Comparative Transcriptomics Reveals Gene Families Associated with Predatory Behavior in Photuris femme fatale Fireflies
Abstract
1. Introduction
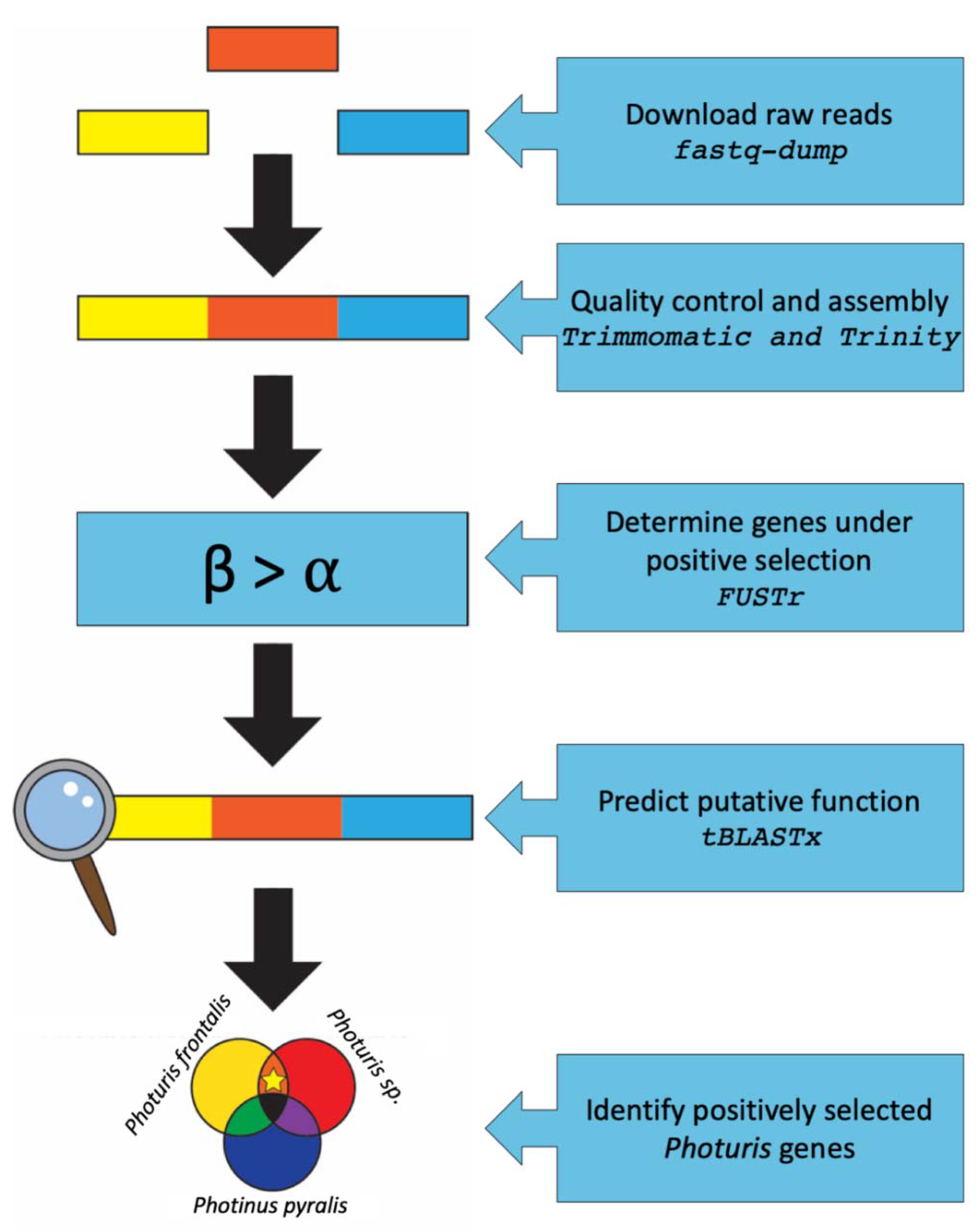
2. Materials and Methods
2.1. Experimental Design
2.2. Transcriptome Assembly and Assessment
2.3. Site-Specific Positive Selection Analysis
2.4. Functional Annotation
3. Results and Discussion
3.1. De Novo Assembly Results in Relatively Complete Transcriptomes for All Three Species
3.2. Species Comparisons Identify Gene Families under Selection
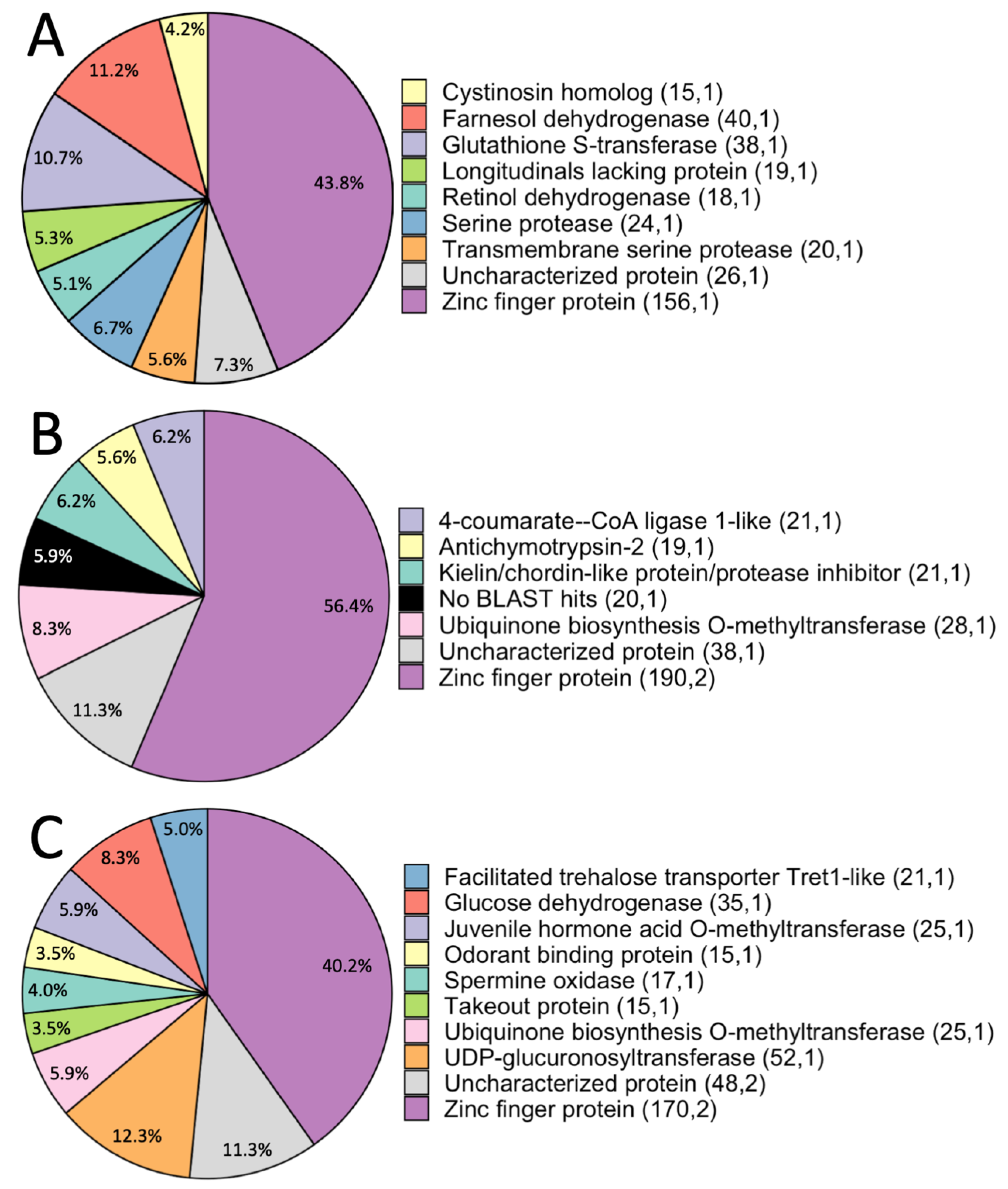
3.2.1. Predatory vs. Non-Predatory Photuris Comparison
Genes Involved in Vision, Digestion and Detoxification, and Egg Provisioning Are Candidates for Adaptation to Predation
Juvenile Hormone, Serine Proteases, and lola May Be Involved in Neural Processes Underlying Predation
3.2.2. Photuris-Photinus Comparisons
Diapause, Immunity, and Venom Proteins Are Identified in Non-Predatory Photuris-Photinus Comparisons
Candidate Genes Identified in the Predatory Pt. sp. and Non-Predatory Pn. pyralis Comparison Are Implicated in Both Predation and in Other Phenotypic Variation
3.2.3. Positively Selected Genes Common to All Comparisons
3.3. Implications for Evolution of Predation in Fireflies
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thomas, A.L.; Davis, S.M.; Dierick, H.A. Of fighting flies, mice, and men: Are some of the molecular and neuronal mechanisms of aggression universal in the animal kingdom? PLoS Genet. 2015, 11, e1005416. [Google Scholar] [CrossRef] [PubMed]
- Konopka, R.J.; Benzer, S. Clock mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1971, 68, 2112–2116. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.C. Courtship among males due to a male-sterile mutation in Drosophila melanogaster. Behav. Genet. 1978, 8, 125–141. [Google Scholar] [CrossRef] [PubMed]
- York, R.A. Assessing the genetic landscape of animal behavior. Genetics 2018, 209, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Zhang, H.; Tang, Z.; Guo, Z.; Yan, J.; Xiao, J.; Luo, Y.; Zhou, Y. Evidence for natural selection of immune genes from Parachromis managuensis by transcriptome sequencing. Biotechnol. Biotech. Eq. 2018, 32, 1431–1439. [Google Scholar] [CrossRef]
- Heras, J.; Aguilar, A. Comparative transcriptomics reveals patterns of adaptive evolution associated with depth and age within marine rockfishes (Sebastes). J. Hered. 2019, 110, 340–350. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, P.; Lascoux, M.; Meagher, T.R.; Liu, J. Rapidly evolving genes and stress adaptation of two desert poplars, Populus euphratica and P. pruinosa. PLoS ONE 2013, 8, e66370. [Google Scholar] [CrossRef]
- Murrell, B.; Moola, S.; Mabona, A.; Weighill, T.; Sheward, D.; Kosakovsky Pond, S.L.; Scheffler, K. FUBAR: A fast, unconstrained bayesian approximation for inferring selection. Mol. Biol. Evol. 2013, 30, 1196–1205. [Google Scholar] [CrossRef]
- Balakrishnan, C.N.; Chapus, C.; Brewer, M.S.; Clayton, D.F. Brain transcriptome of the violet-eared waxbill Uraeginthus granatina and recent evolution in the songbird genome. Open Biol. 2013, 3, 130063. [Google Scholar] [CrossRef]
- Barreto, F.S.; Moy, G.W.; Burton, R.S. Interpopulation patterns of divergence and selection across the transcriptome of the copepod Tigriopus californicus. Mol. Ecol. 2011, 20, 560–572. [Google Scholar] [CrossRef]
- Brewer, M.S.; Carter, R.A.; Croucher, P.J.P.; Gillespie, R.G. Shifting habitats, morphology, and selective pressures: Developmental polyphenism in an adaptive radiation of Hawaiian spiders. Evolution 2015, 69, 162–178. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.M.; Cratsley, C.K. Flash signal evolution, mate choice, and predation in fireflies. Annu. Rev. Entomol. 2008, 53, 293–321. [Google Scholar] [CrossRef] [PubMed]
- Sander, S.E.; Hall, D.W. Variation in opsin genes correlates with signalling ecology in North American fireflies. Mol. Ecol. 2015, 24, 4679–4696. [Google Scholar] [CrossRef] [PubMed]
- Amaral, D.T.; Silva, J.R.; Viviani, V.R. Transcriptomes from the photogenic and non-photogenetic tissues and life stages of the Aspisoma lineatum firefly (Coleoptera: Lampyridae): Implications for the evolutionary origins of bioluminescence and its associated light organs. Gene Rep. 2017, 8, 150–159. [Google Scholar] [CrossRef]
- Fallon, T.R.; Lower, S.E.; Chang, C.-H.; Bessho-Uehara, M.; Martin, G.J.; Bewick, A.J.; Behringer, M.; Debat, H.J.; Wong, I.; Day, J.C.; et al. Firefly genomes illuminate parallel origins of bioluminescence in beetles. eLife 2018, 7. [Google Scholar] [CrossRef]
- Fu, X.; Li, J.; Tian, Y.; Quan, W.; Zhang, S.; Liu, Q.; Liang, F.; Zhu, X.; Zhang, L.; Wang, D.; et al. Long-read sequence assembly of the firefly Pyrocoelia pectoralis genome. Gigascience 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Lloyd, J.E. Studies on the Flash Communication System in Photinus Fireflies; Miscellaneous Publications, Museum of Zoology, University of Michigan: Ann Arbor, MI, USA, 1966. [Google Scholar]
- Stanger-Hall, K.F.; Lloyd, J.E. Flash signal evolution in Photinus fireflies: Character displacement and signal exploitation in a visual communication system. Evolution 2015, 69, 666–682. [Google Scholar] [CrossRef]
- Faust, L.F. Fireflies, Glow-Worms, and Lightning Bugs: Identification and Natural History of the Fireflies of the Eastern and Central United States and Canada; University of Georgia Press: Athens, GA, USA, 2017. [Google Scholar]
- Eisner, T.; Goetz, M.A.; Hill, D.E.; Smedley, S.R.; Meinwald, J. Firefly “femmes fatales” acquire defensive steroids (lucibufagins) from their firefly prey. Proc. Natl. Acad. Sci. USA 1997, 94, 9723–9728. [Google Scholar] [CrossRef]
- Deyrup, S.T.; Risteen, R.G.; Tonyai, K.K.; Farrar, M.A.; D’Antonio, B.E.; Ahmed, Z.B.; Christofel, B.T.; Howells, N.R.; Smedley, S.R. Escape into winter: Does a phenological shift by Ellychnia corrusca (Winter Firefly) shield it from a specialist predator (Photuris)? Northeast. Nat. 2017, 24, B147–B166. [Google Scholar] [CrossRef]
- Faust, L.; De Cock, R.; Lewis, S. Thieves in the night: Kleptoparasitism by fireflies in the genus Photuris Dejean (Coleoptera: Lampyridae). Coleopts Bull. 2012, 66, 1–6. [Google Scholar] [CrossRef]
- Barber, H.S. North American fireflies of the genus Photuris. Smithson. Misc. Collect. 1951, 117, 1–58. [Google Scholar]
- Lloyd, J.E.; Wing, S.R. Nocturnal aerial predation of fireflies by light-seeking fireflies. Science 1983, 222, 634–635. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.E. Aggressive mimicry in Photuris: Firefly femmes fatales. Science 1965, 149, 653–654. [Google Scholar] [CrossRef] [PubMed]
- Zorn, L.P.; Carlson, A.D. Effect of mating on response of female Photuris firefly. Anim. Behav. 1978, 26, 843–847. [Google Scholar] [CrossRef]
- Heckscher, C.M. Photuris mysticalampas (Coleoptera: Lampyridae): A new firefly from peatland floodplain forests of the Delmarva peninsula. Entomol. News 2013, 123, 93–100. [Google Scholar] [CrossRef]
- Crespi, B.J. Shared sociogenetic basis of honey bee behavior and human risk for autism. Proc. Natl. Acad. Sci. USA 2017, 114, 9502–9504. [Google Scholar] [CrossRef] [PubMed]
- McLean, M.; Buck, J.; Hanson, F.E. Culture and larval behavior of Photurid fireflies. Am. Midl. Nat. 1972, 87, 133. [Google Scholar] [CrossRef]
- SRA Toolkit Development Team. SRA Toolkit. Available online: http://ncbi.github.io/sra-tools/ (accessed on 6 June 2020).
- Fallon, T.R.; Li, F.-S.; Vicent, M.A.; Weng, J.-K. Sulfoluciferin is biosynthesized by a specialized luciferin sulfotransferase in fireflies. Biochemistry 2016, 55, 3341–3344. [Google Scholar] [CrossRef]
- Al-Wathiqui, N.; Fallon, T.R.; South, A.; Weng, J.-K.; Lewis, S.M. Molecular characterization of firefly nuptial gifts: A multi-omics approach sheds light on postcopulatory sexual selection. Sci. Rep. 2016, 6, 38556. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotech. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Macmanes, M.D. On the optimal trimming of high-throughput mRNA sequence data. Front. Genet. 2014, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Brewer, M.S. FUSTr: A tool to find gene families under selection in transcriptomes. PeerJ 2018, 6, e4234. [Google Scholar] [CrossRef] [PubMed]
- Köster, J.; Rahmann, S. Snakemake-a scalable bioinformatics workflow engine. Bioinformatics 2018, 34, 3600. [Google Scholar] [CrossRef]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Miele, V.; Penel, S.; Duret, L. Ultra-fast sequence clustering from similarity networks with SiLiX. BMC Bioinform. 2011, 12, 116. [Google Scholar] [CrossRef]
- Yang, Z. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef]
- Hofmann, L.; Tsybovsky, Y.; Alexander, N.S.; Babino, D.; Leung, N.Y.; Montell, C.; Banerjee, S.; von Lintig, J.; Palczewski, K. Structural Insights into the Drosophila melanogaster retinol dehydrogenase, a member of the short-chain dehydrogenase/reductase family. Biochemistry 2016, 55, 6545–6557. [Google Scholar] [CrossRef]
- Sumayao, R., Jr.; Newsholme, P.; McMorrow, T. The role of cystinosin in the intermediary thiol metabolism and redox homeostasis in kidney proximal tubular cells. Antioxid. Basel 2018, 7, 179. [Google Scholar] [CrossRef]
- Shi, H.; Pei, L.; Gu, S.; Zhu, S.; Wang, Y.; Zhang, Y.; Li, B. Glutathione S-transferase (GST) genes in the red flour beetle, Tribolium castaneum, and comparative analysis with five additional insects. Genomics 2012, 100, 327–335. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Hare, J.F.; Eisner, T. Chemical egg defense in Photuris firefly “femmes fatales”. Chemoecology 1999, 9, 177–185. [Google Scholar] [CrossRef]
- Mayoral, J.G.; Nouzova, M.; Navare, A.; Noriega, F.G. NADP+-dependent farnesol dehydrogenase, a corpora allata enzyme involved in juvenile hormone synthesis. Proc. Natl. Acad. Sci. USA 2009, 106, 21091–21096. [Google Scholar] [CrossRef] [PubMed]
- Nijhout, F.H. Insect Hormones; Princeton University Press: Princeton, NJ, USA, 1998. [Google Scholar]
- Li, J.; Choo, Y.M.; Lee, K.S.; Je, Y.H.; Woo, S.D.; Kim, I.; Sohn, H.D.; Jin, B.R. A serine protease gene from the firefly, Pyrocoelia rufa: Gene structure, expression, and enzyme activity. Biotechnol. Lett. 2005, 27, 1051–1057. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Reiser, G. Protease-activated receptors in the brain: Receptor expression, activation, and functions in neurodegeneration and neuroprotection. Brain Res. Rev. 2007, 56, 331–345. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, W.; Reiser, G. Trypsin and trypsin-like proteases in the brain: Proteolysis and cellular functions. Cell Mol. Life Sci. 2008, 65, 237–252. [Google Scholar] [CrossRef]
- Turgeon, V.L.; Houenou, L.J. The role of thrombin-like (serine) proteases in the development, plasticity and pathology of the nervous system. Brain Res. Rev. 1997, 25, 85–95. [Google Scholar] [CrossRef]
- Davies, B.J.; Pickard, B.S.; Steel, M.; Morris, R.G.M.; Lathe, R. Serine proteases in rodent hippocampus. J. Biol. Chem. 1998, 273, 23004–23011. [Google Scholar] [CrossRef]
- Gingrich, M.B.; Traynelis, S.F. Serine proteases and brain damage—Is there a link? Trends Neurosci. 2000, 23, 399–407. [Google Scholar] [CrossRef]
- Almonte, A.G.; Sweatt, J.D. Serine proteases, serine protease inhibitors, and protease-activated receptors: Roles in synaptic function and behavior. Brain Res. 2011, 1407, 107–122. [Google Scholar] [CrossRef]
- Giniger, E.; Tietje, K.; Jan, L.Y.; Jan, Y.N. Lola encodes a putative transcription factor required for axon growth and guidance in Drosophila. Development 1994, 120, 1385–1398. [Google Scholar] [PubMed]
- Sato, K.; Ito, H.; Yokoyama, A.; Toba, G.; Yamamoto, D. Partial proteasomal degradation of Lola triggers the male-to-female switch of a dimorphic courtship circuit. Nat. Commun. 2019, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Patel, S.R.; Cheng, X.; Cho, E.A.; Levitan, I.; Ullenbruch, M.; Phan, S.H.; Park, J.M.; Dressler, G.R. Kielin/chordin-like protein, a novel enhancer of BMP signaling, attenuates renal fibrotic disease. Nat. Med. 2005, 11, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Lin, X.-W.; Geng, S.-L.; Xu, W.-H. TGF-β and BMP signals regulate insect diapause through Smad1-POU-TFAM pathway. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Meekins, D.A.; Kanost, M.R.; Michel, K. Serpins in arthropod biology. Semin. Cell Dev. Biol. 2017, 62, 105–119. [Google Scholar] [CrossRef]
- Cohen, S.S. A Guide to the Polyamines; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Wallace, H.M.; Fraser, A.V.; Hughes, A. A perspective of polyamine metabolism. Biochem. J. 2003, 376, 1–14. [Google Scholar] [CrossRef]
- Zhou, J.-J. Odorant-binding proteins in insects. Vitam. Horm. 2010, 241–272. [Google Scholar] [CrossRef]
- Shinoda, T.; Itoyama, K. Juvenile hormone acid methyltransferase: A key regulatory enzyme for insect metamorphosis. Proc. Natl. Acad. Sci. USA 2003, 100, 11986–11991. [Google Scholar] [CrossRef]
- Cavener, D.R. GMC oxidoreductases: A newly defined family of homologous proteins with diverse catalytic activities. J. Mol. Biol. 1992, 223, 811–814. [Google Scholar] [CrossRef]
- Bak, T.-G. Studies on glucose dehydrogenase of aspergillus oryzae. Biochim. Biophys. Acta Enzymol. 1967, 146, 317–327. [Google Scholar] [CrossRef]
- Murtha, M.T.; Cavener, D.R. Ecdysteroid regulation of glucose dehydrogenase and alcohol dehydrogenase gene expression in Drosophila melanogaster. Dev. Biol. 1989, 135, 66–73. [Google Scholar] [CrossRef]
- Iida, K.; Cavener, D.R. Glucose dehydrogenase is required for normal sperm storage and utilization in female Drosophila melanogaster. J. Exp. Biol. 2004, 207, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Kikuta, S.; Hagiwara-Komoda, Y.; Noda, H.; Kikawada, T. A novel member of the trehalose transporter family functions as an h(+)-dependent trehalose transporter in the reabsorption of trehalose in malpighian tubules. Front. Physiol. 2012, 3, 290. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.H.; Carpenter, J.F.; Crowe, L.M. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 1998, 60, 73–103. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Meech, R.; Mackenzie, P.I. Structure and function of uridine diphosphate glucuronosyltransferases. Clin. Exp. Pharmacol. Physiol. 1997, 24, 907–915. [Google Scholar] [CrossRef]
- Lazard, D.; Zupko, K.; Poria, Y.; Nef, P.; Lazarovits, J.; Horn, S.; Khen, M.; Lancet, D. Odorant signal termination by olfactory UDP glucuronosyl transferase. Nature 1991, 349, 790–793. [Google Scholar] [CrossRef]
- Hopkins, T.L.; Kramer, K.J. Insect cuticle sclerotization. Annu. Rev. Entomol. 1992, 37, 273–302. [Google Scholar] [CrossRef]
- Wiesen, B.; Krug, E.; Fiedler, K.; Wray, V.; Proksch, P. Sequestration of host-plant-derived flavonoids by lycaenid butterfly Polyommatus icarus. J. Chem. Ecol. 1994, 20, 2523–2538. [Google Scholar] [CrossRef]
- Dauwalder, B.; Tsujimoto, S.; Moss, J.; Mattox, W. The Drosophila takeout gene is regulated by the somatic sex-determination pathway and affects male courtship behavior. Genes Dev. 2002, 16, 2879–2892. [Google Scholar] [CrossRef]
- Li, H.; Young, B.J.; Wang, H.; Schooley, D.A. The structure of ubiquinones isolated from developing embryos of Manduca sexta. Insect Biochem. Molec. 1998, 28, 69–73. [Google Scholar] [CrossRef]
- Beyer, R.E. An analysis of the role of coenzyme Q in free radical generation and as an antioxidant. Biochem. Cell Biol. 1992, 70, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Ernster, L.; Dallner, G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys. Acta 1995, 1271, 195–204. [Google Scholar] [CrossRef]
- Laity, J.H.; Lee, B.M.; Wright, P.E. Zinc finger proteins: New insights into structural and functional diversity. Curr. Opin. Struct. Biol. 2001, 11, 39–46. [Google Scholar] [CrossRef]
- Emerson, R.O.; Thomas, J.H. Adaptive evolution in zinc finger transcription factors. PLoS Genet. 2009, e1000325. [Google Scholar] [CrossRef]
- Beier, J.C. Frequent blood-feeding and restrictive sugar-feeding behavior enhance the malaria vector potential of Anopheles gambiae s.l. and An. funestus (Diptera: Culicidae) in western Kenya. J. Med. Entomol. 1996, 33, 613–618. [Google Scholar] [CrossRef]
- Robinson, G.E. Regulation of division of labor in insect societies. Annu. Rev. Entomol. 1992, 37, 637–665. [Google Scholar] [CrossRef]
| Species | N Genes | N Transcripts | GC (%) | Median Length (bp) | Mean Length (bp) | N Bases | N50 |
|---|---|---|---|---|---|---|---|
| Photuris frontalis | 40,547 | 58,028 | 34.65 | 405 | 866.46 | 35,132,369 | 1693 |
| Photuris sp. | 38,303 | 56,626 | 34.43 | 402 | 901.44 | 34,527,893 | 1820 |
| Photinus pyralis | 130,648 | 188,474 | 38.96 | 346 | 634.52 | 82,898,270 | 902 |
| Species | C (%) | S (%) | D (%) | F (%) | M (%) |
|---|---|---|---|---|---|
| Photuris frontalis | 90.9% | 62.2% | 28.7% | 4.9% | 4.2% |
| Photuris sp. | 91.3% | 59.6% | 31.7% | 4.4% | 4.3% |
| Photinus pyralis | 93.5% | 46.3% | 47.2% | 3.9% | 2.6% |
| Comparison | N input Transcripts | N Isoforms Disregarded | N Transcripts Used | N Families | N Families with > 15 Sequences | N Families with β > α |
|---|---|---|---|---|---|---|
| Pt. sp. vs. Pt. frontalis | 114,654 | 29,237 | 46,371 | 36,387 | 44 | 9 |
| Pt. sp. vs. Pn. pyralis | 245,100 | 49,882 | 71,700 | 62,055 | 49 | 12 |
| Pt. frontalis vs. Pn. pyralis | 246,502 | 49,025 | 74,039 | 64,354 | 48 | 8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKinley, C.N.; Lower, S.E. Comparative Transcriptomics Reveals Gene Families Associated with Predatory Behavior in Photuris femme fatale Fireflies. Genes 2020, 11, 627. https://doi.org/10.3390/genes11060627
McKinley CN, Lower SE. Comparative Transcriptomics Reveals Gene Families Associated with Predatory Behavior in Photuris femme fatale Fireflies. Genes. 2020; 11(6):627. https://doi.org/10.3390/genes11060627
Chicago/Turabian StyleMcKinley, Cheyenne N., and Sarah E. Lower. 2020. "Comparative Transcriptomics Reveals Gene Families Associated with Predatory Behavior in Photuris femme fatale Fireflies" Genes 11, no. 6: 627. https://doi.org/10.3390/genes11060627
APA StyleMcKinley, C. N., & Lower, S. E. (2020). Comparative Transcriptomics Reveals Gene Families Associated with Predatory Behavior in Photuris femme fatale Fireflies. Genes, 11(6), 627. https://doi.org/10.3390/genes11060627






