Polychromatic Flow Cytometric Analysis of Stromal Vascular Fraction from Lipoaspirate and Microfragmented Counterparts Reveals Sex-Related Immunophenotype Differences
Abstract
:1. Introduction
2. Results
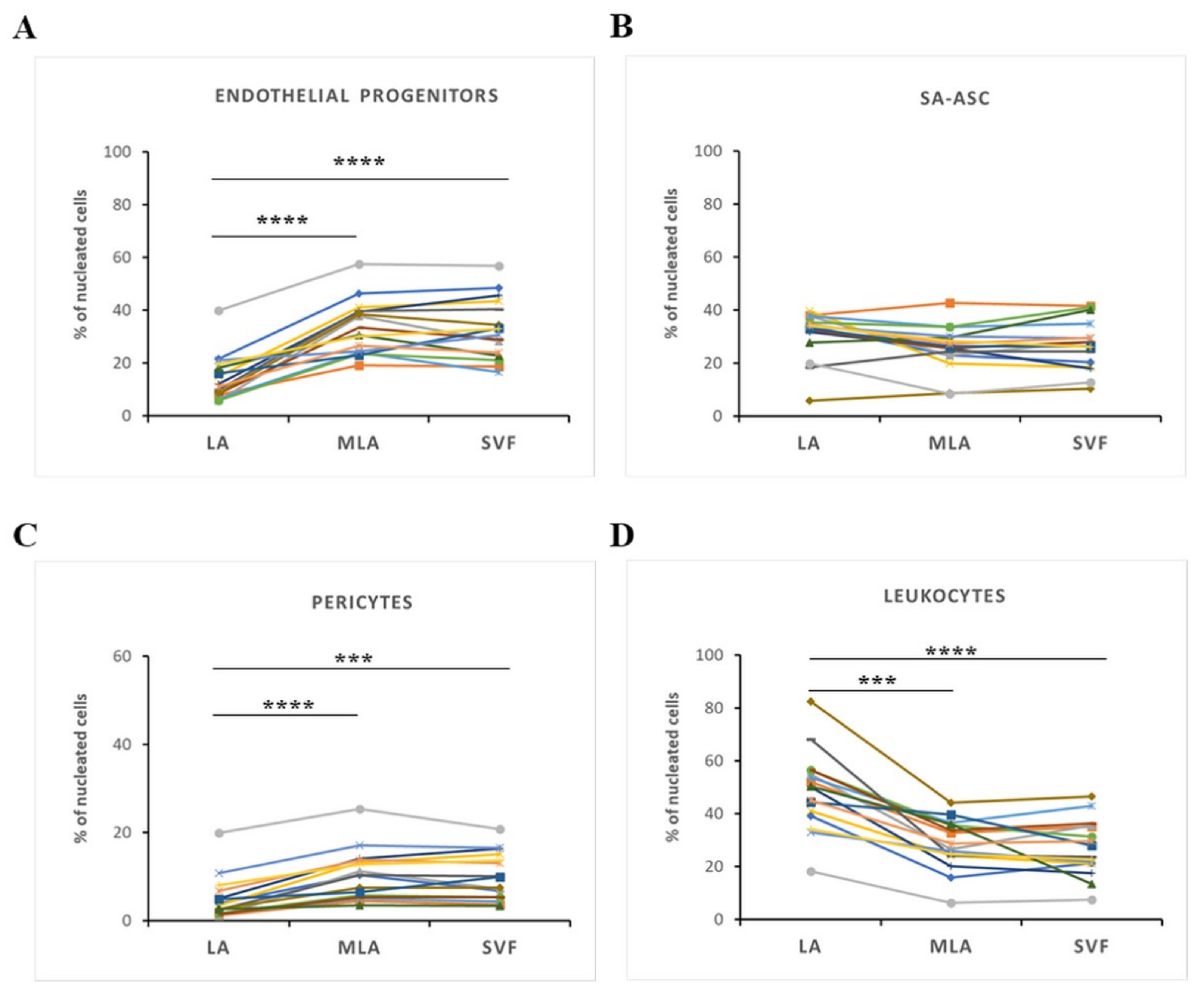
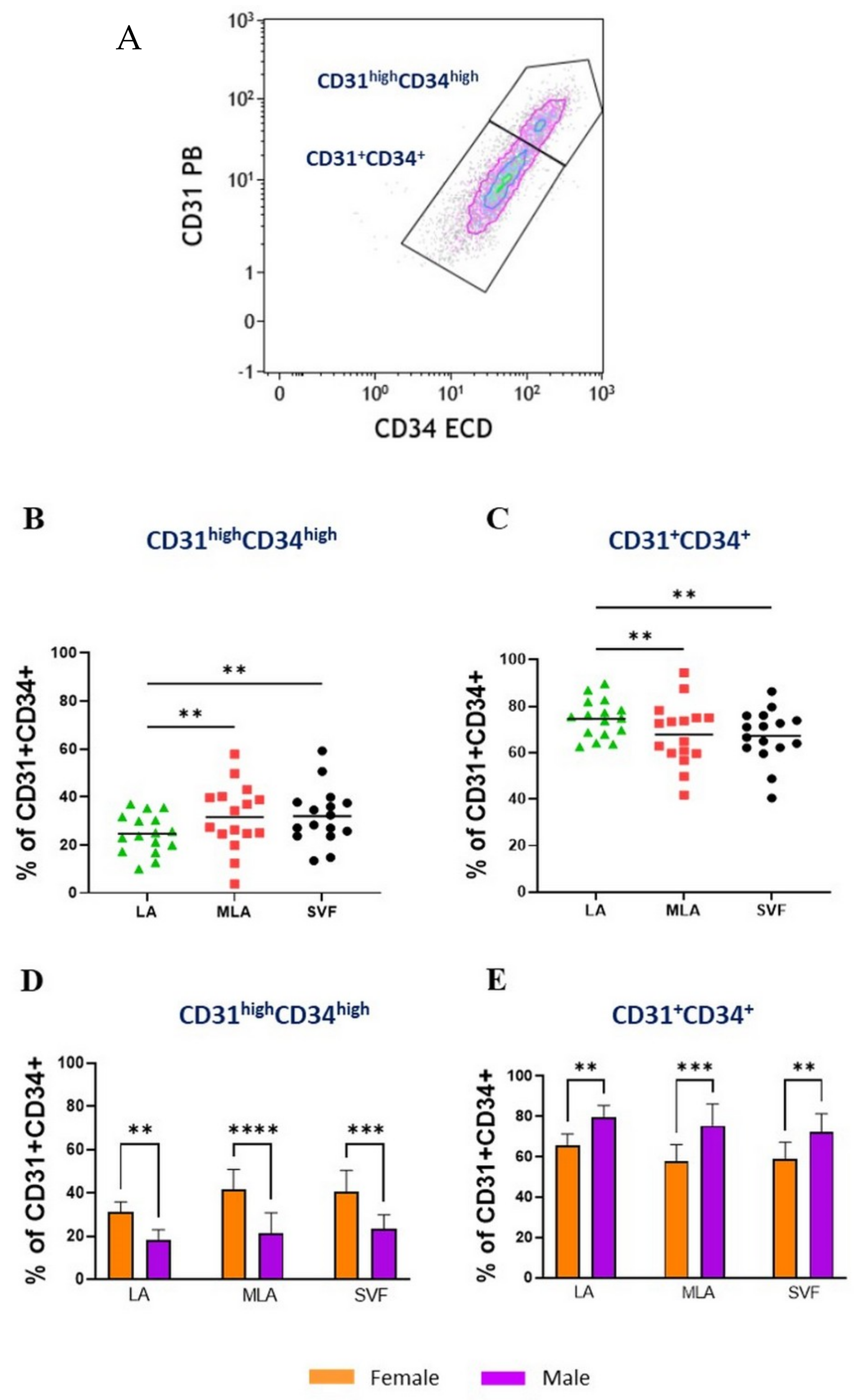
2.1. Immunophenotyping of Stromal Vascular Fraction by Polychromatic Flow Cytometry
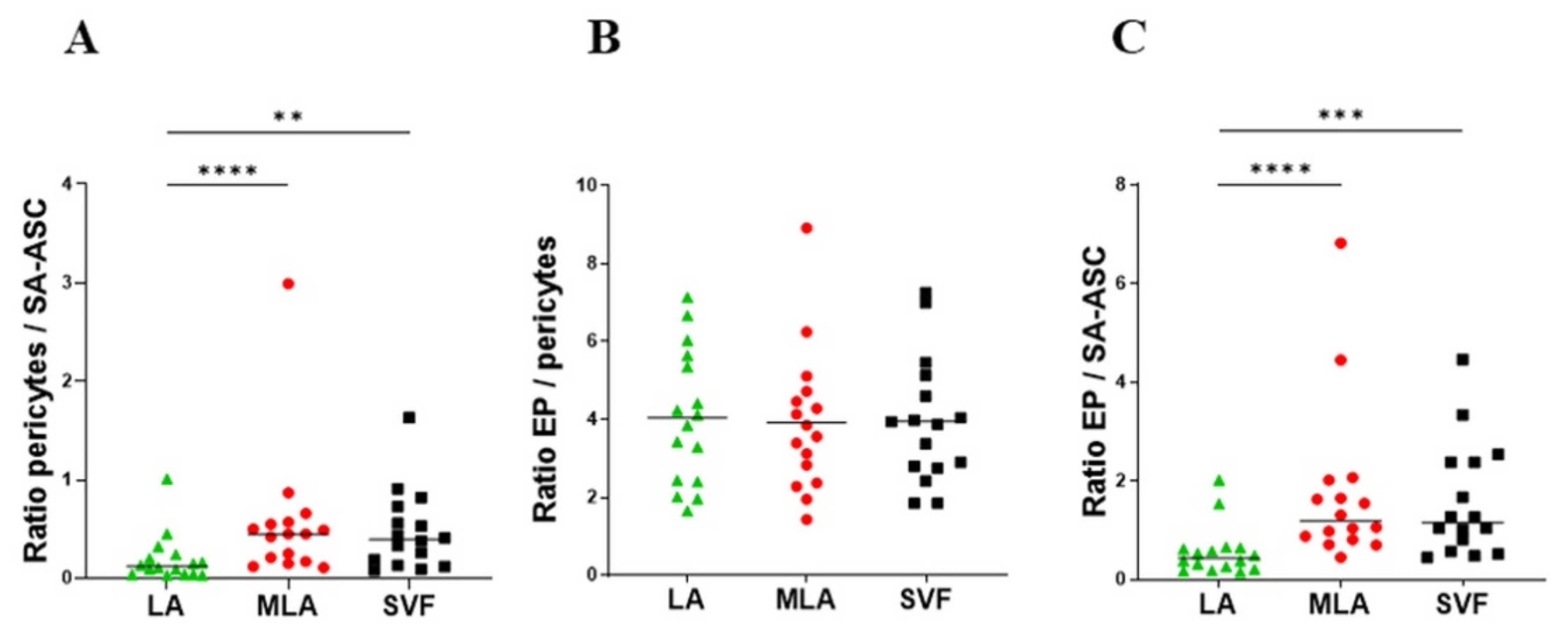
2.2. Regenerative Cell Enrichment of Stromal Vascular Fraction
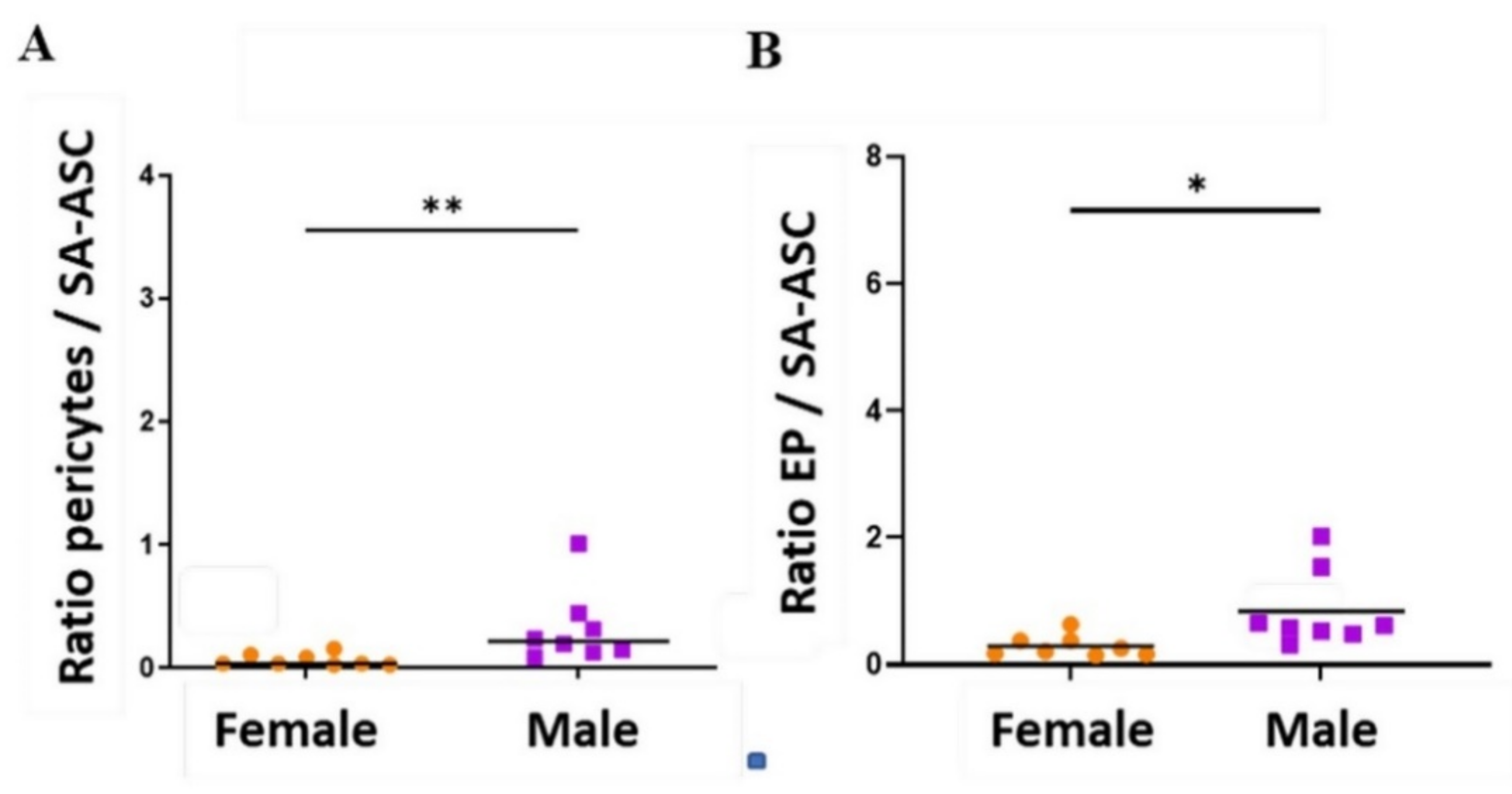
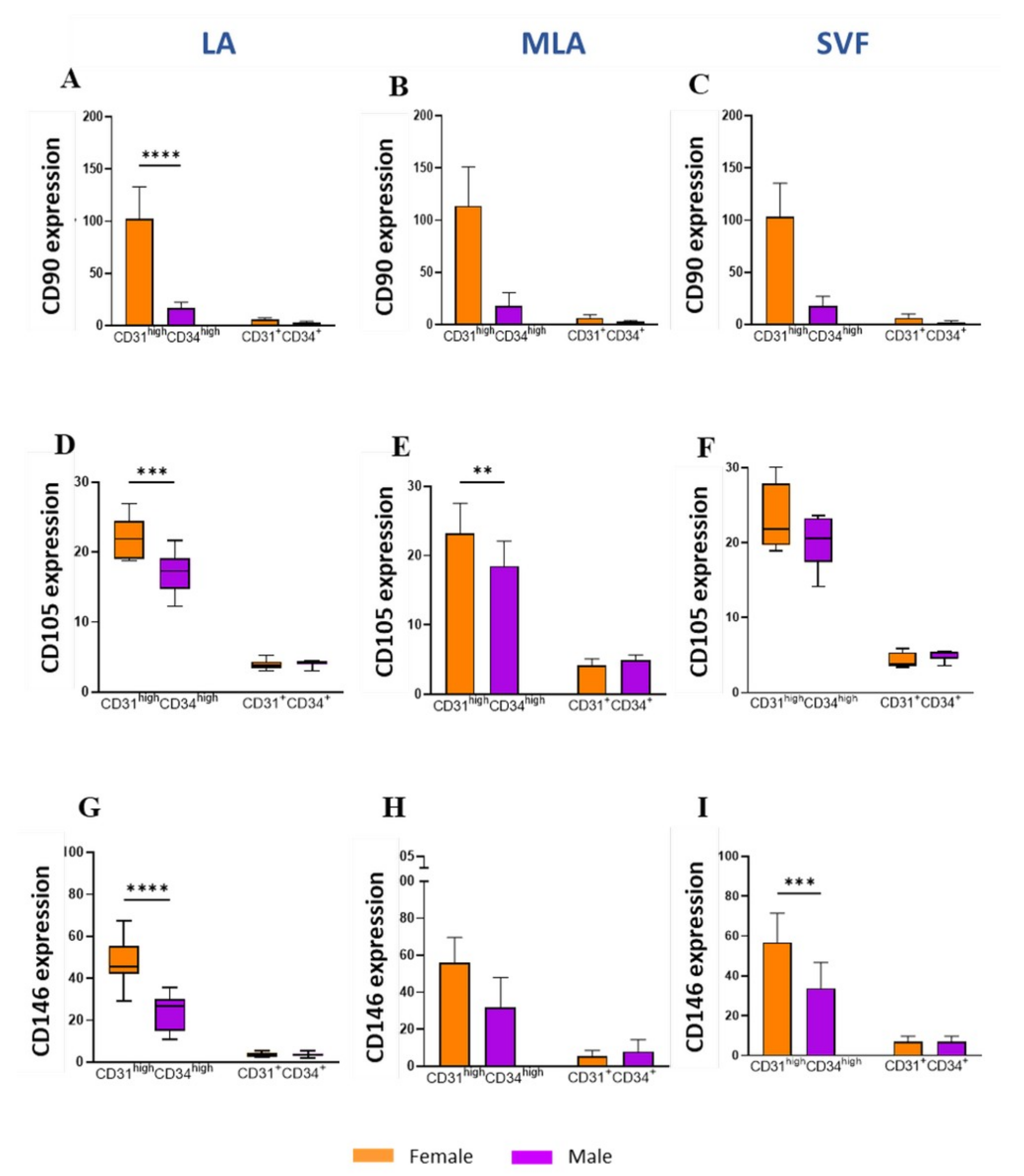
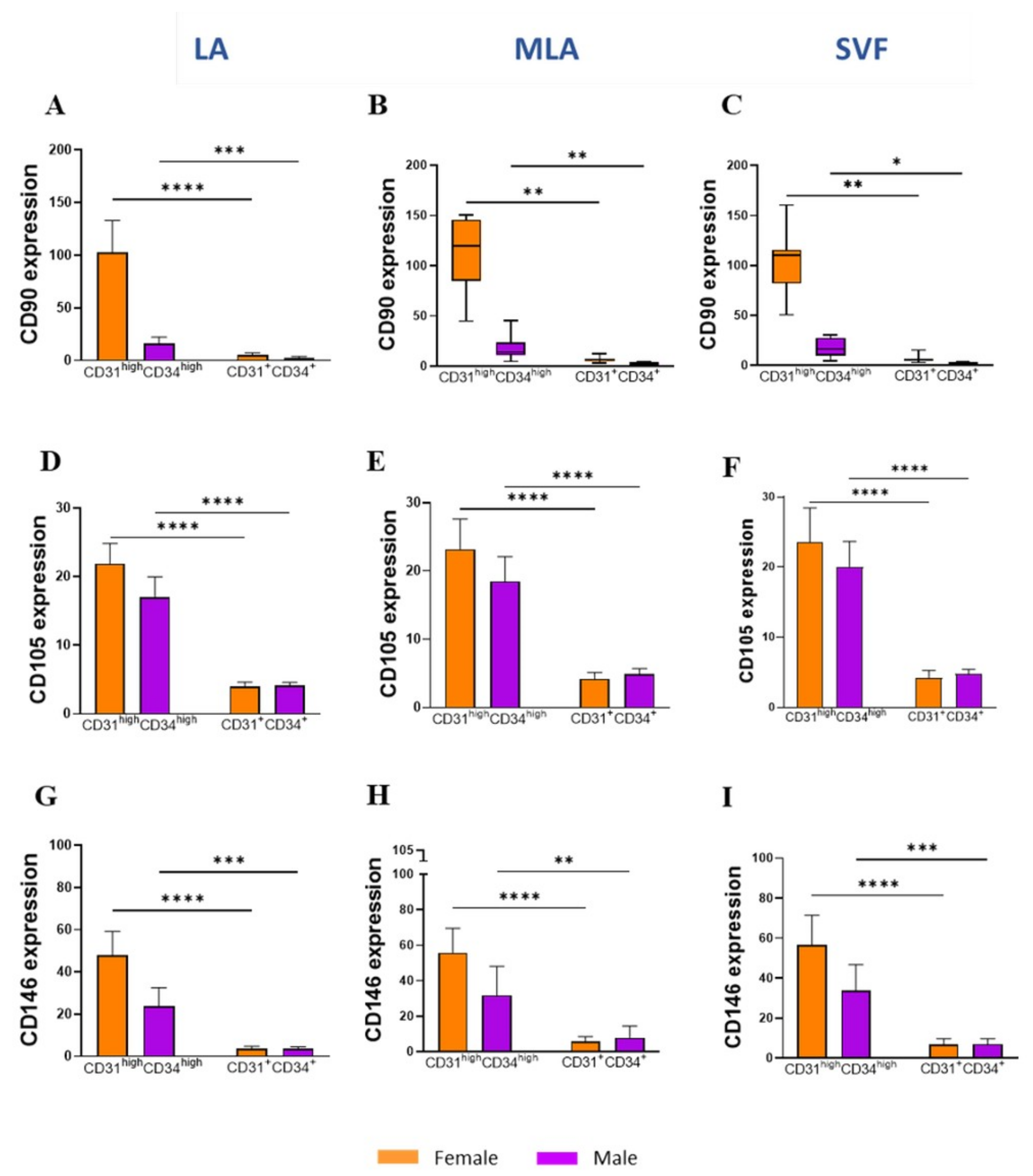
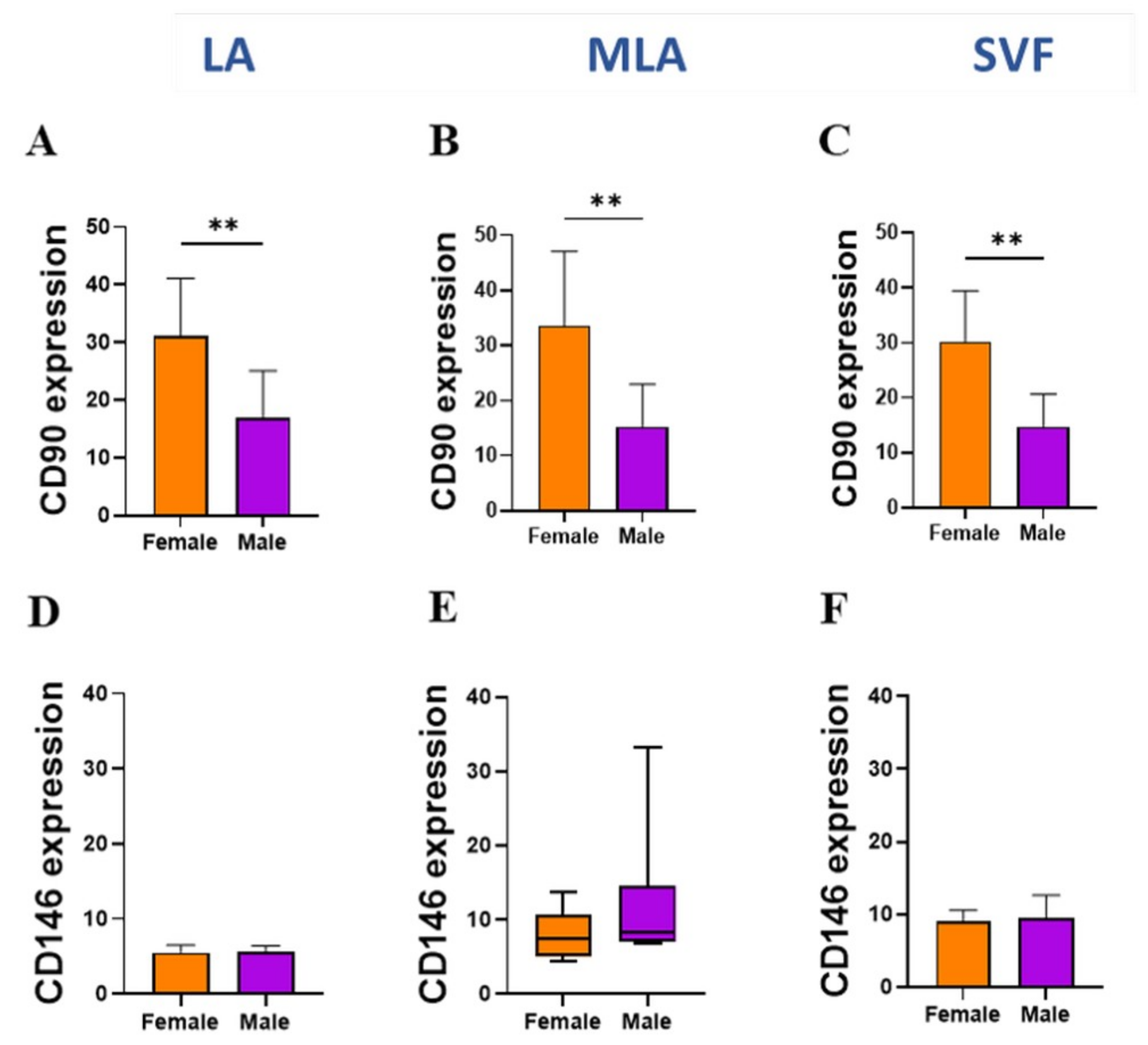
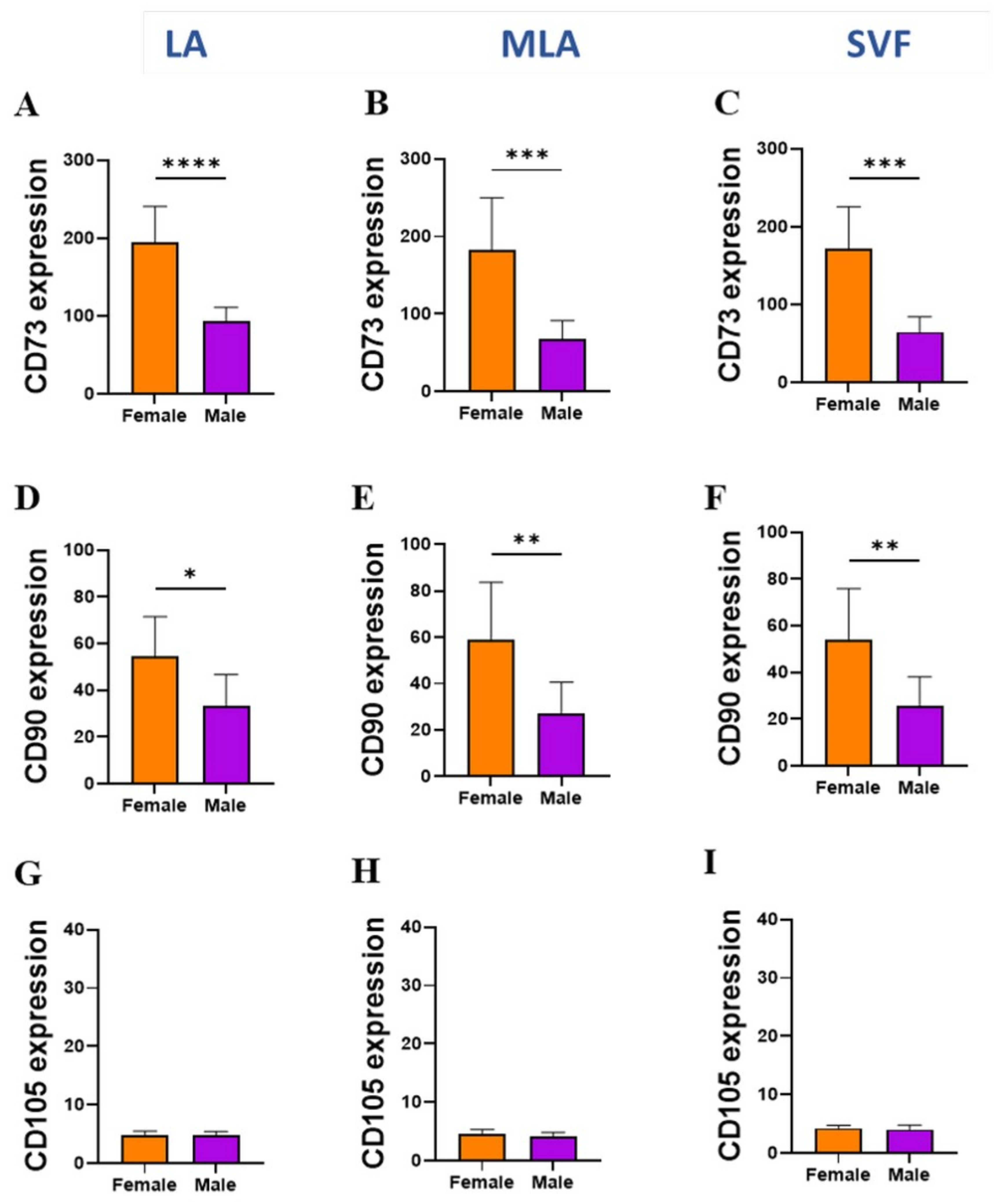
2.3. Sex-Related Differences in the Stromal Progenitor Cells
2.4. Sex-Related Heterogeneity of the Precursor Subpopulations
3. Discussion
4. Materials and Methods
4.1. Patients
- patients with knee osteoarthritis;
- patients older than 18 years and younger than 75 years.
- patients with malignant disease;
- patients with systemic inflammatory diseases (e.g., rheumatoid arthritis);
- patients with grade IV chondromalacia according to the ICRS classification;
- patients with mechanical axis deviation (valgus/varus) of the lower extremities greater than 5 degrees;
- patients with an unstable knee;
- patients with acute meniscal lesions or injuries of other knee structures as the main cause of pain and other symptoms;
- patients with a history of knee surgery;
- patients with mental illness (patients in whom cooperation cannot be expected during the project);
- patients who are found to be unable to respond to follow-up examinations.
4.2. Lipoaspiration and Sample Collection
4.3. Cell Isolation
4.4. Flow Cytometry
4.5. Statistical Analysis
4.6. Ethics Approval
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Primorac, D.; Molnar, V.; Rod, E.; Jeleč, Ž.; Čukelj, F.; Matišić, V.; Vrdoljak, T.; Hudetz, D.; Hajsok, H.; Borić, I. Knee Osteoarthritis: A Review of Pathogenesis and State-Of-The-Art Non-Operative Therapeutic Considerations. Genes 2020, 11, 854. [Google Scholar] [CrossRef] [PubMed]
- Borić, I.; Hudetz, D.; Rod, E.; Jeleč, Ž.; Vrdoljak, T.; Skelin, A.; Polašek, O.; Plečko, M.; Trbojević-Akmačić, I.; Lauc, G.; et al. A 24-Month Follow-Up Study of the Effect of Intra-Articular Injection of Autologous Microfragmented Fat Tissue on Proteoglycan Synthesis in Patients with Knee Osteoarthritis. Genes 2019, 10, 1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudetz, D.; Borić, I.; Rod, E.; Jeleč, Ž.; Kunovac, B.; Polašek, O.; Vrdoljak, T.; Plečko, M.; Skelin, A.; Polančec, D.; et al. Early Results of Intra-Articular Micro-Fragmented Lipoaspirate Treatment in Patients with Late Stages Knee Osteoarthritis: A Prospective Study. Croat. Med. J. 2019, 60, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Hudetz, D.; Borić, I.; Rod, E.; Jeleč, Ž.; Radić, A.; Vrdoljak, T.; Skelin, A.; Lauc, G.; Trbojević-Akmačić, I.; Plečko, M.; et al. The Effect of Intra-Articular Injection of Autologous Microfragmented Fat Tissue on Proteoglycan Synthesis in Patients with Knee Osteoarthritis. Genes 2017, 8, 270. [Google Scholar] [CrossRef] [Green Version]
- Pers, Y.-M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P.; et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Koh, Y.-G.; Kwon, O.-R.; Kim, Y.-S.; Choi, Y.-J.; Tak, D.-H. Adipose-Derived Mesenchymal Stem Cells With Microfracture Versus Microfracture Alone: 2-Year Follow-up of a Prospective Randomized Trial. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-S.; Kim, H.J.; Kim, K.-I.; Kim, G.B.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, S.; Taghiyar, L.; Safari, F.; Baghaban Eslaminejad, M. Regenerative Medicine Applications of Mesenchymal Stem Cells. Adv. Exp. Med. Biol. 2018, 1089, 115–141. [Google Scholar] [CrossRef]
- Primorac, D.; Molnar, V.; Matišić, V.; Hudetz, D.; Jeleč, Ž.; Rod, E.; Čukelj, F.; Vidović, D.; Vrdoljak, T.; Dobričić, B.; et al. Comprehensive Review of Knee Osteoarthritis Pharmacological Treatment and the Latest Professional Societies’ Guidelines. Pharmaceuticals 2021, 14, 205. [Google Scholar] [CrossRef] [PubMed]
- Ceserani, V.; Ferri, A.; Berenzi, A.; Benetti, A.; Ciusani, E.; Pascucci, L.; Bazzucchi, C.; Coccè, V.; Bonomi, A.; Pessina, A.; et al. Angiogenic and Anti-Inflammatory Properties of Micro-Fragmented Fat Tissue and Its Derived Mesenchymal Stromal Cells. Vasc. Cell 2016, 8, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrell, C.R.; Simovic Markovic, B.; Fellabaum, C.; Arsenijevic, A.; Djonov, V.; Volarevic, V. Molecular Mechanisms Underlying Therapeutic Potential of Pericytes. J. Biomed. Sci. 2018, 25, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, A.W.; Péault, B. Perivascular Mesenchymal Progenitors for Bone Regeneration. J. Orthop. Res. 2019, 37, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- De Souza, L.E.B.; Malta, T.M.; Kashima Haddad, S.; Covas, D.T. Mesenchymal Stem Cells and Pericytes: To What Extent Are They Related? Stem Cells Dev. 2016, 25, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Shigeura, T.; Matsumoto, D.; Sato, T.; Takaki, Y.; Aiba-Kojima, E.; Sato, K.; Inoue, K.; Nagase, T.; Koshima, I.; et al. Characterization of Freshly Isolated and Cultured Cells Derived from the Fatty and Fluid Portions of Liposuction Aspirates. J. Cell. Physiol. 2006, 208, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.J.O.; Breuls, R.G.M.; Schouten, T.E.; Jurgens, W.J.F.M.; Bontkes, H.J.; Schuurhuis, G.J.; van Ham, S.M.; van Milligen, F.J. Phenotypical and Functional Characterization of Freshly Isolated Adipose Tissue-Derived Stem Cells. Stem Cells Dev. 2007, 16, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, J.B.; McIntosh, K.; Zvonic, S.; Garrett, S.; Floyd, Z.E.; Kloster, A.; Di Halvorsen, Y.; Storms, R.W.; Goh, B.; Kilroy, G.; et al. Immunophenotype of Human Adipose-Derived Cells: Temporal Changes in Stromal-Associated and Stem Cell-Associated Markers. Stem Cells 2006, 24, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Zimmerlin, L.; Donnenberg, V.S.; Pfeifer, M.E.; Meyer, E.M.; Péault, B.; Rubin, J.P.; Donnenberg, A.D. Stromal Vascular Progenitors in Adult Human Adipose Tissue. Cytom. Part J. Int. Soc. Anal. Cytol. 2010, 77, 22–30. [Google Scholar] [CrossRef]
- Polancec, D.; Zenic, L.; Hudetz, D.; Boric, I.; Jelec, Z.; Rod, E.; Vrdoljak, T.; Skelin, A.; Plecko, M.; Turkalj, M.; et al. Immunophenotyping of a Stromal Vascular Fraction from Microfragmented Lipoaspirate Used in Osteoarthritis Cartilage Treatment and Its Lipoaspirate Counterpart. Genes 2019, 10, 474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal Stem versus Stromal Cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell Committee Position Statement on Nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef]
- Liang, T.; Zhu, L.; Gao, W.; Gong, M.; Ren, J.; Yao, H.; Wang, K.; Shi, D. Coculture of Endothelial Progenitor Cells and Mesenchymal Stem Cells Enhanced Their Proliferation and Angiogenesis through PDGF and Notch Signaling. FEBS Open Bio 2017, 7, 1722–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguirre, A.; Planell, J.A.; Engel, E. Dynamics of Bone Marrow-Derived Endothelial Progenitor Cell/Mesenchymal Stem Cell Interaction in Co-Culture and Its Implications in Angiogenesis. Biochem. Biophys. Res. Commun. 2010, 400, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Bouland, C.; Philippart, P.; Dequanter, D.; Corrillon, F.; Loeb, I.; Bron, D.; Lagneaux, L.; Meuleman, N. Cross-Talk Between Mesenchymal Stromal Cells (MSCs) and Endothelial Progenitor Cells (EPCs) in Bone Regeneration. Front. Cell Dev. Biol. 2021, 9, 674084. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, B.; Shaw, I.; Lesme, H.; Yong, L.; Khan, N.; Tremolada, C.; Péault, B. Higher Pericyte Content and Secretory Activity of Microfragmented Human Adipose Tissue Compared to Enzymatically Derived Stromal Vascular Fraction. Stem Cells Transl. Med. 2018, 7, 876–886. [Google Scholar] [CrossRef] [Green Version]
- Kanayasu-Toyoda, T.; Yamaguchi, T.; Oshizawa, T.; Hayakawa, T. CD31 (PECAM-1)-Bright Cells Derived from AC133-Positive Cells in Human Peripheral Blood as Endothelial-Precursor Cells. J. Cell. Physiol. 2003, 195, 119–129. [Google Scholar] [CrossRef]
- Bieback, K.; Hecker, A.; Schlechter, T.; Hofmann, I.; Brousos, N.; Redmer, T.; Besser, D.; Klüter, H.; Müller, A.M.; Becker, M. Replicative Aging and Differentiation Potential of Human Adipose Tissue-Derived Mesenchymal Stromal Cells Expanded in Pooled Human or Fetal Bovine Serum. Cytotherapy 2012, 14, 570–583. [Google Scholar] [CrossRef]
- Jeske, R.; Yuan, X.; Fu, Q.; Bunnell, B.A.; Logan, T.M.; Li, Y. In Vitro Culture Expansion Shifts the Immune Phenotype of Human Adipose-Derived Mesenchymal Stem Cells. Front. Immunol. 2021, 12, 621744. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Zhu, H.; Zhang, J.; Ouyang, W.; Tang, J.; Zhang, Y.; Qiu, L.; Liu, X.; Ding, Z.; Deng, X. CD73 Expression on Mesenchymal Stem Cells Dictates the Reparative Properties via Its Anti-Inflammatory Activity. Stem Cells Int. 2019, 2019, 8717694. [Google Scholar] [CrossRef]
- Suto, E.G.; Mabuchi, Y.; Toyota, S.; Taguchi, M.; Naraoka, Y.; Itakura, N.; Matsuoka, Y.; Fujii, Y.; Miyasaka, N.; Akazawa, C. Advantage of Fat-Derived CD73 Positive Cells from Multiple Human Tissues, Prospective Isolated Mesenchymal Stromal Cells. Sci. Rep. 2020, 10, 15073. [Google Scholar] [CrossRef]
- Pan, Z.; Zhou, Z.; Zhang, H.; Zhao, H.; Song, P.; Wang, D.; Yin, J.; Zhao, W.; Xie, Z.; Wang, F.; et al. CD90 Serves as Differential Modulator of Subcutaneous and Visceral Adipose-Derived Stem Cells by Regulating AKT Activation That Influences Adipose Tissue and Metabolic Homeostasis. Stem Cell Res. Ther. 2019, 10, 355. [Google Scholar] [CrossRef] [Green Version]
- Moraes, D.A.; Sibov, T.T.; Pavon, L.F.; Alvim, P.Q.; Bonadio, R.S.; Da Silva, J.R.; Pic-Taylor, A.; Toledo, O.A.; Marti, L.C.; Azevedo, R.B.; et al. A Reduction in CD90 (THY-1) Expression Results in Increased Differentiation of Mesenchymal Stromal Cells. Stem Cell Res. Ther. 2016, 7, 97. [Google Scholar] [CrossRef] [Green Version]
- Brooks, A.E.S.; Iminitoff, M.; Williams, E.; Damani, T.; Jackson-Patel, V.; Fan, V.; James, J.; Dunbar, P.R.; Feisst, V.; Sheppard, H.M. Ex Vivo Human Adipose Tissue Derived Mesenchymal Stromal Cells (ASC) Are a Heterogeneous Population That Demonstrate Rapid Culture-Induced Changes. Front. Pharmacol. 2019, 10, 1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz-Rodriguez, F.; Guerrero-Esteo, M.; Botella, L.-M.; Banville, D.; Vary, C.P.H.; Bernabéu, C. Endoglin Regulates Cytoskeletal Organization through Binding to ZRP-1, a Member of the Lim Family of Proteins. J. Biol. Chem. 2004, 279, 32858–32868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, J.; Kurtz, A.; Barutcu, N.; Bodo, J.; Thiel, A.; Dong, J. Concerted Regulation of CD34 and CD105 Accompanies Mesenchymal Stromal Cell Derivation from Human Adventitial Stromal Cell. Stem Cells Dev. 2013, 22, 815–827. [Google Scholar] [CrossRef]
- Zimmerlin, L.; Park, T.S.; Donnenberg, V.S.; Zambidis, E.T.; Donnenberg, A.D. Pericytes: A Ubiquitous Source of Multipotent Adult Tissue Stem Cells. In Stem Cells in Aesthetic Procedures: Art, Science, and Clinical Techniques; Shiffman, M.A., Di Giuseppe, A., Bassetto, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 135–148. ISBN 978-3-642-45207-9. [Google Scholar]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Moravcikova, E.; Meyer, E.M.; Corselli, M.; Donnenberg, V.S.; Donnenberg, A.D. Proteomic Profiling of Native Unpassaged and Culture-Expanded Mesenchymal Stromal Cells (MSC). Cytom. Part A 2018, 93, 894–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kebir, A.; Harhouri, K.; Guillet, B.; Liu, J.W.; Foucault-Bertaud, A.; Lamy, E.; Kaspi, E.; Elganfoud, N.; Vely, F.; Sabatier, F.; et al. CD146 Short Isoform Increases the Proangiogenic Potential of Endothelial Progenitor Cells in Vitro and in Vivo. Circ. Res. 2010, 107, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Bowles, A.C.; Kouroupis, D.; Willman, M.A.; Perucca Orfei, C.; Agarwal, A.; Correa, D. Signature Quality Attributes of CD146+ Mesenchymal Stem/Stromal Cells Correlate with High Therapeutic and Secretory Potency. Stem Cells 2020, 38, 1034–1049. [Google Scholar] [CrossRef]
- Kwok, K.H.M.; Lam, K.S.L.; Xu, A. Heterogeneity of White Adipose Tissue: Molecular Basis and Clinical Implications. Exp. Mol. Med. 2016, 48, e215. [Google Scholar] [CrossRef] [Green Version]
- Aksu, A.E.; Rubin, J.P.; Dudas, J.R.; Marra, K.G. Role of Gender and Anatomical Region on Induction of Osteogenic Differentiation of Human Adipose-Derived Stem Cells. Ann. Plast. Surg. 2008, 60, 306–322. [Google Scholar] [CrossRef]
- Yang, H.J.; Kim, K.-J.; Kim, M.K.; Lee, S.J.; Ryu, Y.H.; Seo, B.F.; Oh, D.-Y.; Ahn, S.-T.; Lee, H.Y.; Rhie, J.W. The Stem Cell Potential and Multipotency of Human Adipose Tissue-Derived Stem Cells Vary by Cell Donor and Are Different from Those of Other Types of Stem Cells. Cells Tissues Organs 2014, 199, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, E.; Casadei, R.; Frabetti, F.; Ventura, C.; Facchin, F.; Canaider, S. Sex-Specific Transcriptome Differences in Human Adipose Mesenchymal Stem Cells. Genes 2020, 11, 909. [Google Scholar] [CrossRef] [PubMed]
- Sammour, I.; Somashekar, S.; Huang, J.; Batlahally, S.; Breton, M.; Valasaki, K.; Khan, A.; Wu, S.; Young, K.C. The Effect of Gender on Mesenchymal Stem Cell (MSC) Efficacy in Neonatal Hyperoxia-Induced Lung Injury. PLoS ONE 2016, 11, e0164269. [Google Scholar] [CrossRef] [Green Version]
- Costa, L.A.; Eiro, N.; Fraile, M.; Gonzalez, L.O.; Saá, J.; Garcia-Portabella, P.; Vega, B.; Schneider, J.; Vizoso, F.J. Functional Heterogeneity of Mesenchymal Stem Cells from Natural Niches to Culture Conditions: Implications for Further Clinical Uses. Cell. Mol. Life Sci. 2021, 78, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal Stem Cells: Immune Evasive, Not Immune Privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Immunophenotype | Lineage Markers | Mesenchymal Stem/Stromal Cell (MSC) Markers |
|---|---|---|
| EP | CD45−CD31+CD34+CD146± | CD73±CD90±CD105± |
| Pericytes | CD45−CD31−CD34−CD146+ | CD73±CD90+CD105− |
| SA-ASC | CD45−CD31−CD34+CD146− | CD73highCD90+CD105− |
| Leukocytes | CD45+CD31−CD34−CD146− | CD73−CD90−CD105− |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zenic, L.; Polancec, D.; Hudetz, D.; Jelec, Z.; Rod, E.; Vidovic, D.; Staresinic, M.; Sabalic, S.; Vrdoljak, T.; Petrovic, T.; et al. Polychromatic Flow Cytometric Analysis of Stromal Vascular Fraction from Lipoaspirate and Microfragmented Counterparts Reveals Sex-Related Immunophenotype Differences. Genes 2021, 12, 1999. https://doi.org/10.3390/genes12121999
Zenic L, Polancec D, Hudetz D, Jelec Z, Rod E, Vidovic D, Staresinic M, Sabalic S, Vrdoljak T, Petrovic T, et al. Polychromatic Flow Cytometric Analysis of Stromal Vascular Fraction from Lipoaspirate and Microfragmented Counterparts Reveals Sex-Related Immunophenotype Differences. Genes. 2021; 12(12):1999. https://doi.org/10.3390/genes12121999
Chicago/Turabian StyleZenic, Lucija, Denis Polancec, Damir Hudetz, Zeljko Jelec, Eduard Rod, Dinko Vidovic, Mario Staresinic, Srecko Sabalic, Trpimir Vrdoljak, Tadija Petrovic, and et al. 2021. "Polychromatic Flow Cytometric Analysis of Stromal Vascular Fraction from Lipoaspirate and Microfragmented Counterparts Reveals Sex-Related Immunophenotype Differences" Genes 12, no. 12: 1999. https://doi.org/10.3390/genes12121999
APA StyleZenic, L., Polancec, D., Hudetz, D., Jelec, Z., Rod, E., Vidovic, D., Staresinic, M., Sabalic, S., Vrdoljak, T., Petrovic, T., Cukelj, F., Molnar, V., Cemerin, M., Matisic, V., Brlek, P., Djukic Koroljevic, Z., Boric, I., Lauc, G., & Primorac, D. (2021). Polychromatic Flow Cytometric Analysis of Stromal Vascular Fraction from Lipoaspirate and Microfragmented Counterparts Reveals Sex-Related Immunophenotype Differences. Genes, 12(12), 1999. https://doi.org/10.3390/genes12121999








