 | ||
| Chicoreus torrefactus | 15,359 | NC_039164 | ||
| Chicoreus asianus | 15,361 | MN793976 | ||
| Boreotrophon candelabrum | 15,265 | NC_046505 | ||
| Ceratostoma rorifluum | 15,338 | MK411750 | ||
| Ceratostoma burnetti | 15,334 | NC_046569 | ||
| Ocinebrellus inornatus | 15,324 | NC_046577 | ||
| Ocinebrellus falcatus | 15,326 | NC_046052 | ||
| Neritimorpha | Neritidae | Nerita chamaeleon | 15,716 | MT161611 |
| Nerita balteata | 15,571 | MN477253 | ||
| Clithon oualaniense | 15,706 | MT568501 | ||
| Clithon sowerbianum | 15,919 | MT230542 | ||
| Clithon retropictus | 15,802 | NC_037238 | ||
| Neritina iris | 15,618 | MW694828 | ||
| Septaria lineata | 15,697 | MW694829 | ||
| Neritina violacea | 15,710 | KY021066 | ||
| Neomphaliones | Peltospiridae | Chrysomallon squamiferum | 15,388 | AP013032 |
| Gigantopelta aegis | 16,097 | MW442948 | ||
| Vetigastropoda | Phasianellidae | Phasianella solida | 16,698 | NC_028709 |
| Phasianella australis | 18,397 | KX298888 | ||
| Angariidae | Angaria neglecta | 19,470 | NC_028707 | |
| Angaria delphinus | 19,554 | NC_031860 | ||
| Haliotidae | Haliotis ovina | 16,531 | NC_056350 | |
| Haliotis tuberculata | 16,521 | FJ599667 | ||
| Haliotis laevigata | 16,545 | NC_024562 | ||
| Trochidae | Stomatella planulata | 17,151 | NC_031861 | |
| Gibbula umbilicalis | 16,277 | NC_035682 | ||
| Umbonium thomasi | 15,998 | MH729882 | ||
| Monodonta labio | 16,440 | MK240320 | ||
| Turbinidae | Bolma rugosa | 17,432 | NC_029366 | |
| Lunella granulate | 17,190 | NC_031857 | ||
| Lunella correensis | 17,308 | MN604179 | ||
| Tegulidae | Tegula lividomaculata | 17,375 | NC_029367 | |
| Tegula brunnea | 17,690 | NC_016954 | ||
| Chlorostoma argyrostomum | 17,780 | KX298892 | ||
| Omphalius rusticus | 18,067 | NC_056356 | ||
| Omphalius nigerrimus | 17,755 | KX298895 |
| Gene | Strand | Location | Length | Codons | Intergenic Nucleotide*(bp) | Anticodon | |
|---|---|---|---|---|---|---|---|
| Start | Stop | ||||||
| cox1 | + | 1 | 1542 | 1542 | ATG/TAA | 74 | |
| cox2 | + | 1617 | 2315 | 699 | ATG/TAA | 21 | |
| trnD | + | 2337 | 2403 | 67 | 58 | GTC | |
| atp8 | + | 2462 | 2650 | 189 | ATG/TAA | 248 | |
| atp6 | + | 2899 | 3393 | 495 | ATG/TAA | 34 | |
| trnT | + | 3428 | 3496 | 69 | 53 | TGT | |
| nad5 | - | 3550 | 5232 | 1683 | ATG/TAA | 39 | |
| trnH | - | 5272 | 5339 | 68 | 30 | GTG | |
| trnQ | - | 5370 | 5438 | 69 | 24 | TTG | |
| nad4 | - | 5463 | 6815 | 1353 | ATA/TAA | 5 | |
| nad4l | - | 6821 | 7087 | 267 | ATG/TAA | 49 | |
| trnS2 | - | 7137 | 7204 | 68 | 17 | ||
| cob | - | 7222 | 8367 | 1146 | ATG/TAG | 20 | |
| nad6 | - | 8388 | 8870 | 483 | ATT/TAA | 24 | |
| trnP | - | 8895 | 8962 | 68 | −47 | TGG | |
| nad1 | - | 8916 | 9881 | 966 | ATT/TAA | 21 | |
| trnL2 | - | 9903 | 9968 | 66 | 4 | ||
| trnL1 | - | 9973 | 10,041 | 69 | −38 | ||
| rrnL | - | 10,004 | 11,269 | 1266 | 87 | ||
| trnV | - | 11,357 | 11,423 | 67 | 0 | TAC | |
| rrnS | - | 11,424 | 12,310 | 887 | 0 | ||
| trnY | - | 12,311 | 12,376 | 66 | 15 | GTA | |
| trnM | - | 12,392 | 12,458 | 67 | −2 | CAT | |
| trnF | - | 12,457 | 12,524 | 68 | 2 | GAA | |
| trnW | - | 12,527 | 12,595 | 69 | 8 | TCA | |
| trnC | - | 12,604 | 12,670 | 67 | 643 | GCA | |
| trnG | + | 13,314 | 13,380 | 67 | 7 | TCC | |
| trnE | + | 13,388 | 13,455 | 68 | 0 | TTC | |
| cox3 | + | 13,456 | 14,235 | 780 | ATG/TAG | 22 | |
| trnR | + | 14,258 | 14,324 | 67 | 1 | TCG | |
| trnN | + | 14,326 | 14,395 | 70 | 76 | GTT | |
| nad3 | + | 14,472 | 14,825 | 354 | ATG/TAA | 7 | |
| trnA | + | 14,833 | 14,900 | 68 | 0 | TGC | |
| trnK | + | 14,901 | 14,973 | 73 | 13 | TTT | |
| trnI | + | 14,987 | 15,054 | 68 | 31 | GAT | |
| trnS1 | + | 15,086 | 15,153 | 68 | 3 | GCT | |
| nad2 | + | 15,157 | 16,254 | 1098 | ATA/TAG | 5 | |
| Lengh (bp) | A (%) | T (%) | G (%) | C (%) | A+T (%) | AT-Skew | GC-Skew | |
|---|---|---|---|---|---|---|---|---|
| Cellana toreuma | 16,260 | 28.9 | 39.5 | 19.9 | 11.7 | 68.4 | −0.155 | 0.261 |
| tRNAs | 1497 | 34.8 | 34.8 | 16.8 | 13.6 | 69.6 | 0.000 | 0.103 |
| rRNAs | 2153 | 41.7 | 29.7 | 14.5 | 14.1 | 71.4 | 0.169 | 0.016 |
| PCGs | 11,133 | 26.5 | 39.8 | 17.3 | 16.4 | 66.3 | −0.201 | 0.027 |
| 1st | 5420 | 30.9 | 36.4 | 21.6 | 11.1 | 67.3 | −0.083 | 0.319 |
| 2nd | 5420 | 28.8 | 38.1 | 19.9 | 13.2 | 66.9 | −0.140 | 0.201 |
| 3rd | 5420 | 27.0 | 43.9 | 18.4 | 10.7 | 70.9 | −0.238 | 0.264 |
| Cellana nigrolineata | 16,153 | 26.5 | 38.0 | 22.7 | 12.7 | 64.6 | −0.179 | 0.283 |
| tRNAs | 1498 | 33.4 | 33.9 | 18.0 | 14.6 | 67.4 | −0.007 | 0.104 |
| rRNAs | 2143 | 41.6 | 27.7 | 14.6 | 16.1 | 69.3 | 0.201 | −0.047 |
| PCGs | 11,046 | 25.4 | 36.8 | 18.9 | 18.8 | 62.2 | −0.184 | 0.003 |
| 1st | 5385 | 28.1 | 36.3 | 23.5 | 12.2 | 64.3 | −0.128 | 0.317 |
| 2nd | 5384 | 26.0 | 37.9 | 22.9 | 13.2 | 63.9 | −0.186 | 0.268 |
| 3rd | 5384 | 25.5 | 40.0 | 21.9 | 12.7 | 65.4 | −0.221 | 0.265 |
| Nacella clypeater | 16,742 | 27.5 | 38.6 | 19.9 | 13.9 | 66.1 | −0.169 | 0.177 |
| tRNAs | 1560 | 32.6 | 33.7 | 19.0 | 14.7 | 66.3 | −0.015 | 0.125 |
| rRNAs | 2222 | 43.1 | 27.5 | 14.6 | 14.8 | 70.6 | 0.221 | −0.006 |
| PCGs | 11,283 | 26.3 | 38.9 | 17.6 | 17.2 | 65.2 | −0.193 | 0.009 |
| 1st | 5581 | 25.8 | 40.4 | 19.2 | 14.7 | 66.1 | −0.221 | 0.132 |
| 2nd | 5581 | 28.2 | 38.3 | 19.8 | 13.7 | 66.5 | −0.151 | 0.182 |
| 3rd | 5580 | 28.4 | 37.3 | 20.9 | 13.4 | 65.7 | −0.134 | 0.217 |
| Nacella concinna | 16,761 | 27.1 | 38.9 | 20.4 | 13.6 | 66.0 | −0.180 | 0.197 |
| tRNAs | 1501 | 32.6 | 34.1 | 18.7 | 14.6 | 66.8 | −0.022 | 0.122 |
| rRNAs | 2216 | 44.0 | 27.1 | 14.2 | 14.7 | 71.1 | 0.237 | −0.017 |
| PCGs | 11,286 | 25.7 | 38.7 | 18.1 | 17.5 | 64.4 | −0.201 | 0.015 |
| 1st | 5587 | 28.2 | 36.4 | 21.3 | 14.1 | 64.6 | −0.127 | 0.202 |
| 2nd | 5587 | 26.5 | 39.5 | 19.9 | 14.1 | 66.0 | −0.198 | 0.171 |
| 3rd | 5587 | 26.6 | 40.8 | 19.9 | 12.7 | 67.4 | −0.212 | 0.220 |
| Nacella magellanica | 16,663 | 27.4 | 38.9 | 20.1 | 13.6 | 66.2 | −0.174 | 0.192 |
| tRNAs | 1500 | 32.4 | 34.1 | 18.8 | 14.7 | 66.5 | −0.026 | 0.124 |
| rRNAs | 2214 | 43.5 | 27.4 | 14.4 | 14.6 | 71.0 | 0.227 | −0.008 |
| PCGs | 11,280 | 26.2 | 39.0 | 17.5 | 17.2 | 65.2 | −0.197 | 0.009 |
| 1st | 5555 | 28.0 | 37.0 | 21.5 | 13.5 | 65.0 | −0.139 | 0.230 |
| 2nd | 5554 | 27.7 | 38.0 | 20.1 | 14.2 | 65.7 | −0.158 | 0.170 |
| 3rd | 5554 | 26.4 | 41.6 | 18.8 | 13.2 | 68.1 | −0.224 | 0.175 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Miao, J.; Ye, Y.; Li, J.; Xu, K.; Guo, B.; Yan, X. Insights into the Deep Phylogeny and Novel Divergence Time Estimation of Patellogastropoda from Complete Mitogenomes. Genes 2022, 13, 1273. https://doi.org/10.3390/genes13071273
Feng J, Miao J, Ye Y, Li J, Xu K, Guo B, Yan X. Insights into the Deep Phylogeny and Novel Divergence Time Estimation of Patellogastropoda from Complete Mitogenomes. Genes. 2022; 13(7):1273. https://doi.org/10.3390/genes13071273
Chicago/Turabian StyleFeng, Jiantong, Jing Miao, Yingying Ye, Jiji Li, Kaida Xu, Baoying Guo, and Xiaojun Yan. 2022. "Insights into the Deep Phylogeny and Novel Divergence Time Estimation of Patellogastropoda from Complete Mitogenomes" Genes 13, no. 7: 1273. https://doi.org/10.3390/genes13071273
APA StyleFeng, J., Miao, J., Ye, Y., Li, J., Xu, K., Guo, B., & Yan, X. (2022). Insights into the Deep Phylogeny and Novel Divergence Time Estimation of Patellogastropoda from Complete Mitogenomes. Genes, 13(7), 1273. https://doi.org/10.3390/genes13071273







