Fetal Hypoxia Impacts on Proliferation and Differentiation of Sca-1+ Cardiac Progenitor Cells and Maturation of Cardiomyocytes: A Role of MicroRNA-210
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal
2.2. Isolation of Primary Cardiac Cells and Sca-1+ Cardiac Progenitor Cells
2.3. In Vitro Cardiac Progenitor Cell Culture, Transfection, and Differentiation
2.4. Antibodies and Flow Cytometry
2.5. MicroRNA Quantitative RT-PCR
2.6. Echocardiography
2.7. Statistical Analysis
3. Results
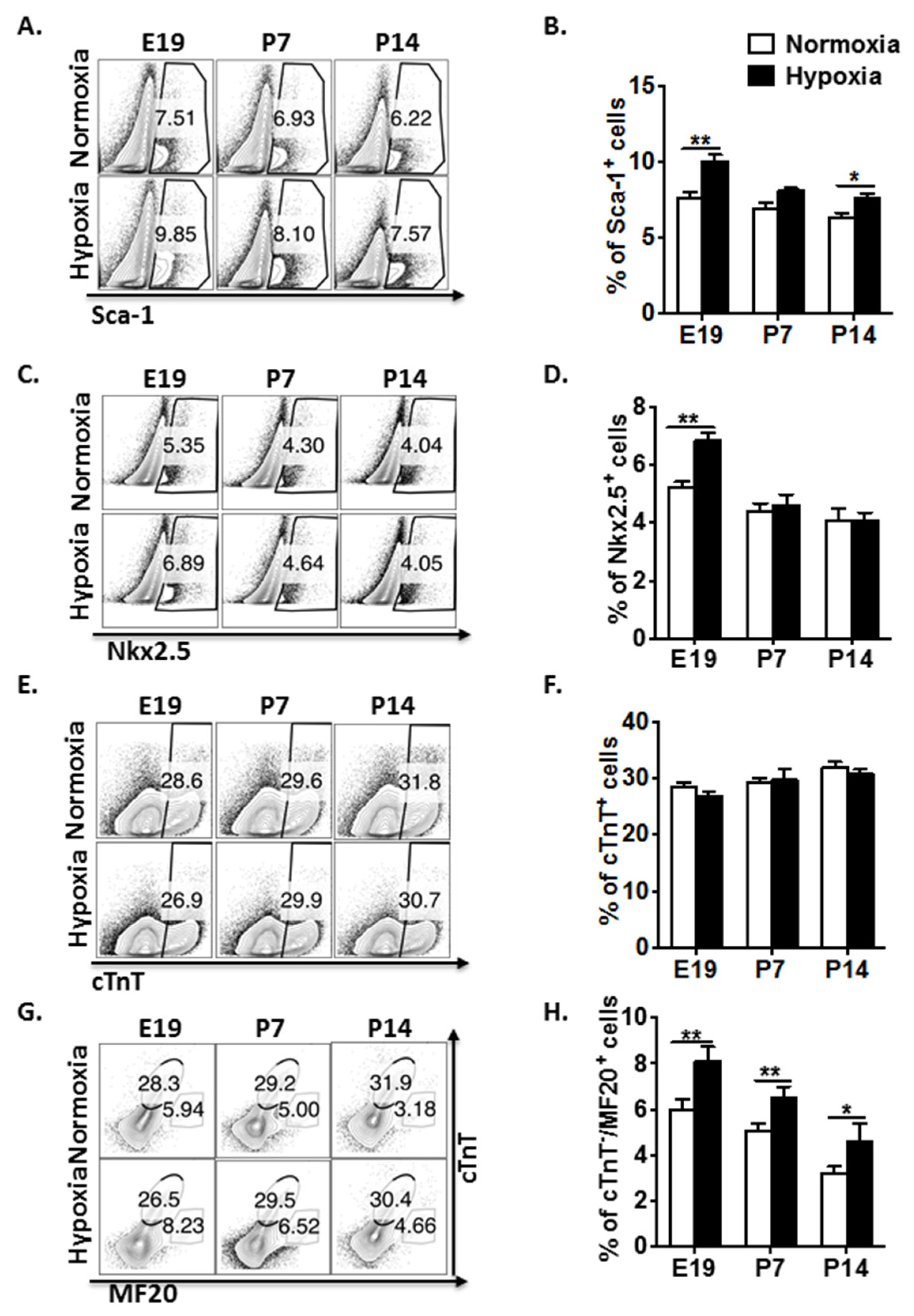
3.1. Fetal Hypoxia Regulates CPC Proliferation and Restrains Cardiomyocyte Maturation in Mouse Fetal and Postnatal Hearts
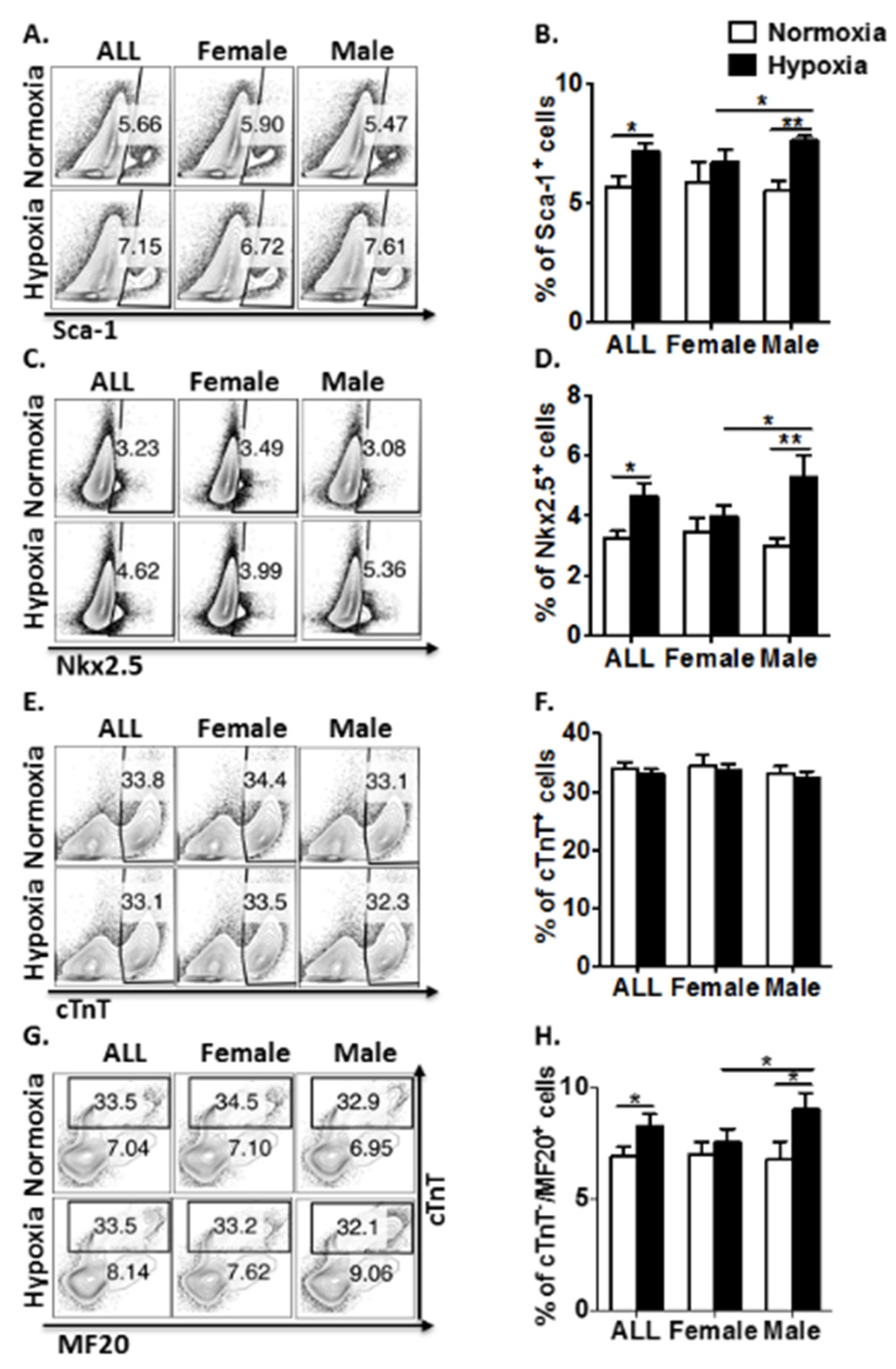
3.2. Antenatal Hypoxia Regulates CPC Proliferation and Restrains Cardiomyocyte Maturation with a Gender-Related Difference in Young Adult Mice
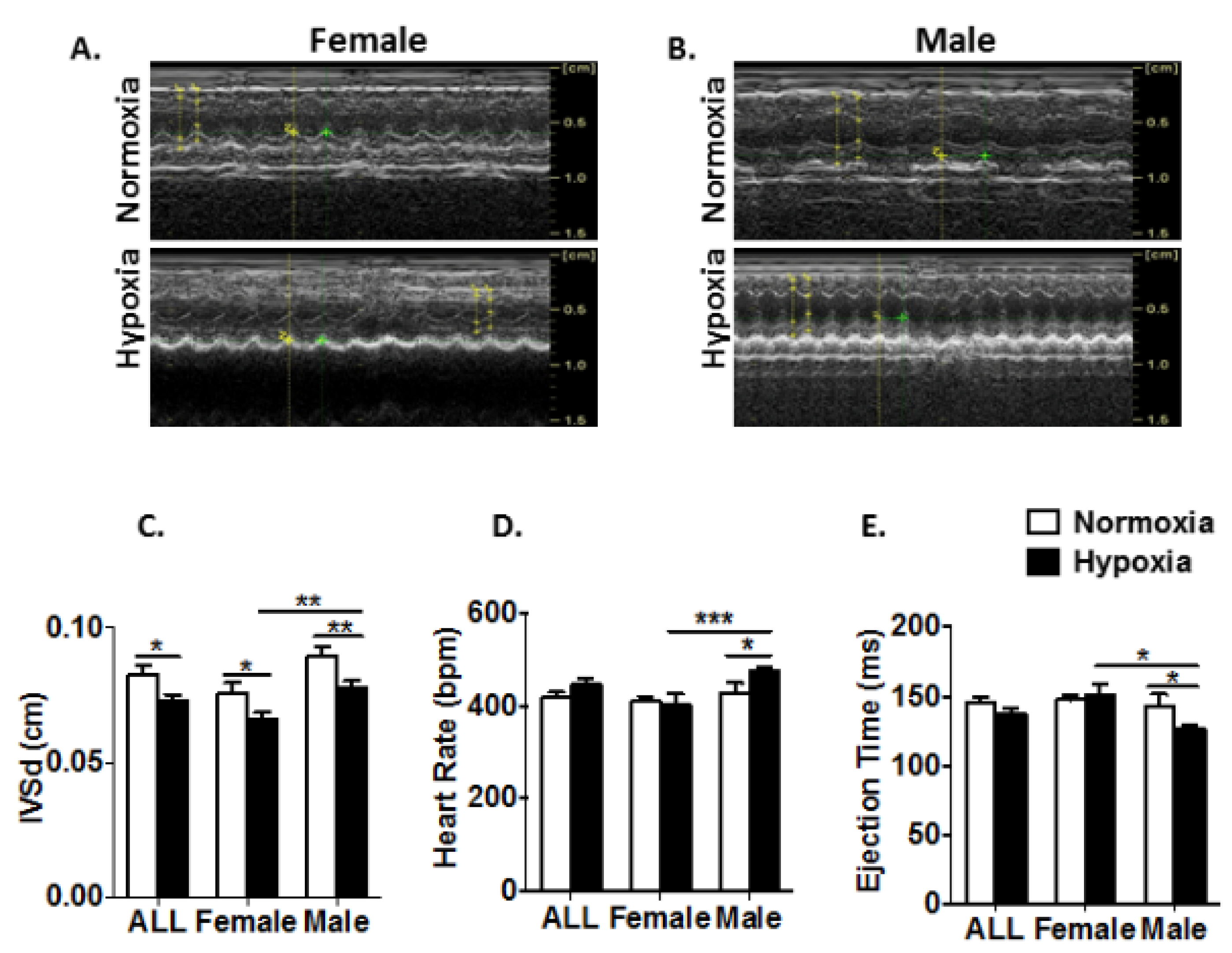
3.3. Prenatal Hypoxia Impairs Heart Function with a Gender-Related Difference in Young Adult Mice
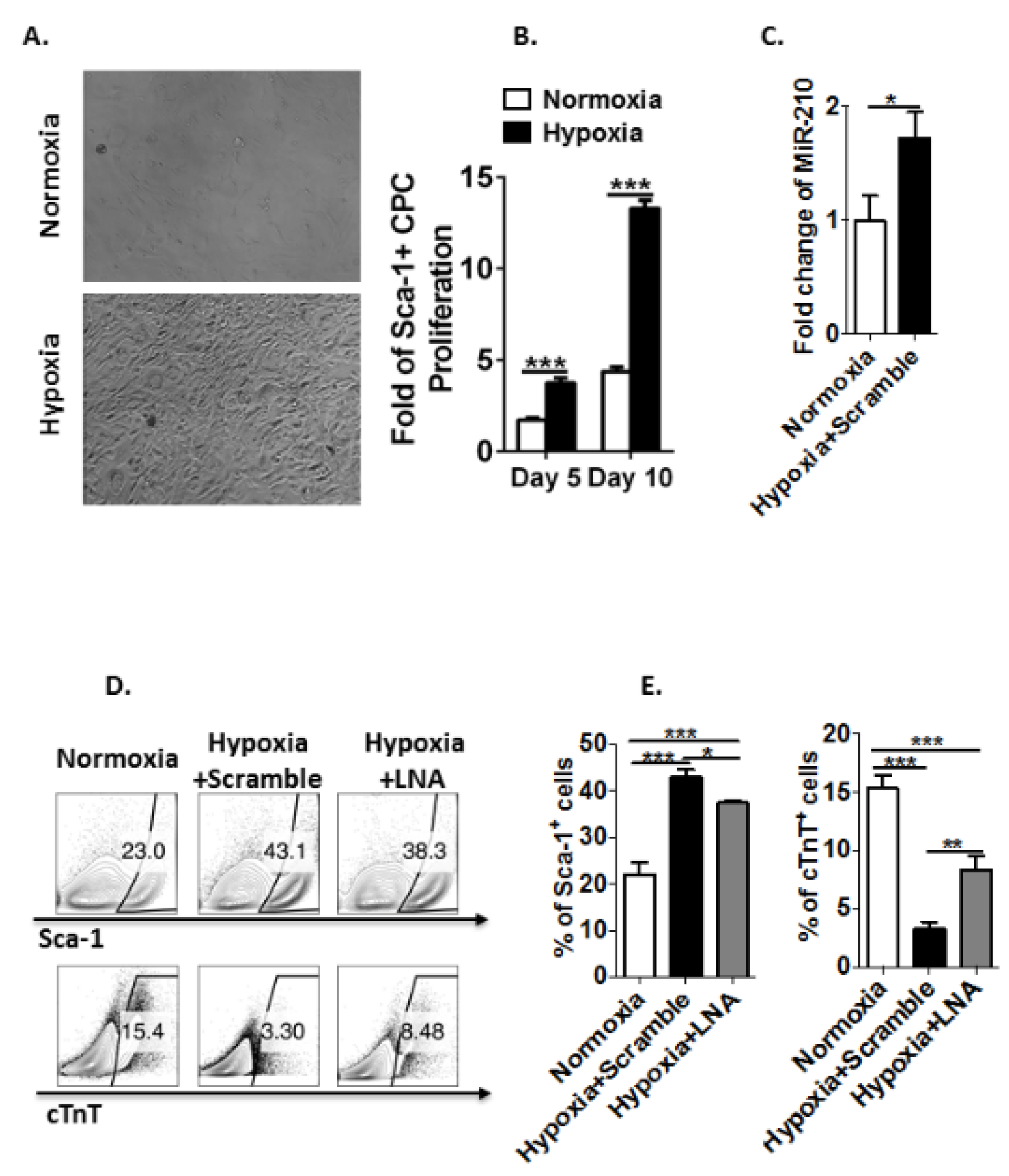
3.4. Hypoxia Induces MicroRNA-210 Expression in Sca-1+ CPCs and Inhibits Their Differentiation to Cardiomyocytes Ex Vivo
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bardot, E.; Calderon, D.; Santoriello, F.; Han, S.; Cheung, K.; Jadhav, B.; Burtscher, I.; Artap, S.; Jain, R.; Epstein, J.; et al. Foxa2 identifies a cardiac progenitor population with ventricular differentiation potential. Nat. Commun. 2017, 8, 14428. [Google Scholar] [CrossRef]
- Yuan, X.; Qi, H.; Li, X.; Wu, F.; Fang, J.; Bober, E.; Dobreva, G.; Zhou, Y.; Braun, T. Disruption of spatiotemporal hypoxic signaling causes congenital heart disease in mice. J. Clin. Investig. 2017, 127, 2235–2248. [Google Scholar] [CrossRef]
- Stothard, K.J.; Tennant, P.W.; Bell, R.; Rankin, J. Maternal overweight and obesity and the risk of congenital anomalies: A systematic review and meta-analysis. JAMA 2009, 301, 636–650. [Google Scholar] [CrossRef]
- Correa, A.; Gilboa, S.M.; Besser, L.M.; Botto, L.D.; Moore, C.A.; Hobbs, C.A.; Cleves, M.A.; Riehle-Colarusso, T.J.; Waller, D.K.; Reece, E.A. Diabetes mellitus and birth defects. Am. J. Obstet. Gynecol. 2008, 199, 237.e1–9. [Google Scholar] [CrossRef]
- Martinez, S.R.; Ma, Q.; Dasgupta, C.; Meng, X.; Zhang, L. MicroRNA-210 suppresses glucocorticoid receptor expression in response to hypoxia in fetal rat cardiomyocytes. Oncotarget 2017, 8, 80249–80264. [Google Scholar] [CrossRef]
- Xiao, D.; Wang, L.; Huang, X.; Li, Y.; Dasgupta, C.; Zhang, L. Protective Effect of Antenatal Antioxidant on Nicotine-Induced Heart Ischemia-Sensitive Phenotype in Rat Offspring. PLoS ONE 2016, 11, e0150557. [Google Scholar] [CrossRef]
- Stout, K. Pregnancy in women with congenital heart disease: The importance of evaluation and counselling. Heart 2005, 91, 713–714. [Google Scholar] [CrossRef]
- Sun, L.; Macgowan, C.K.; Sled, J.G.; Yoo, S.J.; Manlhiot, C.; Porayette, P.; Grosse-Wortmann, L.; Jaeggi, E.; McCrindle, B.W.; Kingdom, J.; et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation 2015, 131, 1313–1323. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, X.; Najafi, N.; Cass, M.; Lin, L.; Cai, C.L.; Chen, J.; Evans, S.M. Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells. Dev. Biol. 2007, 304, 286–296. [Google Scholar] [CrossRef]
- Lien, C.L.; Wu, C.; Mercer, B.; Webb, R.; Richardson, J.A.; Olson, E.N. Control of early cardiac-specific transcription of Nkx2-5 by a GATA-dependent enhancer. Development 1999, 126, 75–84. [Google Scholar]
- Serpooshan, V.; Liu, Y.H.; Buikema, J.W.; Galdos, F.X.; Chirikian, O.; Paige, S.; Paige, S.; Venkatraman, S.; Kumar, A.; Rawnsley, D.R.; et al. Nkx2.5+ Cardiomyoblasts Contribute to Cardiomyogenesis in the Neonatal Heart. Sci. Rep. 2017, 7, 12590. [Google Scholar] [CrossRef]
- Moretti, A.; Caron, L.; Nakano, A.; Lam, J.T.; Bernshausen, A.; Chen, Y.; Qyang, Y.; Bu, L.; Sasaki, M.; Martin-Puig, S.; et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 2006, 127, 1151–1165. [Google Scholar] [CrossRef]
- Pfister, O.; Mouquet, F.; Jain, M.; Summer, R.; Helmes, M.; Fine, A.; Colucci, W.S.; Liao, R. CD31- but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ. Res. 2005, 97, 52–61. [Google Scholar] [CrossRef]
- Takamiya, M.; Haider, K.H.; Ashraf, M. Identification and characterization of a novel multipotent sub-population of Sca-1(+) cardiac progenitor cells for myocardial regeneration. PLoS ONE 2011, 6, e25265. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.; Feng, B.; Wang, X.; He, X.; Hu, R.; Yin, M.; Wang, W.; Fu, W.; Xu, Z. Isolation and characterization of a Sca-1+/CD31- progenitor cell lineage derived from mouse heart tissue. BMC Biotechnol. 2014, 14, 75. [Google Scholar] [CrossRef]
- Banerjee, I.; Fuseler, J.W.; Price, R.L.; Borg, T.K.; Baudino, T.A. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1883–H1891. [Google Scholar] [CrossRef]
- Lescroart, F.; Wang, X.; Lin, X.; Swedlund, B.; Gargouri, S.; Sanchez-Danes, A.; Moignard, V.; Dubois, C.; Paulissen, C.; Kinston, S.; et al. Defining the earliest step of cardiovascular lineage segregation by single-cell RNA-seq. Science 2018, 359, 1177–1181. [Google Scholar] [CrossRef]
- Santini, M.P.; Forte, E.; Harvey, R.P.; Kovacic, J.C. Developmental origin and lineage plasticity of endogenous cardiac stem cells. Development 2016, 143, 1242–1258. [Google Scholar] [CrossRef]
- Kusuma, S.; Peijnenburg, E.; Patel, P.; Gerecht, S. Low oxygen tension enhances endothelial fate of human pluripotent stem cells. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Kimura, W.; Xiao, F.; Canseco, D.C.; Muralidhar, S.; Thet, S.; Zhang, H.M.; Abderrahman, Y.; Chen, R.; Garcia, J.A.; Shelton, J.M.; et al. Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart. Nature 2015, 523, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Meng, X.; Zhang, L. microRNAs and cardiac stem cells in heart development and disease. Drug Discov. Today 2018, 24, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Liu, Y.; Su, Q.; Yang, Y.; Wang, L.; Ma, S.; Yan, J.; Xue, F.; Wang, J. Hypoxia stimulates proliferation of rat neural stem/progenitor cells by regulating mir-21, an in vitro study. Neurosci. Lett. 2017, 661, 71–76. [Google Scholar] [CrossRef]
- Xiao, J.; Pan, Y.; Li, X.H.; Yang, X.Y.; Feng, Y.L.; Tan, H.H.; Jiang, L.; Feng, J.; Yu, X.Y. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis. 2016, 7, e2277. [Google Scholar] [CrossRef]
- Deng, S.; Zhao, Q.; Zhou, X.; Zhang, L.; Bao, L.; Zhen, L.; Zhang, Y.; Fan, H.; Liu, Z.; Yu, Z. Neonatal Heart-Enriched miR-708 Promotes Differentiation of Cardiac Progenitor Cells in Rats. Int. J. Mol. Sci. 2016, 17, 875. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, J.; Cui, J.; Sun, M.; Du, W.; Chen, T.; Ming, X.; Zhang, L.; Tian, J.; Li, J.; et al. MiR218 Modulates Wnt Signaling in Mouse Cardiac Stem Cells by Promoting Proliferation and Inhibiting Differentiation through a Positive Feedback Loop. Sci. Rep. 2016, 6, 20968. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Deng, W.; Long, X.; Zhao, R.; Wang, Y.; Chen, W.; Xu, G.; Sheng, J.; Wang, D.; Cao, S. miR-21 increases c-kit(+) cardiac stem cell proliferation in vitro through PTEN/PI3K/Akt signaling. PeerJ 2017, 5, e2859. [Google Scholar] [CrossRef]
- Kulshreshtha, R.; Ferracin, M.; Wojcik, S.E.; Garzon, R.; Alder, H.; Agosto-Perez, F.J.; Davuluri, R.; Liu, C.G.; Croce, C.M.; Negrini, M.; et al. A microRNA signature of hypoxia. Mol. Cell. Biol. 2007, 27, 1859–1867. [Google Scholar] [CrossRef]
- Huang, X.; Le, Q.T.; Giaccia, A.J. MiR-210--micromanager of the hypoxia pathway. Trends Mol. Med. 2010, 16, 230–237. [Google Scholar] [CrossRef]
- Zhang, P.; Ke, J.; Li, Y.; Huang, L.; Chen, Z.; Huang, X.; Zhang, L.; Xiao, D. Long-term exposure to high altitude hypoxia during pregnancy increases fetal heart susceptibility to ischemia/reperfusion injury and cardiac dysfunction. Int. J. Cardiol. 2019, 274, 7–15. [Google Scholar] [CrossRef]
- Smits, A.M.; van Vliet, P.; Metz, C.H.; Korfage, T.; Sluijter, J.P.; Doevendans, P.A.; Goumans, M.J. Human cardiomyocyte progenitor cells differentiate into functional mature cardiomyocytes: An in vitro model for studying human cardiac physiology and pathophysiology. Nat. Protoc. 2009, 4, 232–243. [Google Scholar] [CrossRef]
- Nakada, Y.; Canseco, D.C.; Thet, S.; Abdisalaam, S.; Asaithamby, A.; Santos, C.X.; Shah, A.M.; Zhang, H.; Faber, J.E.; Kinter, M.T.; et al. Hypoxia induces heart regeneration in adult mice. Nature 2017, 541, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Laugwitz, K.L.; Moretti, A.; Lam, J.; Gruber, P.; Chen, Y.; Woodard, S.; Lin, L.Z.; Cai, C.L.; Lu, M.M.; Reth, M.; et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 2005, 433, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Khattar, P.; Friedrich, F.W.; Bonne, G.; Carrier, L.; Eschenhagen, T.; Evans, S.M.; Schwartz, K.; Fiszman, M.Y.; Vilquin, J.T. Distinction between two populations of islet-1-positive cells in hearts of different murine strains. Stem Cells Dev. 2011, 20, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Stacy, V.; De Matteo, R.; Brew, N.; Sozo, F.; Probyn, M.E.; Harding, R.; Black, M.J. The influence of naturally occurring differences in birthweight on ventricular cardiomyocyte number in sheep. Anat. Rec. 2009, 292, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Zhang, L. Prenatal hypoxia causes a sex-dependent increase in heart susceptibility to ischemia and reperfusion injury in adult male offspring: Role of protein kinase C epsilon. J. Pharmacol. Exp. Ther. 2009, 330, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Netuka, I.; Szarszoi, O.; Maly, J.; Besik, J.; Neckar, J.; Kolar, F.; Ostadalova, I.; Pirk, J.; Ostadal, B. Effect of perinatal hypoxia on cardiac tolerance to acute ischaemia in adult male and female rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 714–719. [Google Scholar] [CrossRef]
- Patterson, A.J.; Chen, M.; Xue, Q.; Xiao, D.; Zhang, L. Chronic prenatal hypoxia induces epigenetic programming of PKCε gene repression in rat hearts. Circ. Res. 2010, 107, 365–373. [Google Scholar] [CrossRef]
- Bellio, M.A.; Pinto, M.T.; Florea, V.; Barrios, P.A.; Taylor, C.N.; Brown, A.B.; Lamondin, C.; Hare, J.M.; Schulman, I.H.; Rodrigues, C.O. Hypoxic Stress Decreases c-Myc Protein Stability in Cardiac Progenitor Cells Inducing Quiescence and Compromising Their Proliferative and Vasculogenic Potential. Sci. Rep. 2017, 7, 9702. [Google Scholar] [CrossRef]
- Miao, C.Y.; Zuberbuhler, J.S.; Zuberbuhler, J.R. Prevalence of congenital cardiac anomalies at high altitude. J. Am. Coll. Cardiol. 1988, 12, 224–228. [Google Scholar] [CrossRef]
- Giordano, F.J. Oxygen, oxidative stress, hypoxia, and heart failure. J. Clin. Investig. 2005, 115, 500–508. [Google Scholar] [CrossRef]
- Williams, S.J.; Hemmings, D.G.; Mitchell, J.M.; McMillen, I.C.; Davidge, S.T. Effects of maternal hypoxia or nutrient restriction during pregnancy on endothelial function in adult male rat offspring. J. Physiol. 2005, 565, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Bergeron, D.L.; Simon, M.C. Hypoxia-inducible factor and the development of stem cells of the cardiovascular system. Stem Cells 2001, 19, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Rios, C.; D’Ippolito, G.; Curtis, K.M.; Delcroix, G.J.; Gomez, L.A.; El Hokayem, J.; Rieger, M.; Parrondo, R.; de Las Pozas, A.; Perez-Stable, C.; et al. Low Oxygen Modulates Multiple Signaling Pathways, Increasing Self-Renewal, While Decreasing Differentiation, Senescence, and Apoptosis in Stromal MIAMI Cells. Stem Cells Dev. 2016, 25, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Giussani, D.A.; Niu, Y.; Herrera, E.A.; Richter, H.G.; Camm, E.J.; Thakor, A.S.; Kane, A.D.; Hansell, J.A.; Brain, K.L.; Skeffington, K.L.; et al. Heart disease link to fetal hypoxia and oxidative stress. Adv. Exp. Med. Biol. 2014, 814, 77–87. [Google Scholar]
- Hernandez, I.; Baio, J.M.; Tsay, E.; Martinez, A.F.; Fuentes, T.I.; Bailey, L.L.; Hasaniya, N.W.; Kearns-Jonker, M. Short-term hypoxia improves early cardiac progenitor cell function in vitro. Am. J. Stem Cells 2018, 7, 1–17. [Google Scholar]
- Ream, M.; Ray, A.M.; Chandra, R.; Chikaraishi, D.M. Early fetal hypoxia leads to growth restriction and myocardial thinning. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R583–R595. [Google Scholar] [CrossRef]
- Ostadal, B.; Ostadal, P. Sex-based differences in cardiac ischaemic injury and protection: Therapeutic implications. Br. J. Pharmacol. 2014, 171, 541–554. [Google Scholar] [CrossRef]
- Zhao, X.; Eghbali-Webb, M. Gender-related differences in basal and hypoxia-induced activation of signal transduction pathways controlling cell cycle progression and apoptosis, in cardiac fibroblasts. Endocrine 2002, 18, 137–145. [Google Scholar] [CrossRef]
- Liu, N.; Bezprozvannaya, S.; Williams, A.H.; Qi, X.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008, 22, 3242–3254. [Google Scholar] [CrossRef]
- Fasanaro, P.; D’Alessandra, Y.; Di Stefano, V.; Melchionna, R.; Romani, S.; Pompilio, G.; Capogrossi, M.C.; Martelli, F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J. Biol. Chem. 2008, 283, 15878–15883. [Google Scholar] [CrossRef]
- Cicchillitti, L.; Di Stefano, V.; Isaia, E.; Crimaldi, L.; Fasanaro, P.; Ambrosino, V.; Antonini, A.; Capogrossi, M.C.; Gaetano, C.; Piaggio, G.; et al. Hypoxia-inducible factor 1-alpha induces miR-210 in normoxic differentiating myoblasts. J. Biol. Chem. 2012, 287, 44761–44771. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ding, L.; Bennewith, K.L.; Tong, R.T.; Welford, S.M.; Ang, K.K.; Story, M.; Le, Q.T.; Giaccia, A.J. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol. Cell. 2009, 35, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Mutharasan, R.K.; Nagpal, V.; Ichikawa, Y.; Ardehali, H. microRNA-210 is upregulated in hypoxic cardiomyocytes through Akt- and p53-dependent pathways and exerts cytoprotective effects. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1519–H1530. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.X.; Zhou, J.; Tong, R.Q.; Wang, B.; Chen, X.L.; Zhuang, Y.Y.; Xia, F.; Wei, X.D. Hypoxia-induced miR210 contributes to apoptosis of mouse spermatocyte GC2 cells by targeting Kruppellike factor 7. Mol. Med. Rep. 2019, 19, 271–279. [Google Scholar]
- Endo, K.; Naito, Y.; Ji, X.; Nakanishi, M.; Noguchi, T.; Goto, Y.; Nonogi, H.; Ma, X.; Weng, H.; Hirokawa, G.; et al. MicroRNA 210 as a biomarker for congestive heart failure. Biol. Pharm. Bull. 2013, 36, 48–54. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Zhang, P.; Zhang, L. Fetal Hypoxia Impacts on Proliferation and Differentiation of Sca-1+ Cardiac Progenitor Cells and Maturation of Cardiomyocytes: A Role of MicroRNA-210. Genes 2020, 11, 328. https://doi.org/10.3390/genes11030328
Meng X, Zhang P, Zhang L. Fetal Hypoxia Impacts on Proliferation and Differentiation of Sca-1+ Cardiac Progenitor Cells and Maturation of Cardiomyocytes: A Role of MicroRNA-210. Genes. 2020; 11(3):328. https://doi.org/10.3390/genes11030328
Chicago/Turabian StyleMeng, Xianmei, Peng Zhang, and Lubo Zhang. 2020. "Fetal Hypoxia Impacts on Proliferation and Differentiation of Sca-1+ Cardiac Progenitor Cells and Maturation of Cardiomyocytes: A Role of MicroRNA-210" Genes 11, no. 3: 328. https://doi.org/10.3390/genes11030328
APA StyleMeng, X., Zhang, P., & Zhang, L. (2020). Fetal Hypoxia Impacts on Proliferation and Differentiation of Sca-1+ Cardiac Progenitor Cells and Maturation of Cardiomyocytes: A Role of MicroRNA-210. Genes, 11(3), 328. https://doi.org/10.3390/genes11030328





