Abstract
Acidithiobacillus species are fundamental players in biofilm formation by acidophile bioleaching communities. It has been previously reported that Acidithiobacillus ferrooxidans possesses a functional quorum sensing mediated by acyl-homoserine lactones (AHL), involved in biofilm formation, and AHLs naturally produced by Acidithiobacillus species also induce biofilm formation in Acidithiobacillus thiooxidans. A c-di-GMP pathway has been characterized in Acidithiobacillus species but it has been pointed out that the c-di-GMP effector PelD and pel-like operon are only present in the sulfur oxidizers such as A. thiooxidans. PEL exopolysaccharide has been recently involved in biofilm formation in this Acidithiobacillus species. Here, by comparing wild type and ΔpelD strains through mechanical analysis of biofilm-cells detachment, fluorescence microscopy and qPCR experiments, the structural role of PEL exopolysaccharide and the molecular network involved for its biosynthesis by A. thiooxidans were tackled. Besides, the effect of AHLs on PEL exopolysaccharide production was assessed. Mechanical resistance experiments indicated that the loss of PEL exopolysaccharide produces fragile A. thiooxidans biofilms. qRT-PCR analysis established that AHLs induce the transcription of pelA and pelD genes while epifluorescence microscopy studies revealed that PEL exopolysaccharide was required for the development of AHL-induced biofilms. Altogether these results reveal for the first time that AHLs positively regulate pel genes and participate in the molecular network for PEL exopolysaccharide biosynthesis by A. thiooxidans.
1. Introduction
The biomining industry takes advantage of the metabolism of leaching microorganisms which mediate the dissolution of metal sulfides through their ability to oxidize ferrous iron and reduced inorganic sulfur compounds (RISCs). Bioleaching has been successfully used for the recovery of cobalt, gold, nickel, zinc and it is currently used for the recovery of copper from low-grade ores [1,2]. However, the leaching of metal sulfides under uncontrolled circumstances creates environmental pollution in the form of acid mine/rock drainage (AMD/ARD) [3]. It has been reported that biofilm formation by bacterial cells on minerals is a key step for leaching performance due to the formation of a thin reaction space between ore and cells, which is filled by extracellular polymeric substances including exopolysaccharides (EPS), proteins, lipids and uronic acids [4,5]. Understanding the molecular events involved in biofilm formation by acidophilic species may help to develop improvements in biomining technologies or to mitigate AMD/ARD. Due to their role in bioleaching, bacteria belonging to Acidithiobacillus genus were the first acidophilic species to be characterized and considered to be pivotal players for the biomining process [6,7].
In bacteria, biofilm formation is mainly controlled by two specific and widespread phenomena named quorum sensing (QS) [8,9] and the cyclic diguanylate (c-di-GMP) pathway [9,10,11,12]. QS mechanisms have been studied for several decades in Gram-negative and Gram-positive bacteria and in addition they also have been identified in eukaryotic fungi and the Protista kingdom [13,14]. QS is defined as a cell-cell communication process that regulates gene expression in a cell-density-dependent manner. This is achieved through the secretion of diffusible autoinducers (AIs), allowing the expression of different behaviours more suitable for the cell population rather than individual cells [8]. These include virulence, EPS biosynthesis and biofilm formation. Different QS systems have been reported, and characterized AIs have been described as specific molecular players for intraspecies as well as interspecies cell-cell communication [15,16,17,18,19]. Canonical QS systems in Gram-positive bacteria involve oligopeptides as AI and a specific two-component system for the signal transduction [20]. QS type AI-1, which is mediated by N-acyl-homoserine lactone (AHL) molecules through its binding to transcriptional regulators belonging to the LuxR-like protein family, is one of the best characterized QS systems in Gram-negative bacteria [20]. A type AI-1 QS system was reported in Acidithiobacillus ferrooxidans [21]. It includes canonical afeR and afeI genes, encoding for the transcriptional regulator AfeR and the AHL synthase AfeI. Since AfeI is capable of driving the biosynthesis of nine different AHLs, it has been suggested that cross-communication could occur between A. ferrooxidans and other bacterial species belonging to the bioleaching ecological niche [22]. In this way, it has been reported that attachment to pyrite by the sulfur oxidizer Acidithiobacillus thiooxidans requires a pre-colonization step mediated by iron oxidizing species [23]. In addition, the use of synthetic QS molecules revealed that AHLs naturally produced by iron/sulfur oxidizer A. ferrooxidans promote biofilm formation not only in A. ferrooxidans [24], but also in the sulfur oxidizer A. thiooxidans [23]. An initial bioinformatics analysis suggested that the A. ferrooxidans QS regulon may comprise 75 genes some of them involved in polysaccharide biosynthesis [25]. Moreover, DNA microarray experiments performed in A. ferrooxidans by using an AHL super-agonist analog indicated that 42.5% of predicted QS regulon genes are related to biofilm formation and to the biosynthesis, polymerization and secretion of exopolysaccharides [26]. Nevertheless, the molecular network involved in biofilm formation by Acidithiobacillus species, from the addition of exogenous AHL to EPS production upon cell attachment on pyrite or sulfur surfaces, remains to be deciphered.
The second messenger c-di-GMP has emerged as a central metabolite that controls several phenotypes in bacteria, including motility and biofilm formation [11,12]. This messenger is synthesized by diguanylate cyclase (DGC) enzymes and degraded by c-di-GMP specific phosphodiesterases (PDEs) [11,12]. The signal transduction is carried out by several families of proteins and RNA receptors [27]. One of the first c-di-GMP effector proteins to be characterized was the inner-membrane protein PelD from Pseudomonas aeruginosa [28]. The PelD protein is part of a multiproteic complex involved in the biosynthesis and export of PEL, an exopolysaccharide involved in pellicle formation at the air/surface interface of P. aeruginosa static liquid cultures [28]. The unique architecture and export mechanism of the PEL polysaccharide synthase, as well as the structural composition of PEL exopolysaccharide have recently been deciphered in P. aeruginosa [29,30,31]. PEL is a cationic exopolysaccharide, mainly composed of N-acetylgalactosamine and N-acetylglucosamine subunits [29], whose translocation across the outer membrane requires functional PelB and PelC proteins [30], and the binding of c-di-GMP to PelD for recruiting PelF and promoting its glycosil-transferase activity through quaternary rearrangements of the PEL polysaccharide synthase PelDEFG [31,32]. Functional c-di-GMP pathways have been reported and partially characterized in three Acidithiobacillus species, and directly related to exopolysaccharide production and biofilm formation [33,34,35]. In addition, by analysing 35 chromosomal replicons [36] it has been recently reported that the c-di-GMP pathway is widespread among the Acidithiobacillus species complex. Castro et al. [36] also corroborated that: (i) the c-di-GMP network is highly diverse, depending on both species and strains, the most complex c-di-GMP metabolism pathways being identified in A. thiooxidans strains, which can harbour up to 40 DGC and PDE encoding genes [36]; (ii) despite a wide diversity of c-di-GMP effectors such as the transcriptional regulator FleQ [37] and PilZ domain, initially identified in the cellulose synthase subunit BcsA [38], PelD and the pel operon were identified in very few species of acidophile. Thus, two operons, encoding for cellulose (bcs operon) and PEL exopolysaccharide (pel operon) biosynthetic pathways have been suggested to be involved in biofilm formation in Acidithiobacilus species [34,35]. However, while the bcs operon is widespread in iron/sulfur-oxidizing species, the pel operon [39] has been identified only in the sulfur-oxidizing species A. caldus and A. thiooxidans [30,34,35]. Indeed, a pel-like operon was identified in A. caldus and A. thiooxidans, and the construction of an A. thiooxidans ΔpelD null-mutant strain revealed that PEL exopolysaccharide is involved in its biofilm architecture [35]. Therefore, PEL was the first exopolysaccharide experimentally linked with biofilm formation by Acidithiobacillus species [35].
During the last decade, several reports have revealed that QS and the c-di-GMP pathway form intricate molecular networks that can integrate data on population density and environmental conditions in different bacterial species [40,41,42,43,44]. Indeed, pellicle formation at the air–surface interface of a bacterial culture, which is supported by the production of PEL and BEP exopolysaccharides in P. aeruginosa and Burkholderia cenocepacia, respectively, is induced by high intracellular levels of c-di-GMP, but it is negatively regulated by two different QS pathways decreasing c-di-GMP content either deactivating DGC or activating PDE enzymes [40,42,44,45]. However, despite the results reported by Diaz et al. [35], the identification of the molecular players involved in biosynthesis of PEL exopolysaccharide and biofilm formation by Acidithiobacillus is still mostly incomplete. In order to gain insights into the regulatory network involved in the biosynthesis of PEL exopolysaccharide by Acidithiobacillus species, and also to assess if QS signalling, c-di-GMP and PEL exopolysaccharide are interconnected during biofilm formation by A. thiooxidans ATCC 19377T, fluorescence microscopy and qPCR experiments were performed, taking advantage of the ΔpelD null-mutant strain [35]. The results of the present work demonstrated that in our experimental conditions transcription levels of pel genes were increased when A. thiooxidans planktonic cells were exposed to QS signalling molecule 3-oxo-C8-AHL, while fluorescent lectin binding analysis (FLBA) combined with epifluorescence microscopy clearly indicated that PEL exopolysaccharide was required for the development of AHL-induced biofilms. Therefore, this work provides the first evidence that QS signalling molecules are positively linked to the transcription of pel genes including the c-di-GMP effector encoding gene pelD and PEL exopolysaccharide biosynthesis during biofilm formation in Acidithiobacillus species. Nevertheless, further studies are still necessary to fully decipher the interplay of QS and c-di-GMP molecular pathways in Acidithiobacillus species.
2. Materials and Methods
2.1. Strains and Growth Conditions
A. thiooxidans ATCC 19377T parental strain and null-mutant ΔpelD derived strain whose biofilm architecture is modified by this deletion [35] were routinely grown with agitation (150 rpm) at 30 °C under aerobic conditions in Mackintosh (MAC) medium at pH 4.5 [46], supplemented with 5% w/v sulfur (S°) prills as energy substrate. For fluorescence microscopy experiments and mechanical strength analysis, S°-coupons (0.5 cm2, obtained by S° melting and fusion) were added to the MAC medium. The null-mutant strain ΔpelD was maintained in MAC medium supplemented with 150 μg/mL kanamycin. For A. thiooxidans growth in the presence of AHL signalling molecules (5 µM final concentration), 3-oxo-C8-AHL and C8-AHL (SIGMA®, Oakville, ON, Canada) were added from 50 mM stock solutions in 100% DMSO.
2.2. Visualization of A. thiooxidansT Biofilms
Colonized S°-coupons obtained from three independent cultures of A. thiooxidansT cells grown in the presence of 3-oxo-C8-AHL (5 µM), C8-AHL (5 µM) or 0.01% DMSO were extracted from 5-days growth cultures and washed as described [35]. FLBA was done as described by Zhang et al. [47]. Coupons were then incubated in darkness for 1 h in lectin buffer (10 mM NaH2PO4 pH 7.2, 150 mM NaCl), supplemented with different FITC-conjugated lectins at 50 µg/mL (Table S1) (EY Laboratories®, San Mateo, CA, USA). For FITC-conjugate GS-II lectin, CaCl2 (0.5 mM) was added into the lectin buffer. After incubation, S°-coupons were washed with the same lectin buffer and counterstained with 4,6-diamidino-2-phenylindole (DAPI) at 1 mg/mL in 2% formaldehyde, to fix the samples [23]. Finally, coupons were washed with sterile water, dried at room temperature, mounted with a drop of an anti-fading agent (Citifluor® AF2) and imaged by epifluorescence microscopy. Images were taken with an inverted Axiovert-100 MBP microscope (Zeiss®) equipped with an HBO 100 mercury vapour lamp, filters for DAPI (Ex 358 nm/Em 461 nm) and FITC (Ex 490 nm/Em 505–545), a Zeiss® filterset 49 air-objective (Zeiss® EC epiplan NEOFLUAR 420363/9901) and a digital microscope camera (Zeiss® AxioCam® MRm). All images were acquired by viewing five different coupons from each independent culture and processed using the software Axio-Vision 4.2 (Zeiss®).
2.3. Transcriptional Analyses
Real Time RT-PCR experiments (qRT-PCR) were performed as previously described [35]. Total RNA was extracted from both planktonic and biofilm cells obtained from A. thiooxidansT cultures grown in the presence of 3-oxo-C8-AHL (5 µM) or 0.01% DMSO during five days. Planktonic cells were directly collected by centrifugation for 15 min at 6000× g while biofilm cells were previously separated from S°-prills by 10 min incubation with 0.05% Triton X-100 and vortexing [35]. cDNA was synthetized from 1 µg of total RNA obtained from both cell sub-populations by using reverse transcriptase (Promega, Madison, WI, USA) and random primers (Promega, Madison, WI, USA). Transcriptional levels of pel (pelA, pelD and wcaG) and flaA genes were measured with specific primers (Table S2), including both 16S rDNA gene and the methionine aminopeptidase encoding gene map, used as housekeeping genes for data normalization [48].
2.4. Mechanical Resistance of A. thiooxidans Biofilms
To investigate the role of both AHL signalling molecules and PEL exopolysaccharide on biofilm formation by A. thiooxidansT, a specific assay to measure mechanical resistance of attached cells was developed. First, to eliminate any remaining planktonic cells, colonized S°-coupons were extracted from three independent 5-days growth cultures of A. thiooxidansT cells and washed twice with aqueous H2SO4 pH 2. The mechanical resistance of attached cells was assessed by incubating the colonized S°-coupons in a Triton X-100 solution (0.05% Triton X-100, pH 2) and vortexing for 10 min. Afterwards, the A. thiooxidans cells released were quantified at different times of incubation by separating free A. thiooxidans cells from colonized S°-coupons through centrifugation at low velocity (1000× g). Suspensions of released cells were diluted in acidic water (pH 2.0, H2SO4) and counted in a Petroff-Hausser counting chamber. The number of released cells was normalized by the mass of S°-coupons.
2.5. Bioinformatics Search for a LuxR-Like Protein
The search for a LuxR-like orthologs in A. thiooxidans proteomes was performed by Blastp [49] with defaults parameters (e-value < 0.05) using AfeR (A. ferrooxidans), SdiA (Escherichia coli) and RpaR (Rhodopseudomonas palustris) as queries against the new available genome sequences of A. thiooxidans. Functional protein domains from queries and subject proteins were validated against the Conserved Domain Database (CDD) v.3.18 and Pfam Database V.32.0 using CD-search [50] with defaults parameters (e-value < 0.01).
3. Results
3.1. N-Acetyl-Galactosamine and N-Acetyl-Glucosamine Are Structural Blocks of PEL Exopolysaccharide in A. thiooxidansT
To gain some insights about the sugar composition of the A. thiooxidans cell surface and to understand how PEL exopolysaccharide is involved, FLBA was performed using twelve different lectins. In our experimental conditions, most of the tested lectins did not bind A. thiooxidans wild type cells (Table S1). However, binding signals were observed with lectins AAL, BPA, ConA and GS-II, which bind l-Fucose α(1,6) N-Acetyl-d-Glucosamine, N-acetyl-d-galactosamine, internal d-mannose and d-Glucose and N-acetyl-d-glucosamine, respectively (Figure 1 and Figure S1).
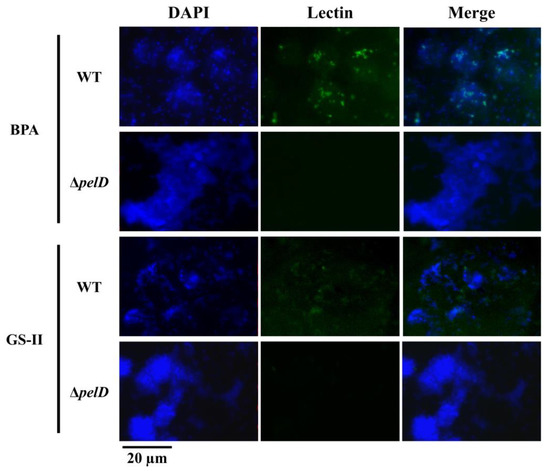
Figure 1.
Analysis of PEL exopolysaccharide composition by using epifluorescence microscopy coupled to FLBA. S°-coupons colonized by A. thiooxidansT (WT) or mutant derived (ΔpelD) cells were extracted from 5-days growth cultures and incubated with FITC-conjugated BPA or GS-II lectins. Then, they were stained with DAPI before microscopy imaging. Size bars represent 20 µm.
In agreement with Diaz et al. [35], DAPI staining corroborated that wild type and ΔpelD null-mutant cells are capable of forming biofilms on S°-coupons (Figure 2 and Figure S2) and definitively pointed out that A. thiooxidans cells can also adhere to S°-coupons independently of PEL. AAL, BPA and GS-II clearly indicated that the glycoconjugate composition of biofilm cell surfaces is different in the two strains. Positive binding signals were obtained with the aforementioned three lectins for the wild type strain, while no (BPA, GS-II) or decreased (AAL) fluorescence signals were obtained for ΔpelD null-mutant strain (Figure 2 and Figure S2), indicating that the PEL exopolysaccharide of A. thiooxidans is most likely composed of N-acetyl-d-galactosamine and N-acetyl-d-glucosamine.
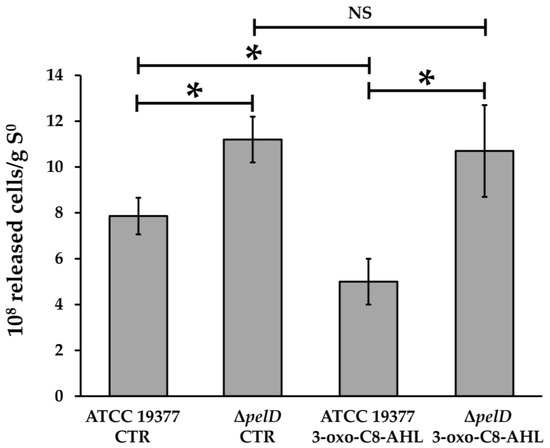
Figure 2.
The presence of PEL exopolysaccharide offers a stronger embedment into the biofilm matrix for At. thiooxidans cells. Inoculated S°-coupons extracted from 5-days growth cultures were treated with 0.05% Triton X-100 and vortexed during 10 min. Number of cells released from the wild type and ΔpelD null-mutant biofilms subjected to mechanical stress was determined with a Petroff-Hausser counting chamber and normalized against mass of sulfur. Significant differences calculated by a one-way ANOVA test (p < 0.05) are noted (*). CTR, DMSO 0.01% without AHL. NS, No significant difference.
3.2. The Loss of PEL Exopolysaccharide Produces Fragile Biofilms in A. thiooxidans
Diaz et al. [35] have previously reported that ΔpelD mutation affects biofilm architecture. In order to assess the relevance of PEL exopolysaccharide for A. thiooxidans biofilm architecture and function, wild type and ΔpelD null-mutant biofilms were subjected to mechanical stress by vortexing colonized S°-coupons. As shown in Figure 2, the number of cells released at the end of vortexing (10 min) from control ΔpelD biofilms without 3-OH-C8-AHL was 29.9% higher than those released from wild type biofilms. This increase was also observed at different times of the vortexing assay (Figure S2).
Concordantly, and compared to the ΔpelD null-mutant strain, the presence of PEL exopolysaccharide reduced the release of wild type cells from S°-coupons in presence of the QS signalling molecule 3-oxo-C8-AHL by 53.3% (Figure 2). Moreover, a significant decrease (26.4%) was also observed for WT cells grown with 3-oxo-C8-AHL compared to WT cells without QS signalling molecules and the highest releases were observed for ΔpelD biofilms with or without 3-oxo-C8-AHL (Figure 2). Altogether, these results clearly show that the strength of A. thiooxidans cells embedding and the mechanical resistance of the biofilm matrix are correlated to the presence of PEL exopolysaccharide. In addition, our results highlight for the first time a role for an AHL signalling molecule in PEL biosynthesis by this acidophile species.
3.3. PEL Biosynthesis by A. thiooxidans Requires QS Signalling Molecules
Ueda and Wood [40] reported that QS mediated by the Las system negatively modulates pellicle/biofilm formation in P. aeruginosa. In addition, while sulfur-oxidizing species such as A. thiooxidans and A. caldus do not possess any canonical genes for QS [51,52], it has been reported that biofilm formation in A. thiooxidansT can be induced by the addition of QS signalling molecules 3-oxo-C8-AHL or C8-AHL [23]. Thus, we decided to assess the influence of the addition of these AHLs on PEL production and biofilm formation, by comparing the A. thiooxidans ΔpelD null-mutant and WT strains. Despite the basal level of adhered cells observed in both controls without QS signalling molecules and all ΔpelD experiments that corroborated that A. thiooxidans can adhere to S°-coupons independently of PEL, results obtained by fluorescence microscopy clearly indicated that the formation of the AHL-induced biofilm by A. thiooxidans requires the presence of the c-di-GMP effector PelD (Figure 3).
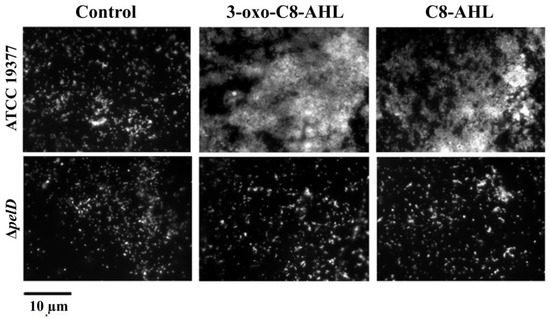
Figure 3.
The deletion of pelD interferes with A. thiooxidans biofilm response to both QS signalling molecules 3-oxo-C8-AHL and C8-AHL. S°-coupons were inoculated with A. thiooxidans ATCC 19,377 or mutant derived ΔpelD strains and extracted from 5-days growth cultures. Then they were washed and stained with 0.01% DAPI. Finally, S°-coupons were viewed by fluorescence microscopy. Size bars represent 10 µm. Control, DMSO 0.01% without AHL.
This indicates that these QS signalling molecules have a positive effect on PEL biosynthesis and suggests that it could regulate the expression of the PEL apparatus. In order to test this hypothesis, transcriptional analyses of three genes belonging to the A. thiooxidans pel operon (pelA, desacetylase PelA; pelD, c-di-GMP effector protein PelD; wcaG, UDP-Glucose-4-epimerase) were performed in the presence of 3-oxo-C8-AHL (5 μM), since it appeared to induce the highest biofilm formation in this microorganism (Figure 3). No difference was measured for pelA, pelD and wcaG transcription levels by comparing biofilm cells with or without addition of 3-oxo-C8-AHL (Figure 4 and Figure S3). However by comparing data obtained from planktonic and attached cells in controls (CTR) experiments, it is possible to point out that basal transcription levels are higher in biofilm cells compared to planktonic. This supports the idea that AHL effect on pelA and pelD transcriptions takes place early in A. thiooxidans planktonic cells to promote the shift of lifestyle from planktonic to adhered as it was reported by a global transcriptional analysis in A. ferrooxidans performed to assess the transcriptional effect of an AHL analogue on biofilm formation by this Acidithiobacillus species [26].
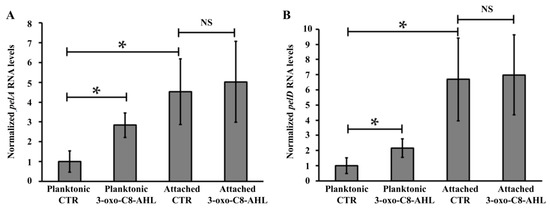
Figure 4.
Effects of 3-oxo-C8-AHL addition on transcription levels of pelA and pelD genes. Transcript levels of pelA (A) and pelD (B) genes were measured by qRT-PCR. Total RNAs were obtained from 5-days growth cultures. Data were normalized using DNA 16S and map genes. Values represent the average of 4 independent experiments ± standard deviation. Significant differences calculated by a one-way ANOVA test (p < 0.05) are noted (*). CTR, DMSO 0.01% without AHL. NS, No significant difference.
However, in planktonic cells, the transcription levels of the pelA and pelD genes were increased 2.2- and 2.8-fold in presence of 3-oxo-C8-AHL (5 μM), compared to control assays without its addition (Figure 4), while no significant change was observed for the last gene of the pel operon, wcaG (Figure S3). Because it has been reported that motility and biofilm are two phenotypes regulated in an opposite manner by intracellular levels of c-di-GMP, the transcription levels of flaA, a flagellin-like encoding gene present in A. thiooxidans ATCC 19,377 genome, was also assessed. However, none differences were observed by comparing WT and ΔpelD null-mutant planktonic cells grown with or without AHL (Figure S4).
4. Discussion
PEL was recently identified as a structural exopolysaccharide for biofilm formation by A. thiooxidans and it was reported that biofilm structures are different in A. thiooxidans WT strain compared to ΔpelD null-mutant strain that overexpressed a filamentous appendix [35]. However, a better characterization of the molecular events involved in the regulation of PEL biosynthesis is still required to understand its role in biofilm formation and architecture of these acidophilic species. To address this question, the ΔpelD null-mutant strain developed by Diaz et al. [35] was used to analyse the effect of QS signalling molecules, extracellular glycoconjugate diversity, mechanical resistance of biofilms, and to gain more precise new insights into the molecular network involved in the regulation of PEL exopolysaccharide biosynthesis in Acidithiobacillus species that can only oxidize RISCs.
In agreement with results obtained for P. aeruginosa [29], PEL exoplysaccharide from A. thiooxidans appears to be mainly composed of N-acetyl-d-galactosamine and N-acetyl-d-glucosamine. The presence of an additional wcaG gene encoding for an UDP-glucose-4-epimerase [35], downstream of the canonical pel operon, which is overexpressed in biofilm cells compared to planktonic cells (Figure S3), strongly suggested that the formation of UDP-N-acetyl-galactosamine from UDP-N-acetyl-glucosamine could be catalysed by this enzyme, as it has been recently reported by Whitfield et al. [31]. These data point out that the biofilm architecture differences observed between wild type and ΔpelD null-mutant biofilms recently reported by Diaz et al. [35] can be directly related to the presence of PEL exopolysaccharide and its main structural components N-acetyl-d-galactosamine and N-acetyl-d-glucosamine.
Altogether, the results obtained here strongly suggested that the QS molecules 3-oxo-C8-AHL and C8-AHL positively regulate PEL biofilm production by increasing the transcription levels of PEL-apparatus encoding genes including the c-di-GMP binding protein PelD. Interestingly, this result differs with the work of Ueda and Wood [40], which reported that QS negatively regulated PEL production in P. aeruginosa by decreasing c-di-GMP biosynthesis, but it agrees with Pérez-Mendoza et al. [53] who revealed that the ExpR/SinI QS system mediated by AHLs positively regulates the transcription of the bgsA gene encoding for a c-di-GMP effector protein involved in the synthesis of MLG exopolysaccharides by Sinorhizobium meliloti.
On the other hand, qPCR results reported in this work also revealed that transcription levels of flaA do not change in A. thiooxidans WT and ΔpelD cells grown with or without AHL molecules. Then the hypothesis suggesting that the overexpressed filamentous appendix observed in biofilm produced by A. thiooxidans ΔpelD strain could correspond to a mesh of entangled flagella [35] can be discarded. The ability of the A. thiooxidans ΔpelD cells to still be adhered on S°-coupons could be related to the presence of the bcs operon identified in the genome sequence of A. thiooxidansT [34,35] and then cellulose could be responsible for this AHL independent adherence.
How the addition of AHLs increases pel genes mRNAs and enhances attachment to surfaces is a matter of discussion and is still an open question as illustrated by Figure 5.
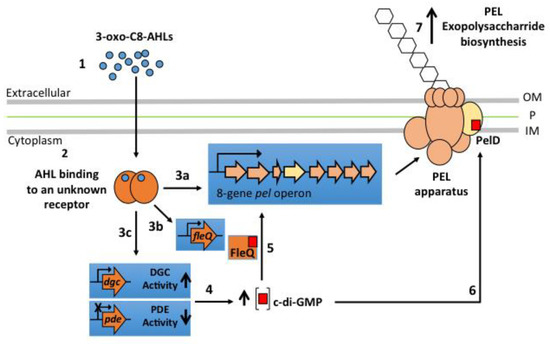
Figure 5.
Working model for the regulation of PEL exopolysaccharide biosynthesis by obligate sulfur-oxidizing Acidithiobacillus species based on experimental data previously reported and obtained here with A. thiooxidans. The binding of 3-oxo-C8-AHL (1) by an unknown AHL receptor that could act as a positive transcriptional regulator (2) can directly promote pel operon expression (3a; Figure 3). But it may also induce the transcription of FleQ (3b) and diguanilate cyclase (DGC) (3c) encoding genes and/or repress the transcription of phosphodiesterases (PDE) encoding genes (3c). The new balance between DGC and PDE activities generate an increase of intracellular c-di-GMP levels (4). Then, c-di-GMP can bind to its specific receptors FleQ (5) and/or PelD (6) promoting the biosynthesis of PEL exopolysaccharide (7). OM, Outer membrane; P, Peptidoglycan; IM, Inner membrane.
Recently, it has been proposed that the flexibility to QS-signalling molecules such as AHLs is mainly due to variability of AHL-receptor proteins [54]. Valdés et al. [51,52] reported that sulfur-oxidizing species A. thiooxidans and A. caldus do not possess any canonical genes for QS system. However, it is well established now that A. thiooxidans is capable to sense AHL signalling molecules to induce biofilm formation [23] (this work) This indicates that an unknown AHL-receptor protein has to exist in this acidophile species and may act as a transcriptional regulator either directly to promote the transcription of pel genes (Figure 5, #3a), or indirectly to induce the transcription of DGC and PDE encoding genes involved in c-di-GMP metabolism (Figure 5, #3c) but also c-di-GMP protein receptors encoding genes such as the transcriptional regulator FleQ (Figure 5, #3b). Further studies are necessary to identify and characterize this unknown AHL-receptor protein. Nevertheless, by performing a bioinformatics analysis on the newly available A. thiooxidans genome sequences (strains A01, CLST, DXS-W, BY-02, A02, DMC, GD1-3, JYC-17, and ZBY) in which a pel operon has been identified (personal communication), we have recently identified a SdiA-like protein with a high e-value (Figure S5) that appears as a strong candidate to play the molecular role of QS transcriptional regulator. Effectively, SdiA is an orphan QS transcriptional regulator, not associated with an AHL synthase, that binds AHLs and has been related to motility and biofilm formation in Escherichia coli and Salmonella enterica serovar Thyphimurium [55,56]. Interestingly, Prescott and Decho [54] have proposed the orphan QS transcriptional regulators as key molecular players for flexibility and adaptability of QS, especially to maintain or develop cell-cell communication during the dynamic evolution of a biofilm community. Indeed, a bioinformatic analysis of the new available genome sequence on ten different genome sequences also revealed that A. thiooxidans and A. caldus possess several copies of the fleQ gene [57]. Then, a possible mechanism of action of the 3-oxo-C8-AHL could be promotion of transcription of a FleQ encoding gene, which in turn could induce PEL exopolysaccharide biosynthesis, as occurs in P. aeruginosa [37]. The existence of interplays between QS and c-di-GMP pathways has been demonstrated in other bacterial species [40,41], in which certain QS molecules regulate activity levels of DCG and PDE enzymes. Therefore, we can also hypothesize that in A. thiooxidans some of the genes targeted by the binary complex (3-oxo-C8-AHL/transcriptional regulator) may encode for proteins with DGC and/or PDE activities, producing an increase in intracellular c-di-GMP levels and consequently PEL-biofilm formation.
5. Conclusions
Here we report that PEL-biofilm contributes to A. thiooxidans cells resistance to mechanical stress. We also reveal that the positive effect induced by QS signalling molecules on biofilm formation by A. thiooxidans is directly mediated by PEL exopolysaccharide. We highlight that AHL molecules induce the transcription of several genes belonging to the pel operon including pelD, which encodes the c-di-GMP effector protein PelD. Finally, these results offer the first opportunity to propose a working model (Figure 5) that will enable further molecular characterization of the regulating network involved in PEL-biofilm formation by obligate sulfur-oxidizing Acidithiobacillus species.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/12/1/69/s1, Table S1: Summary results of fluorescence binding analysis obtained with twelve different lectins; Table S2: Primers used in this work; Figure S1: Analysis of PEL exopolysaccharide glycoconjugate composition by using epifluorescence microscopy coupled to FLBA; Figure S2: The loss of PEL exopolysaccharide amplifies the release of A. thiooxidans attached cells from S°-coupons; Figure S3: Addition of QS molecule 3-oxo-C8-AHL has no significant effect on transcription levels of wcaG from A. thiooxidansT; Figure S4: Transcriptional analysis of flaA gene from A. thiooxidansT; Figure S5: Bioinformatic characterization of WP_024895086.1, a SdiA-like protein present in the new available genome sequences of A. thiooxidans.
Author Contributions
N.G. conceived the research. M.D., N.G. and M.V. designed the experiments. M.D. and D.S.M. performed the experiments. M.C. was in charge of bioinformatics analysis. M.V. helped with microscopy imaging. Manuscript was written by N.G., M.D., M.C. and M.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FONDECYT Grant 1160702 (April 2016–March 2020) from ANID.
Acknowledgments
To Wolfgang Sand, Biofilm Centre, Universität Duisburg-Essen for hosting MD for microscopy and biofilm analyses. M.D. acknowledges CONICYT to support his doctoral studies (scholarship 21120064, 2012). We also thank Diego Rojas Muñoz for his scientific comments related to LuxR-like protein and Tim Rudge, for proofreading the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Rohwerder, T.; Sand, W. Oxidation of Inorganic Sulfur Compounds in Acidophilic Prokaryotes. Eng. Life Sci. 2007, 7, 301–309. [Google Scholar] [CrossRef]
- Sand, W.; Jozsa, P.G.; Kovacs, Z.M.; Sasaran, N.; Schippers, A. Long-term evaluation of acid rock drainage mitigation measures in large lysimeters. J. Geochem. Explor. 2007, 92, 205–211. [Google Scholar] [CrossRef]
- Rohwerder, T.; Gehrke, T.; Kinzler, K.; Sand, W. Bioleaching review part A: Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation. Appl. Microbiol. Biotechnol. 2003, 63, 239–248. [Google Scholar] [CrossRef]
- Harneit, K.; Göksel, A.; Kock, D.; Klock, J.-H.; Gehrke, T.; Sand, W. Adhesion to metal sulfide surfaces by cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans and Leptospirillum ferrooxidans. Hydrometallurgy 2006, 83, 245–254. [Google Scholar] [CrossRef]
- Baker, B.J.; Banfield, J.F. Microbial communities in acid mine drainage. FEMS Microbiol. Ecol. 2003, 44, 139–152. [Google Scholar] [CrossRef]
- Nuñez, H.; Moya-Beltrán, A.; Covarrubias, P.C.; Issotta, F.; Cárdenas, J.P.; González, M.; Atavales, J.; Acuña, L.G.; Johnson, D.B.; Quatrini, R. Molecular Systematics of the Genus Acidithiobacillus: Insights into the Phylogenetic Structure and Diversification of the Taxon. Front. Microbiol. 2017, 8, 30. [Google Scholar] [CrossRef]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Camilli, A.; Bassler, B.L. Bacterial small-molecule signaling pathways. Science 2006, 311, 1113–1116. [Google Scholar] [CrossRef]
- Hengge, R. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 2009, 7, 263–273. [Google Scholar] [CrossRef]
- Römling, U.; Galperin, M.Y.; Gomelsky, M. Cyclic di-GMP: The first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 2013, 77, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Jenal, U.; Reinders, A.; Lori, C. Cyclic di-GMP: Second messenger extraordinaire. Nat. Rev. Microbiol. 2017, 15, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Asghar, A.; Groth, M.; Siol, O.; Gaube, F.; Enzensperger, C.; Glöckner, G.; Winckler, T. Developmental gene regulation by an ancient intercellular communication system in social amoebae. Protist 2012, 163, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Barriuso, J.; Hogan, D.A.; Keshavarz, T.; Martínez, M.J. Role of quorum sensing and chemical communication in fungal biotechnology and pathogenesis. FEMS Microbiol. Rev. 2018, 42, 627–638. [Google Scholar] [CrossRef]
- Flavier, A.B.; Clough, S.J.; Schell, M.A.; Denny, T.P. Identification of 3-hydroxypalmitic acid methyl ester as a novel autoregulator controlling virulence in Ralstonia solanacearum. Mol. Microbiol. 1997, 26, 251–259. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum Sensing in Bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef]
- Pereira, C.S.; Thompson, J.A.; Xavier, K.B. AI-2-mediated signalling in bacteria. FEMS Microbiol. Rev. 2013, 37, 156–181. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, L.H.; Cámara, M.; He, Y.W. The DSF Family of Quorum Sensing Signals: Diversity, Biosynthesis, and Turnover. Trends Microbiol. 2016, 25, 293–303. [Google Scholar] [CrossRef]
- Lin, J.; Cheng, J.; Wang, Y.; Shen, X. The Pseudomonas Quinolone Signal (PQS): Not just for Quorum Sensing anymore. Front. Cell Infect. Microbiol. 2018, 8, 230. [Google Scholar] [CrossRef]
- Ng, W.L.; Bassler, B.L. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 2009, 43, 197–222. [Google Scholar] [CrossRef]
- Farah, C.; Vera, M.; Morin, D.; Haras, D.; Jerez, C.A.; Guiliani, N. Evidence for a functional quorum-sensing type AI-1 system in the extremophilic bacterium Acidithiobacillus ferrooxidans. Appl. Environ. Microbiol. 2005, 71, 7033–7040. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, S.; Banderas, A.; Jerez, C.A.; Guiliani, N. Cell-cell communication in Bacteria. In Microbial Processing of Metal Sulfides; Donati, E.R., Sand, W., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 253–264. [Google Scholar]
- Bellenberg, S.; Díaz, M.; Noël, N.; Sand, W.; Poetsch, A.; Guiliani, N.; Vera, M. Biofilm formation, communication and interactions of leaching bacteria during colonization of pyrite and sulfur surfaces. Res. Microbiol. 2014, 165, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Bellenberg, S.; Mamani, S.; Ruiz, L.; Echeverria, A.; Soulere, L.; Doutheau, A.; Demergasso, C.; Sand, W.; Queneau, Y.; et al. AHL signaling molecules with a large acyl chain enhance biofilm formation on sulfur and metal sulfides by the bioleaching bacterium Acidithiobacillus ferrooxidans. Appl. Microbiol. Biotechnol. 2013, 97, 3729–3737. [Google Scholar] [CrossRef]
- Banderas, A.; Guiliani, N. Bioinformatic prediction of gene functions regulated by quorum sensing in the bioleaching bacterium Acidithiobacillus ferrooxidans. Int. J. Mol. Sci. 2013, 14, 16901–16916. [Google Scholar] [CrossRef] [PubMed]
- Mamani, S.; Moiner, D.; Denis, Y.; Soulere, L.; Queneau, Y.; Talla, E.; Bonnefoy, V.; Guiliani, N. Insights into the Quorum Sensing Regulon of the Acidophilic Acidithiobacillus ferrooxidans Revealed by Transcriptomic in the Presence of an Acyl Homoserine Lactone Superagonist Analog. Front. Microbiol. 2016, 7, 1365. [Google Scholar] [CrossRef] [PubMed]
- Hengge, R. Trigger phosphodiesterases as a novel class of c-di-GMP effector proteins. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150498. [Google Scholar] [CrossRef]
- Lee, V.T.; Matewish, J.M.; Kessler, J.L.; Hyodo, M.; Hayakawa, Y.; Lory, S. A cyclic-di-GMP receptor required for bacterial exopolysaccharide production. Mol. Microbiol. 2007, 65, 1474–1484. [Google Scholar] [CrossRef]
- Jennings, L.K.; Storek, K.M.; Ledvina, H.E.; Coulon, C.; Marmont, L.S.; Sadovskaya, I.; Secor, P.R.; Tseng, B.S.; Scian, M.; Filloux, A.; et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA 2015, 112, 11353–11358. [Google Scholar] [CrossRef]
- Marmont, L.S.; Rich, J.D.; Whitney, J.C.; Whitfield, G.B.; Almblad, H.; Robinson, H.; Parsek, M.R.; Harrison, J.J.; Howell, P.L. Oligomeric lipoprotein PelC guides Pel polysaccharide export across the outer membrane of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2017, 114, 2892–2897. [Google Scholar] [CrossRef]
- Whitfield, G.B.; Marmont, L.S.; Ostaszewski, A.; Rich, J.D.; Whitney, J.C.; Parsek, M.R.; Harrison, J.J.; Howell, P.L. Pel polysaccharide biosynthesis requires an inner membrane complex comprised of PelD, PelE, PelF, and PelG. J. Bacteriol. 2020, 202, e00684-19. [Google Scholar] [CrossRef]
- Franklin, M.J.; Nivens, D.E.; Weadge, J.T.; Howell, P.L. Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, Alginate, Pel, and Psl. Front. Microbiol. 2011, 2, 167. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.M.; Castro, M.; Barriga, A.; Jerez, C.A.; Guiliani, N. The extremophile Acidithiobacillus ferrooxidans possesses a c-di-GMP signalling pathway that could play a significant role during bioleaching of minerals. Lett. Appl. Microbiol. 2012, 54, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Deane, S.M.; Ruiz, L.; Rawlings, D.E.; Guiliani, N. Diguanylate cyclase null mutant reveals that C-Di-GMP pathway regulates the motility and adherence of the extremophile bacterium Acidithiobacillus caldus. PLoS ONE 2015, 10, e0116399. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.; Castro, M.; Copaja, S.; Guiliani, N. Biofilm formation by the acidophile bacterium Acidithiobacillus thiooxidans involves c-di-GMP pathway and Pel exopolysaccharide. Genes 2018, 9, 113. [Google Scholar] [CrossRef]
- Castro, M.; Díaz, M.; Moya, A.; Guiliani, N. Cyclic di-GMP Signaling in Extreme Acidophilic Bacteria. In Microbial Cyclic Di-Nucleotide Signaling; Chou, S.H., Guiliani, N., Lee, V.T., Römling, U., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2020; ISBN 978-3-030-33307-2/978-3-030-33308-9. [Google Scholar] [CrossRef]
- Hickman, J.W.; Harwood, C.S. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol. Microbiol. 2008, 69, 376–389. [Google Scholar] [CrossRef]
- Amikam, D.; Galperin, M. PilZ domain is part of the bacterial c-di-GMP binding protein. Bioinformatics 2006, 22, 3–6. [Google Scholar] [CrossRef]
- Friedman, F.; Kolter, R. Genes involved in matrix formation in Pseudomonas aeruginosa PA 14 biofilms. Mol. Microbiol. 2004, 51, 675–690. [Google Scholar] [CrossRef]
- Ueda, A.; Wood, T.K. Connecting quorum sensing, c-di-GMP, pel polysaccharide, and biofilm formation in Pseudomonas aeruginosa through tyrosine phosphatase TpbA (PA 3885). PLoS Pathog. 2009, 5, e1000483. [Google Scholar] [CrossRef]
- Srivastava, D.; Waters, C.M. A tangled web: Regulatory connections between Quorum Sensing and Cyclic di-GMP. J. Bacteriol. 2012, 194, 4485–4493. [Google Scholar] [CrossRef]
- Deng, Y.; Schmid, N.; Wang, C.; Wang, J.; Pessi, G.; Wu, D.; Lee, J.; Aguilar, C.; Ahrens, C.H.; Chang, C.; et al. Cis-2-dodecenoic acid receptor RpfR links quorum-sensing signal perception with regulation of virulence through cyclic dimeric guanosine monophosphate turnover. Proc. Natl. Acad. Sci. USA 2012, 109, 15479–15484. [Google Scholar] [CrossRef]
- Lin Chua, S.; Liu, Y.; Li, Y.; Jun Ting, H.; Kohli, G.S.; Cai, Z.; Suwanchaikasem, P.; Kau Kit Goh, K.; Pin Ng, S.; Tolker-Nielsen, T.; et al. Reduced intracellular c-di-GMP content increases expression of Quorum Sensing-regulated genes in Pseudomonas aeruginosa. Front. Cell Infect. Microbiol. 2017, 7, 451. [Google Scholar] [CrossRef] [PubMed]
- Schmid, N.; Suppiger, A.; Steiner, E.; Pessi, G.; Kaever, V.; Fazli, M.; Tolker-Nielsen, T.; Jenal, U.; Eberl, L. High intracellular c-di-GMP levels antagonize quorum sensing and virulence gene expression in Burkholderia cenocepacia H 111. Microbiology 2017, 63, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.M.; Fazli, M.; Schmid, N.; Shilling, R.; Suppiger, A.; Givskov, M.; Eberl, L.; Tolker-Nielsen, T. Key Players and Individualists of Cyclic-di-GMP Signaling in Burkholderia cenocepacia. Front. Microbiol. 2019, 9, 3286. [Google Scholar] [CrossRef] [PubMed]
- Mackintosh, M.E. Nitrogen Fixation by Thiobacillus ferrooxidans. J. Gen. Microbiol. 1978, 105, 215–218. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Neu, T.R.; Bellenberg, S.; Kuhlicke, U.; Sand, W.; Vera, M. Use of lectins to in situ visualize glycoconjugates of extracellular polymeric substances in acidophilic archaeal biofilms. Microb. Biotechnol. 2015, 8, 448–461. [Google Scholar] [CrossRef]
- Nieto, P.A.; Covarrubias, P.C.; Jedlicki, E.; Holmes, D.S.; Quatrini, R. Selection and evaluation of reference genes for improved interrogation of microbial transcriptomes: Case study with the extremophile Acidithiobacillus ferrooxidans. BMC Mol. Biol. 2009, 10, 63. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Lu, S.; Anderson, J.B.; Chitsaz, F.; Derbyshire, M.K.; De Weese-Scott, C.; Fong, J.H.; Geer, L.Y.; Geer, R.C.; Gonzales, N.R.; et al. CDD: A Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 2011, 39, 225–229. [Google Scholar] [CrossRef]
- Valdés, J.; Pedroso, I.; Quatrini, R.; Holmes, D.S. Comparative genome analysis of Acidithiobacillus ferrooxidans, A. thiooxidans and A. caldus: Insights into their metabolism and ecophysiology. Hydrometallurgy 2008, 94, 180–184. [Google Scholar] [CrossRef]
- Valdés, J.; Ossandon, F.; Quatrini, R.; Dopson, M.; Holmes, D.S. Draft genome sequence of the extremely acidophilic biomining bacterium Acidithiobacillus thiooxidans ATCC 19377 provides insights into the evolution of the Acidithiobacillus genus. J. Bacteriol. 2011, 193, 7003–7004. [Google Scholar] [CrossRef]
- Pérez-Mendoza, D.; Rodríguez-Carvajal, M.A.; Romero-Jiménez, L.; De Araujo Farias, G.; Lloret, J.; Gallegos, M.T.; Sanjuána, J. Novel mixed-linkage β-glucan activated by c-di-GMP in Sinorhizobium meliloti. Proc. Natl. Acad. Sci. USA 2015, 112, E757–E765. [Google Scholar] [CrossRef] [PubMed]
- Prescott, R.D.; Decho, A.W. Flexibility and Adaptability of Quorum Sensing in Nature. Trends Microbiol. 2020, 28, 436–444. [Google Scholar] [CrossRef]
- Smith, J.L.; Fratamico, P.M.; Yan, X. Eavesdropping by Bacteria: The Role of SdiA in Escherichia coli and Salmonella enterica Serovar Typhimurium Quorum Sensing. Foodborne Pathog. Dis. 2011, 8, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.E.; Patankar, A.V. Orphan LuxR regulators of quorum sensing. FEMS Microbiol. Rev. 2009, 33, 739–756. [Google Scholar] [CrossRef]
- Moya-Beltrán, A.; Rojas-Villalobos, C.; Diaz, M.; Guiliani, N.; Quatrini, R.; Castro, M. Nucleotide Second Messenger-Based Signaling in Extreme Acidophiles of the Acidithiobacillus Species Complex: Partition Between the Core and Variable Gene Complements. Front. Microbiol. 2019, 7, 381. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).