Inorganic Nitrogen Transport and Assimilation in Pea (Pisum sativum)
Abstract
:1. Introduction
2. Transporter Families
2.1. NRT1.1/NPFs including Transceptors
2.2. NRT2 and NAR2 Families
2.3. CLC Family
2.4. SLAC/SLAH Family
2.5. AMT1 and AMT2 Families
3. Assimilatory Steps and Enzymes
3.1. NIA, NITR2, and NiR Families
3.2. Glutamine Synthetases (GS1 and GS2)
4. Regulatory Components
4.1. NLPs
4.2. CIPK23 and CBLs
4.3. CPKIII
4.4. CNGC15
4.5. PP2C
4.6. CLE/CEP
4.7. MiRNAs
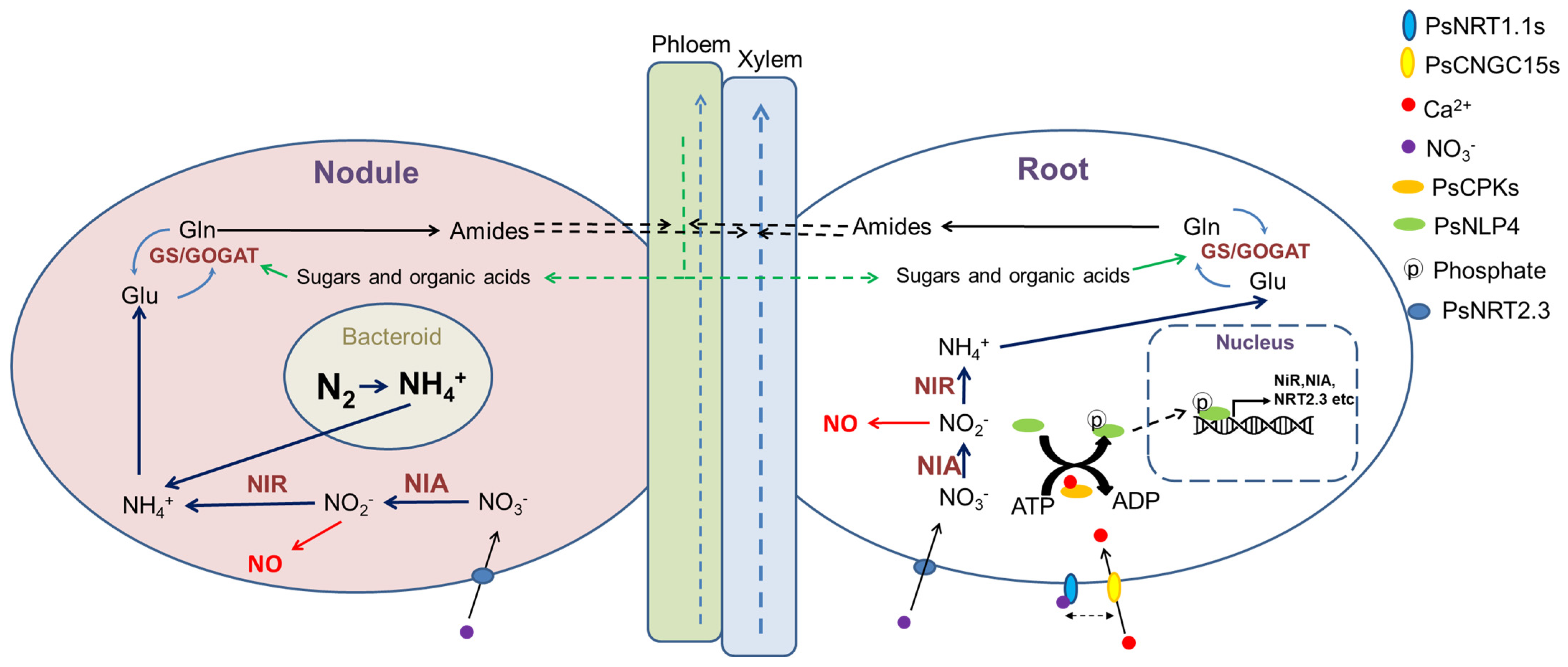
5. Nodulation
6. Conclusions
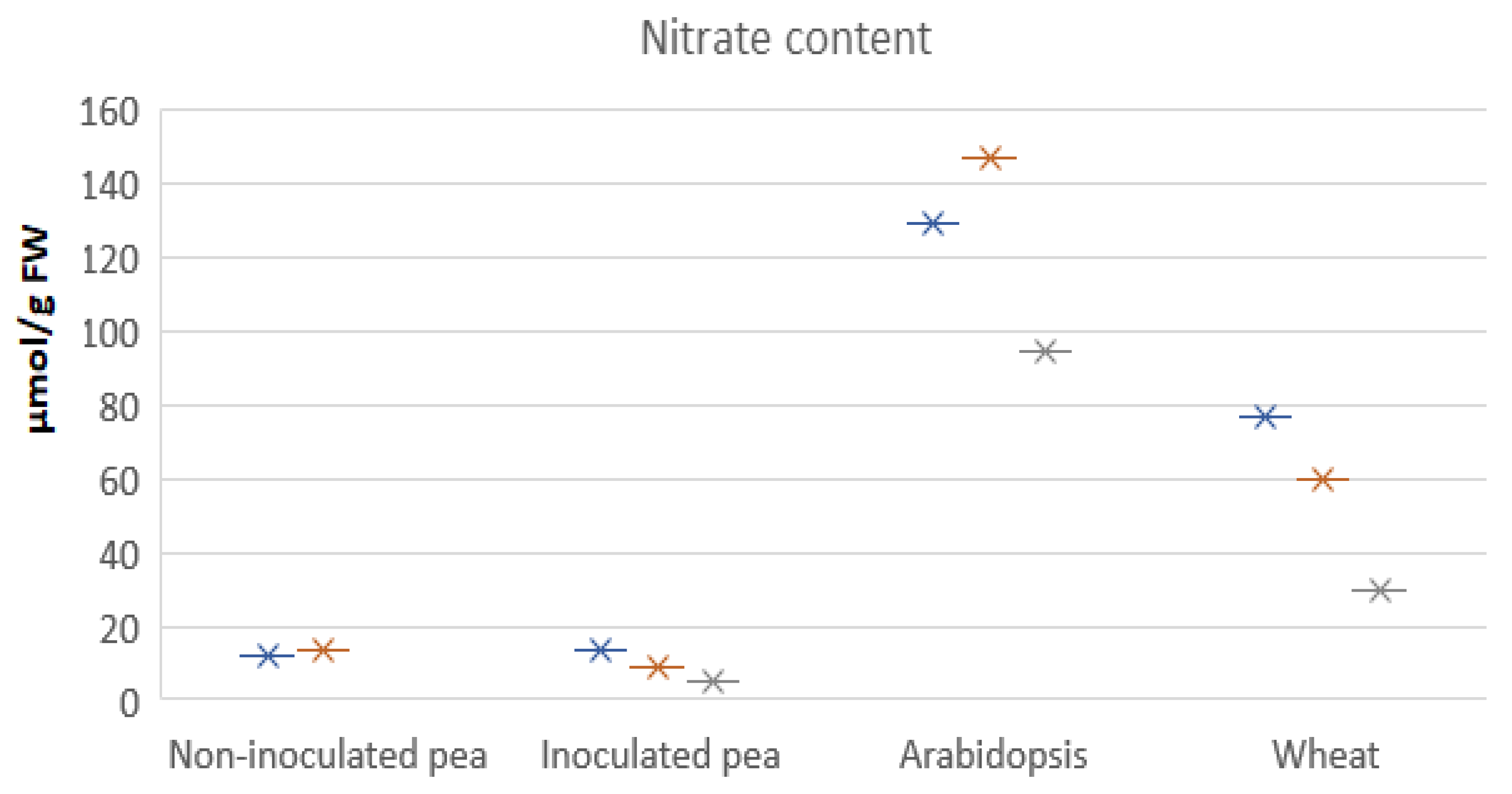
- Pea has lower numbers of NRT2-type nitrate transporters, implying less reliance on nitrate from soil.
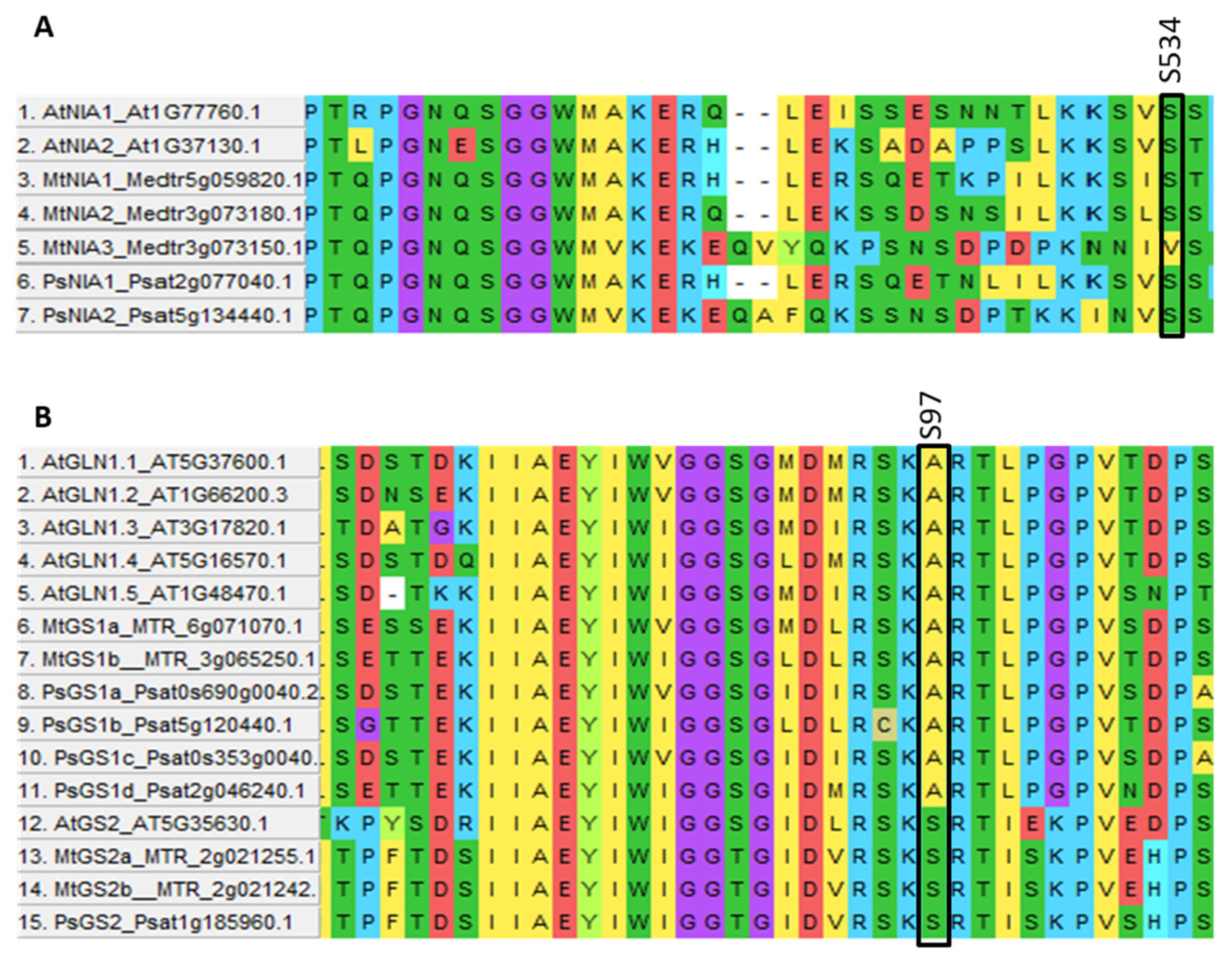
- Most post-translational regulatory sites for phosphorylation and nitrosylation are conserved in pea, such as NRT1.1, AMT1, NIA, GS2 and NLP.
- Pea has fewer CIPKs.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kreplak, J.; Madoui, M.A.; Capal, P.; Novak, P.; Labadie, K.; Aubert, G.; Bayer, P.E.; Gali, K.K.; Syme, R.A.; Main, D.; et al. A reference genome for pea provides insight into legume genome evolution. Nat. Genet. 2019, 51, 1411. [Google Scholar] [CrossRef]
- Pellizzaro, A.; Alibert, B.; Planchet, E.; Limami, A.M.; Morere-Le Paven, M.C. Nitrate transporters: An overview in legumes. Planta 2017, 246, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Valkov, V.C.M. Nitrate Transport and Signaling. In The Lotus Japonicus Genome, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 125–136. [Google Scholar]
- Christensen, T.M.I.E.; Jochimsen, B.U. Enzymes of Ureide Synthesis in Pea and Soybean. Plant Physiol. 1983, 72, 56–59. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.D.; Liu, C.W.; Chen, Y.; Miller, A.J. Nitrogen sensing in legumes. J. Exp. Bot. 2017, 68, 1919–1926. [Google Scholar] [CrossRef]
- Corratge-Faillie, C.; Lacombe, B. Substrate (un)specificity of Arabidopsis NRT1/PTR FAMILY (NPF) proteins. J. Exp. Bot. 2017, 68, 3107–3113. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.H.; Lin, S.H.; Hu, H.C.; Tsay, Y.F. CHL1 Functions as a Nitrate Sensor in Plants. Cell 2009, 138, 1184–1194. [Google Scholar] [CrossRef] [Green Version]
- Wen, Z.Y.; Tyerman, S.D.; Dechorgnat, J.; Ovchinnikova, E.; Dhugga, K.S.; Kaiser, B.N. Maize NPF6 Proteins Are Homologs of Arabidopsis CHL1 That Are Selective for Both Nitrate and Chloride. Plant Cell 2017, 29, 2581–2596. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Q.Y.; Chen, Y.; Liu, C.W.; Robson, F.; Roy, S.; Cheng, X.F.; Wen, J.Q.; Mysore, K.; Miller, A.J.; Murray, J.D. MtNPF6.5 mediates chloride uptake and nitrate preference in Medicago roots. EMBO J. 2021, 40, e106847. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Lin, S.H.; Cheng, L.H.; Wu, J.J.; Lin, Y.C.; Tsay, Y.F. Potential transceptor AtNRT1.13 modulates shoot architecture and flowering time in a nitrate-dependent manner. Plant Cell 2021, 33, 1492–1505. [Google Scholar] [CrossRef]
- Fan, S.C.; Lin, C.S.; Hsu, P.K.; Lin, S.H.; Tsay, Y.F. The Arabidopsis Nitrate Transporter NRT1.7, Expressed in Phloem, Is Responsible for Source-to-Sink Remobilization of Nitrate. Plant Cell 2009, 21, 2750–2761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, P.K.; Tsay, Y.F. Two Phloem Nitrate Transporters, NRT1.11 and NRT1.12, Are Important for Redistributing Xylem-Borne Nitrate to Enhance Plant Growth. Plant Physiol. 2013, 163, 844–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.Y.; Tsay, Y.F. Arabidopsis Nitrate Transporter NRT1.9 Is Important in Phloem Nitrate Transport. Plant Cell 2011, 23, 1945–1957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, J.M.; Schinke, A.L.; Brooks, M.D.; Pasquino, A.; Leonelli, L.; Varala, K.; Safi, A.; Krouk, G.; Krapp, A.; Coruzzi, G.M. Transient genome-wide interactions of the master transcription factor NLP7 initiate a rapid nitrogen-response cascade. Nat. Commun. 2020, 11, 1157. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Crawford, N.M. Genetic identification of a gene involved in constitutive, high-affinity nitrate transport in higher plants. Proc. Natl. Acad. Sci. USA 1996, 93, 9297–9301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chopin, F.; Orsel, M.; Dorbe, M.F.; Chardon, F.; Truong, H.N.; Miller, A.J.; Krapp, A.; Daniel-Vedele, F. The Arabidopsis ATNRT2.7 nitrate transporter controls nitrate content in seeds. Plant Cell 2007, 19, 1590–1602. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Tang, Z.; Tan, Y.; Zhang, Y.; Luo, B.; Yang, M.; Lian, X.; Shen, Q.; Miller, A.J.; Xu, G. Correction for Fan et al., Overexpression of a pH-sensitive nitrate transporter in rice increases crop yields. Proc. Natl. Acad. Sci. USA 2017, 114, E7650. [Google Scholar]
- Imsande, J.; Edwards, D.G. Decreased Rates of Nitrate Uptake during Pod Fill by Cowpea, Green Gram, and Soybean. Agron. J. 1988, 80, 789–793. [Google Scholar] [CrossRef]
- Wirth, J.; Chopin, F.; Santoni, V.; Viennois, G.; Tillard, P.; Krapp, A.; Lejay, L.; Daniel-Vedele, F.; Gojon, A. Regulation of root nitrate uptake at the NRT2.1 protein level in Arabidopsis thaliana. J. Biol. Chem. 2007, 282, 23541–23552. [Google Scholar] [CrossRef] [Green Version]
- Orsel, M.; Chopin, F.; Leleu, O.; Smith, S.J.; Krapp, A.; Daniel-Vedele, F.; Miller, A.J. Characterization of a two-component high-affinity nitrate uptake system in Arabidopsis. Physiology and protein-protein interaction. Plant Physiol. 2006, 142, 1304–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Angeli, A.; Monachello, D.; Ephritikhine, G.; Frachisse, J.M.; Thomine, S.; Gambale, F.; Barbier-Brygoo, H. The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 2006, 442, 939–942. [Google Scholar] [CrossRef]
- von der Fecht-Bartenbach, J.; Bogner, M.; Dynowski, M.; Ludewig, U. CLC-b-mediated NO-3/H+ exchange across the tonoplast of Arabidopsis vacuoles. Plant Cell Physiol. 2010, 51, 960–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zifarelli, G.; Pusch, M. CLC transport proteins in plants. FEBS Lett. 2010, 584, 2122–2127. [Google Scholar] [CrossRef] [Green Version]
- Lv, Q.-D.; Tang, R.-J.; Liu, H.; Gao, X.-S.; Li, Y.-Z.; Zheng, H.-Q.; Zhang, H.-X. Cloning and molecular analyses of the Arabidopsis thaliana chloride channel gene family. Plant Sci. 2009, 176, 650–661. [Google Scholar] [CrossRef]
- Gloser, V.; Dvorackova, M.; Mota, D.H.; Petrovic, B.; Gonzalez, P.; Geilfus, C.M. Early Changes in Nitrate Uptake and Assimilation Under Drought in Relation to Transpiration. Front. Plant Sci. 2020, 11, 602065. [Google Scholar] [CrossRef] [PubMed]
- Hervas, A.L.; FLluch, C. Nitrate reduction in pea plants: Effects of nitrate application and rhizobium strains. Soil Biol. Biochem. 1991, 23, 695–699. [Google Scholar] [CrossRef]
- Ligero, F.; Lluch, C.; Hervas, A.; Olivares, J.; Bedmar, E.J. Effect of nodulation on the expression of nitrate reductase activity in pea cultivars. New Phytol. 1987, 107, 53–61. [Google Scholar] [CrossRef]
- Meng, S.; Peng, J.S.; He, Y.N.; Zhang, G.B.; Yi, H.Y.; Fu, Y.L.; Gong, J.M. Arabidopsis NRT1.5 Mediates the Suppression of Nitrate Starvation-Induced Leaf Senescence by Modulating Foliar Potassium Level. Mol. Plant. 2016, 9, 461–470. [Google Scholar] [CrossRef] [Green Version]
- Criado, M.V.; Caputo, C.; Roberts, I.N.; Castro, M.A.; Barneix, A.J. Cytokinin-induced changes of nitrogen remobilization and chloroplast ultrastructure in wheat (Triticum aestivum). J. Plant Physiol. 2009, 166, 1775–1785. [Google Scholar] [CrossRef]
- Imran, M.; Sun, X.; Hussain, S.; Ali, U.; Rana, M.S.; Rasul, F.; Saleem, M.H.; Moussa, M.G.; Bhantana, P.; Afzal, J.; et al. Molybdenum-Induced Effects on Nitrogen Metabolism Enzymes and Elemental Profile of Winter Wheat (Triticum aestivum L.) under Different Nitrogen Sources. Int. J. Mol. Sci. 2019, 20, 3009. [Google Scholar] [CrossRef] [Green Version]
- Wallace, W. Distribution of nitrate assimilation between the root and shoot of legumes and a comparison with wheat. Physiol. Plant 1986, 66, 630–636. [Google Scholar] [CrossRef]
- Hedrich, R.; Geiger, D. Biology of SLAC1-type anion channels—From nutrient uptake to stomatal closure. New Phytol. 2017, 216, 46–61. [Google Scholar] [CrossRef] [Green Version]
- Vahisalu, T.; Kollist, H.; Wang, Y.F.; Nishimura, N.; Chan, W.Y.; Valerio, G.; Lamminmaki, A.; Brosche, M.; Moldau, H.; Desikan, R.; et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 2008, 452, 487–491. [Google Scholar] [CrossRef]
- Negi, J.; Matsuda, O.; Nagasawa, T.; Oba, Y.; Takahashi, H.; Kawai-Yamada, M.; Uchimiya, H.; Hashimoto, M.; Iba, K. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 2008, 452, 483–486. [Google Scholar] [CrossRef]
- Gazzarrini, S.; Lejay, L.; Gojon, A.; Ninnemann, O.; Frommer, W.B.; von Wiren, N. Three Functional Transporters for Constitutive, Diurnally Regulated, and Starvation-Induced Uptake of Ammonium into Arabidopsis Roots. Plant Cell 1999, 11, 937–947. [Google Scholar] [CrossRef] [Green Version]
- Neuhauser, B.; Dynowski, M.; Ludewig, U. Channel-like NH3 flux by ammonium transporter AtAMT2. FEBS Lett. 2009, 583, 2833–2838. [Google Scholar] [CrossRef] [Green Version]
- Giehl, R.F.H.; Laginha, A.M.; Duan, F.; Rentsch, D.; Yuan, L.; von Wiren, N. A Critical Role of AMT2;1 in Root-To-Shoot Translocation of Ammonium in Arabidopsis. Mol. Plant 2017, 10, 1449–1460. [Google Scholar] [CrossRef] [PubMed]
- Lanquar, V.; Loque, D.; Hormann, F.; Yuan, L.X.; Bohner, A.; Engelsberger, W.R.; Lalonde, S.; Schulze, W.X.; von Wiren, N.; Frommer, W.B. Feedback Inhibition of Ammonium Uptake by a Phospho-Dependent Allosteric Mechanism in Arabidopsis. Plant Cell 2009, 21, 3610–3622. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.Y.; Chen, Y.N.; Wang, H.Y.; Liu, Z.T.; Frommer, W.B.; Ho, C.H. Feedback inhibition of AMT1 NH4(+)-transporters mediated by CIPK15 kinase. BMC Biol. 2020, 18, 196. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.Q.; Crawford, N.M. ldentif ication of the Arabidopsis CHL3 Gene as the Nitrate Reductase Structural Gene MA2. Plant Cell 1991, 3, 461–471. [Google Scholar] [PubMed] [Green Version]
- Yu, X.; Sukumaran, S.; Marton, L. Differential Expression of the Arabidopsis Nia1 and Nia2 Genes1. Plant Physiol. 1998, 116, 1091–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, S.; Konishi, M.; Yanagisawa, S.; Omata, T. Nitrite transport activity of a novel HPP family protein conserved in cyanobacteria and chloroplasts. Plant Cell Physiol. 2014, 55, 1311–1324. [Google Scholar] [CrossRef] [Green Version]
- Crete, P.; Caboche, M.; Meyer, C. Nitrite reductase expression is regulated at the post-transcriptional level by the nitrogen source in Nicotiana plumbaginifolia and Arabidopsis thaliana. Plant J. 1997, 11, 625–634. [Google Scholar] [CrossRef]
- Han, M.; Wong, J.L.; Su, T.; Beatty, P.H.; Good, A.G. Identification of Nitrogen Use Efficiency Genes in Barley: Searching for QTLs Controlling Complex Physiological Traits. Front. Plant Sci. 2016, 7, 1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seabra, A.R.; Carvalho, H.G. Glutamine synthetase in Medicago truncatula, unveiling new secrets of a very old enzyme. Front. Plant Sci. 2015, 6, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodenas, R.; Vert, G. Regulation Of Root Nutrient Transporters By CIPK23: “One Kinase To Rule Them All”. Plant Cell Physiol. 2021, 62, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, C.; Tian, L.; Hou, C.; Tian, W.; Hu, B.; Zhang, Q.; Ren, Z.; Niu, Q.; Song, J.; et al. A transceptor-channel complex couples nitrate sensing to calcium signaling in Arabidopsis. Mol. Plant 2021, 14, 774–786. [Google Scholar] [CrossRef]
- Griesmann, M.; Chang, Y.; Liu, X.; Song, Y.; Haberer, G.; Crook, M.B.; Billault-Penneteau, B.; Lauressergues, D.; Keller, J.; Imanishi, L.; et al. Phylogenomics reveals multiple losses of nitrogen-fixing root nodule symbiosis. Science 2018, 361, eaat1743. [Google Scholar] [CrossRef] [Green Version]
- Schauser, L.; Roussis, A.; Stiller, J.; Stougaard, J. A plant regulator controlling development of symbiotic root nodules. Nature 1999, 402, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lee, T.; Schiessl, K.; Oldroyd, G.E.D. Processing of NODULE INCEPTION controls the transition to nitrogen fixation in root nodules. Science 2021, 374, 629–632. [Google Scholar] [CrossRef]
- Liu, C.W.; Breakspear, A.; Guan, D.; Cerri, M.R.; Jackson, K.; Jiang, S.; Robson, F.; Radhakrishnan, G.V.; Roy, S.; Bone, C.; et al. NIN Acts as a Network Hub Controlling a Growth Module Required for Rhizobial Infection. Plant Physiol. 2019, 179, 1704–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, X.; Luo, J. Evolutionary analyses of NIN-like proteins in plants and their roles in nitrate signaling. Cell Mol. Life Sci. 2019, 76, 3753–3764. [Google Scholar] [CrossRef]
- Lin, J.S.; Li, X.L.; Luo, Z.P.; Mysore, K.S.; Wen, J.Q.; Xie, F. NIN interacts with NLPs to mediate nitrate inhibition of nodulation in Medicago truncatula. Nat. Plants 2018, 4, 942. [Google Scholar] [CrossRef]
- Jiang, S.J.; Gao, M.; Pecrix, J.; Wen, Y.; Mysore, J.; Xu, K.; Sanchez-Canizares, P.; Ruan, C.; Li, Y.; Zhu, Q.; et al. NIN-like protein transcription factors regulate leghemoglobin genes in legume nodules. Science 2021, 374, 625–628. [Google Scholar] [CrossRef]
- Yan, D.; Easwaran, V.; Chau, V.; Okamoto, M.; Ierullo, M.; Kimura, M.; Endo, A.; Yano, R.; Pasha, A.; Gong, Y.; et al. NIN-like protein 8 is a master regulator of nitrate-promoted seed germination in Arabidopsis. Nat. Commun. 2016, 7, 13179. [Google Scholar] [CrossRef]
- Marchive, C.; Roudier, F.; Castaings, L.; Brehaut, V.; Blondet, E.; Colot, V.; Meyer, C.; Krapp, A. Nuclear retention of the transcription factor NLP7 orchestrates the early response to nitrate in plants. Nat. Commun. 2013, 4, 1713. [Google Scholar] [CrossRef]
- Suzuki, W.; Konishi, M.; Yanagisawa, S. The evolutionary events necessary for the emergence of symbiotic nitrogen fixation in legumes may involve a loss of nitrate responsiveness of the NIN transcription factor. Plant Signal. Behav. 2013, 8, e25975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, H.; Nosaki, S.; Suzuki, T.; Ito, M.; Miyakawa, T.; Nomoto, M.; Tada, Y.; Miura, K.; Tanokura, M.; Kawaguchi, M.; et al. Different DNA-binding specificities of NLP and NIN transcription factors underlie nitrate-induced control of root nodulation. Plant Cell 2021, 33, 2340–2359. [Google Scholar] [CrossRef]
- Nishida, H.; Tanaka, S.; Handa, Y.; Ito, M.; Sakamoto, Y.; Matsunaga, S.; Betsuyaku, S.; Miura, K.; Soyano, T.; Kawaguchi, M.; et al. A NIN-like protein mediates nitrate-induced control of root nodule symbiosis in Lotus japonicus. Nat. Commun. 2018, 9, 499. [Google Scholar] [CrossRef]
- Riveras, E.; Alvarez, J.M.; Vidal, E.A.; Oses, C.; Vega, A.; Gutierrez, R.A. The Calcium Ion Is a Second Messenger in the Nitrate Signaling Pathway of Arabidopsis. Plant Physiol. 2015, 169, 1397–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.H.; Niu, Y.; Konishi, M.; Wu, Y.; Du, H.; Sun Chung, H.; Li, L.; Boudsocq, M.; McCormack, M.; Maekawa, S.; et al. Discovery of nitrate-CPK-NLP signalling in central nutrient-growth networks. Nature 2017, 545, 311–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borisov, A.Y.; Madsen, L.H.; Tsyganov, V.E.; Umehara, Y.; Voroshilova, V.A.; Batagov, A.O.; Sandal, N.; Mortensen, A.; Schauser, L.; Ellis, N.; et al. The sym35 gene required for root nodule development in pea is an ortholog of nin from Lotus japonicus. Plant Physiol. 2003, 131, 1009–1017. [Google Scholar] [CrossRef] [Green Version]
- Ohkubo, Y.; Kuwata, K.; Matsubayashi, Y. A type 2C protein phosphatase activates high-affinity nitrate uptake by dephosphorylating NRT2.1. Nat. Plants 2021, 7, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Leran, S.; Edel, K.H.; Pervent, M.; Hashimoto, K.; Corratge-Faillie, C.; Offenborn, J.N.; Tillard, P.; Gojon, A.; Kudla, J.; Lacombe, B. Nitrate sensing and uptake in Arabidopsis are enhanced by ABI2, a phosphatase inactivated by the stress hormone abscisic acid. Sci. Signal. 2015, 8, ra43. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, K.; Niu, X.; Wang, Q.; Wan, Y.; Yang, F.; Li, G.; Wang, Y.; Wang, R. Genome-wide Identification of PP2C Genes and Their Expression Profiling in Response to Drought and Cold Stresses in Medicago truncatula. Sci. Rep. 2018, 8, 12841. [Google Scholar] [CrossRef] [Green Version]
- Tovar-Mendez, A.; Miernyk, J.A.; Hoyos, E.; Randall, D.D. A functional genomic analysis of Arabidopsis thaliana PP2C clade D. Protoplasma 2014, 251, 265–271. [Google Scholar] [CrossRef]
- Taleski, M.; Imin, N.; Djordjevic, M.A. CEP peptide hormones: Key players in orchestrating nitrogen-demand signalling, root nodulation, and lateral root development. J. Exp. Bot. 2018, 69, 1829–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mens, C.; Hastwell, A.H.; Su, H.; Gresshoff, P.M.; Mathesius, U.; Ferguson, B.J. Characterisation of Medicago truncatula CLE34 and CLE35 in nitrate and rhizobia regulation of nodulation. New Phytol. 2021, 229, 2525–2534. [Google Scholar] [CrossRef]
- Zhu, F.G.; Deng, J.; Chen, H.; Liu, P.; Zheng, L.H.; Ye, Q.Y.; Li, R.; Brault, M.; Wen, J.Q.; Frugier, F.; et al. A CEP Peptide Receptor-Like Kinase Regulates Auxin Biosynthesis and Ethylene Signaling to Coordinate Root Growth and Symbiotic Nodulation inMedicago truncatula([OPEN]). Plant Cell 2020, 32, 2855–2877. [Google Scholar] [CrossRef]
- Moreau, C.; Gautrat, P.; Frugier, F. Nitrate-induced CLE35 signaling peptides inhibit nodulation through the SUNN receptor and miR2111 repression. Plant Physiol. 2021, 185, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Gautrat, P.; Laffont, C.; Frugier, F.; Ruffel, S. Nitrogen Systemic Signaling: From Symbiotic Nodulation to Root Acquisition. Trends Plant Sci. 2021, 26, 392–406. [Google Scholar] [CrossRef]
- Zhang, M.B.; Su, H.N.; Gresshoff, P.M.; Ferguson, B.J. Shoot-derived miR2111 controls legume root and nodule development. Plant Cell Environ. 2021, 44, 1627–1641. [Google Scholar] [CrossRef]
- Streeter, J.; Wong, P.P. Inhibition of legume nodule formation and N2 fixation by nitrate. Crit. Rev. Plant Sci. 1988, 7, 1–23. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Mens, C.; Hastwell, A.H.; Zhang, M.B.; Su, H.A.; Jones, C.H.; Chu, X.T.; Gresshoff, P.M. Legume nodulation: The host controls the party. Plant Cell Environ. 2019, 42, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laffont, C.; Ivanovici, A.; Gautrat, P.; Brault, M.; Djordjevic, M.A.; Frugier, F. The NIN transcription factor coordinates CEP and CLE signaling peptides that regulate nodulation antagonistically. Nat. Commun. 2020, 11, 3167. [Google Scholar] [CrossRef]
- Lebedeva, M.A.; Yashenkova, Y.S.; Dodueva, I.E.; Lutova, L.A. Molecular Dialog between Root and Shoot via Regulatory Peptides and Its Role in Systemic Control of Plant Development. Russ. J. Plant Physiol. 2020, 67, 985–1002. [Google Scholar] [CrossRef]
- Cowden, R.J.; Shah, A.N.; Lehmann, L.M.; Kiaer, L.P.; Henriksen, C.B.; Ghaley, B.B. Nitrogen Fertilizer Effects on Pea-Barley Intercrop Productivity Compared to Sole Crops in Denmark. Sustainability 2020, 12, 9335. [Google Scholar] [CrossRef]
| Name | Gene Abbrev. | Accession Number |
|---|---|---|
| NLPs | PsSYM35/PsNIN | Psat2g001120.1 |
| PsNLP1 | Psat0s498g0160.3 | |
| PsNLP2 | Psat7g208800.1 | |
| PsNLP3 | Psat5g291840.4 | |
| PsNLP4 | Psat6g197400.1 | |
| NRT2s | PsNRT2.1 | Psat4g155600.1 |
| PsNRT2.2 | Psat7g149120.1 | |
| PsNRT2.3 | Psat4g113000.1 | |
| CLE/CEP/SUNNs | Pscam040153 | |
| Pscam040702 | Psat7g164920.1 | |
| Pscam040984 | ||
| Pscam041632 | ||
| HAR1/SUNN | SYM29 | Psat7g183240.1 |
| PLENTY/RDN | PsRDN1 | Psat0s3806g0040.1 |
| PsRDN2 | Psat4g145800.4 | |
| PsRDN3 | Psat6g028240.1 | |
| TML | PsTML | Psat3g176480.1 |
| NIA | PsNIA1 | Psat2g077040.1 |
| PsNIA2 | Psat5g134440.1 | |
| NIR | PsNIR | Psat7g123960.1 |
| NITR2 | PsNITR2 | chr4LG4-85657742..85658907 |
| AtCIPK23 | PsCIPK1 | Psat1g020800.1 |
| GS1 | PsGS1a | Psat0s690g0040.2 |
| PsGS1b | Psat5g120440.1 | |
| PsGS1c | Psat0s353g0040.1 | |
| PsGS1d | Psat2g046240.1 | |
| GS2 | PsGS2 | Psat1g185960.1 |
| GOGAT | PsGLU1 | Psat3g078160.3 |
| PsGLT1 | Psat6g037200.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, B.; Chen, Y.; Xie, F.; Murray, J.D.; Miller, A.J. Inorganic Nitrogen Transport and Assimilation in Pea (Pisum sativum). Genes 2022, 13, 158. https://doi.org/10.3390/genes13010158
Gu B, Chen Y, Xie F, Murray JD, Miller AJ. Inorganic Nitrogen Transport and Assimilation in Pea (Pisum sativum). Genes. 2022; 13(1):158. https://doi.org/10.3390/genes13010158
Chicago/Turabian StyleGu, Benguo, Yi Chen, Fang Xie, Jeremy D. Murray, and Anthony J. Miller. 2022. "Inorganic Nitrogen Transport and Assimilation in Pea (Pisum sativum)" Genes 13, no. 1: 158. https://doi.org/10.3390/genes13010158
APA StyleGu, B., Chen, Y., Xie, F., Murray, J. D., & Miller, A. J. (2022). Inorganic Nitrogen Transport and Assimilation in Pea (Pisum sativum). Genes, 13(1), 158. https://doi.org/10.3390/genes13010158






