Multiple Sex Chromosomes and Evolutionary Relationships in Amazonian Catfishes: The Outstanding Model of the Genus Harttia (Siluriformes: Loricariidae)
Abstract
1. Introduction
2. Materials and Methods
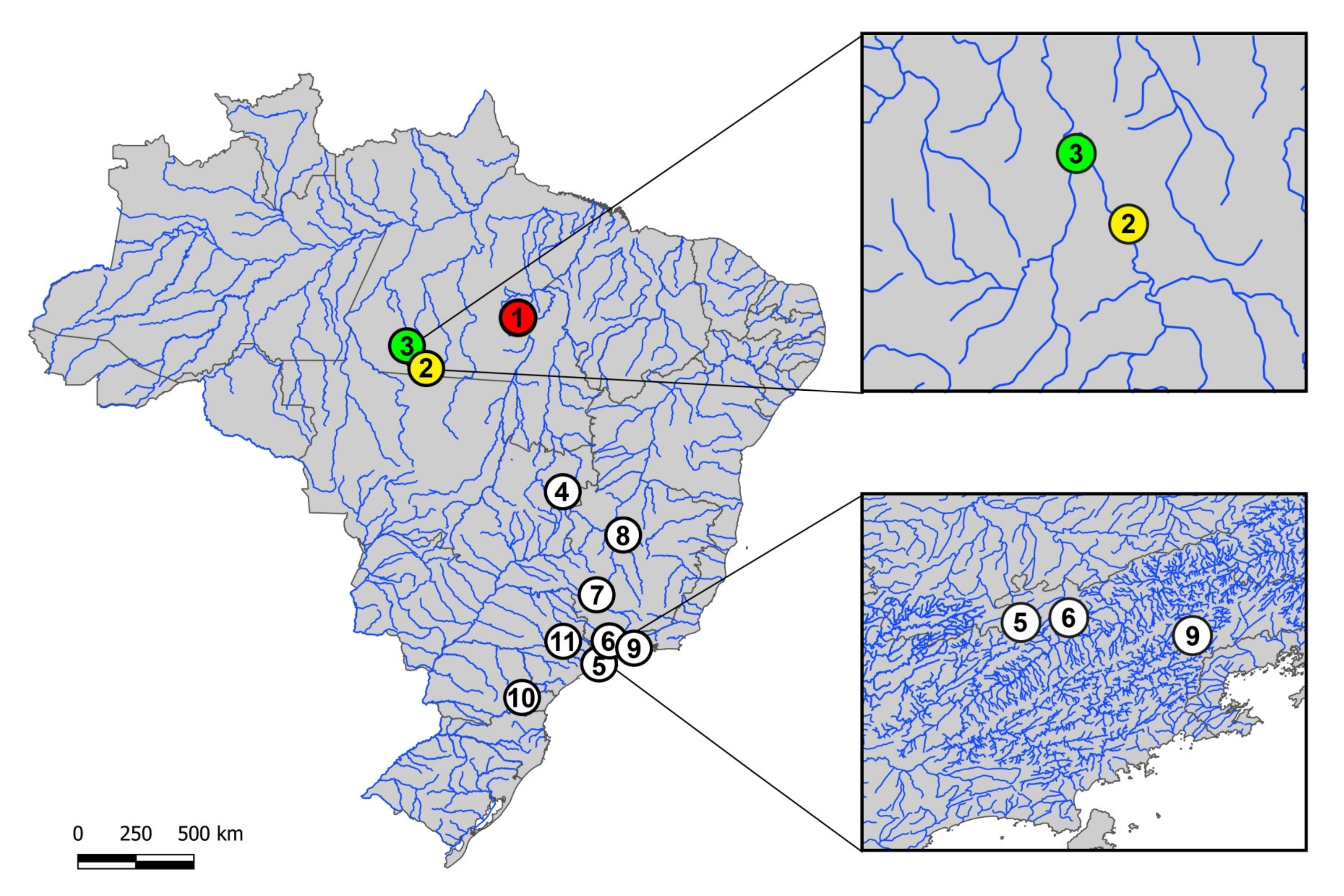
2.1. Sampling
2.2. Chromosome Preparation and C-Banding
2.3. Fluorescence In Situ Hybridization (FISH) for Repetitive DNA Mapping
2.4. Comparative Genomic Hybridization (CGH)
2.5. Microscopic Analysis and Image Processing
3. Results
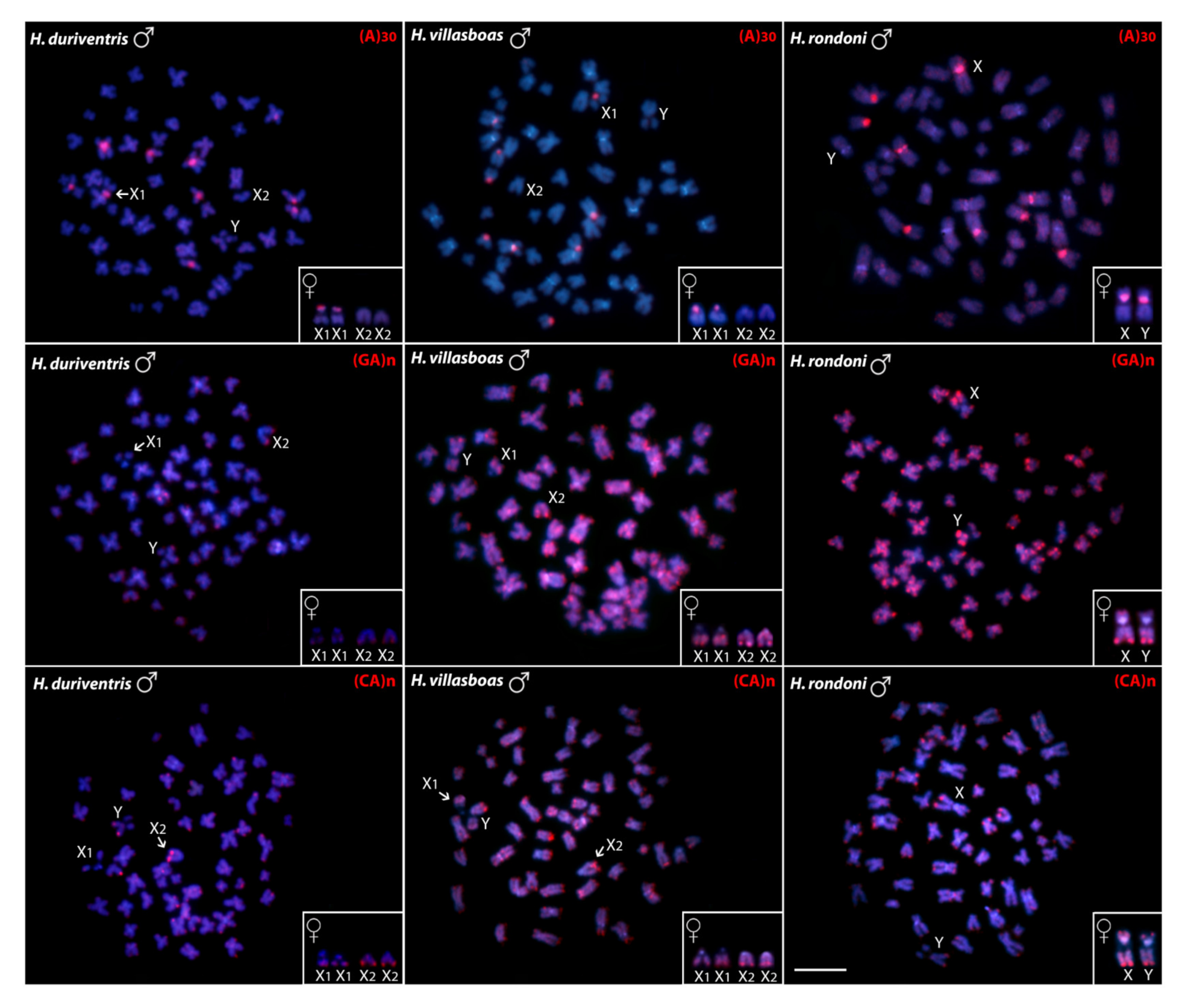
3.1. Karyotypes, C-banding and Sex Chromosomes
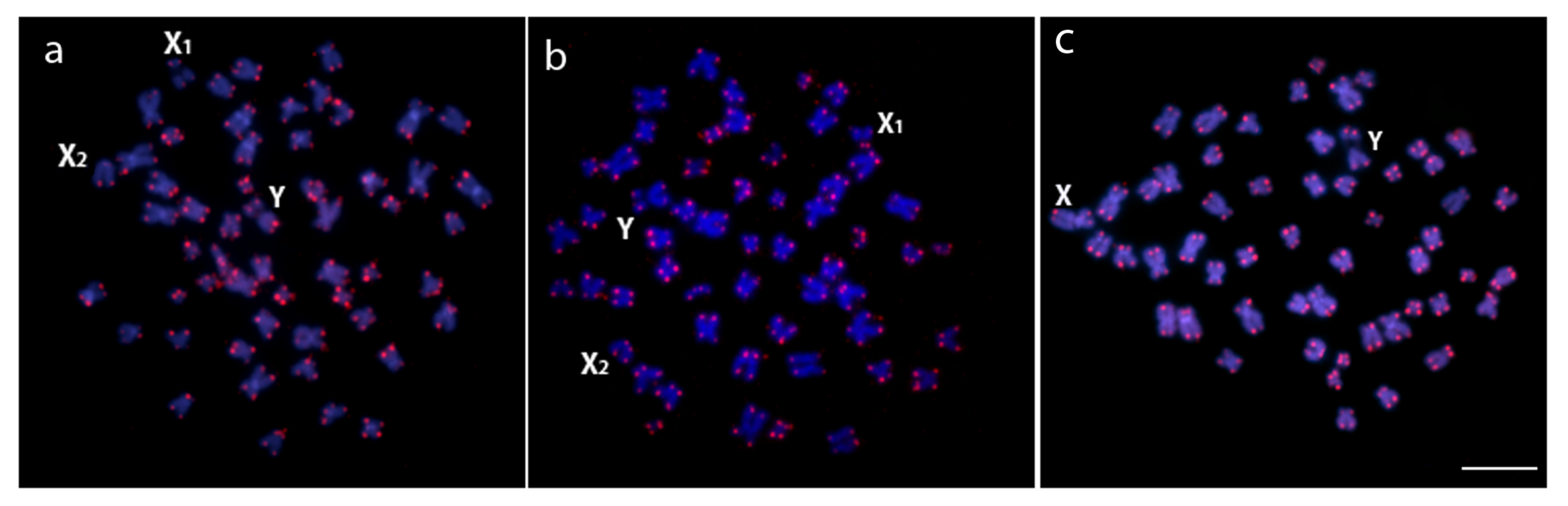
3.2. Chromosomal Distribution of rDNAs, Microsatellite Motifs and Telomeric Repeats
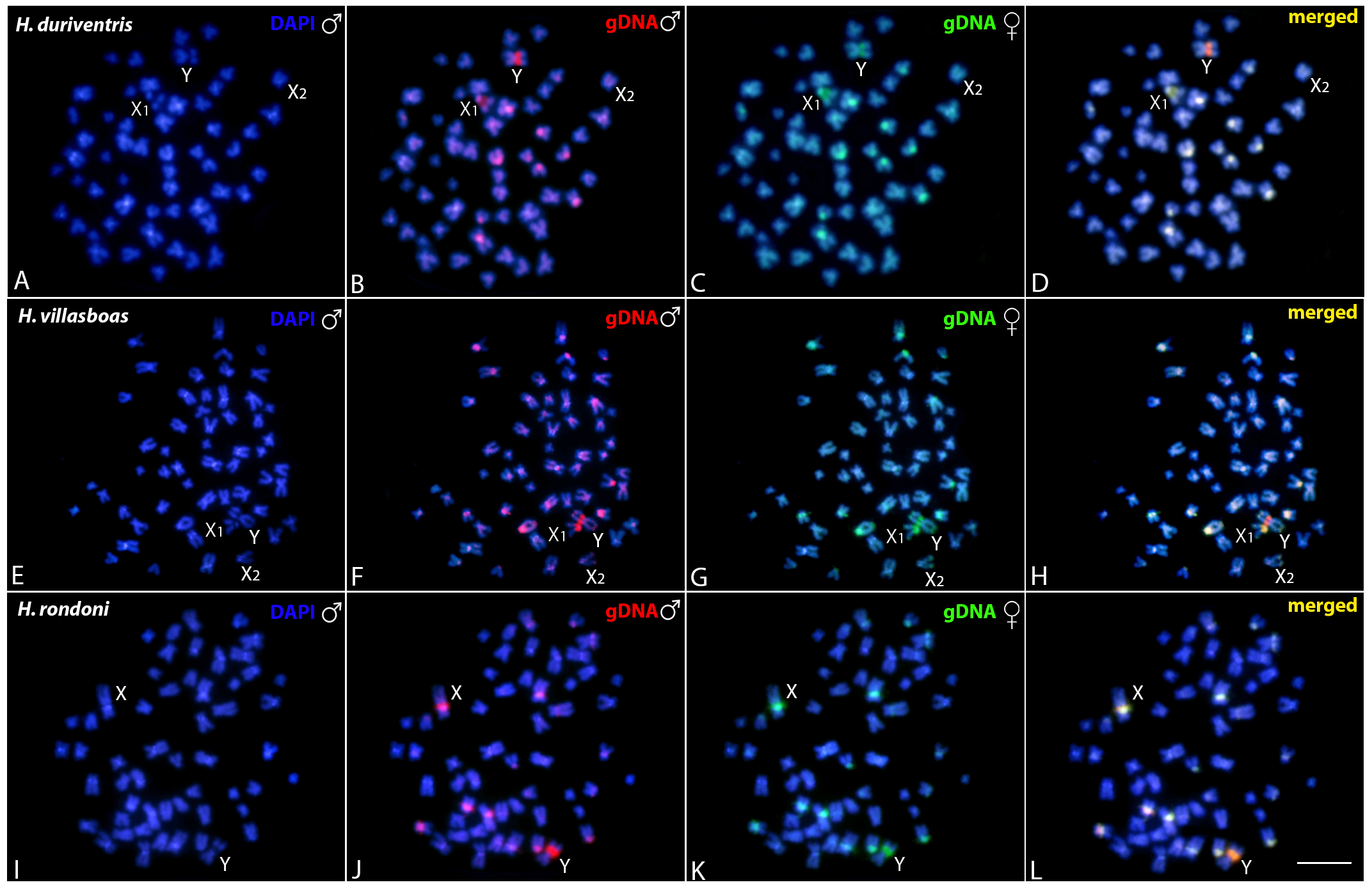
3.3. Comparative Genomic Hybridization (CGH)
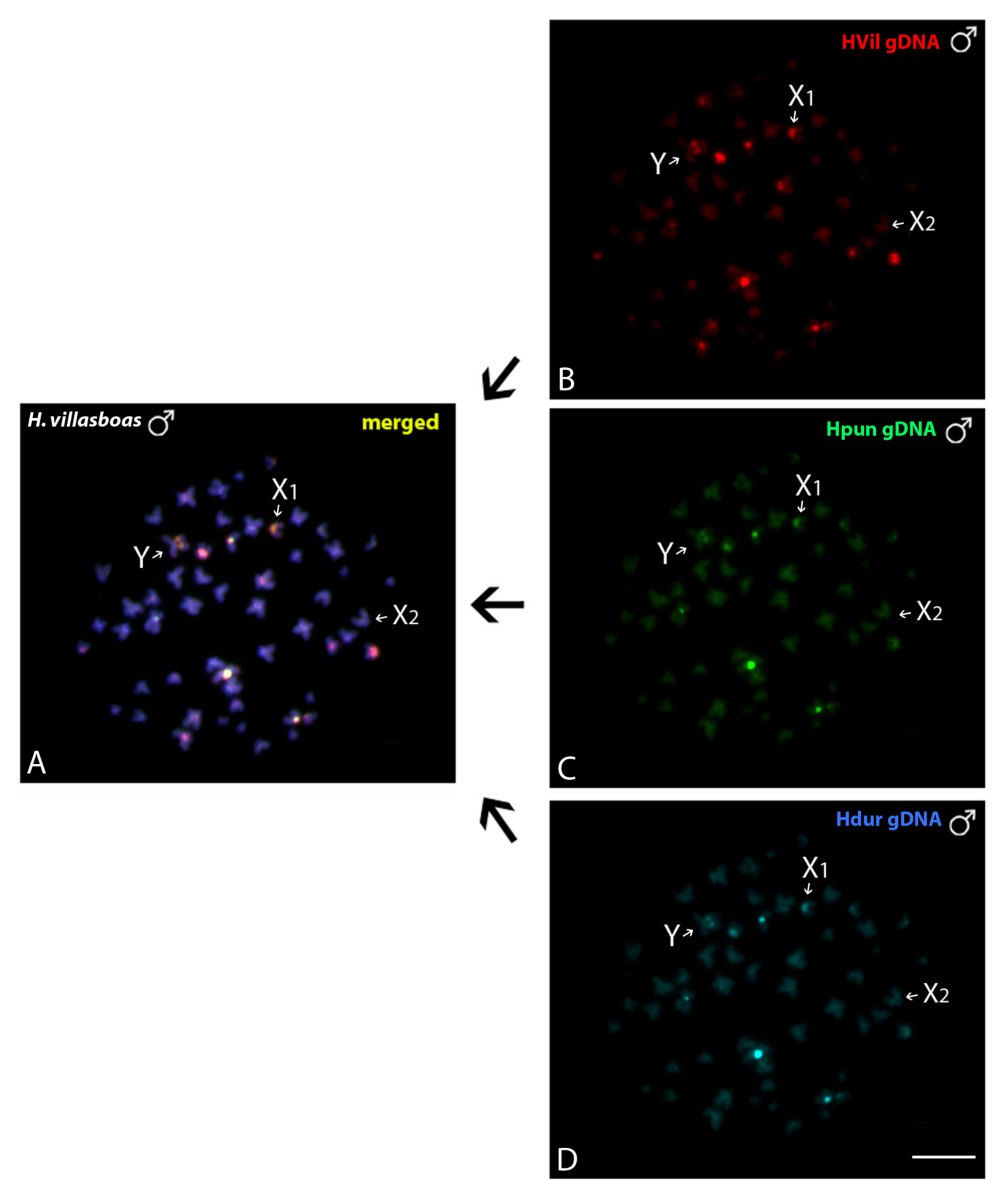
3.3.1. Intraspecific Genomic Relationships: Detecting Male-Specific Regions
3.3.2. Interspecific Genomic Relationships, Focusing on the Multiple X1X2Y Sex System
4. Discussion
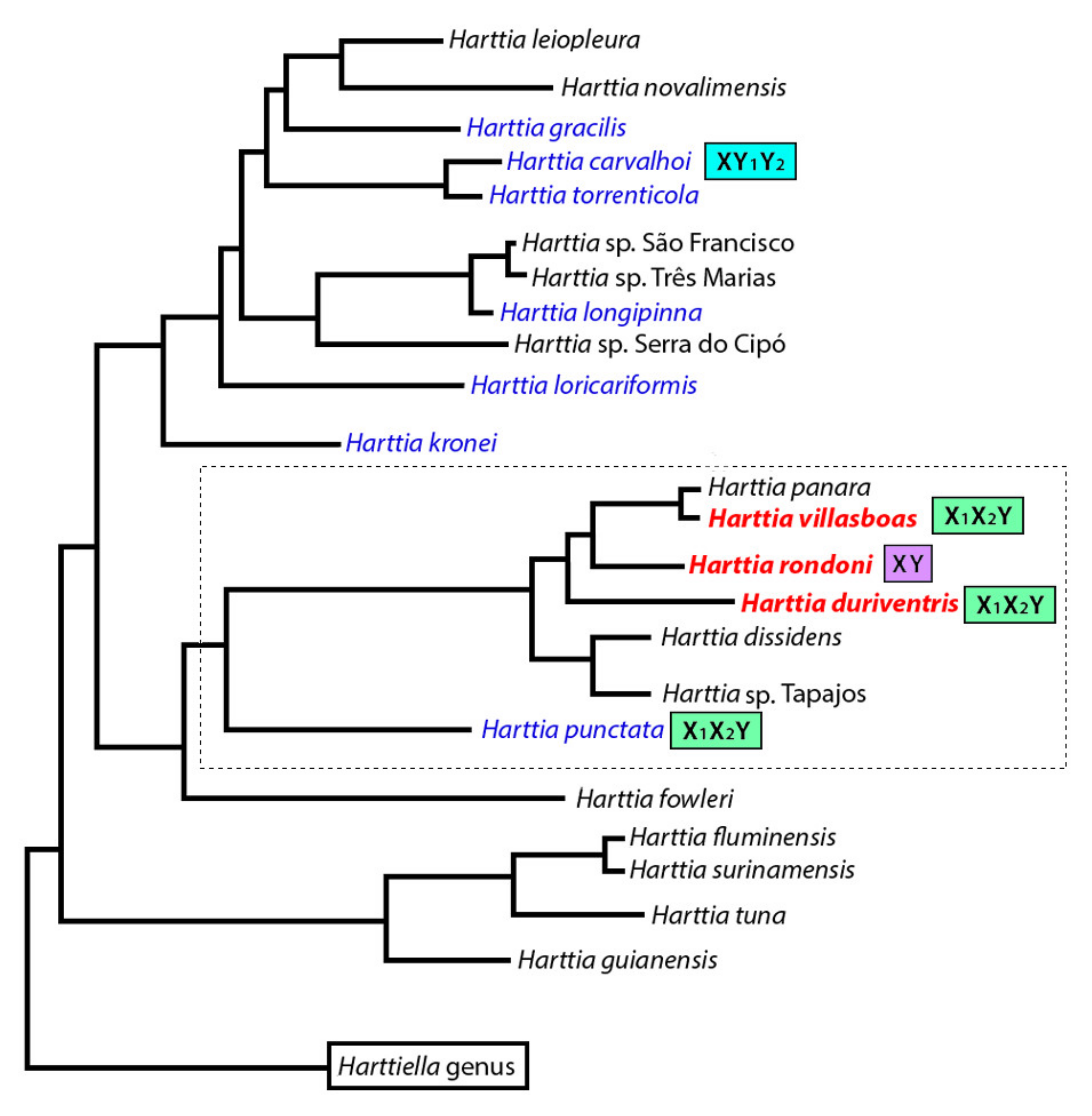
4.1. Evolutionary Relationships among Harttia Species
4.2. The Genus Harttia as A Repository of Multiple Sex Chromosome Systems
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferraris, C.J. Checklist of Catfishes, Recent and Fossil (Osteichthyes: Siluriformes), and Catalogue of Siluriform Primary Types; Magnolia Press: Auckland, New Zealand, 2007; ISBN 9781869770587. [Google Scholar]
- Fricke, R.; Eschmeyer, W.N.; van der Laan, R. Eschmeyer’s Catalog of Fishes: Genera, Species. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 12 February 2020).
- Traldi, J.B.; Lui, R.L.; Martinez, J.d.F.; Vicari, M.R.; Nogaroto, V.; Filho, O.M.; Blanco, D.R. Chromosomal distribution of the retroelements Rex1, Rex3 and Rex6 in species of the genus Harttia and Hypostomus (Siluriformes: Loricariidae). Neotrop. Ichthyol. 2019, 17, 1–10. [Google Scholar] [CrossRef]
- De Oliveira, J.C.; Oyakawa, O.T. New loricariid fishes from headwaters on Serra da Mantiqueira and Complexo do Espinhaço, Minas Gerais state, Brazil (Teleostei: Siluriformes: Loricariidae). Zootaxa 2019, 4586, 401–424. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.L.; Oliveira, C.; Foresti, F. Karyotype variability in eight species of the subfamilies Loricariinae and Ancistrinae (Teleostei, Siluriformes, Loricariidae). Caryologia 2003, 56, 57–63. [Google Scholar] [CrossRef]
- Kavalco, K.F.; Pazza, R.; Bertollo, L.A.C.; Moreira-Filho, O. Heterochromatin characterization of four fish species of the family Loricariidae (Siluriformes). Hereditas 2004, 141, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Kavalco, K.F.; Pazza, R.; Bertollo, L.A.C.; Moreira-Filho, O. Karyotypic diversity and evolution of Loricariidae (Pisces, Siluriformes). Heredity 2005, 94, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Centofante, L.; Bertollo, L.A.C.; Moreira-Filho, O. Cytogenetic characterization and description of an XX/XY1Y2 sex chromosome system in catfish Harttia carvalhoi (Siluriformes, Loricariidae). Cytogenet. Genome Res. 2006, 112, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.M. Estudos Cromossômicos e Moleculares em Loricariinae Com Ênfase em Espécies de Rineloricaria (Siluriformes, Loricariidae): Uma Perspectiva Evolutiva; Universidade de São Paulo: São Paulo, Brazil, 2010. [Google Scholar]
- Blanco, D.R.; Vicari, M.R.; Artoni, R.F.; Traldi, J.B.; Moreira-Filho, O. Chromosomal Characterization of Armored Catfish Harttia longipinna (Siluriformes, Loricariidae): First Report of B Chromosomes in the Genus. Zool. Sci. 2012, 29, 604–609. [Google Scholar] [CrossRef]
- Blanco, D.R.; Vicari, M.R.; Lui, R.L.; Bertollo, L.A.C.; Traldi, J.B.; Moreira-Filho, O. The role of the Robertsonian rearrangements in the origin of the XX/XY1Y2 sex chromosome system and in the chromosomal differentiation in Harttia species (Siluriformes, Loricariidae). Rev. Fish Biol. Fish. 2013, 23, 127–134. [Google Scholar] [CrossRef]
- Blanco, D.R.; Vicari, M.R.; Lui, R.L.; Artoni, R.F.; de Almeida, M.C.; Traldi, J.B.; Margarido, V.P.; Moreira-Filho, O. Origin of the X1X1X2X2/X1X2Y sex chromosome system of Harttia punctata (Siluriformes, Loricariidae) inferred from chromosome painting and FISH with ribosomal DNA markers. Genetica 2014, 142, 119–126. [Google Scholar] [CrossRef]
- Blanco, D.R.; Vicari, M.R.; Lui, R.L.; Traldi, J.B.; Bueno, V.; Martinez, J.d.F.; Brandão, H.; Oyakawa, O.T.; Moreira Filho, O. Karyotype Diversity and Evolutionary Trends in Armored Catfish Species of the Genus Harttia (Siluriformes: Loricariidae). Zebrafish 2017, 14, 169–176. [Google Scholar] [CrossRef]
- Cioffi, M.B.; Bertollo, L.A.C.; Garrido-Ramos, M.A.; Genome Dynamics, V. Chromosomal Distribution and Evolution of Repetitive DNAs in Fish. Genome Dyn. 2012, 7, 197–221. [Google Scholar] [PubMed]
- Barby, F.F.; Bertollo, L.A.C.; de Oliveira, E.A.; Yano, C.F.; Hatanaka, T.; Ráb, P.; Sember, A.; Ezaz, T.; Artoni, R.F.; Liehr, T.; et al. Emerging patterns of genome organization in Notopteridae species (Teleostei, Osteoglossiformes) as revealed by Zoo-FISH and Comparative Genomic Hybridization (CGH). Sci. Rep. 2019, 9, 1112. [Google Scholar] [CrossRef] [PubMed]
- de Menezes Cavalcante Sassi, F.; Aguiar de Oliveira, E.; Bertollo, L.A.C.; Nirchio, M.; Hatanaka, T.; Marinho, M.M.F.; Moreira-Filho, O.; Aroutiounian, R.; Liehr, T.; Al-Rikabi, A.B.H.; et al. Chromosomal evolution and evolutionary relationships of Lebiasina species (Characiformes, Lebiasinidae). Int. J. Mol. Sci. 2019, 20, 2944. [Google Scholar]
- de Moraes, R.L.R.; Sember, A.; Bertollo, L.A.; De Oliveira, E.A.; Rab, P.; Hatanaka, T.; Marinho, M.M.; Liehr, T.; Al-Rikabi, A.B.; Feldberg, E.; et al. Comparative cytogenetics and neo-Y formation in small-sized fish species of the genus Pyrrhulina (Characiformes, Lebiasinidae). Front. Genet. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Toma, G.A.; De Moraes, R.L.R.; Sassi, F.D.M.C.; Bertollo, L.A.C.; De Oliveira, E.A.; Rab, P.; Sember, A.; Liehr, T.; Hatanaka, T.; Viana, P.F.; et al. Cytogenetics of the small-sized fish, Copeina guttata (Characiformes, Lebiasinidae): Novel insights into the karyotype differentiation of the family. PLoS ONE 2019, 14, 1–13. [Google Scholar] [CrossRef]
- Covain, R.; Fisch-Muller, S.; Oliveira, C.; Mol, J.H.; Montoya-Burgos, J.I.; Dray, S. Molecular phylogeny of the highly diversified catfish subfamily Loricariinae (Siluriformes, Loricariidae) reveals incongruences with morphological classification. Mol. Phylogenetics Evol. 2016, 94, 492–517. [Google Scholar] [CrossRef]
- Bertollo, L.A.C.; Cioffi, M.d.B.; Galetti, P.M.; Filho, O.M. Contributions to the cytogenetics of the Neotropical fish fauna. Comp. Cytogenet. 2017, 11, 665–690. [Google Scholar] [CrossRef]
- Sumner, A.T. A simple technique for demonstrating centromeric heterochromatin. Exp. Cell Res. 1972, 75, 304–306. [Google Scholar] [CrossRef]
- Pendás, A.M.; Móran, P.; Freije, J.P.; Garcia-Vásquez, E. Chromosomal location and nucleotide sequence of two tandem repeats of the Atlantic salmon 5S rDNA. Cytogenet. Genome Res. 1994, 67, 31–36. [Google Scholar] [CrossRef]
- Cioffi, M.d.B.; Martins, C.; Centofante, L.; Jacobina, U.P.; Bertollo, L.A.C. Chromosomal variability among allopatric populations of Erythrinidae fish Hoplias malabaricus: Mapping of three classes of repetitive DNAs. Cytogenet. Genome Res. 2009, 125, 132–141. [Google Scholar] [CrossRef]
- Kubat, Z.; Hobza, R.; Vyskot, B.; Kejnovský, E. Microsatellite accumulation in the Y chromosome of Silene latifolia. Genome 2008, 51, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Yano, C.F.; Bertollo, L.A.C.; Cioffi, M.B. Fish-FISH: Molecular cytogenetics in fish species. In Fluorescence in Situ Hybridization (FISH)- Application Guide; Liehr, T., Ed.; Springer: Berlin, Germany, 2017; pp. 429–444. [Google Scholar]
- Sambrook, J.; Russell, D.W. Molecular Cloning, A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Zwick, M.S.; Hanson, R.E.; Mcknight, T.D.; Islam-Faridi, M.H.; Stelly, D.M.; Wing, R.A.; Price, H.J. A rapid procedure for the isolation of C 0 t-1 DNA from plants. Genome 1997, 40, 138–142. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, R.L.R.; Bertollo, L.A.C.; Marinho, M.M.F.; Yano, C.F.; Hatanaka, T.; Barby, F.F.; Troy, W.P.; Cioffi, M.D.B. Evolutionary relationships and cytotaxonomy considerations in the genus Pyrrhulina (Characiformes, Lebiasinidae). Zebrafish 2017, 14, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.M.C.; Hatanaka, T.; Moraes, R.L.R.; Toma, G.A.; Oliveira, E.A.; Liehr, T.; Rab, P.; Bertollo, L.A.C.; Viana, P.F.; Feldberg, E.; et al. An insight into the chromosomal evolution of Lebiasinidae (Teleostei, Characiformes). Genes 2020, 11, 365. [Google Scholar]
- Symonová, R.; Sember, A.; Majtánová, Z.; Ráb, P. Characterization of fish genome by GISH and CGH. In Fish Cytogenetic Techniques. Ray-Fin Fishes and Chondrichthyans; Ozouf-Costaz, C., Pisano, E., Foresti, F., de Almeida-Toledo, L.F., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 118–131. [Google Scholar]
- Levan, A.; Fredga, K.; Sandberg, A.A. Nomenclature for centromeric position on chromosomes. Hereditas 1964, 52, 201–220. [Google Scholar] [CrossRef]
- Karr, J.; James, F.C. Eco-morphological configurations and convergent evolution in species and communities. In Ecology and Evolution of Communities; Cody, M.L., Diamond, J.M., Eds.; Harvard University Press: Cambridge, UK, 1975; pp. 258–291. [Google Scholar]
- Wainwright, P.C.; Reilly, S.M. Ecological Morphology: Integrative Organismal Biology; University of Chicago Press: Chicago, IL, USA, 1994. [Google Scholar]
- Pazza, R.; Kavalco, K.F.; Bertollo, L.A.C. Chromosome polymorphism in Astyanax fasciatus (Teleostei, Characidae). 1. Karyotype analysis, Ag-NORs and mapping of the 18S and 5S ribosomal genes in sympatric karyotypes and their possible hybrid forms. Cytogenet. Genome Res. 2006, 112, 313–319. [Google Scholar] [CrossRef]
- Ferreira-Neto, M.; Artoni, R.; Vicari, M.; Moreira Filho, O.; Camacho, J.; Bakkali, M.; Oliveira, C.; Foresti, F. Three sympatric karyomorphs in the fish Astyanax fasciatus (Teleostei, Characidae) do not seem to hybridize in natural populations. Comp. Cytogenet. 2012, 6, 29–40. [Google Scholar] [CrossRef]
- Pazza, R.; Dergam, J.A.; Kavalco, K.F. Trends in karyotype evolution in Astyanax (Teleostei, Characiformes, Characidae): Insights from molecular data. Front. Genet. 2018, 9, 131. [Google Scholar] [CrossRef]
- Bertollo, L.A.C.; Born, G.G.; Dergam, J.A.; Fenocchio, A.S.; Moreira-Filho, O. A biodiversity approach in the neotropical Erythrinidae fish, Hoplias malabaricus. Karyotypic survey, geographic distribution of cytotypes and cytotaxonomic considerations. Chromosome Res. 2000, 8, 603–613. [Google Scholar] [CrossRef]
- Vicari, M.R.; Artoni, R.F.; Bertollo, L.A.C. Comparative cytogenetics of Hoplias malabaricus (Pisces, Erythrinidae): A population analysis in adjacent hydrographic basins. Genet. Mol. Biol. 2005, 110, 103–110. [Google Scholar] [CrossRef]
- Ibagón, N.; Maldonado-Ocampo, J.A.; Cioffi, M.d.B.; Dergam, J.A. Chromosomal Diversity of Hoplias malabaricus (Characiformes, Erythrinidae) Along the Magdalena River (Colombia—Northern South America) and Its Significance for the Neotropical Region. Zebrafish 2020, 17, 211–219. [Google Scholar] [CrossRef]
- Lundberg, J.G.; Marshall, L.G.; Guerrero, J.; Horton, B.; Malabarba, M.; Wesselingh, F. The stage for Neotropical fish diversification: A history of tropical South American rivers. In Phylogeny and Classification of Neotropical Fishes; Malabarba, L.R., Reis, R.E., Vari, R.P., Lucena, C.A.S., Eds.; Edipucrs Porto Alegre: Porto Alegre, Brazil, 1998; Volume 27, pp. 13–48. [Google Scholar]
- Oyakawa, O.T.; Fichberg, I.; Py-Daniel, L.R. Three new species of Harttia (Loricariidae: Loricariinae) from Serra do Cachimbo, Rio Xingu basin, Pará, Northern Brazil. Zootaxa 2018, 4387, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Birindelli, J.L.O.; Britski, H.A. New species of the genus Leporinus Agassiz (Characiformes: Anostomidae) from the rio Curuá, rio Xingu basin, Serra do Cachimbo, Brazil, with comments on Leporinus reticulatus. Neotrop. Ichthyol. 2009, 7, 1–10. [Google Scholar] [CrossRef]
- Netto-Ferreira, A.L. Three new species of Lebiasina (Characiformes: Lebiasinidae) from the Brazilian shield border at Serra do Cachimbo, Pará, Brazil. Neotrop. Ichthyol. 2012, 10, 487–498. [Google Scholar] [CrossRef]
- Varella, H.R.; Sabaj Pérez, M.H. A titan among dwarfs: Apistogramma kullanderi, new species (Teleostei: Cichlidae). Ichthyol. Explor. Freshw. 2014, 25, 243–258. [Google Scholar]
- Meyne, J.; Ratliff, R.L.; Moyzis, R.K. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc. Natl. Acad. Sci. USA 1989, 86, 7049–7053. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, N.L.; Al-Rikabi, A.B.H.; Bertollo, L.A.C.; Ezaz, T.; Yano, C.F.; de Oliveira, E.A.; Hatanaka, T.; de Bello Cioffi, M. Early Stages of XY Sex Chromosomes Differentiation in the Fish Hoplias malabaricus (Characiformes, Erythrinidae) Revealed by DNA Repeats Accumulation. Curr. Genom. 2017, 19, 216–226. [Google Scholar] [CrossRef]
- Gammerdinger, W.J.; Kocher, T.D. Unusual Diversity of Sex Chromosomes in African Cichlid Fishes. Genes 2018, 9, 480. [Google Scholar] [CrossRef]
- Kottler, V.; Schartl, M. The Colorful Sex Chromosomes of Teleost Fish. Genes 2018, 9, 233. [Google Scholar] [CrossRef]
- Schartl, M.; Schmid, M.; Nanda, I. Dynamics of vertebrate sex chromosome evolution: From equal size to giants and dwarfs. Chromosoma 2016, 125, 553–571. [Google Scholar] [CrossRef]
- Guiguen, Y.; Pasquier, J.; Fostier, A.; Bobe, J. Evolution of Sex Hormone Binding Globulins Reveals Early Gene Duplication at the Root of Vertebrates. bioRxiv 2020. [Google Scholar] [CrossRef]
- Arai, R. Fish Karyotypes: A Check List; Springer Science & Business Media: Heidelberg, Germany, 2011. [Google Scholar]
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Pennell, M.W.; Kirkpatrick, M.; Otto, S.P.; Vamosi, J.C.; Peichel, C.L.; Valenzuela, N.; Kitano, J. Y Fuse? Sex Chromosome Fusions in Fishes and Reptiles. PLoS Genet. 2015, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kitano, J.; Peichel, C.L. Turnover of sex chromosomes and speciation in fishes. Environ. Biol. Fishes 2012, 94, 549–558. [Google Scholar] [CrossRef]
- Cioffi, M.B.; Bertollo, L.A.C. Initial steps in XY chromosome differentiation in Hoplias malabaricus and the origin of an X1X2Y sex chromosome system in this fish group. Heredity 2010, 105, 554–561. [Google Scholar] [CrossRef]
- Cioffi, M.B.; Camacho, J.P.M.; Bertollo, L.A.C. Repetitive DNAs and differentiation of sex chromosomes in neotropical fishes. Cytogenet. Genome Res. 2011, 132, 188–194. [Google Scholar] [CrossRef]
- Bitencourt, J.A.; Sampaio, I.; Ramos, R.T.C.; Vicari, M.R.; Affonso, P.R.A.D.M. First Report of Sex Chromosomes in Achiridae (Teleostei: Pleuronectiformes) with Inferences about the Origin of the Multiple X1X1X2X2/X1X2Y System and Dispersal of Ribosomal Genes in Achirus achirus. Zebrafish 2017, 14, 90–95. [Google Scholar] [CrossRef]
- Ferreira, M.; Garcia, C.; Matoso, D.A.; de Jesus, I.S.; Feldberg, E. A new multiple sex chromosome system X1X1X2X2/X1Y1X2Y2 in Siluriformes: Cytogenetic characterization of Bunocephalus coracoideus (Aspredinidae). Genetica 2016, 144, 591–599. [Google Scholar] [CrossRef]
- Xu, D.; Sember, A.; Zhu, Q.; De Oliveira, E.A.; Liehr, T.; Al-Rikabi, A.B.H.; Xiao, Z.; Song, H.; De Bello Cioffi, M. Deciphering the Origin and Evolution of the X1X2Y System in Two Closely-Related Oplegnathus Species (Oplegnathidae and Centrarchiformes). Int. J. Mol. Sci. 2019, 20, 3571. [Google Scholar] [CrossRef]
- Reed, K.M.; Phillips, R.B. Polymorphism of the nucleolus organizer region (NOR) on the putative sex chromosomes of Arctic char (Salvelinus alpinus) is not sex related. Chromosome Res. 1997, 5, 221–227. [Google Scholar] [CrossRef]
- Charlesworth, D.; Charlesworth, B.; Marais, G. Steps in the evolution of heteromorphic sex chromosomes. Heredity 2005, 95, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.B.; Moreira-Filho, O.; Almeida-Toledo, L.F.; Bertollo, L.A.C. The contrasting role of heterochromatin in the differentiation of sex chromosomes: An overview from Neotropical fishes. J. Fish Biol. 2012, 80, 2125–2139. [Google Scholar] [CrossRef]
- Phillips, B.C.; Edmands, S. Does the speciation clock tick more slowly in the absence of heteromorphic sex chromosomes? BioEssays 2012, 34, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Poltronieri, J.; Marquioni, V.; Bertollo, L.A.C.; Kejnovsky, E.; Molina, W.F.; Liehr, T.; Cioffi, M.B. Comparative chromosomal mapping of microsatellites in Leporinus species (Characiformes, Anostomidae): Unequal accumulation on the W chromosomes. Cytogenet. Genome Res. 2013, 142, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Sabath, N.; Itescu, Y.; Feldman, A.; Meiri, S.; Mayrose, I.; Valenzuela, N. Sex determination, longevity, and the birth and death of reptilian species. Ecol. Evol. 2016, 6, 5207–5220. [Google Scholar] [CrossRef] [PubMed]
- Yano, C.F.; Bertollo, L.A.C.; Liehr, T.; Troy, W.P.; Cioffi, M.D.B. W Chromosome Dynamics in Triportheus Species (Characiformes, Triportheidae): An Ongoing Process Narrated by Repetitive Sequences. J. Hered. 2016, 107, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Viana, P.F.; Ezaz, T.; de Bello Cioffi, M.; Liehr, T.; Al-Rikabi, A.; Goll, L.G.; Rocha, A.M.; Feldberg, E. Landscape of snake’ sex chromosomes evolution spanning 85 MYR reveals ancestry of sequences despite distinct evolutionary trajectories. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Filho, O.; Bertollo, L.A.C.; Galetti, P.M. Distribution of sex chromosome mechanisms in neotropical fish and description of a ZZ/ZW system in Parodon hilarii (Parodontidae). Caryologia 1993, 46, 115–125. [Google Scholar] [CrossRef][Green Version]
- Ramirez, J.L.; Birindelli, J.L.; Carvalho, D.C.; Affonso, P.R.; Venere, P.C.; Ortega, H.; Carrillo-Avila, M.; Rodríguez-Pulido, J.A.; Galetti, P.M., Jr. Revealing hidden diversity of the underestimated neotropical ichthyofauna: DNA barcoding in the recently described genus Megaleporinus (Characiformes: Anostomidae). Front. Genet. 2017, 8, 149. [Google Scholar] [CrossRef]
- Schemberger, M.O.; Bellafronte, E.; Nogaroto, V.; Almeida, M.C.; Schühli, G.S.; Artoni, R.F.; Moreira-Filho, O.; Vicari, M.R. Differentiation of repetitive DNA sites and sex chromosome systems reveal closely related group in Parodontidae (Actinopterygii: Characiformes). Genetica 2011, 139, 1499–1508. [Google Scholar] [CrossRef]
- Pucci, M.B.; Nogaroto, V.; Bertollo, L.A.C.; Moreira-Filho, O.; Vicari, M.R. The karyotypes and evolution of ZZ/ZW sex chromosomes in the genus Characidium (Characiformes, Crenuchidae). Comp. Cytogenet. 2018, 12, 421. [Google Scholar] [CrossRef] [PubMed]
- Yano, C.F.; Bertollo, L.A.C.; Ezaz, T.; Trifonov, V.; Sember, A.; Liehr, T.; Cioffi, M.B. Highly conserved Z and molecularly diverged W chromosomes in the fish genus Triportheus (Characiformes, Triportheidae). Heredity 2017, 118, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.D.B.; Yano, C.F.; Sember, A.; Bertollo, L.A.C. Chromosomal evolution in lower vertebrates: Sex chromosomes in neotropical fishes. Genes 2017, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Takehana, Y.; Naruse, K.; Hamaguchi, S.; Sakaizumi, M. Evolution of ZZ/ZW and XX/XY sex-determination systems in the closely related medaka species, Oryzias hubbsi and O. dancena. Chromosoma 2007, 116, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Takehana, Y.; Naruse, K.; Hamaguchi, S.; Sakaizumi, M. Evidence for different origins of sex chromosomes in closely related oryzias fishes: Substitution of the master sex-determining gene. Genetics 2007, 177, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Mank, J.E.; Avise, J.C. Evolutionary diversity and turn-over of sex determination in teleost fishes. Sex. Dev. 2009, 3, 60–67. [Google Scholar] [CrossRef]
- Schultheis, C.; Böhne, A.; Schartl, M.; Volff, J.N.; Galiana-Arnoux, D. Sex determination diversity and sex chromosome evolution in poeciliid fish. Sex. Dev. 2009, 3, 68–77. [Google Scholar] [CrossRef]
- Cioffi, M.B.; Liehr, T.; Trifonov, V.; Molina, W.F.; Bertollo, L.A.C. Independent sex chromosome evolution in lower vertebrates: A molecular cytogenetic overview in the erythrinidae fish family. Cytogenet. Genome Res. 2013, 141, 186–194. [Google Scholar] [CrossRef]
- Schartl, M. Sex chromosome evolution in non-mammalian vertebrates. Curr. Opin. Genet. Dev. 2004, 14, 634–641. [Google Scholar] [CrossRef]

| Species | Locality | N |
|---|---|---|
| 1. H. duriventris | Parauapebas River, Canaã dos Carajás-PA (Brazil) (6°30’06.5’’ S 50°02’35.3’’ W) | 08♀, 07♂ |
| 2. H. villasboas | Curuá River, Cachoeira da Serra-PA (Brazil) (8°44’09.0’’ S 54°57’46.0’’ W) | 34♀, 38♂ |
| 3. H. rondoni | 13 de Maio River, Cachoeira da Serra-PA (Brazil) (8°38’53.0’’ S 55°01’41.0’’ W) | 15♀, 14♂ |
| 4. H. punctata | Itiquira river, Formosa—GO (Brazil) (15°19’25’’ S 47°25’26’’ W) | 10♀, 12♂ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sassi, F.d.M.C.; Deon, G.A.; Moreira-Filho, O.; Vicari, M.R.; Bertollo, L.A.C.; Liehr, T.; de Oliveira, E.A.; Cioffi, M.B. Multiple Sex Chromosomes and Evolutionary Relationships in Amazonian Catfishes: The Outstanding Model of the Genus Harttia (Siluriformes: Loricariidae). Genes 2020, 11, 1179. https://doi.org/10.3390/genes11101179
Sassi FdMC, Deon GA, Moreira-Filho O, Vicari MR, Bertollo LAC, Liehr T, de Oliveira EA, Cioffi MB. Multiple Sex Chromosomes and Evolutionary Relationships in Amazonian Catfishes: The Outstanding Model of the Genus Harttia (Siluriformes: Loricariidae). Genes. 2020; 11(10):1179. https://doi.org/10.3390/genes11101179
Chicago/Turabian StyleSassi, Francisco de M. C., Geize A. Deon, Orlando Moreira-Filho, Marcelo R. Vicari, Luiz A. C. Bertollo, Thomas Liehr, Ezequiel Aguiar de Oliveira, and Marcelo B. Cioffi. 2020. "Multiple Sex Chromosomes and Evolutionary Relationships in Amazonian Catfishes: The Outstanding Model of the Genus Harttia (Siluriformes: Loricariidae)" Genes 11, no. 10: 1179. https://doi.org/10.3390/genes11101179
APA StyleSassi, F. d. M. C., Deon, G. A., Moreira-Filho, O., Vicari, M. R., Bertollo, L. A. C., Liehr, T., de Oliveira, E. A., & Cioffi, M. B. (2020). Multiple Sex Chromosomes and Evolutionary Relationships in Amazonian Catfishes: The Outstanding Model of the Genus Harttia (Siluriformes: Loricariidae). Genes, 11(10), 1179. https://doi.org/10.3390/genes11101179








