B Chromosomes and Cytogenetic Characteristics
Abstract
:1. Introduction
2. Materials and Methods
3. Results
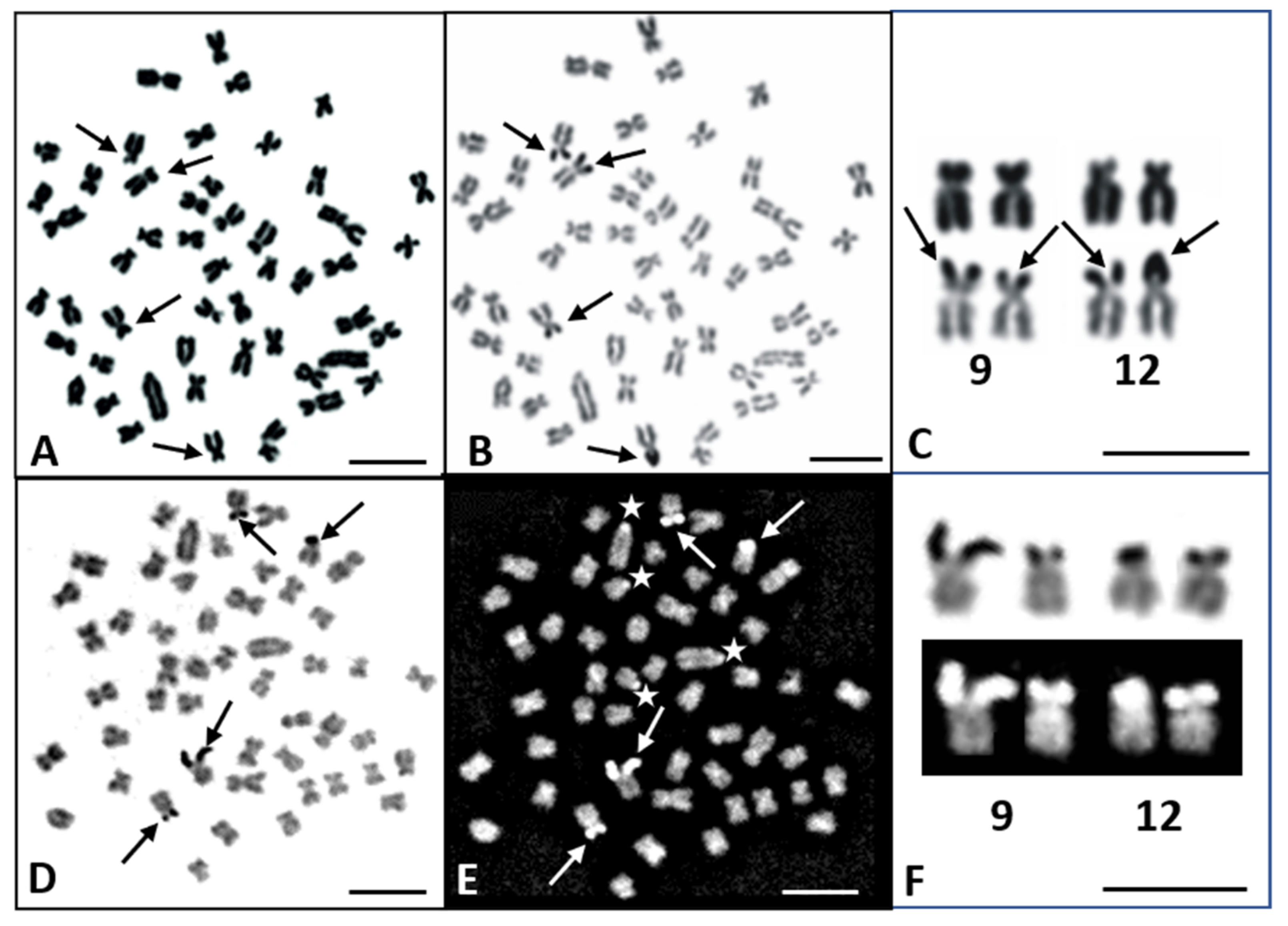
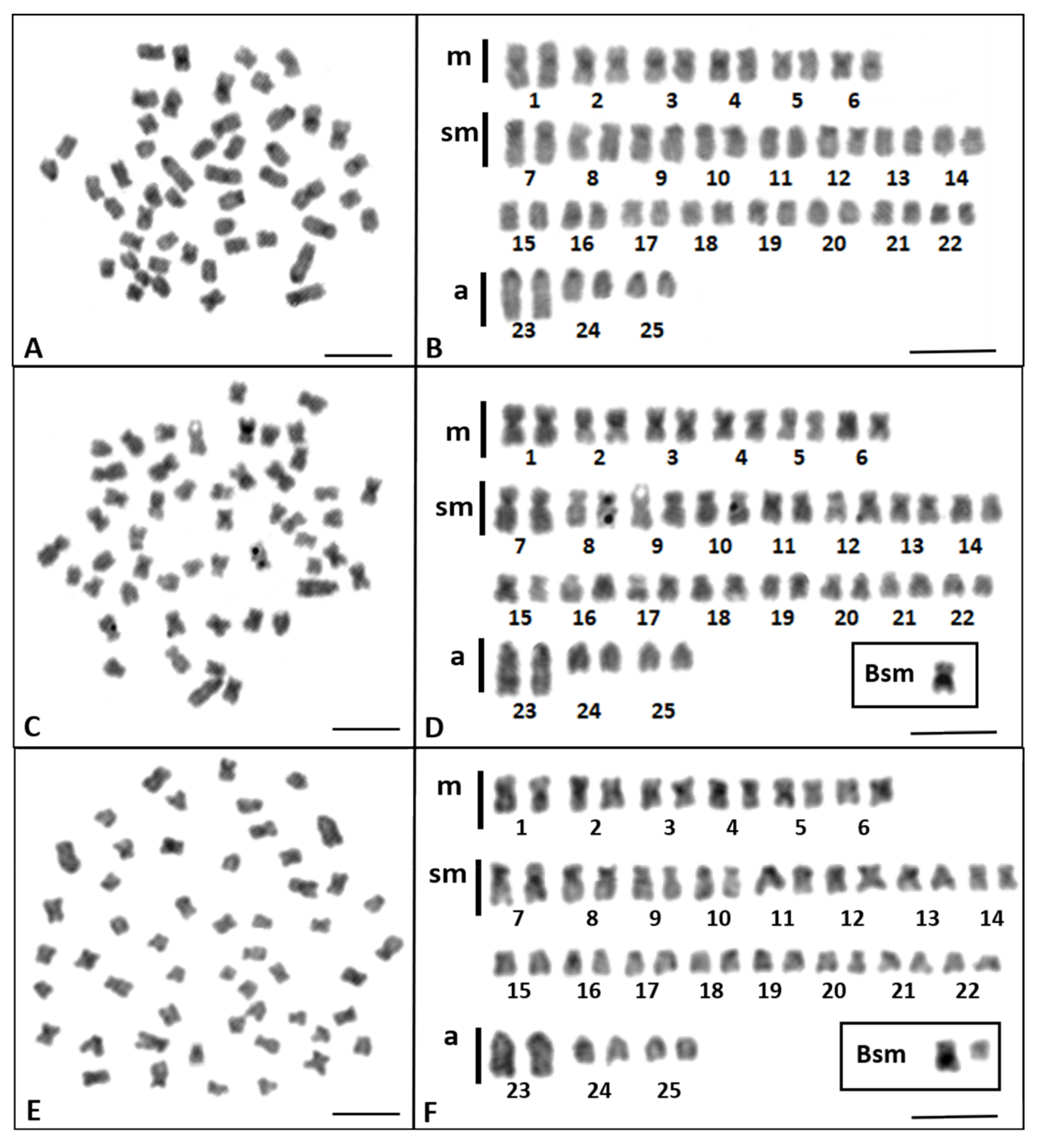
3.1. Karyotype and Banding Patterns
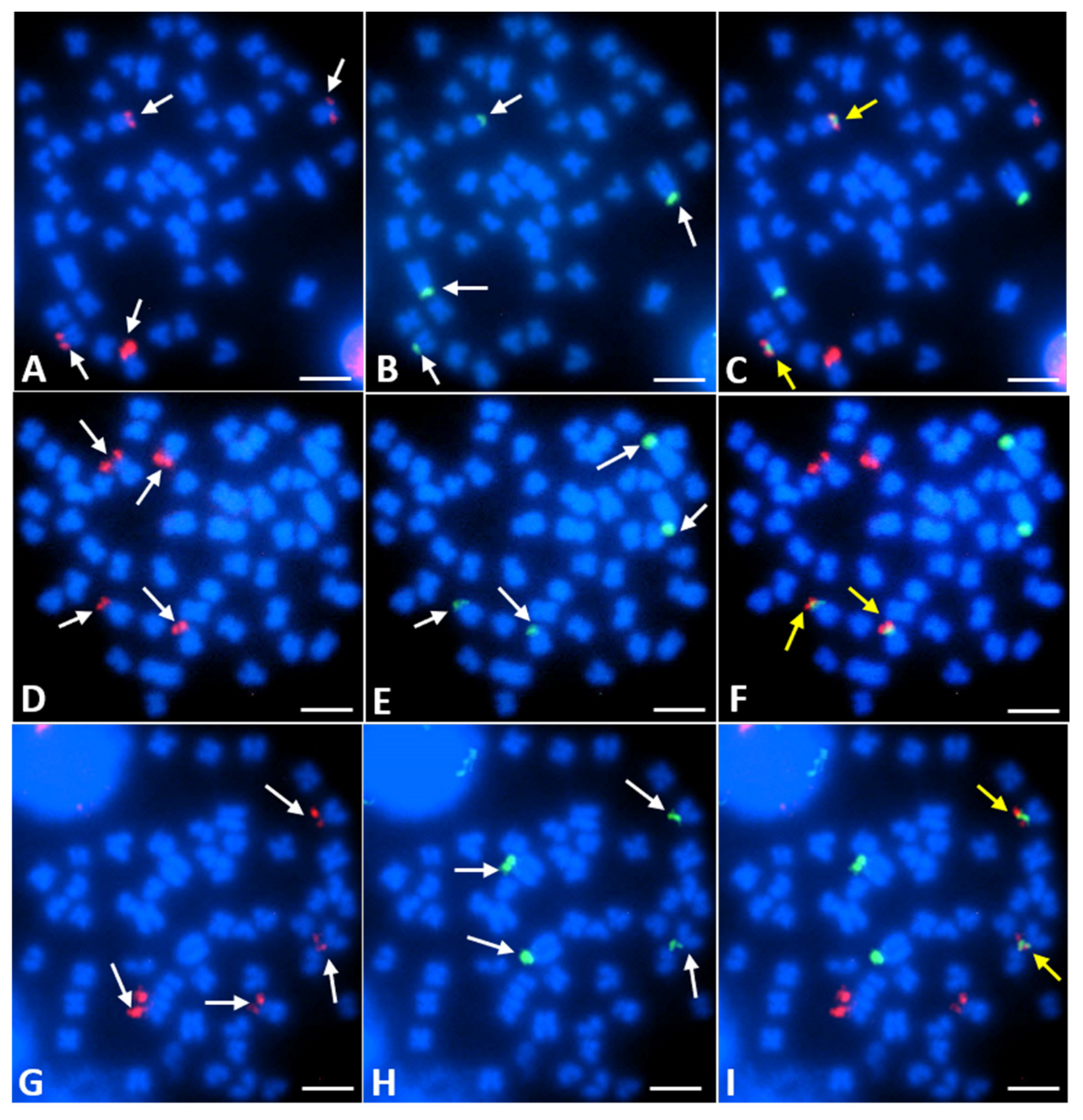
3.2. FISH Mapping of 28S and 5S rDNA Loci
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Camacho, J.P.M. B Chromosomes. How widely distributed are B chromosomes? In The Evolution of the Genome; Gregory, T.R., Ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; pp. 223–286. [Google Scholar]
- D’Ambrosio, U.; Alonso-Lifante, M.P.; Barros, K.; Kovarik, A.; De Xacars, G.M.; Garcia, S. B-chrom: A database on B-chromosomes of plants, animals and fungi. New Phytol. 2017, 216, 635–642. [Google Scholar] [CrossRef] [Green Version]
- Utsunomia, R.; Silva, D.M.Z.D.A.; Ruiz-Ruano, F.J.; Araya-Jaime, C.; Pansonato-Alves, J.C.; Scacchetti, P.C.; Hashimoto, D.T.; Oliveira, C.; Trifonov, V.A.; Porto-Foresti, F.; et al. Uncovering the Ancestry of B Chromosomes in Moenkhausia sanctaefilomenae (Teleostei, Characidae). PLoS ONE 2016, 11, e0150573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noleto, R.B.; Vicari, M.R.; Cestari, M.M.; Artoni, R.F. Variable B chromosomes frequencies between males and females of two species of pufferfishes (Tetraodontiformes). Rev. Fish Biol. Fish. 2012, 22, 343–349. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Martins, C. The Modern View of B Chromosomes under the Impact of High Scale Omics Analyses. Cells 2019, 8, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- B-Chrom database. Available online: http://www.bchrom.csic.es/ (accessed on 4 November 2020).
- Vujošević, M.; Rajičić, M.; Blagojević, J. B Chromosomes in Populations of Mammals Revisited. Genes 2018, 9, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konerat, J.T.; Bueno, V.; Baumgartner, L.; Martins-Santos, I.C.; Margarido, V.P. B chromosome and NORs polymorphism in Callichthys callichthys (Linnaeus, 1758) (Siluriformes: Callichthyidae) from upper Paraná River, Brazil. Neotrop. Ichthyol. 2014, 12, 603–609. [Google Scholar] [CrossRef] [Green Version]
- Penitente, M.; Natal Daniel, S.; Elda Sobrinho Scudeler, P.; Foresti, F.; Porto-Foresti, F. B chromosome variants in Prochilodus lineatus (Characiformes, Prochilodontidae) analyzed by microdissection and chromosome painting techniques. Caryologia 2016, 69, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Nanda, I.; Schlupp, I.; Lamatsch, D.K.; Lampert, K.P.; Schmid, M.; Schartl, M. Stable inheritance of host species-derived microchromosomes in the gynogenetic fish Poecilia formosa. Genetics 2007, 177, 917–926. [Google Scholar] [CrossRef] [Green Version]
- Swarça, A.C.; Fenocchio, A.S.; Dias, A.L. An update Cytogenetic Review for Species of the Families Pseudopimelodidae, Pimelodidae and Heptapteridae (Pisces, Siluriformes). Suggestion of a Cytotaxonomical Classification. Caryologia 2007, 60, 338–348. [Google Scholar] [CrossRef]
- Castro, J.P.; Moura, M.O.; Moreira-Filho, O.; Shibatta, O.A.; Santos, M.H.; Nogaroto, V.; Vicari, M.R.; Almeida, M.C.; Artoni, R.F. Diversity of the Astyanax scabripinnis species complex (Teleostei: Characidae) in the Atlantic Forest, Brazil: Species limits and evolutionary inferences. Rev. Fish Biol. Fish. 2015, 25, 231–244. [Google Scholar] [CrossRef]
- Schönhuth, S.; Vukić, J.; Šanda, R.; Yang, L.; Mayden, R.L. Phylogenetic relationships and classification of the Holarctic family Leuciscidae (Cypriniformes: Cyprinoidei). Mol. Phylogenet. Evol. 2018, 127, 781–799. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, C.G.; Lamatsch, D.K.; Steinlein, C.; Engel, W.; Schartl, M.; Schmid, M. The giant B chromosome of the cyprinid fish Alburnus alburnus harbours a retrotransposon-derived repetitive DNA sequence. Chromosome Res. 2003, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Ráb, P.; Roth, P. Chromosome studies in European Leuciscine fishes (Pisces, Cyprinidae). Aneuploidy due to B-chromosome in Rutilus rutilus. Folia Zool. 2007, 38, 333–337. [Google Scholar]
- Vujosevic, M.; Zivkovic, S.; Rimsa, D.; Jurisic, S.; Cakic, P. The chromosomes of 9 fish species from Dunav basin in Yugoslavia. Ichthyologia 1983, 15, 29–40. [Google Scholar]
- Schmid, M.; Ziegler, C.G.; Steinlein, C.; Nanda, I.; Schartl, M. Cytogenetics of the bleak (Alburnus alburnus), with special emphasis on the B chromosomes. Chromosome Res. 2006, 14, 231–242. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhang, Q.Y.; Zhang, J.; Zhou, L.; Li, Z.; Zhang, X.J.; Wang, D.; Gui, J.F. Extra microchromosomes play male determination role in polyploid gibel carp. Genetics 2016, 203, 1415–1424. [Google Scholar] [CrossRef] [Green Version]
- Przybył, A.; Przybylski, M.; Spóz, A.; Juchno, D.; Szabelska, A.; Kowalewska, K.; Boroń, A. Sex, size and ploidy ratios of Carassius gibelio from Poland. Aquat. Invasions 2020, 15, 335–354. [Google Scholar] [CrossRef]
- Kucinski, M.; Demska-Zakes, K.; Zarski, D.; Liszewski, T.; Fopp-Bayat, D.; Jankun, M.; Furgala-Selezniow, G. The morphological, histological and cytogenetic characteristics of goldfish Carassius auratus (L.) × common carp Cyprinus carpio (L.) hybrids. Caryologia 2015, 68, 77–83. [Google Scholar] [CrossRef]
- Valente, G.T.; Conte, M.A.; Fantinatti, B.E.A.; Cabral-de-Mello, D.C.; Carvalho, R.F.; Vicari, M.R.; Kocher, T.D.; Martins, C. Origin and Evolution of B Chromosomes in the Cichlid Fish Astatotilapia latifasciata Based on Integrated Genomic Analyses. Mol. Biol. Evol. 2014, 31, 2061–2072. [Google Scholar] [CrossRef] [Green Version]
- Martinez, P.A.; Araújo, W.C.; Molina, W.F. Derived cytogenetic traits, multiple NORs and B chromosomes in the compact karyotype of Canthigaster figueiredoi (Tetraodontiformes). Mar. Genom. 2010, 3, 85–89. [Google Scholar] [CrossRef]
- Serrano-Freitas, É.A.; Silva, D.M.Z.A.; Ruiz-Ruano, F.J.; Utsunomia, R.; Araya-Jaime, C.; Oliveira, C.; Camacho, J.P.M.; Foresti, F. Satellite DNA content of B chromosomes in the characid fish Characidium gomesi supports their origin from sex chromosomes. Mol. Genet. Genom. 2020, 295, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Terai, Y.; Mizoiri, S.; Aibara, M.; Nishihara, H.; Watanabe, M.; Kuroiwa, A.; Hirai, H.; Hirai, Y.; Matsuda, Y.; et al. B Chromosomes Have a Functional Effect on Female Sex Determination in Lake Victoria Cichlid Fishes. PLoS Genet. 2011, 7, e1002203. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.M.Z.A.; Pansonato-Alves, J.C.; Utsunomia, R.; Araya-Jaime, C.; Ruiz-Ruano, F.J.; Daniel, S.N.; Hashimoto, T.; Oliveira, C.; Camacho, J.P.M.; Porto-Foresti, F.; et al. Delimiting the Origin of a B Chromosome by FISH Mapping, Chromosome Painting and DNA Sequence Analysis in Astyanax paranae (Teleostei, Characiformes). PLoS ONE 2014, 9, e94896. [Google Scholar] [CrossRef] [Green Version]
- Arkhipchuk, V.V. Chromosome Database; Database of Dr. Victor Arkhipchuk; 1999; Available online: www.fishbase.org (accessed on 5 November 2020).
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Publications Kottelat, Cornol and Freyhof: Berlin, Germany, 2007; p. 646. ISBN 978-2-8399-0298-4. [Google Scholar]
- Barshiene, J.V. Karyotype studies in the under mouth Chondrostoma nasus. Citologia 1977, 19, 390–392. (In Russian) [Google Scholar]
- Vetešník, L.; Halačka, K.; Papoušek, I.; Mendel, J.; Šımková, A. The first record of a natural hybrid of the roach Rutilus rutilus and nase Chondrostoma nasus in the Danube River Basin, Czech Republic: Morphological, karyological and molecular characteristics. J. Fish Biol. 2009, 74, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.; Collares-Pereira, M.J. NOR polymorphism in the Iberian species Chondrostoma lusitanicum (Pisces: Cyprinidae). Genetica 1996, 98, 59–63. [Google Scholar] [CrossRef]
- Collares-Pereira, M.J.; Ráb, P. NOR polymorphism in the Iberian species Chondrostoma lusitanicum (Pisces: Cyprinidae) re-examination by FISH. Genetica 1999, 105, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.S.A.; Ráb, P.; Collares-Pereira, M.J. Chromosomes of European cyprinid fishes: Comparative cytogenetics and chromosomal characteristics of ribosomal DNAs in nine Iberian chondrostomine species (Leuciscinae). Genetica 2012, 140, 485–495. [Google Scholar] [CrossRef]
- Boroń, A.; Porycka, K.; Ito, D.; Abe, S.; Kirtiklis, L. Comparative molecular cytogenetic analysis of three Leuciscus species (Pisces, Cyprinidae) using chromosome banding and FISH with rDNA. Genetica 2009, 135, 199–207. [Google Scholar] [CrossRef]
- Levan, A.; Fredga, K.; Sandberg, A.A. Nomenclature for centromeric position on chromosomes. Hereditas 1964, 52, 201–220. [Google Scholar] [CrossRef]
- Sola, L.; Rossi, A.R.; Iaselli, V.; Rasch, E.M.; Monaco, P.J. Cytogenetics of bisexual/unisexual species of Poecilia. II. Analysis of heterochromatin and nucleolar organizer regions in Poecilia maxicana by C-banding and DAPI, quinacrine, chromomycin and silver staining. Cytogenet. Cell Genet. 1992, 60, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Sumner, A.T. A simple technique for demonstrating centromeric heterochromatin. Exp. Cell Res. 1972, 75, 304–306. [Google Scholar] [CrossRef]
- Kirtiklis, L.; Porycka, K.; Boron, A.; Coutanceau, J.-P.; Dettai, A. Use of the chromosomal co-location of the minor 5S and the major 28S rDNA as a cytogenetic marker within the genus Leuciscus (Pisces, Cyprinidae). Folia Biol. 2010, 58, 245–249. [Google Scholar] [CrossRef]
- Ráb, P.; Collares-Pereira, M.J. Chromosomes of European cyprinid fishes (Cyprinidae, Cypriniformes). Folia Zool. 1995, 44, 193–214. [Google Scholar]
- Esmaeili, H.R.; Zareian, H.; Gholamhosseini, A.; Ebrahimi, M.; Gholami, Z.; Teimori, A.; Ansari, T.H. Karyotype Analysis of the King Nase Fish, Chondrostoma regium (Heckel, 1843) (Actinopterygii: Cyprinidae) from Iran. Turk J. Fish Aquat. Sci. 2010, 10, 477–481. [Google Scholar] [CrossRef]
- Maistro, E.L.; Foresti, F.; Oliveira, C.; Almeida-Toledo, L.F. Occurrence of macro B chromosomes in Astyanax scabripinnis paranae (Pisces, Characiformes, Characidae). Genetica 1992, 87, 101–106. [Google Scholar] [CrossRef]
- Salvador, L.B.; Moreira-Filho, O. B chromosomes in Astyanax scabripinnis (Pisces, Characidae). Heredity 1992, 69, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, C.A.; Martins-Santos, I.C. Sympatric occurrence of three cytotypes and four morphological types of B chromosomes of Astyanax scabripinnis (Pisces, Characiformes) in the River Ivai Basin, state of Parana, Brazil. Genetica 2005, 124, 301–306. [Google Scholar] [CrossRef]
- Camacho, J.P.M.; Sharbel, T.F.; Bekeboom, L.W. B-chromosome evolution. Phil. Trans. R. Soc. 2000, 355, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Ráb, P.; Karakousis, Y.; Rábová, M.; Economidis, P.S. Banded karyotype of cyprinid fish Leuciscus borysthenicus. Ichthyol. Res. 1996, 43, 463–468. [Google Scholar] [CrossRef]
- Pereira, C.; Neto, A.; Collares-Pereira, M.J. Cytogenetic survey of species of two distinct genera of Iberian nases (Cyprinidae, Leuciscinae) that hybridize extensively in nature. I. Evidence of a similar and conserved chromosome pattern with some few species-specific markers at macro-structural level. Genetica 2009, 137, 285–291. [Google Scholar] [CrossRef]
- Monteiro, R.; Carvalho, C.; Collares-Pereira, M.J. Karyotype and genome size of Iberochondrostoma almacai (Teleostei, Cyprinidae) and comparison with the sister-species I. lusitanicum. Genet. Mol. Biol. 2009, 32, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Houben, A. B chromosomes—A matter of chromosome drive. Front. Plant Sci. 2017, 8, 210. [Google Scholar] [CrossRef] [Green Version]

| Number of Fish and Sex | n | Number of Metaphase Plates with the Indicated Number of B Chromosomes | ||
|---|---|---|---|---|
| 0 (2n = 50) | 1 (2n = 51) | 2 (2n = 52) | ||
| Five juveniles | 193 | 193 | - | - |
| Female | 41 | 41 | - | - |
| Three juveniles | 174 | 152 | 22 | - |
| * Three juveniles | 74 | 61 | 13 | - |
| Four juveniles | 170 | 108 | 33 | 29 |
| Three males | 88 | 60 | 17 | 11 |
| Female | 56 | 48 | - | 8 |
| Number of Fish and Sex | Number of Metaphase Plates with the Indicated Number of | Co-Localization of 28S and 5S rDNA Sites | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 28S rDNA Sites | 5S rDNA Sites | |||||||||
| n | 2 | 3 | 4 | n | 2 | 3 | 4 | 1 | 2 | |
| Three juveniles | 45 | 4 | - | 41 | 45 | 2 | - | 43 | 4 | 41 |
| Two females | 30 | 4 | 2 | 24 | 39 | 3 | 2 | 34 | 8 | 22 |
| Three males | 45 | 5 | 3 | 37 | 56 | - | 3 | 53 | 4 | 41 |
| 120 | 13 | 5 | 102 | 140 | 5 | 5 | 130 | 16 | 104 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boroń, A.; Grabowska, A.; Spóz, A.; Przybył, A.
B Chromosomes and Cytogenetic Characteristics
Boroń A, Grabowska A, Spóz A, Przybył A.
B Chromosomes and Cytogenetic Characteristics
Boroń, Alicja, Anna Grabowska, Aneta Spóz, and Anna Przybył.
2020. "B Chromosomes and Cytogenetic Characteristics





