A Third MLPH Variant Causing Coat Color Dilution in Dogs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. MLPH Exon Sequencing
2.3. Whole-Genome Database Query
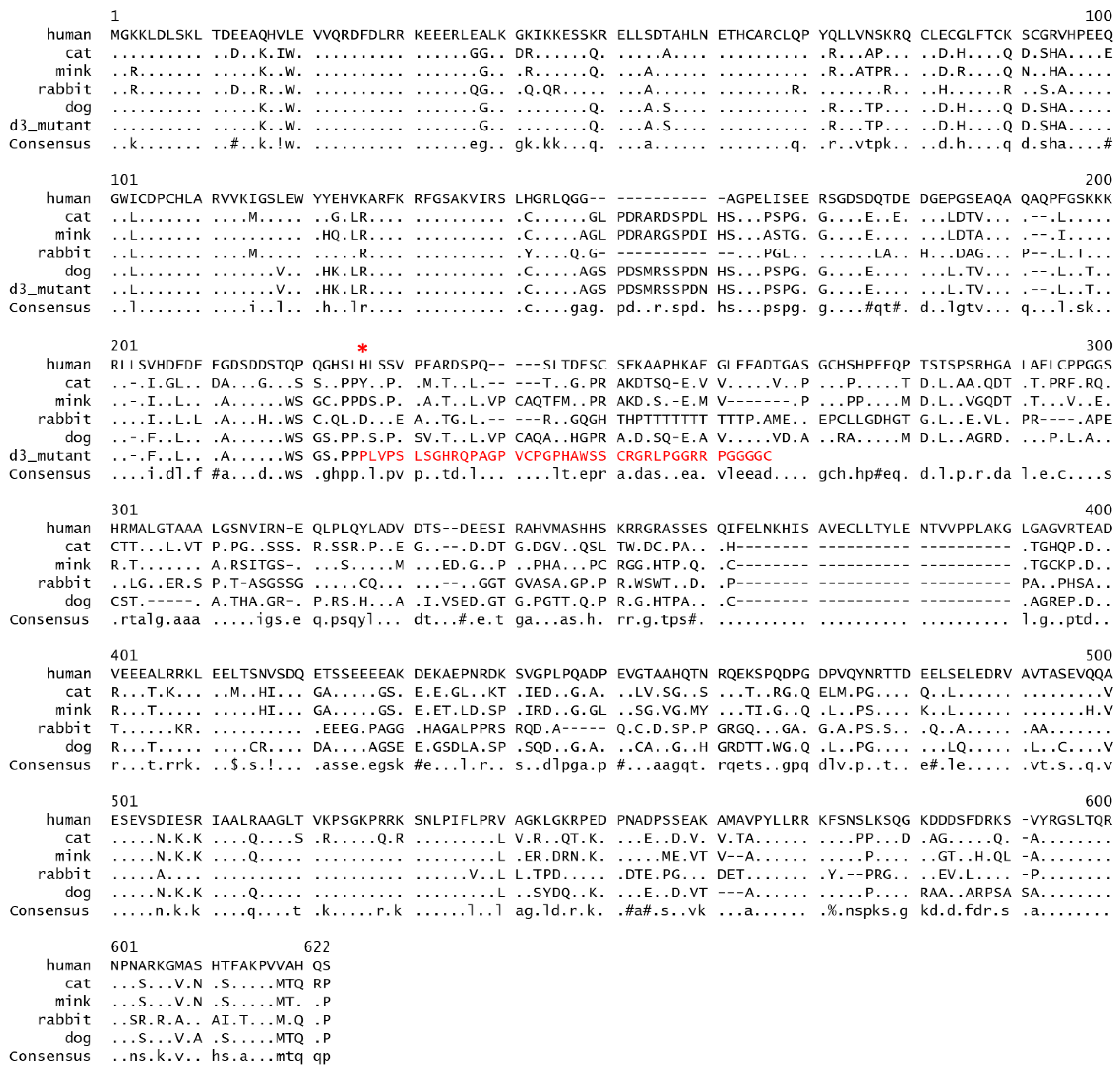
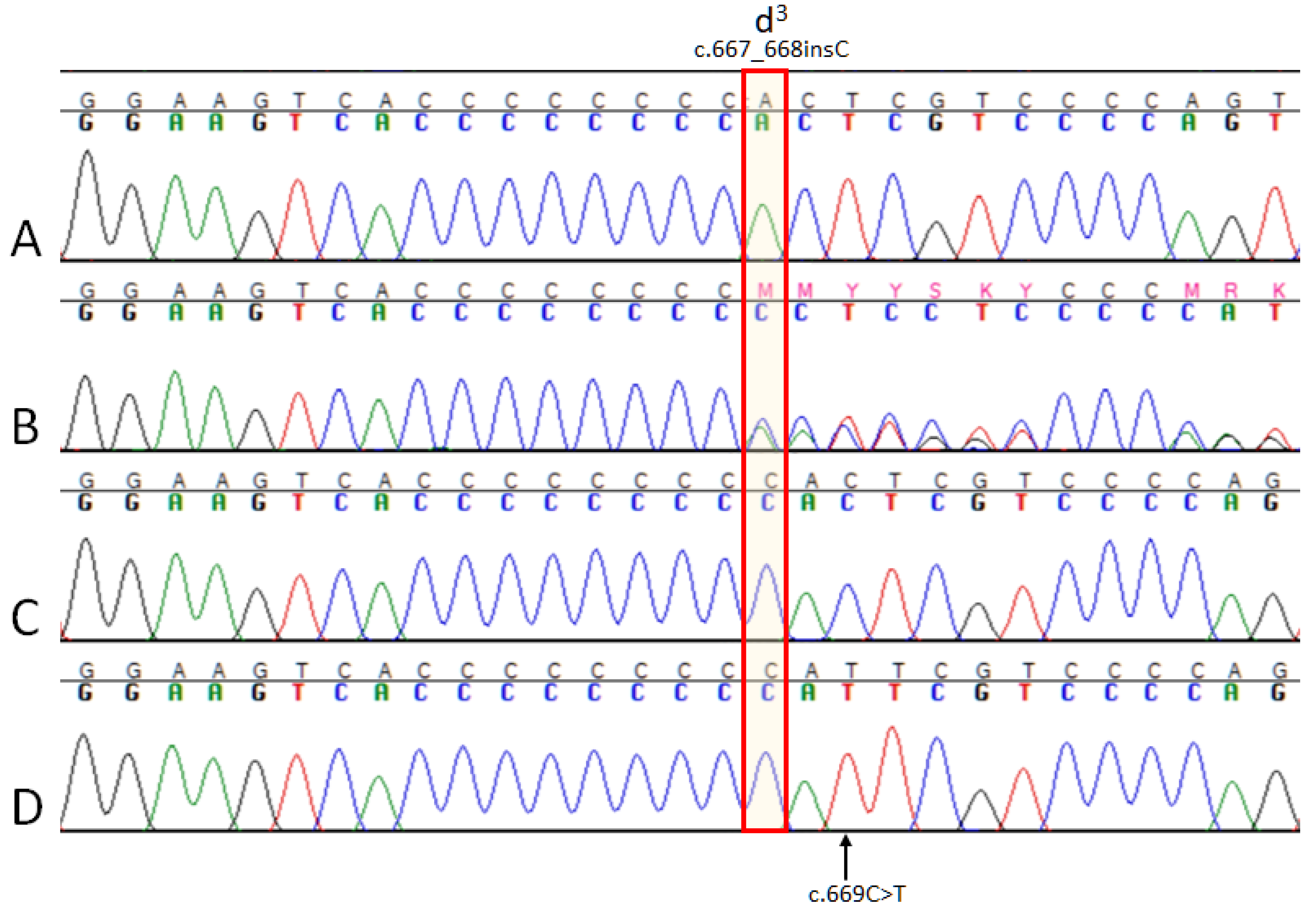
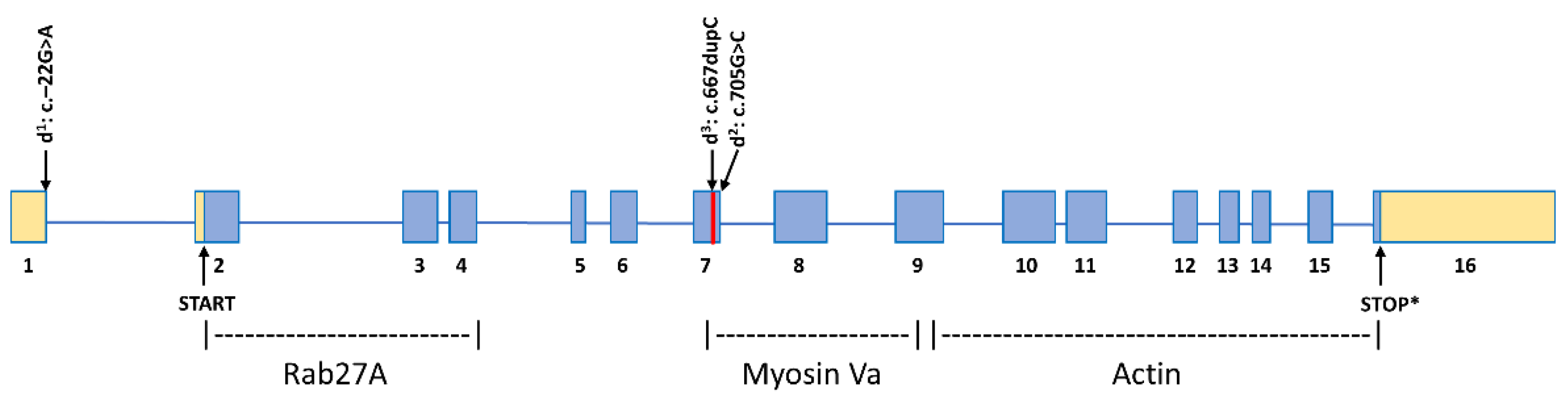
3. Results
3.1. MLPH Exon Sequencing
3.2. Variant Database Search
3.3. MLPH Genotyping
3.4. Genotype-Phenotype Association
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jo, C.; Park, H.I.; Jung, H.J.; Park, J.I.; Lee, J.E.; Myung, C.H.; Hwang, J.S. A novel function of Prohibitin on melanosome transport in melanocytes. Theranostics 2020, 10, 3880–3891. [Google Scholar] [CrossRef] [PubMed]
- Drögemüller, C.; Philipp, U.; Haase, B.; Günzel-Apel, A.R.; Leeb, T. A noncoding melanophilin gene (MLPH) SNP at the splice donor of exon 1 represents a candidate causal mutation for coat color dilution in dogs. J. Hered. 2007, 98, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Kehl, A.; Jagannathan, V.; Leeb, T. A novel MLPH variant in dogs with coat colour dilution. Anim. Genet. 2018, 49, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Weich, K.; Affolter, V.; York, D.; Rebhun, R.; Grahn, R.; Kallenberg, A.; Bannasch, D. Pigment Intensity in Dogs is Associated with a Copy Number Variant Upstream of KITLG. Genes 2020, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Dreger, D.L.; Hooser, B.N.; Hughes, A.M.; Ganesan, B.; Donner, J.; Anderson, H.; Holtvoigt, L.; Ekenstedt, K.J. True Colors: Commercially-acquired morphological genotypes reveal hidden allele variation among dog breeds, informing both trait ancestry and breed potential. PLoS ONE 2019, 14, e0223995. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, V.; Drögemüller, C.; Leeb, T. Dog Biomedical Variant Database Consortium (DBVDC). A comprehensive biomedical variant catalogue based on whole genome sequences of 582 dogs and eight wolves. Anim. Genet. 2019, 50, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Golden Helix GenomeBrowse® visualization tool (Version 2.x). Available online: http://www.goldenhelix.com (accessed on 11 April 2020).
- Clark, L.A.; Wahl, J.M.; Rees, C.A. Murphy KE. Retrotransposon insertion in SILV is responsible for merle patterning of the domestic dog. Proc. Natl. Acad. Sci. USA 2006, 103, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Langevin, M.; Synkova, H.; Jancuskova, T.; Pekova, S. Merle phenotypes in dogs—SILV SINE insertions from Mc to Mh. PLoS ONE 2018, 13, e0198536. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, S.M.; Berryere, T.G.; Goldfinch, A.D. TYRP1 and MC1R genotypes and their effects on coat color in dogs. Mamm. Genome 2002, 13, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Hrckova, T.E.; Majchrakovam, Z.; Bielikovam, M.; Soltysm, K.; Turnam, J.; Dudasm, A. A novel mutation in the TYRP1 gene associated with brown coat colour in the Australian Shepherd Dog Breed. Anim. Genet. 2017, 48, 626. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.E.; Schofield, E.; Mellersh, C.S.; Burmeister, L.M. A novel TYRP1 variant is associated with liver and tan coat colour in Lancashire Heelers. Anim. Genet. 2019, 50, 783. [Google Scholar] [CrossRef] [PubMed]
- Everts, R.E.; Rothuizen, J.; van Oost, B.A. Identification of a premature stop codon in the melanocyte-stimulating hormone receptor gene (MC1R) in Labrador and Golden retrievers with yellow coat colour. Anim. Genet. 2000, 31, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, S.M.; Berryere, T.G.; Ellinwood, N.M.; Kerns, J.A.; Barsh, G.S. MC1R studies in dogs with melanistic mask or brindle patterns. J. Hered. 2003, 94, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Dreger, D.L.; Schmutz, S.M. A new mutation in MC1R explains a coat color phenotype in 2 “old” breeds: Saluki and Afghan hound. J. Hered. 2010, 10, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Dürig, N.; Letko, A.; Lepori, V.; Hadji Rasouliha, S.; Loechel, R.; Kehl, A.; Hytönen, M.K.; Lohi, H.; Mauri, N.; Dietrich, J.; et al. Two MC1R loss-of-function alleles in cream-coloured Australian Cattle Dogs and white Huskies. Anim. Genet. 2018, 49, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; David, V.A.; Eizirik, E.; Schäffer, A.A.; Neelam, B.A.; Roelke, M.E.; Hannah, S.S.; O’Brien, S.J. Menotti-Raymond, M. A homozygous single-base deletion in MLPH causes the dilute coat color phenotype in the domestic cat. Genomics 2006, 88, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Lehner, S.; Gähle, M.; Dierks, C.; Stelter, R.; Gerber, J.; Brehm, R.; Distl, O. Two-exon skipping within MLPH is associated with coat color dilution in rabbits. PLoS ONE 2013, 8, e84525. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sartelet, A.; Tamma, N.; Coppieters, W.; Georges, M.; Charlier, C. Reverse genetic screen for loss-of-function mutations uncovers a frameshifting deletion in the melanophilin gene accountable for a distinctive coat color in Belgian Blue cattle. Anim. Genet. 2016, 47, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Vaez, M.; Follett, S.A.; Bed’hom, B.; Gourichon, D.; Tixier-Boichard, M.; Burke, T. A single point-mutation within the melanophilin gene causes the lavender plumage colour dilution phenotype in the chicken. BMC Genet. 2008, 9. [Google Scholar] [CrossRef] [PubMed]
- Cirera, S.; Markakis, M.N.; Christensen, K.; Anistoroaei, R. New insights into the melanophilin (MLPH) gene controlling coat color phenotypes in American mink. Gene 2013, 527, 48–54. [Google Scholar] [CrossRef] [PubMed]


| Breed | Color | D/D | D/d1 | D/d3 | d1/d3 | d1/d1 | d3/d3 |
|---|---|---|---|---|---|---|---|
| Hungarian pumi | Black | 5 | 4 | 2 | 0 | 0 | 0 |
| Hungarian pumi | Grey | 0 | 0 | 0 | 3 | 0 | 0 |
| Hungarian mudi | Black * | 5 | 3 | 6 | 0 | 0 | 0 |
| Hungarian mudi | Grey * | 0 | 0 | 0 | 2 | 0 | 2 |
| Hungarian mudi | Cream * | 0 | 0 | 1 | 0 | 0 | 1 # |
| Wolf-dog hybrid | Black | 0 | 1 | 0 | 0 | 0 | 0 |
| Wolf-dog hybrid | Grey | 0 | 0 | 0 | 1 | 1 | 3 |
| Wolf-dog hybrid | Other | 1 | 0 | 0 | 0 | 0 | 0 |
| Chihuahua | Grey | 0 | 0 | 0 | 1 | 0 | 0 |
| Italian greyhound | Grey | 0 | 0 | 0 | 1 | 0 | 0 |
| Pekingese | White | 0 | 0 | 0 | 1 # | 0 | 0 |
| Pekingese | Brown | 0 | 1 | 0 | 0 | 0 | 0 |
| Shih Tzu | White | 1 | 0 | 0 | 0 | 0 | 0 |
| Shih Tzu | Grey | 0 | 0 | 0 | 0 | 1 ** | |
| Shih Tzu | Non-dilute | 0 | 2 ** | 0 | 0 | 0 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Buren, S.L.; Minor, K.M.; Grahn, R.A.; Mickelson, J.R.; Grahn, J.C.; Malvick, J.; Colangelo, J.R.; Mueller, E.; Kuehnlein, P.; Kehl, A. A Third MLPH Variant Causing Coat Color Dilution in Dogs. Genes 2020, 11, 639. https://doi.org/10.3390/genes11060639
Van Buren SL, Minor KM, Grahn RA, Mickelson JR, Grahn JC, Malvick J, Colangelo JR, Mueller E, Kuehnlein P, Kehl A. A Third MLPH Variant Causing Coat Color Dilution in Dogs. Genes. 2020; 11(6):639. https://doi.org/10.3390/genes11060639
Chicago/Turabian StyleVan Buren, Samantha L., Katie M. Minor, Robert A. Grahn, James R. Mickelson, Jennifer C. Grahn, Julia Malvick, Jennifer R. Colangelo, Elisabeth Mueller, Petra Kuehnlein, and Alexandra Kehl. 2020. "A Third MLPH Variant Causing Coat Color Dilution in Dogs" Genes 11, no. 6: 639. https://doi.org/10.3390/genes11060639
APA StyleVan Buren, S. L., Minor, K. M., Grahn, R. A., Mickelson, J. R., Grahn, J. C., Malvick, J., Colangelo, J. R., Mueller, E., Kuehnlein, P., & Kehl, A. (2020). A Third MLPH Variant Causing Coat Color Dilution in Dogs. Genes, 11(6), 639. https://doi.org/10.3390/genes11060639






