Cross-Species BAC Mapping Highlights Conservation of Chromosome Synteny across Dragon Lizards (Squamata: Agamidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Sample Collection
2.2. Cell Culture and Chromosome Preparation
2.3. Fluorescence In Situ Hybridization (FISH) and Image Analysis
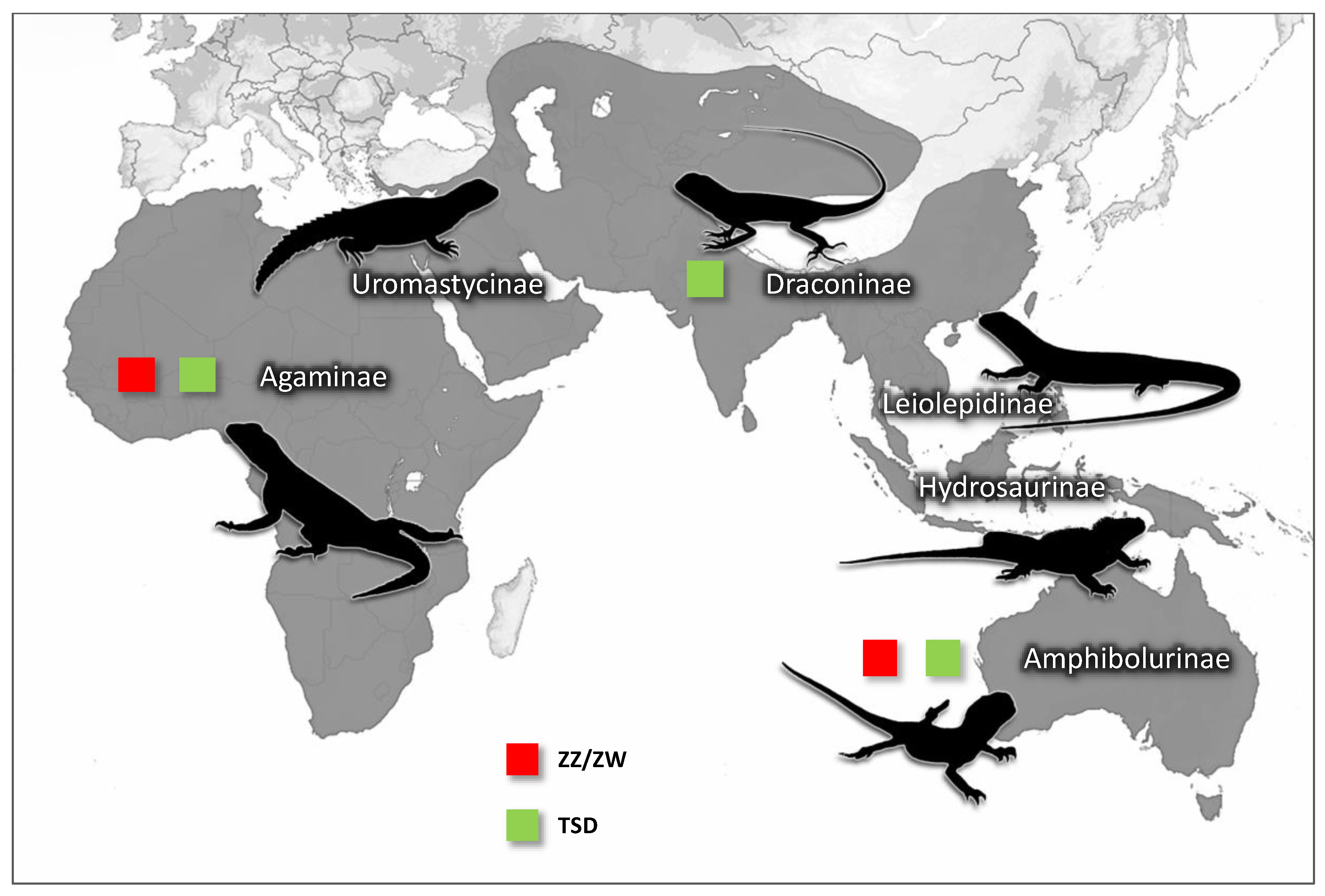
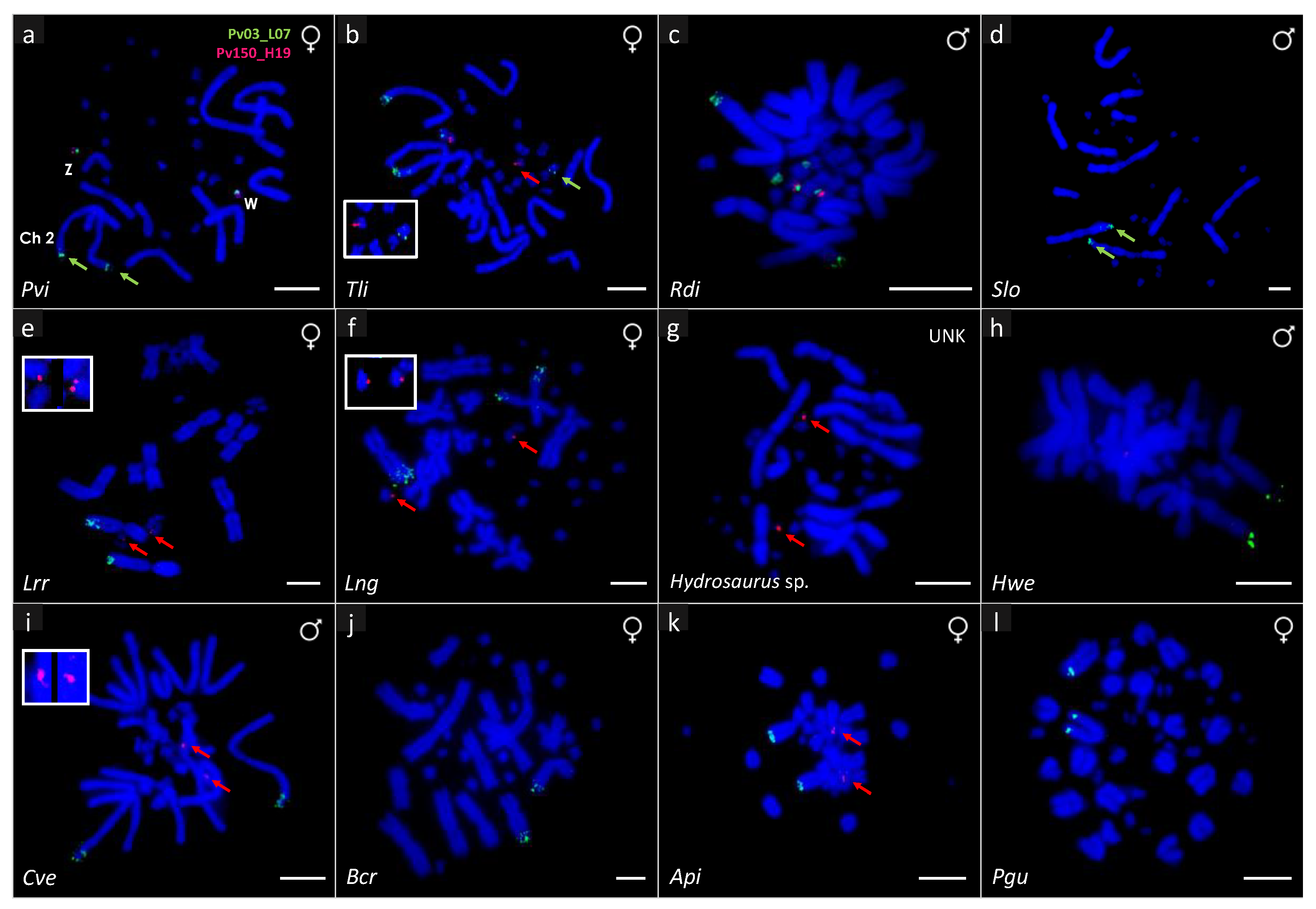
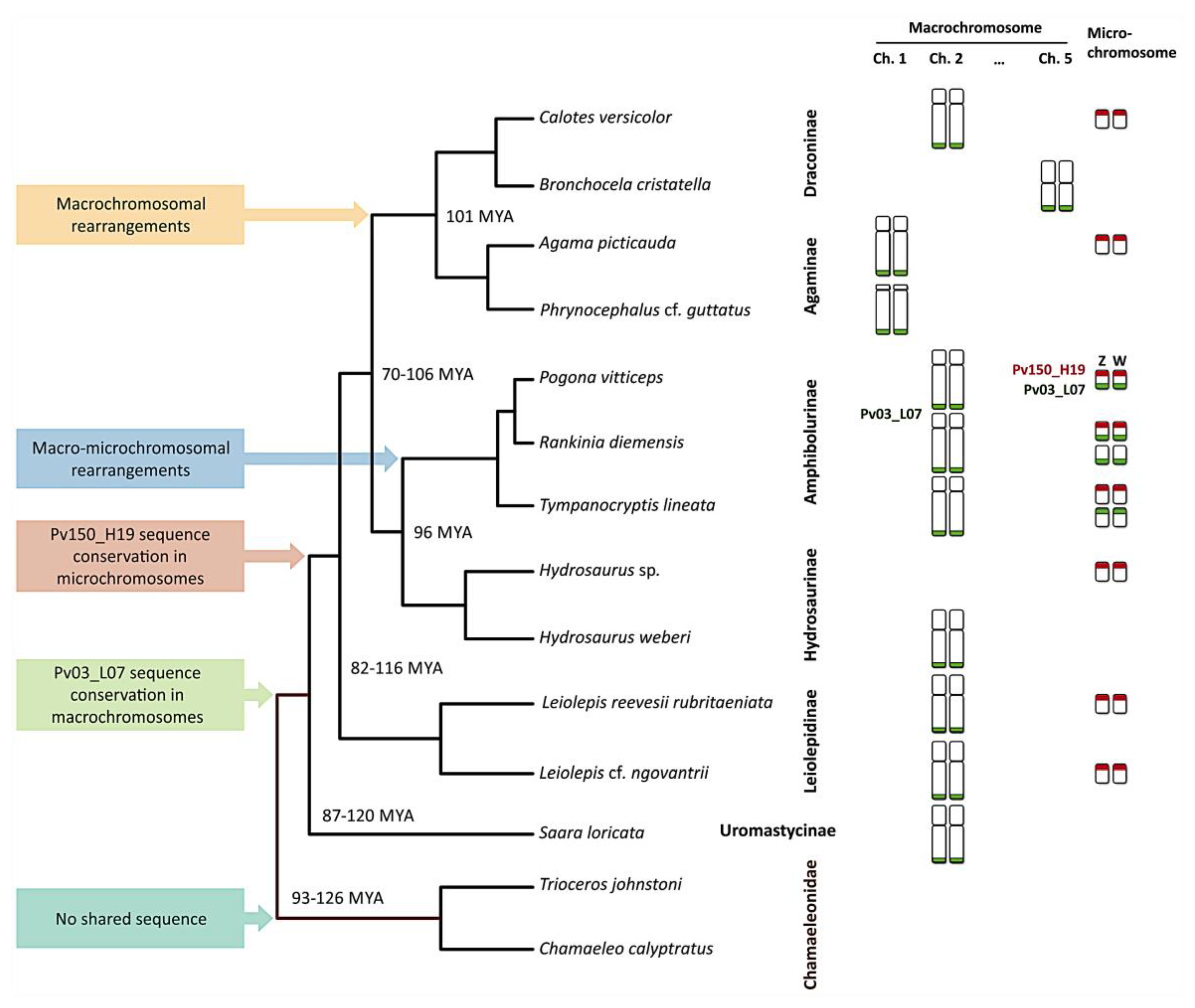
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alam, S.M.I.; Sarre, S.D.; Gleeson, D.; Georges, A.; Ezaz, T. Did lizards follow unique pathways in sex chromosome evolution? Genes 2018, 9, 239. [Google Scholar] [CrossRef] [PubMed]
- Ezaz, T.; Sarre, S.D.; O’Meally, D.; Graves, J.A.; Georges, A. Sex chromosome evolution in lizards: Independent origins and rapid transitions. Cytogenet. Genome Res. 2009, 127, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, N.; Lance, V. Temperature-Dependent Sex Determination in Vertebrates; Smithsonian Books: Washington, DC, USA, 2004. [Google Scholar]
- Olmo, E.; Signorino, G. Chromorep: A Reptile Chromosomes Database. Available online: www.chromorep.univpm.it (accessed on 30 March 2018).
- Ezaz, T.; Srikulnath, K.; Graves, J.A.M. Origin of amniote sex chromosomes: An ancestral super-sex chromosome, or common requirements? J. Hered. 2017, 108, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Uetz, P.; Freed, P.; Hošek, J. The Reptile Database. Available online: http://reptile-database.org (accessed on 14 February 2020).
- Ezaz, T.; Quinn, A.E.; Sarre, S.D.; O’Meally, D.; Georges, A.; Graves, J.A.M. Molecular marker suggests rapid changes of sex-determining mechanisms in Australian dragon lizards. Chromosome Res. 2009, 17, 91–98. [Google Scholar] [CrossRef]
- Deakin, J.E.; Ezaz, T. Understanding the evolution of reptile chromosomes through applications of combined cytogenetics and genomics approaches. Cytogenet. Genome Res. 2019, 157, 7–20. [Google Scholar] [CrossRef]
- Deakin, J.E.; Potter, S.; O’Neill, R.; Ruiz-Herrera, A.; Cioffi, M.B.; Eldridge, M.D.; Fukui, K.; Marshall Graves, J.A.; Griffin, D.; Grutzner, F. Chromosomics: Bridging the gap between genomes and chromosomes. Genes 2019, 10, 627. [Google Scholar] [CrossRef]
- Deakin, J.E.; Ezaz, T. Tracing the evolution of amniote chromosomes. Chromosoma 2014, 123, 201–216. [Google Scholar] [CrossRef]
- O’Meally, D.; Ezaz, T.; Georges, A.; Sarre, S.D.; Graves, J.A.M. Are some chromosomes particularly good at sex? Insights from amniotes. Chromosome Res. 2012, 20, 7–19. [Google Scholar] [CrossRef]
- Matsubara, K.; Tarui, H.; Toriba, M.; Yamada, K.; Nishida-Umehara, C.; Agata, K.; Matsuda, Y. Evidence for different origin of sex chromosomes in snakes, birds, and mammals and step-wise differentiation of snake sex chromosomes. Proc. Natl. Acad. Sci. USA 2006, 103, 18190–18195. [Google Scholar] [CrossRef]
- Ezaz, T.; Moritz, B.; Waters, P.; Marshall Graves, J.A.; Georges, A.; Sarre, S.D. The ZW sex microchromosomes of an Australian dragon lizard share no homology with those of other reptiles or birds. Chromosome Res. 2009, 17, 965–973. [Google Scholar] [CrossRef]
- Kawai, A.; Ishijima, J.; Nishida, C.; Kosaka, A.; Ota, H.; Kohno, S.-I.; Matsuda, Y. The ZW sex chromosomes of Gekko hokouensis (Gekkonidae, Squamata) represent highly conserved homology with those of avian species. Chromosoma 2009, 118, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Alföldi, J.; di Palma, F.; Grabherr, M.; Williams, C.; Kong, L.; Mauceli, E.; Russell, P.; Lowe, C.B.; Glor, R.E.; Jaffe, J.D.; et al. The genome of the green anole lizard and a comparative analysis with birds and mammals. Nature 2011, 477, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Srikulnath, K.; Matsubara, K.; Uno, Y.; Nishida, C.; Olsson, M.; Matsuda, Y. Identification of the linkage group of the Z sex chromosomes of the sand lizard (Lacerta agilis, Lacertidae) and elucidation of karyotype evolution in lacertid lizards. Chromosoma 2014, 123, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Srikulnath, K.; Nishida, C.; Matsubara, K.; Uno, Y.; Thongpan, A.; Suputtitada, S.; Apisitwanich, S.; Matsuda, Y. Karyotypic evolution in squamate reptiles: Comparative gene mapping revealed highly conserved linkage homology between the butterfly lizard (Leiolepis reevesii rubritaeniata, Agamidae, Lacertilia) and the Japanese four-striped rat snake (Elaphe quadrivirgata, Colubridae, Serpentes). Chromosome Res. 2009, 17, 975–986. [Google Scholar] [PubMed]
- Srikulnath, K.; Uno, Y.; Nishida, C.; Matsuda, Y. Karyotype evolution in monitor lizards: Cross-species chromosome mapping of cDNA reveals highly conserved synteny and gene order in the Toxicofera clade. Chromosome Res. 2013, 21, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Harlow, P. Temperature-dependent sex determination in lizards. In Temperature-Dependent Sex Determination in Vertebrates; Valenzuela, N., Lance, V., Eds.; Smithsonian Books: Washington, DC, USA, 2004; pp. 42–52. [Google Scholar]
- Ezaz, T.; Quinn, A.E.; Miura, I.; Sarre, S.D.; Georges, A.; Graves, J.A.M. The dragon lizard Pogona vitticeps has ZZ/ZW micro-sex chromosomes. Chromosome Res. 2005, 13, 763–776. [Google Scholar] [CrossRef]
- Harlow, P.S. The Ecology of Sex-Determining Mechanisms in Australian Agamid Lizards. Ph.D. Thesis, Macquarie University, Sydney, Australia, 2001. [Google Scholar]
- Holleley, C.E.; O’Meally, D.; Sarre, S.D.; Graves, J.A.M.; Ezaz, T.; Matsubara, K.; Azad, B.; Zhang, X.; Georges, A. Sex reversal triggers the rapid transition from genetic to temperature-dependent sex. Nature 2015, 523, 79–82. [Google Scholar] [CrossRef]
- Quinn, A.E.; Georges, A.; Sarre, S.D.; Guarino, F.; Ezaz, T.; Graves, J.A.M. Temperature sex reversal implies sex gene dosage in a reptile. Science 2007, 316, 411. [Google Scholar] [CrossRef]
- Pyron, R.A.; Burbrink, F.T.; Wiens, J.J. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 2013, 13, 93. [Google Scholar] [CrossRef]
- Zheng, Y.; Wiens, J.J. Combining phylogenomic and supermatrix approaches, and a time-calibrated phylogeny for squamate reptiles (lizards and snakes) based on 52 genes and 4162 species. Mol. Phylogenet. Evol. 2016, 94, 537–547. [Google Scholar] [CrossRef]
- Rovatsos, M.; Altmanová, M.; Johnson Pokorná, M.; Velenský, P.; Sánchez Baca, A.; Kratochvíl, L. Evolution of karyotypes in chameleons. Genes 2017, 8, 382. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.V.; Banks, J.L.; Diaz, R.E.; Trainor, P.A.; Gamble, T. Dynamic sex chromosomes in Old World chameleons (Squamata: Chamaeleonidae). J. Evol. Biol. 2018, 31, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Altmanová, M.; Rovatsos, M.; Johnson Pokorná, M.; Veselý, M.; Wagner, F.; Kratochvíl, L. All iguana families with the exception of basilisks share sex chromosomes. Zoology 2018, 126, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.V.; Guzmán-Méndez, I.A.; Gamble, T.; Blumer, M.; Pinto, B.J.; Kratochvíl, L.; Rovatsos, M. Escaping the evolutionary trap? Sex chromosome turnover in basilisks and related lizards (Corytophanidae: Squamata). Biol. Lett. 2019, 15, 20190498. [Google Scholar] [CrossRef] [PubMed]
- Hugall, A.F.; Foster, R.; Hutchinson, M.; Lee, M.S.Y. Phylogeny of Australasian agamid lizards based on nuclear and mitochondrial genes: Implications for morphological evolution and biogeography. Biol. J. Linn. Soc. 2008, 93, 343–358. [Google Scholar] [CrossRef]
- Grismer, J.L.; Bauer, A.M.; Grismer, L.L.; Thirakhupt, K.; Aowphol, A.; Oaks, J.R.; Wood, P.L., Jr.; Onn, C.K.; Thy, N.; Cota, M.; et al. Multiple origins of parthenogenesis, and a revised species phylogeny for the Southeast Asian butterfly lizards, Leiolepis. Biol. J. Linn. Soc. 2014, 113, 1080–1093. [Google Scholar] [CrossRef]
- Miller, K.L.; Rico, S.C.; Muletz-Wolz, C.R.; Campana, M.G.; McInerney, N.; Augustine, L.; Frere, C.; Peters, A.M.; Fleischer, R.C. Parthenogenesis in a captive Asian water dragon (Physignathus cocincinus) identified with novel microsatellites. PLoS ONE 2019, 14, e0217489. [Google Scholar] [CrossRef]
- Blackburn, D.G. Evolutionary origins of viviparity in the Reptilia. I. Sauria. Amphibia-Reptilia 1982, 3, 185–205. [Google Scholar] [CrossRef]
- Nakhasi, U. Karyotypic homology and evolution of the Agamid lizards. Cytologia 1980, 45, 211–219. [Google Scholar]
- Witten, G.J. Some karyotypes of Australian agamids (Reptilia: Lacertilia). Aust. J. Zool. 1983, 31, 533–540. [Google Scholar] [CrossRef]
- Adegoke, J. Studies on the chromosomes of the rainbow lizard Agama agama agama (L.) with notes on polypoidy in the spermatocytes. Cytologia 1988, 53, 233–239. [Google Scholar] [CrossRef]
- Viets, B.E.; Ewert, M.A.; Talent, L.G.; Nelson, C.E. Sex-determining mechanisms in squamate reptiles. J. Exp. Zool. 1994, 270, 45–56. [Google Scholar] [CrossRef]
- Henle, K. A brief review of the origin and use of ‘stellio’ in herpetology and a comment on the nomenclature and taxonomy of agamids of the genus Agama (sensu lato). Herpetozoa 1995, 8, 3–9. [Google Scholar]
- Zeng, X.M.; Wang, Y.Z.; Liu, Z.J.; Fang, Z.L.; Wu, G.F. Karyotypes on nine species in the genus Phrynocephalus, with discussion of karyotypic evolution of Chinese Phrynocephalus. Acta. Zool. Sin. 1997, 43, 399–410. [Google Scholar]
- Kritpetcharat, O.; Kritpetcharat, C.; Luangpirom, A.; Watcharanon, P. Karyotype of four Agamidae species from the Phu Phan national park in Thailand. Sci. Asia 1999, 25, 185–188. [Google Scholar] [CrossRef]
- Pokorná, M.; Kratochvíl, L. Phylogeny of sex-determining mechanisms in squamate reptiles: Are sex chromosomes an evolutionary trap? Zool. J. Linn. Soc. 2009, 156, 168–183. [Google Scholar] [CrossRef]
- Srikulnath, K.; Uno, Y.; Matsubara, K.; Thongpan, A.; Suputtitada, S.; Apisitwanich, S.; Nishida, C.; Matsuda, Y. Chromosomal localization of the 18S-28S and 5S rRNA genes and (TTAGGG)n sequences of butterfly lizards (Leiolepis belliana belliana and Leiolepis boehmei, Agamidae, Squamata). Genet. Mol. Biol. 2011, 34, 583–586. [Google Scholar] [CrossRef]
- Baig, K.J.; Wagner, P.; Ananjeva, N.B.; Boehme, W. A morphology-based taxonomic revision of Laudakia Gray, 1845 (Squamata: Agamidae). Vertebr. Zool. 2012, 62, 213–260. [Google Scholar]
- Inamdar Doddamani, L.S.; Vani, V.; Seshagiri, P.B. A tropical oviparous lizard, Calotes versicolor, exhibiting a potentially novel FMFM pattern of temperature-dependent sex determination. J. Exp. Zool. A Ecol. Genet. Physiol. 2012, 317, 32–46. [Google Scholar] [CrossRef]
- Phimphan, S.; Tanomtong, A.; Patawang, I.; Kaewsri, S.; Jantarat, S.; Sanoamuang, L.O. Cytogenetic Study of Northeastern Butterfly Lizard, Leiolepis reevesii rubritaeniata (Squamata, Agamidae) in Northeast Thailand. Cytologia 2013, 78, 133–140. [Google Scholar] [CrossRef][Green Version]
- Utong, J.A.M.; Abukashawa, S.M.A. Characterization of the agamid lizard genus Uromastyx from Eastern Sudan based on morphology, karyotypes and mitochondrial DNA. Asian. Herpetol. Res. 2013, 4, 268–281. [Google Scholar]
- Gamble, T.; Coryell, J.; Ezaz, T.; Lynch, J.; Scantlebury, D.P.; Zarkower, D. Restriction site-associated DNA sequencing (RAD-seq) reveals an extraordinary number of transitions among gecko sex-determining systems. Mol. Biol. Evol. 2015, 32, 1296–1309. [Google Scholar] [CrossRef] [PubMed]
- Grismer, J.L.; Grismer, L.L. Who’s your mommy? Identifying maternal ancestors of asexual species of Leiolepis Cuvier, 1829 and the description of a new endemic species of asexual Leiolepis Cuvier, 1829 from Southern Vietnam. Zootaxa 2010, 2433, 47–61. [Google Scholar] [CrossRef]
- Midtgaard, R. RepFocus—A Survey of the Reptiles of the World; 1st ed.; E-book Published by the Author, Middelfart, Denmark. 2019. Available online: www.repfocus.dk/Agamidae.html (accessed on 14 April 2020).
- Pokorná, M.; Altmanová, M.; Kratochvíl, L. Multiple sex chromosomes in the light of female meiotic drive in amniote vertebrates. Chromosome Res. 2014, 22, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Quinn, A.; Georges, A.; Sarre, S.; Guarino, F.; Ezaz, T.; Graves, J. Sex chromosomes and incubation temperature interact to determine sex of a reptile. IAHS Proc. Rep. 2007, 316, 411. [Google Scholar]
- Sarre, S.D.; Georges, A.; Quinn, A. The ends of a continuum: Genetic and temperature-dependent sex determination in reptiles. BioEssays 2004, 26, 639–645. [Google Scholar] [CrossRef]
- Georges, A.; Ezaz, T.; Quinn, A.E.; Sarre, S.D. Are reptiles predisposed to temperature-dependent sex determination? Sex. Dev. 2010, 4, 7–15. [Google Scholar] [CrossRef]
- Janes, D.E.; Organ, C.L.; Fujita, M.K.; Shedlock, A.M.; Edwards, S.V. Genome evolution in Reptilia, the sister group of mammals. Ann. Rev. Genomics. Hum. Genet. 2010, 11, 239–264. [Google Scholar] [CrossRef]
- O’Meally, D. Evolution of Reptile Sex Chromosomes. Ph.D. Thesis, The Australian National University, Canberra, Australia, 2010. [Google Scholar]
- Ezaz, T.; Azad, B.; O’Meally, D.; Young, M.J.; Matsubara, K.; Edwards, M.J.; Zhang, X.; Holleley, C.E.; Deakin, J.E.; Graves, J.A.M.; et al. Sequence and gene content of a large fragment of a lizard sex chromosome and evaluation of candidate sex differentiating gene R-spondin 1. BMC Genomics 2013, 14, 899. [Google Scholar] [CrossRef]
- Domaschenz, R.; Livernois, A.M.; Rao, S.; Ezaz, T.; Deakin, J.E. Immunofluorescent staining reveals hypermethylation of microchromosomes in the central bearded dragon, Pogona vitticeps. Mol. Cytogenet. 2015, 8, 104. [Google Scholar] [CrossRef]
- Young, M.J.; O’Meally, D.; Sarre, S.D.; Georges, A.; Ezaz, T. Molecular cytogenetic map of the central bearded dragon, Pogona vitticeps (Squamata: Agamidae). Chromosome Res. 2013, 21, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Deakin, J.E.; Edwards, M.J.; Patel, H.; O’Meally, D.; Lian, J.; Stenhouse, R.; Ryan, S.; Livernois, A.M.; Azad, B.; Holleley, C.E.; et al. Anchoring genome sequence to chromosomes of the central bearded dragon (Pogona vitticeps) enables reconstruction of ancestral squamate macrochromosomes and identifies sequence content of the Z chromosome. BMC Genomics 2016, 17, 447. [Google Scholar] [CrossRef] [PubMed]
- Georges, A.; Li, Q.; Lian, J.; O’Meally, D.; Deakin, J.; Wang, Z.; Zhang, P.; Fujita, M.; Patel, H.R.; Holleley, C.E.; et al. High-coverage sequencing and annotated assembly of the genome of the Australian dragon lizard Pogona vitticeps. GigaScience 2015, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; O’Meally, D.; Sarre, S.D.; Georges, A.; Srikulnath, K.; Ezaz, T. ZW sex chromosomes in Australian dragon lizards (Agamidae) originated from a combination of duplication and translocation in the nucleolar organising region. Genes 2019, 10, 861. [Google Scholar] [CrossRef] [PubMed]
- Srikulnath, K.; Azad, B.; Singchat, W.; Ezaz, T. Distribution and amplification of interstitial telomeric sequences (ITSs) in Australian dragon lizards support frequent chromosome fusions in Iguania. PLoS ONE 2019, 14, e0212683. [Google Scholar] [CrossRef] [PubMed]
- Ezaz, T.; O’Meally, D.; Quinn, A.E.; Sarre, S.D.; Georges, A.; Graves, J.A.M. A simple non-invasive protocol to establish primary cell lines from tail and toe explants for cytogenetic studies in Australian dragon lizards (Squamata: Agamidae). Cytotechnology 2008, 58, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasertsri, N.; Uno, Y.; Peyachoknagul, S.; Prakhongcheep, O.; Baicharoen, S.; Charernsuk, S.; Nishida, C.; Matsuda, Y.; Koga, A.; Srikulnath, K. Highly species-specific centromeric repetitive DNA sequences in lizards: Molecular cytogenetic characterization of a novel family of satellite DNA sequences isolated from the water monitor lizard (Varanus salvator macromaculatus, Platynota). J. Hered. 2013, 104, 798–806. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Augstenová, B.; Clemente, L.; Auer, M.; Fritz, U.; Praschag, P.; Protiva, T.; Velenský, P.; Kratochvíl, L.; Rovatsos, M. Turtles of the genera Geoemyda and Pangshura (Testudines: Geoemydidae) lack differentiated sex chromosomes: The end of a 40-year error cascade for Pangshura. PeerJ 2019, 7, e6241. [Google Scholar] [CrossRef]
- Townsend, T.M.; Mulcahy, D.G.; Noonan, B.P.; Sites, J.W., Jr.; Kuczynski, C.A.; Wiens, J.J.; Reeder, T.W. Phylogeny of iguanian lizards inferred from 29 nuclear loci, and a comparison of concatenated and species-tree approaches for an ancient, rapid radiation. Mol. Phylogenet. Evol. 2011, 61, 363–380. [Google Scholar] [CrossRef]
- Voss, S.R.; Kump, D.K.; Putta, S.; Pauly, N.; Reynolds, A.; Henry, R.J.; Basa, S.; Walker, J.A.; Smith, J.J. Origin of amphibian and avian chromosomes by fission, fusion, and retention of ancestral chromosomes. Genome Res. 2011, 21, 1306–1312. [Google Scholar] [CrossRef]
- Iannucci, A.; Altmanová, M.; Ciofi, C.; Ferguson-Smith, M.; Milan, M.; Pereira, J.C.; Pether, J.; Rehák, I.; Rovatsos, M.; Stanyon, R.; et al. Conserved sex chromosomes and karyotype evolution in monitor lizards (Varanidae). Heredity 2019, 123, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Barby, F.F.; Bertollo, L.A.C.; de Oliveira, E.A.; Yano, C.F.; Hatanaka, T.; Ráb, P.; Sember, A.; Ezaz, T.; Artoni, R.F.; Liehr, T.; et al. Emerging patterns of genome organization in Notopteridae species (Teleostei, Osteoglossiformes) as revealed by Zoo-FISH and Comparative Genomic Hybridization (CGH). Sci. Rep. 2019, 9, 1112. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.B.; Ráb, P.; Ezaz, T.; Bertollo, L.A.C.; Lavoué, S.; Oliveira, E.A.; Sember, A.; Molina, W.F.; Souza, F.H.S.; Majtánová, Z.; et al. Deciphering the evolutionary history of arowana fishes (Teleostei, Osteoglossiformes, Osteoglossidae): Insight from comparative cytogenomics. Int. J. Mol. Sci. 2019, 20, 4296. [Google Scholar] [CrossRef] [PubMed]
- Kasai, F.; Garcia, C.; Arruga, M.; Ferguson-Smith, M. Chromosome homology between chicken (Gallus gallus domesticus) and the red-legged partridge (Alectoris rufa); evidence of the occurrence of a neocentromere during evolution. Cytogenet. Genome Res. 2003, 102, 326–330. [Google Scholar] [CrossRef]
- Shibusawa, M.; Nishida-Umehara, C.; Masabanda, J.; Griffin, D.K.; Isobe, T.; Matsuda, Y. Chromosome rearrangements between chicken and guinea fowl defined by comparative chromosome painting and FISH mapping of DNA clones. Cytogenet. Genome Res. 2002, 98, 225–230. [Google Scholar] [CrossRef]
- Shetty, S.; Griffin, D.K.; Graves, J.A.M. Comparative painting reveals strong chromosome homology over 80 million years of bird evolution. Chromosome Res. 1999, 7, 289–295. [Google Scholar] [CrossRef]
- Montiel, E.E.; Badenhorst, D.; Lee, L.S.; Literman, R.; Trifonov, V.; Valenzuela, N. Cytogenetic insights into the evolution of chromosomes and sex determination reveal striking homology of turtle sex chromosomes to amphibian autosomes. Cytogenet. Genome Res. 2016, 148, 292–304. [Google Scholar] [CrossRef]
| Taxon | SDM | 2n | M + m | Sex | Mapping | |
|---|---|---|---|---|---|---|
| Pv03_L07 | Pv150_H19 | |||||
| Family: Agamidae | ||||||
| Subfamily Amphibolurinae | ||||||
| Pogona vitticeps | GSD—ZW | 32 | 12 + 20 | 1 F | 2qtel + ZW micro sex chromosome | ZW micro sex chromosome |
| Tympanocryptis lineata | UNK | 32 | 12 + 20 | 1 M, 1 F | 2qtel + 1 pair of micros | 1 pair of micros |
| Rankinia diemensis | UNK | 32 | 12 + 20 | 1 M, 1 F | 2qtel + 2 pairs of micros | 1 pair of micros |
| Subfamily Agaminae | ||||||
| Agama picticauda | TSD | 44 | 20 + 24 | 1 M, 1 F | 1qtel | 1 pair of micros |
| Phrynocephalus cf. guttatus | UNK | 46 | 22 + 24 | 1 M, 1 F | 1qtel | No hybridization |
| Subfamily Draconinae | ||||||
| Calotes versicolor | TSD | 34 | 12 + 22 | 1 M, 1 F | 2qtel | 1 pair of micros |
| Bronchocela cristatella | UNK | 34 | 14 + 20 | 1 F | 5qtel | No hybridization |
| Subfamily Hydrosaurinae | ||||||
| Hydrosaurus sp. | UNK | 36 | 12 + 24 | 1 UNK | No hybridization | 1 pair of micros |
| Hydrosaurus weberi | UNK | 36 | 12 + 24 | 1 M | 2qtel | No hybridization |
| Subfamily Leiolepidinae | ||||||
| Leiolepis reevesii rubritaeniata | UNK | 36 | 12 + 24 | 1 F | 2qtel | 1 pair of micros |
| Leiolepis cf. ngovantrii | OP | 36 | 12 + 24 | 1 F | 2qtel | 1 pair of micros |
| Subfamily Uromastycinae | ||||||
| Saara loricata | UNK | 36 | 12 + 24 | 1 M, 1 F | 2qtel | No hybridization |
| Family: Chamaeleonidae | ||||||
| Chamaeleo calyptratus | XY | 24 | 12 + 12 | 1 M, 1 F | No hybridization | No hybridization |
| Trioceros johnstoni | UNK | 36 | 14 + 22 | 1 M, 1 F | No hybridization | No hybridization |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, S.M.I.; Altmanová, M.; Prasongmaneerut, T.; Georges, A.; Sarre, S.D.; Nielsen, S.V.; Gamble, T.; Srikulnath, K.; Rovatsos, M.; Kratochvíl, L.; et al. Cross-Species BAC Mapping Highlights Conservation of Chromosome Synteny across Dragon Lizards (Squamata: Agamidae). Genes 2020, 11, 698. https://doi.org/10.3390/genes11060698
Alam SMI, Altmanová M, Prasongmaneerut T, Georges A, Sarre SD, Nielsen SV, Gamble T, Srikulnath K, Rovatsos M, Kratochvíl L, et al. Cross-Species BAC Mapping Highlights Conservation of Chromosome Synteny across Dragon Lizards (Squamata: Agamidae). Genes. 2020; 11(6):698. https://doi.org/10.3390/genes11060698
Chicago/Turabian StyleAlam, Shayer Mahmood Ibney, Marie Altmanová, Tulyawat Prasongmaneerut, Arthur Georges, Stephen D. Sarre, Stuart V. Nielsen, Tony Gamble, Kornsorn Srikulnath, Michail Rovatsos, Lukáš Kratochvíl, and et al. 2020. "Cross-Species BAC Mapping Highlights Conservation of Chromosome Synteny across Dragon Lizards (Squamata: Agamidae)" Genes 11, no. 6: 698. https://doi.org/10.3390/genes11060698
APA StyleAlam, S. M. I., Altmanová, M., Prasongmaneerut, T., Georges, A., Sarre, S. D., Nielsen, S. V., Gamble, T., Srikulnath, K., Rovatsos, M., Kratochvíl, L., & Ezaz, T. (2020). Cross-Species BAC Mapping Highlights Conservation of Chromosome Synteny across Dragon Lizards (Squamata: Agamidae). Genes, 11(6), 698. https://doi.org/10.3390/genes11060698








