Telomere Dynamics in the Diploid and Triploid Rainbow Trout (Oncorhynchus mykiss) Assessed by Q-FISH Analysis
Abstract
1. Introduction
2. Materials and Method
2.1. Fish Origin
2.2. Egg Incubation and Fish Rearing
2.3. Preparation of Interphase Spreads
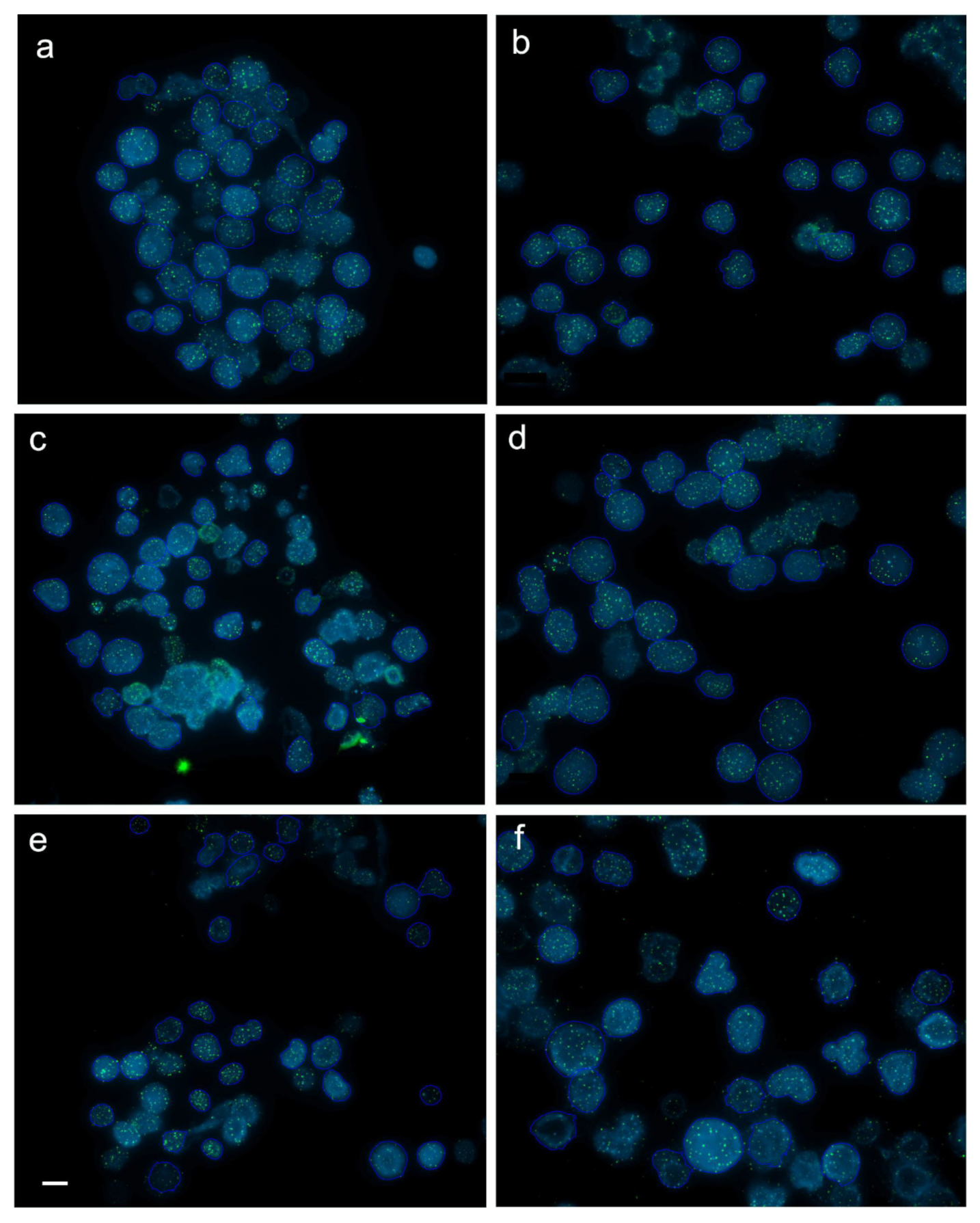
2.4. Interphase Quantitative Fluorescence In Situ Hybridization (Q-FISH)
2.5. Statistical Analysis
3. Results
3.1. Body Weight and Length and Gonadal Development
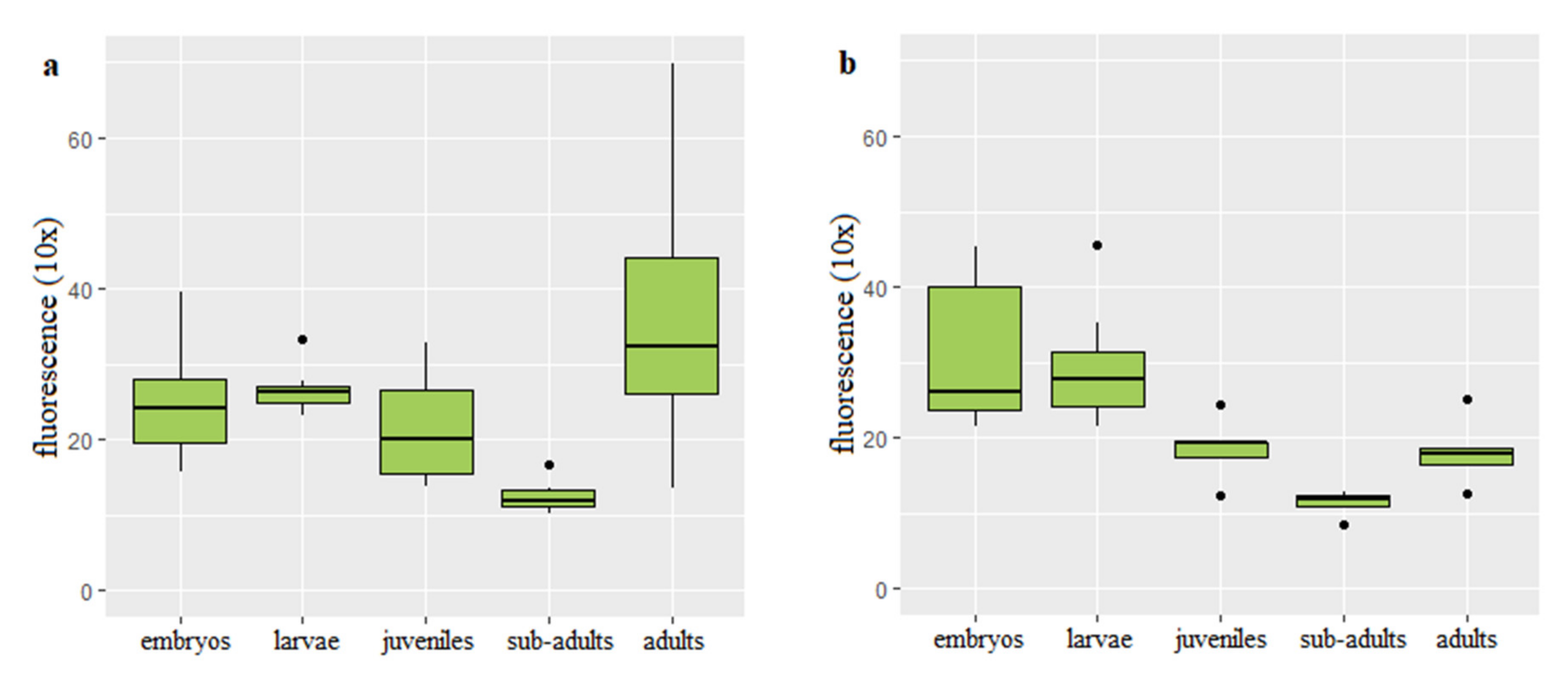
3.2. Dynamics of Telomere Length in Rainbow Trout
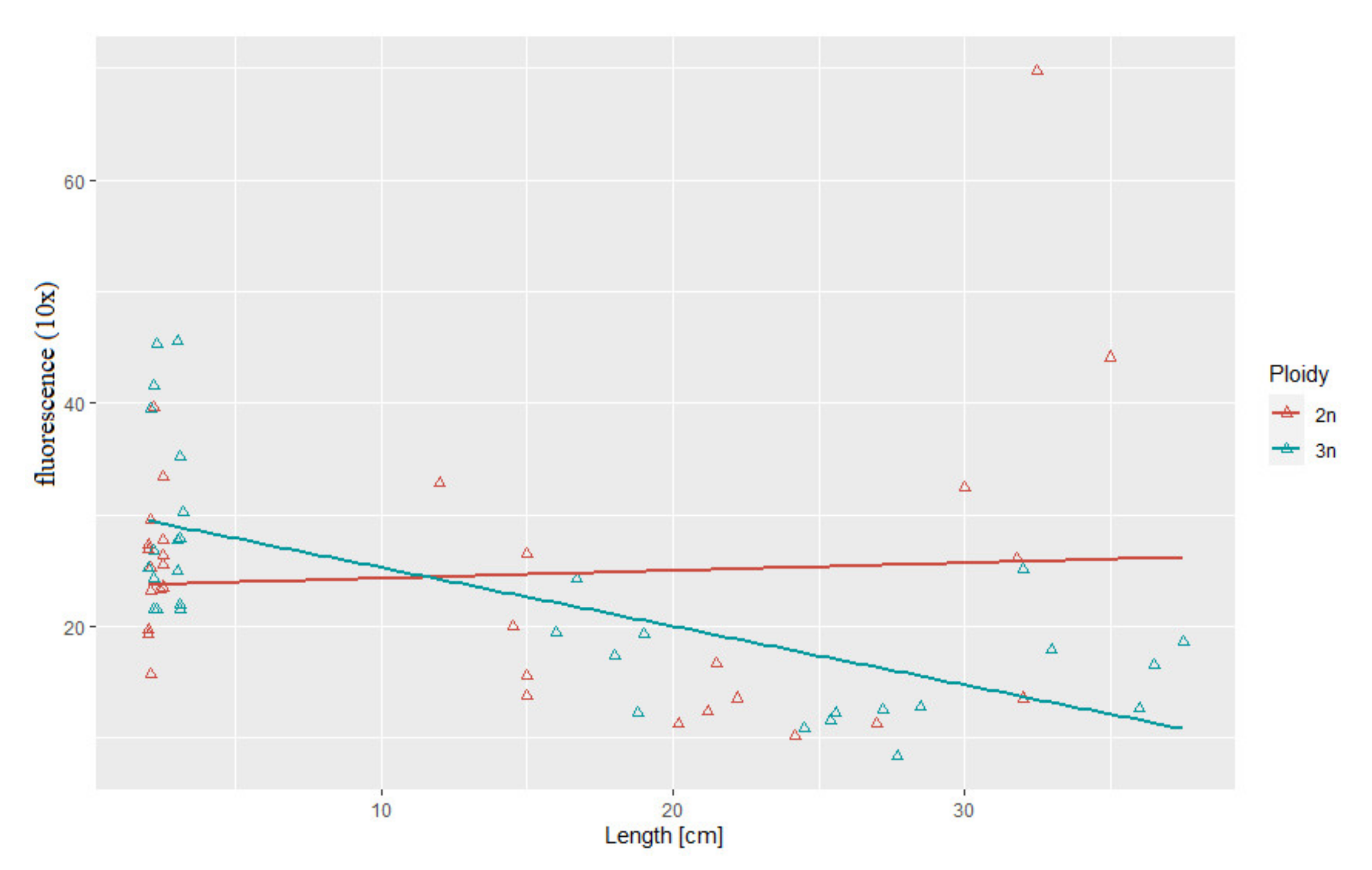
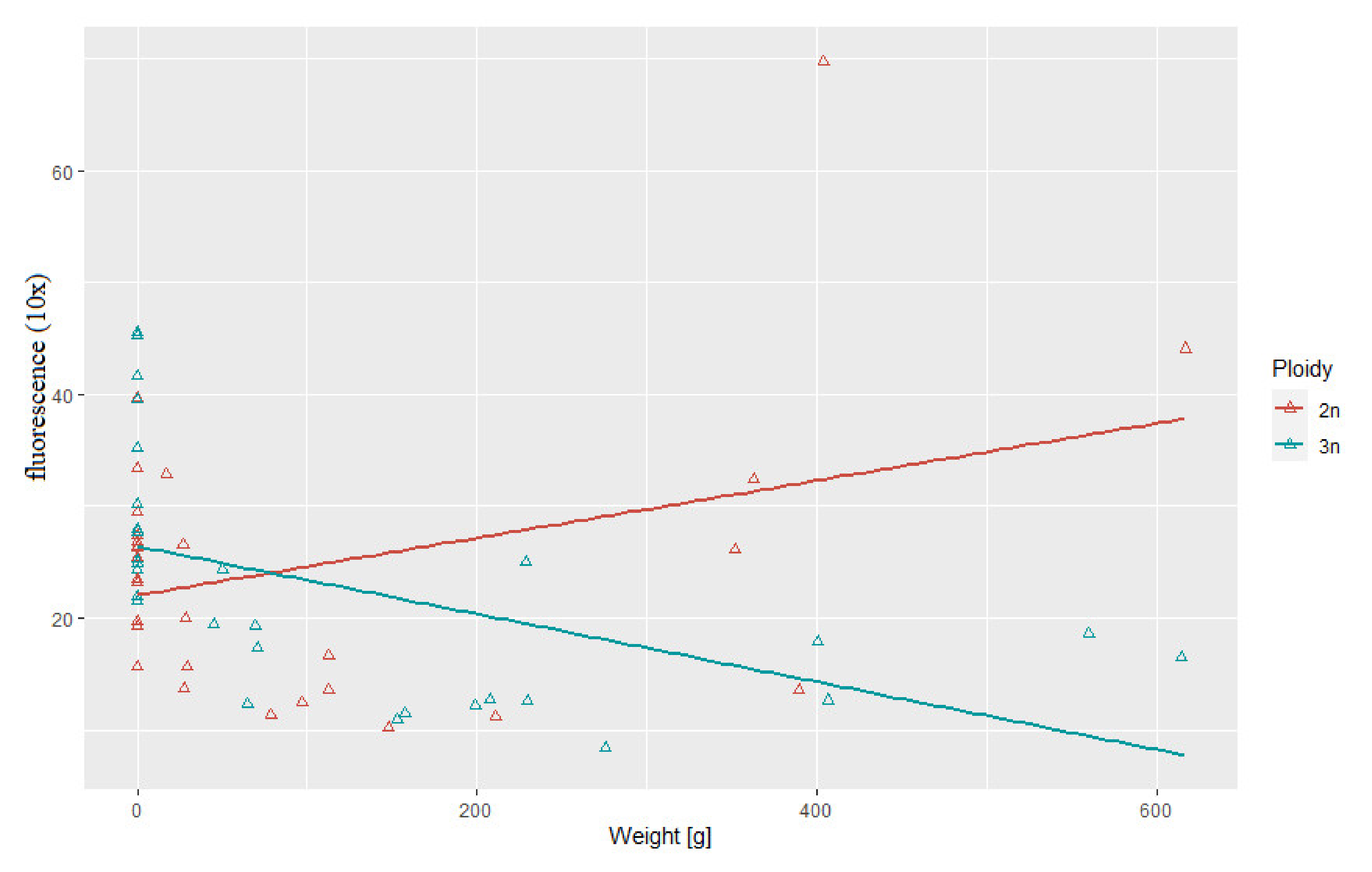
3.3. Correlation between Body Weight and Length and Telomere Length-Related Fluorescence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meyne, J.; Ratliff, R.L.; Moyzis, R.K. Conservation of the human telomere sequence (TTAGGG) n among vertebrates. Proc. Natl. Acad. Sci. USA 1989, 86, 7049–7053. [Google Scholar] [CrossRef]
- Bolzán, A.D.; Bianchi, M.S. Telomeres, interstitial telomeric repeat sequences, and chromosomal aberrations. Mutat. Res. Rev. Mutat. Res. 2006, 612, 189–214. [Google Scholar] [CrossRef]
- De Lange, T. Protection of mammalian telomeres. Oncogene 2002, 21, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Olovnikov, A.M. A theory of marginotomy: The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J. Theor. Biol. 1973, 41, 181–190. [Google Scholar] [CrossRef]
- Von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemato, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell Biochem. 2004, 266, 37–56. [Google Scholar] [CrossRef]
- Klapper, W.; Heidorn, K.; Kühne, K.; Parwaresch, R.; Krupp, G. Telomerase activity in ‘immortal’fish 1. FEBS Lett. 1998, 434, 409–412. [Google Scholar] [CrossRef]
- Elmore, L.W.; Norris, M.W.; Sircar, S.; Bright, A.T.; McChesney, P.A.; Winn, R.N.; Holt, S.E. Upregulation of telomerase function during tissue regeneration. Exp. Biol. Med. 2008, 233, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Lund, T.C.; Glass, T.J.; Tolar, J.; Blazar, B.R. Expression of telomerase and telomere length are unaffected by either age or limb regeneration in Danio rerio. PLoS ONE 2009, 4, e7688. [Google Scholar] [CrossRef]
- Gomes, N.M.; Shay, J.W.; Wright, W.E. Telomere biology in Metazoa. FEBS Lett. 2010, 584, 3741–3751. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Wapstra, E.; Friesen, C. Ectothermic telomeres: It’s time they came in from the cold. In Philosophical Transactions of the Royal Society B: Biological Sciences 373; The Royal Society: Lodon, UK, 2018. [Google Scholar]
- Scott, N.M.; Haussmann, M.F.; Elsey, R.M.; Trosclair, P.L.; Vleck, C.M. Telomere length shortens with body length in Alligator mississippiensis. Southeast Nat. 2006, 5, 685–692. [Google Scholar] [CrossRef][Green Version]
- Bronikowski, A.M. The evolution of aging phenotypes in snakes: A review and synthesis with new data. Age 2008, 30, 169. [Google Scholar] [CrossRef] [PubMed]
- Plot, V.; Criscuolo, F.; Zahn, S.; Georges, J.J.-Y. Telomeres, Age and Reproduction in a long-lived reptile. PLoS ONE 2012, 7, e40855. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Nakamura, K.I.; Izumiyama-Shimomura, N.; Ishii, A.; Tsuchida, S.; Takubo, K.; Ishikawa, N. The teleost Oryzias latipes shows telomere shortening with age despite considerable telomerase activity throughout life. Mech. Ageing Dev. 2008, 129, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.; Reichwald, K.; Lechel, A.; Graf, M.; Kirschner, J.; Dorn, A.; Terzbasi, E.; Wellner, J.; Platzer, M.; Rudolph, K.; et al. Telomeres shorten while Tert expression increases during ageing of the short-lived fish Nothobranchius furzeri. Mech. Ageing Dev. 2009, 130, 290–296. [Google Scholar] [CrossRef]
- Horn, T.; Gemmell, N.J.; Robertson, B.C.; Bridges, C.R. Telomere length change in European sea bass (Dicentrarchus labrax). Aust J. Zool. 2008, 56, 207–210. [Google Scholar] [CrossRef]
- Gao, J.; Munch, S.B. Does reproductive investment decrease telomere length in Menidia menidia? PLoS ONE 2015, 10, e0125674. [Google Scholar] [CrossRef]
- Anchelin, M.; Murcia, L.; Alcaraz-Pérez, F.; García-Navarro, E.M.; Cayuela, M.L. Behaviour of telomere and telomerase during aging and regeneration in zebrafish. PLoS ONE 2011, 6, e16955. [Google Scholar] [CrossRef]
- Monaghan, P.; Ozanne, S.E. Somatic growth and telomeric dynamics in vertebrates: Relationships, mechanisms and consequences. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160446. [Google Scholar] [CrossRef]
- Barrett, E.L.B.; Richardson, D.S. Sex differences in telomeres and lifespan. Aging Cell 2011, 10, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.; Borrás, C.; Gambini, J.; Sastre, J.; Pallardó, F.V. Why females live longer than males? Importance of the upregulation of longevity-associated genes by oestrogenic compounds. FEBS Lett. 2005, 12, 2541–2545. [Google Scholar] [CrossRef]
- Lindsay, W.R.; Friesen, C.R.; Sihlbom, C.; Bergström, J.; Berger, E.; Wilson, M.R.; Olsson, M. Vitellogenin offsets oxidative costs of reproduction in female painted dragon lizards. J. Exp. Biol. 2020, 223, jeb221630. [Google Scholar] [CrossRef]
- Piferrer, F.; Beaumont, A.; Falguière, J.C.; Flajšhans, M.; Haffray, P.; Colombo, L. Polyploid fish and shellfish: Production, biology and applications to aquaculture for performance improvement and genetic containment. Aquaculture 2009, 293, 125–156. [Google Scholar] [CrossRef]
- Tivary, B.K.; Kirubagaran, R.; Ray, A.K. The biology of triploid fish. Rev. Fish Biol. Fish. 2004, 14, 391–402. [Google Scholar]
- Cormier, S.M.; Neiheisel, T.W.; Williams, D.E.; Tiersch, T.R. Natural occurrence of triploidy in a wild brown bullhead. Trans. Am. Fish. Soc. 1993, 122, 390–392. [Google Scholar] [CrossRef]
- Fernandes-Matioli, F.M.C.; Almeida-Toled, L.F.; Toledo-Filho, S.A. Natural triploidy in the Neotropical species Gymnotus carapo (Pisces: Gymnotiformes). Caryologia 1998, 51, 319–322. [Google Scholar] [CrossRef]
- Ocalewicz, K.; Dobosz, S. Karyotype variation in the albino rainbow trout (Oncorhynchus mykiss (Walbaum)). Genome 2009, 52, 347–352. [Google Scholar] [CrossRef]
- Pandian, T.J.; Koteeswaran, R. Ploidy induction and sex control in fish. Hydrobiologia 1998, 384, 167–243. [Google Scholar] [CrossRef]
- Poon, S.S.; Lansdorp, P.M. Quantitative fluorescence in situ hybridization (Q-FISH). Curr. Protoc. Cell Biol. 2001, 12, 18.4.1–18.4.21. [Google Scholar] [CrossRef]
- Montpetit, A.J.; Alhareeri, A.A.; Montpetit, M.; Starkweather, A.R.; Elmore, L.W.; Filler, K.; Mohanraj, L.; Burton, C.W.; Menzies, V.S.; Lyon, D.E.; et al. Telomere length: A review of methods for measurement. Nurs. Res. 2014, 63, 289. [Google Scholar] [CrossRef]
- Polonis, M.; Fujimoto, T.; Dobosz, S.; Zalewski, T.; Ocalewicz, K. Genome incompatibility between rainbow trout (Oncorhynchus mykiss) and sea trout (Salmo trutta) and induction of the interspecies gynogenesis. J. Appl. Genet. 2018, 59, 91–97. [Google Scholar] [CrossRef]
- Ocalewicz, K.; Furgala-Slezniow, G.; Szmyt, M.; Lisboa, R.; Kuciński, M.; Lejk, A.M.; Jankun, M. Pericentromeric location of the telomeric DNA sequences on the European grayling chromosomem. Genetica 2013, 141, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Ocalewicz, K.; Woznicki, P.; Jankun, M. Mapping of rRNA genes and telomeric sequences in Danube salmon (Hucho hucho) chromosomes using primed in situ labeling technique (PRINS). Genetica 2008, 134, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Ocalewicz, K.; Sliwinska, A.; Jankun, M. Autosomal localization of Interstitial Telomeric Site (ITS) in brook trout, Salvelinus fontinalis (Pisces, Salmonidae). Cytogenet. Genome Res. 2004, 105, 79–82. [Google Scholar] [CrossRef]
- Jankun, M.; Woznicki, P.; Ocalewicz, K.; Furgala-Selezniow, G. Chromosomal evolution in the three species of Holarctic fish of the genus Coregonus (Salmoniformes). Adv. Limnol. 2007, 60, 25–37. [Google Scholar]
- Pauliny, A.; Devlin, R.H.; Johnsson, J.I.; Blomqvist, D. Rapid growth accelerates telomere attrition in a transgenic fish. BMC Evol. Biol. 2015, 15, 159. [Google Scholar] [CrossRef]
- McLennan, D.; Armstrong, J.D.; Stewart, D.C.; Mckelvey, S.; Boner, W.; Monaghan, P.; Metcalfe, N.B. Shorter juvenile telomere length is associated with higher survival to spawning in migratory Atlantic salmon. Funct. Ecol. 2017, 31, 2070–2079. [Google Scholar] [CrossRef]
- McLennan, D.; Armstrong, J.D.; Stewart, D.C.; Mckelvey, S.; Boner, W.; Monaghan, P.; Metcalfe, N.B. Telomere elongation during early development is independent of environmental temperatures in Atlantic salmon. J. Exp. Biol. 2018, 221. [Google Scholar] [CrossRef]
- Fauconneau, B.; Kaushik, S.J.; Blanc, J.M. Uptake and metabolization of dissolved compounds in rainbow trout (Salmo gairdneri R.) fry. Comp. Biochem. Physiol. 1989, 93, 839–843. [Google Scholar] [CrossRef]
- Oliva-Teles, A.; Kaushik, S.J. Nitrogen and energy metabolism during the early ontogeny of diploid and triploid rainbow trout (Salmo gairdneri R.). Comp. Biochem. Physiol. 1987, 87, 157–160. [Google Scholar] [CrossRef]
- Oliva-Teles, A.; Kaushik, S.J. Effect of temperature on utilization of endogenous energy reserves during embryonic development of diploid and triploid rainbow trout (Salmo gairdneri R.). Aquaculture 1990, 84, 373–382. [Google Scholar] [CrossRef]
- Shrimpton, J.M.; Heath, J.W.; Devlin, R.H.; Heath, D.D. Effect of triploidy on growth and ionoregulatory performance in ocean-type Chinook salmon: A quantitative genetics approach. Aquaculture 2012, 362, 248–254. [Google Scholar] [CrossRef]
- Leggatt, R.A.; Scheer, K.W.; Afonso, L.O.; Iwama, G.K. Triploid and diploid rainbow trout do not differ in their stress response to transportation. N. Am. J. Aquac. 2006, 68, 1–8. [Google Scholar] [CrossRef]
- Chalmers, L.; Vera, L.M.; Taylor, J.F.; Adams, A.; Migaud, H. Comparative ploidy response to experimental hydrogen peroxide exposure in Atlantic salmon (Salmo salar). Fish Shelfish Immun. 2018, 81, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Risques, R.A.; Promislow, D.E. All’s well that ends well: Why large species have short telomeres. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160448. [Google Scholar] [CrossRef]
- Pauliny, A.; Larsson, K.; Blomqvist, D. Telomere dynamics in a long-lived bird, the barnacle goose. BMC Evol. Biol. 2012, 12, 1–8. [Google Scholar] [CrossRef]
- Rollings, N.; Uhrig, E.J.; Krohmer, R.W.; Waye, H.L.; Mason, R.T.; Olsson, M.; Whittington, C.M.; Friesen, C.R. Age-related sex differences in body condition and telomere dynamics of red-sided garter snakes. Proc. R. Soc. B 2017, 284, 61. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Yamazaki, H.; Nakamura, K.I.; Izumiyama-Shimomura, N.; Aida, J.; Suzuki, H.; Tsuchida, S.; Matsuura, M.; Takubo, K.; Ishikawa, N. Telomere attrition and restoration in the normal teleost Oryzias latipes are linked to growth rate and telomerase activity at each life stage. Aging 2016, 8, 62. [Google Scholar] [CrossRef]
- Biessmann, H.; Mason, J.M. Telomere maintenance without telomerase. Chromosoma 1997, 106, 63–69. [Google Scholar] [CrossRef]
- Henson, J.D.; Neumann, A.A.; Yeager, T.R.; Reddel, R.R. Alternative lengthening of telomeres in mammalian cells. Oncogene 2002, 21, 598–610. [Google Scholar] [CrossRef] [PubMed]
| Stage of Development | Ploidy | Length (cm) | Weight (g) | ||
|---|---|---|---|---|---|
| Mean | ±SD | Mean | ±SD | ||
| Embryos | 2n | 2.08 * | 0.07 | 0.12 | 0.009 |
| 3n | 2.19 * | 0.10 | 0.12 | 0.010 | |
| Larvae | 2n | 2.43 * | 3.14 | 0.12 * | 0.017 |
| 3n | 3.08 * | 0.07 | 0.21 * | 0.021 | |
| Juveniles (one year old) | 2n | 14.30 * | 1.30 | 26.12 * | 5.01 |
| 3n | 17.70 * | 1.31 | 60.28 * | 11.80 | |
| Subadults (two years old) | 2n | 22.72 * | 2.49 | 126.83 * | 47.10 |
| 3n | 26.48 * | 1.55 | 204.00 * | 46.08 | |
| Adults (three years old) | 2n | 32.26 | 1.80 | 425.20 | 109.20 |
| 3n | 35.00 | 2.37 | 442.40 | 151.76 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panasiak, L.; Dobosz, S.; Ocalewicz, K. Telomere Dynamics in the Diploid and Triploid Rainbow Trout (Oncorhynchus mykiss) Assessed by Q-FISH Analysis. Genes 2020, 11, 786. https://doi.org/10.3390/genes11070786
Panasiak L, Dobosz S, Ocalewicz K. Telomere Dynamics in the Diploid and Triploid Rainbow Trout (Oncorhynchus mykiss) Assessed by Q-FISH Analysis. Genes. 2020; 11(7):786. https://doi.org/10.3390/genes11070786
Chicago/Turabian StylePanasiak, Ligia, Stefan Dobosz, and Konrad Ocalewicz. 2020. "Telomere Dynamics in the Diploid and Triploid Rainbow Trout (Oncorhynchus mykiss) Assessed by Q-FISH Analysis" Genes 11, no. 7: 786. https://doi.org/10.3390/genes11070786
APA StylePanasiak, L., Dobosz, S., & Ocalewicz, K. (2020). Telomere Dynamics in the Diploid and Triploid Rainbow Trout (Oncorhynchus mykiss) Assessed by Q-FISH Analysis. Genes, 11(7), 786. https://doi.org/10.3390/genes11070786




