Autophagy Genes for Wet Age-Related Macular Degeneration in a Finnish Case-Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Treatment
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Li, J.Q.; Welchowski, T.; Schmid, M.; Mauschitz, M.M.; Holz, F.G.; Finger, R.P. Prevalence and incidence of age-related macular degeneration in Europe: A systematic review and meta-analysis. Br. J. Ophthalmol. 2020, 104, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Colijn, J.M.; Buitendijk, G.H.S.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.J.; Korb, C.; Erke, M.G.; et al. Prevalence of age-related macular degeneration in Chinese populations worldwide: A systematic review and meta-analysis. Clin. Exp. Ophthalmol. 2019, 47, 1019–1027. [Google Scholar]
- Blasiak, J.; Salminen, A.; Kaarniranta, K. Potential of epigenetic mechanisms in AMD pathology. Front. Biosci. (Schol. Ed). 2013, 5, 12–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Gharahkhani, P.; Mitchell, P.; Liew, G.; Hewitt, A.W.; MacGregor, S. Genome-wide meta-analysis identifies novel loci associated with age-related macular degeneration. J. Hum. Genet. 2020, 65, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef] [Green Version]
- Blasiak, J.; Watala, C.; Tuuminen, R.; Kivinen, N.; Koskela, A.; Uusitalo-Järvinen, H.; Tuulonen, A.; Winiarczyk, M.; Mackiewicz, J.; Zmorzyński, S.; et al. Expression of VEGFA-regulating miRNAs and mortality in wet AMD. J. Cell. Mol. Med. 2019, 23, 8464–8471. [Google Scholar] [CrossRef] [Green Version]
- Seddon, J.M.; Rosner, B. Validated Prediction Models for Macular Degeneration Progression and Predictors of Visual Acuity Loss Identify High-Risk Individuals. Am. J. Ophthalmol. 2019, 198, 223–261. [Google Scholar] [CrossRef]
- Karesvuo, P.; Hakkala, L.; Kaarniranta, K.; Uusitalo, H.; Ojamo, M.; Tuuminen, R. Correlation between the rate of intravitreal injections, use of aflibercept as a second-line treatment and visual impairment for wet AMD in Finland. Acta Ophthalmol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Taipale, C.; Lindholm, J.M.; Kaarniranta, K.; Tuuminen, R. Comparison of Two Different Treat-and-Extend Protocols with Aflibercept in Wet Age-Related Macular Degeneration: Two-Year Results. Adv. Ther. 2020, 37, 2256–2266. [Google Scholar] [CrossRef]
- Schroeder, M.; Westborg, I.; Lövestam, A.M. Twelve per cent of 6142 eyes treated for neovascular age-related macular degeneration (nAMD) presented with low visual outcome within 2 years. Analysis from the Swedish Macula Registry (SMR). Acta Ophthalmol. 2020, 98, 274–278. [Google Scholar] [CrossRef] [Green Version]
- Kataja, M.; Hujanen, P.; Huhtala, H.; Kaarniranta, K.; Tuulonen, A.; Uusitalo-Jarvinen, H. Outcome of anti-vascular endothelial growth factor therapy for neovascular age-related macular degeneration in real-life setting. Br. J. Ophthalmol. 2018, 102, 959–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuuminen, R.; Sipilä, R.; Komulainen, J.; Saarela, V.; Kaarniranta, K.; Tuulonen, A. The first ophthalmic Choosing Wisely recommendations in Finland for glaucoma and wet age-related macular degeneration. Acta Ophthalmol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Valverde-Megías, A.; Veganzones-de-Castro, S.; Donate-López, J.; Maestro-de-las-Casas, M.L.; Megías-Fresno, A.; García-Feijoo, J. ARMS2 A69S polymorphism is associated with the number of ranibizumab injections needed for exudative age-related macular degeneration in a pro re nata regimen during 4 years of follow-up. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Lorés-Motta, L.; Riaz, M.; Grunin, M.; Corominas, J.; van Asten, F.; Pauper, M.; Leenders, M.; Richardson, A.J.; Muether, P.; Cree, A.J.; et al. Association of genetic variants with response to anti-vascular endothelial growth factor therapy in age-related macular degeneration. JAMA Ophthalmol. 2018, 136, 875–884. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Sinha, D.; Blasiak, J.; Kauppinen, A.; Veréb, Z.; Salminen, A.; Boulton, M.E.; Petrovski, G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy 2013, 9, 973–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Cross, S.D.; Stanton, J.B.; Marmorstein, A.D.; Le, Y.Z.; Marmorstein, L.Y. Early AMD-like defects in the RPE and retinal degeneration in aged mice with RPE-specific deletion of Atg5 or Atg7. Mol. Vis. 2017, 23, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W.; Ding, J.; et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 2014, 10, 1989–2005. [Google Scholar] [CrossRef] [Green Version]
- Felszeghy, S.; Viiri, J.; Paterno, J.J.; Hyttinen, J.M.T.; Koskela, A.; Chen, M.; Leinonen, H.; Tanila, H.; Kivinen, N.; Koistinen, A.; et al. Loss of NRF-2 and PGC-1α genes leads to retinal pigment epithelium damage resembling dry age-related macular degeneration. Redox Biol. 2019, 20, 1–12. [Google Scholar] [CrossRef]
- Gurubaran, I.S.; Viiri, J.; Koskela, A.; Hyttinen, J.M.T.; Paterno, J.J.; Kis, G.; Antal, M.; Urtti, A.; Kauppinen, A.; Felszeghy, S.; et al. Mitophagy in the Retinal Pigment Epithelium of Dry Age-Related Macular Degeneration Investigated in the NFE2L2/PGC-1α-/- Mouse Model. Int. J. Mol. Sci. 2020, 21, 1976. [Google Scholar] [CrossRef] [Green Version]
- Lahiri, V.; Hawkins, W.D.; Klionsky, D.J. Watch What You (Self-) Eat: Autophagig Mechanisms that Modulate Metabolism. Cell Metab. 2019, 29, 803–826. [Google Scholar] [CrossRef] [Green Version]
- Blasiak, J.; Pawlowska, E.; Szczepanska, J.; Kaarniranta, K. Interplay between Autophagy and the Ubiquitin-Proteasome System and Its Role in the Pathogenesis of Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 210. [Google Scholar] [CrossRef] [Green Version]
- Baek, A.; Yoon, S.; Kim, J.; Baek, Y.M.; Park, H.; Lim, D.; Chung, H.; Kim, D.E. Autophagy and KRT8/keratin 8 protect degeneration of retinal pigment epithelium under oxidative stress. Autophagy 2017, 13, 248–263. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Mitter, S.K.; Qi, X.; Beli, E.; Rao, H.V.; Ding, J.; Ip, C.S.; Gu, H.; Akin, D.; Dunn, W.A., Jr.; et al. Oxidative stress-mediated NFkappaB phosphorylation upregulates p62/SQSTM1 and promotes retinal pigmented epithelial cell survival through increased autophagy. PLoS ONE 2017. [Google Scholar] [CrossRef]
- Yao, J.; Jia, L.; Khan, N.; Lin, C.; Mitter, S.K.; Boulton, M.E.; Dunaief, J.L.; Klionsky, D.J.; Guan, J.L.; Thompson, D.A.; et al. Deletion of autophagy inducer RB1CC1 results in degeneration of the retinal pigment epithelium. Autophagy 2015, 11, 939–953. [Google Scholar] [CrossRef] [Green Version]
- Kaarniranta, K.; Paananen, J.; Nevalainen, T.; Sorri, I.; Seitsonen, S.; Immonen, I.; Salminen, A.; Pulkkinen, L.; Uusitupa, M. Adiponectin receptor 1 gene (ADIPOR1) variant is associated with advanced age-related macular degeneration in Finnish population. Neurosci. Lett. 2012, 513, 233–237. [Google Scholar] [CrossRef]
- Tuuminen, R.; Uusitalo-Järvinen, H.; Aaltonen, V.; Hautala, N.; Kaipiainen, S.; Laitamäki, N.; Ollila, M.; Rantanen, J.; Välimäki, S.; Sipilä, R.; et al. The Finnish national guideline for diagnosis, treatment and follow-up of patients with wet age-related macular degeneration. Acta Ophthalmol. 2017, 95 (Suppl. A105), 1–9. [Google Scholar] [CrossRef] [Green Version]
- Heesterbeek, T.J.; Lorés-Motta, L.; Hoyng, C.B.; Lechanteur, Y.T.E.; den Hollander, A.I. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol. Opt. 2020, 40, 40–70. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Chen, J.; Hua, J.; Hu, X.; Jian, S.; Zheng, G.; Wang, J.; Li, H.; Yang, J.; Hejtmancik, J.F.; et al. MITF protects against oxidative damage-induced retinal degeneration by regulating the NRF2 pathway in the retinal pigment epithelium. Redox Biol. 2020, 34. [Google Scholar] [CrossRef]
- Cobos, E.; Recalde, S.; Anter, J.; Hernandez-Sanchez, M.; Barreales, C.; Olavarrieta, L.; Valverde, A.; Suarez-Figueroa, M.; Cruz, F.; Abraldes, M.; et al. Association between CFH, CFB, ARMS2, SERPINF1, VEGFR1 and VEGF polymorphisms and anatomical and functional response to ranibizumab treatment in neovascular age-related macular degeneration. Acta Ophthalmol. 2018, 96, 201–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smailhodzic, D.; Muether, P.S.; Chen, J.; Kwestro, A.; Zhang, A.Y.; Omar, A.; Van de Ven, J.P.; Keunen, J.E.; Kirchhof, B.; Hoyng, C.B.; et al. Cumulative effect of risk alleles in CFH, ARMS2, and VEGFA on the response to ranibizumab treatment in age-related macular degeneration. Ophthalmology 2012, 119, 2304–2311. [Google Scholar] [CrossRef]
- Chakravarthy, U.; Wong, T.Y.; Fletcher, A.; Piault, E.; Evans, C.; Zlateva, G.; Buggage, R.; Pleil, A.; Mitchell, P. Clinical risk factors for age-related macular degeneration: A systematic review and meta-analysis. BMC Ophthalmol. 2010. [Google Scholar] [CrossRef]
- Saunier, V.; Merle, B.M.J.; Delyfer, M.N.; Cougnard-Grégoire, A.; Rougier, M.B.; Amouyel, P.; Lambert, J.C.; Dartigues, J.F.; Korobelnik, J.F.; Delcourt, C. Incidence of and Risk Factors Associated with Age-Related Macular Degeneration: Four-Year Follow-up from the ALIENOR Study. JAMA Ophthalmol. 2018, 136, 473–481. [Google Scholar] [CrossRef]
- Al-Holou, S.N.; Tucker, W.R.; Agrón, E.; Clemons, T.E.; Cukras, C.; Ferris, F.L., III; Chew, E.Y. Age-Related Eye Disease Study 2 Research Group. The Association of Statin Use with Age-Related Macular Degeneration Progression: The Age-Related Eye Disease Study 2 Report Number 9. Ophthalmology 2015, 122, 2490–2496. [Google Scholar] [CrossRef] [Green Version]
- Ouimet, M. Autophagy in obesity and atherosclerosis: Interrelationships between cholesterol homeostasis, lipoprotein metabolism and autophagy in macrophages and other systems. Biochim. Biophys. Acta 2013, 1831, 1124–1133. [Google Scholar] [CrossRef]
- Barbero-Camps, E.; Roca-Agujetas, V.; Bartolessis, I.; de Dios, C.; Fernández-Checa, J.C.; Marí, M.; Morales, A.; Hartmann, T.; Colell, A. Cholesterol impairs autophagy-mediated clearance of amyloid β while promoting its secretion. Autophagy 2018, 14, 1129–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Small, K.W.; Garabetian, C.A.; Shaya, F.S. Macular degeneration and aspirin use. Retina 2017, 37, 1630–1635. [Google Scholar] [CrossRef]
- Ying, G.S.; Maguire, M.G.; Daniel, E.; Grunwald, J.E.; Ahmed, O.; Martin, D.F.; Comparison of Age-Related Macular Degeneration Treatments Trials Research Group. Association between Antiplatelet or Anticoagulant Drugs and Retinal or Subretinal Hemorrhage in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2016, 123, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Modjtahedi, B.S.; Fong, D.S.; Jorgenson, E.; Van Den Eeden, S.K.; Quinn, V.; Slezak, J.M. The Relationship Between Nonsteroidal Anti-inflammatory Drug Use and Age-related Macular Degeneration. Am. J. Ophthalmol. 2018, 188, 111–122. [Google Scholar] [CrossRef]
- Rim, T.H.; Yoo, T.K.; Kwak, J.; Lee, J.S.; Kim, S.H.; Kim, D.W.; Kim, S.S. Long-Term Regular Use of Low-Dose Aspirin and Neovascular Age-Related Macular Degeneration: National Sample Cohort 2010–2015. Ophthalmology 2019, 126, 274–282. [Google Scholar] [CrossRef]
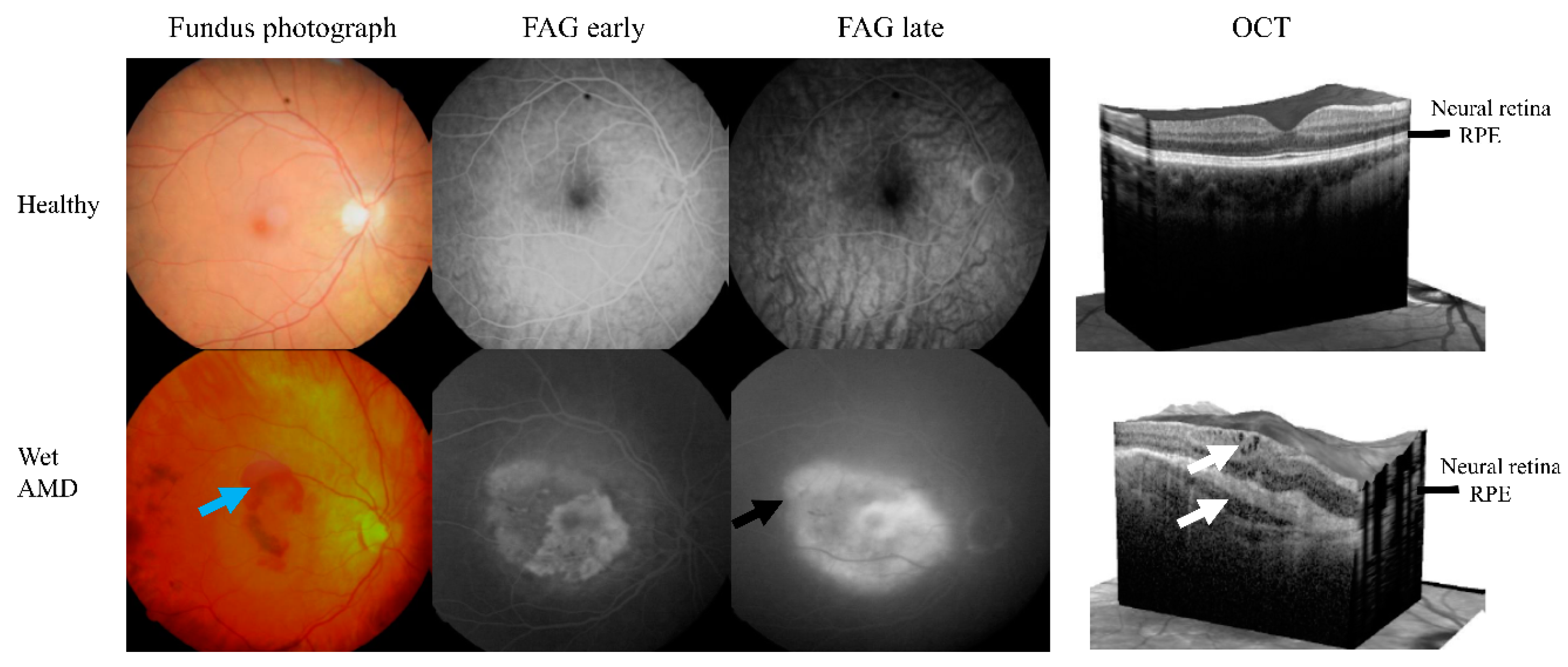
| Feature | Control (n = 161) | Wet AMD (n = 225) | p3 |
|---|---|---|---|
| Age (years) | 74.1 ± 6.3 | 78.4 ± 6.9 | 0.000 |
| Sex (male/female) | 52/109 | 70/155 | 0.368 |
| BMI (mean) | 25.7 ± 4.5 1 | 26.2 ± 4.1 2 | 0.161 |
| Smoking | 0.001 | ||
| Non-smoker | 93 (57.8%) | 140 (62.2%) | |
| Occasionally | 16 (9.9%) | 26 (11.6%) | |
| Smoker | 6 (3.7%) | 27 (12.0%) | |
| No information | 46 (28.6%) | 32 (14.2%) | |
| Medication | |||
| Blood pressure | 115 (79.3%) 1 | 164 (76.3%) 2 | 0.340 |
| Anti-cholesterol | 77 (53.1%) 1 | 93 (43.5%) 2 | 0.011 |
| Anticoagulant | 43 (29.5%) 1 | 64 (30.2%) 2 | 0.832 |
| Antiplatelet | 66 (44.6%) 1 | 109 (50.9%) 2 | 0.094 |
| Polymorphism | Gene | Change | Location within Gene |
|---|---|---|---|
| rs2295080 | mTOR—Mechanistic Target of Rapamycin Kinase | g.11262571G > T, C | 5′UTR (promoter) |
| rs11121704 | g.11233902C > A, T | Intron | |
| rs1057079 | c.1437C > T | Exon | |
| rs1064261 | c.2997C > T | Exon | |
| rs573775 | ATG5—Autophagy Related 5 | g. 106316991G > A | Intron |
| rs11246867 | ULK1—Unc-51 Like Autophagy Activating Kinase 1 | g. 131893472G > A | 2 KB Upstream Variant |
| rs3088051 | ULK1—Unc-51 Like Autophagy Activating Kinase 1 | g. 131922463T > C | 3′UTR |
| rs10902469 | g. 131893588G > A, C | 2 KB Upstream Variant | |
| rs73105013 | MAP1LC3A—Microtubule Associated Protein 1 Light Chain 3 α | g. 179837731T > A, C | Intron |
| rs10277 | SQSTM1—Sequestosome 1 | g. 179837731T > A, C | 3′UTR |
| Polymorphism | Control | AMD | ||
|---|---|---|---|---|
| Chi-square | p1 | Chi-square | p1 | |
| rs2295080 | 6.720 | 0.035 | 1.201 | 0.548 |
| rs11121704 | 6.090 | 0.048 | 2.192 | 0.334 |
| rs1057079 | 1.464 | 0.481 | 0.673 | 0.714 |
| rs1064261 | 5.081 | 0.079 | 0.898 | 0.638 |
| rs573775 | 0.456 | 0.796 | 6.555 | 0.038 |
| rs11246867 | 1.044 | 0.593 | 0.109 | 0.947 |
| rs3088051 | 0.220 | 0.896 | 0.401 | 0.818 |
| rs73105013 | 1.884 | 0.390 | 1.987 | 0.370 |
| rs10277 | 0.668 | 0.716 | 0.033 | 0.984 |
| rs10902469 | 1.044 | 0.593 | 0.109 | 0.947 |
| Genotype/Allele | Frequency | Crude OR (95% CI) | p | Adjusted OR 1 (95% CI) | p | |
|---|---|---|---|---|---|---|
| Control | AMD | |||||
| rs2295080 148/216 (Control/AMD cases) | ||||||
| GG | 0.12 | 0.12 | 1.07 (0.57–2.00) | 0.836 | 1.28 (0.65–2.53) | 0.474 |
| GT | 0.32 | 0.42 | 1.51 (0.98–2.34) | 0.062 | 1.49 (0.94–2.38) | |
| TT | 0.56 | 0.46 | 0.661 (0.435–1.004) 0.664 (0.454–0.971) 2 | 0.052 0.035 | 0.621 (0.398–0.968) 2 0.617 (0.393–0.968)0.651 | 0.035 0.036 |
| G | 0.22 | 0.27 | 1.296 (0.941–1.785) | 0.113 | ||
| T | 0.28 | 0.23 | 0.772 (0.560–1.063) | 0.113 | 0.709 (0.503–0.998) 2 0.703 (0.493–0.995)0.725 | 0.048 0.049 |
| rs11121704 152/209 (Control/AMD cases) | ||||||
| CC | 0.07 | 0.09 | 1.15 (0.53–2.52) | 0.720 | 1.23 (0.54–2.82) | 0.616 |
| CT | 0.26 | 0.33 | 1.489 (0.940–2.357) 1.481 (0.936–2.345) 2 | 0.089 0.093 2 | 1.609 (0.985–2.628) 2 1.634 (0.982–2.717)0.459 | 0.056 0.059 |
| TT | 0.67 | 0.58 | 0.671 (0.436–1.034) 0.663 (0.433–1.016) 2 | 0.071 0.059 2 | 0.613 (0.386–0.972) 2 0.605 (0.375–0.975)0.717 | 0.037 0.039 |
| C | 0.16 | 0.21 | 1.34 (0.94–1.91) | 0.102 | 1.447 (0.993–2.109) 2 1.467 (1.016–2.119)0.479 | 0.054 0.041 |
| T | 0.33 | 0.29 | 0.74 (0.52–1.06) | 0.102 | 0.691 (0.474–1.007) 2 0.681 (0.474–0.985)0.809 | 0.054 0.041 |
| rs1057079 161/217 (Control/AMD cases) | ||||||
| CC | 0.07 | 0.10 | 1.47 (0.71–3.04) | 0.293 | 1.69 (0.77–3.71) | 0.191 |
| CT | 0.33 | 0.41 | 1.37 (0.90–2.10) | 0.141 | 1.39 (0.88–2.18) | 0.153 |
| TT | 0.60 | 0.49 | 0.653 (0.434–0.984) 0.654 (0.435–0.982) 2 | 0.041 0.041 2 | 0.621 (0.402–0.961) 2 0.620 (0.396–0.972)0.675 | 0.032 0.037 |
| C | 0.20 | 0.25 | 1.413 (1.021–1.955) 1.403 (1.017–1.936) 2 | 0.037 0.039 2 | 1.500 (1.059–2.123) 2 1.507 (1.059–2.144)0.612 | 0.022 0.023 |
| T | 0.30 | 0.25 | 0.708 (0.511–0.979) 0.707 (0.513–976) 2 | 0.037 0.035 2 | 0.667 (0.471–0.944) 2 0.661 (0.470–0.929)0.869 | 0.022 0.017 |
| rs1064261 138/215 (Control/AMD cases) | ||||||
| GG | 0.09 | 0.09 | 0.94 (0.45–1.96) | 0.874 | 0.98 (0.45–2.13) | 0.961 |
| GA | 0.30 | 0.37 | 1.42 (0.90–2.24) | 0.131 | 1.50 (0.92–2.44) | 0.101 |
| AA | 0.61 | 0.53 | 0.74 (0.48–1.14) | 0.174 | 0.70 (0.44–1.10) | 0.123 |
| G | 0.19 | 0.23 | 1.19 (0.85–1.68) | 0.313 | 1.25 (0.87–1.80) | 0.229 |
| A | 0.30 | 0.27 | 0.84 (0.59–1.18) | 0.313 | 0.80 (0.55–1.15) | 0.230 |
| rs573775 160/214 (Control/AMD cases) | ||||||
| AA | 0.14 | 0.07 | 0.492 (0.253–0.954) 0.487 (0.246–0.964) 2 | 0.036 0.039 2 | 0.611 (0.304–1.227) | 0.165 |
| AG | 0.44 | 0.53 | 1.42 (0.94–1.14) | 0.092 2 | 1.31 (0.85–2.03) | 0.221 |
| GG | 0.42 | 0.40 | 0.92 (0.61–1.39) | 0.699 | 0.92 (0.59–1.43) | 0.717 |
| A | 0.29 | 0.30 | 0.90 (0.67–1.21) | 0.493 | 0.94 (0.68–1.29) | 0.707 |
| G | 0.21 | 0.20 | 1.11 (0.82–1.50) | 0.493 | 1.06 (0.77–1.46) | 0.707 |
| rs11246867 161/217 (Control/AMD cases) | ||||||
| AA | 0.00 | 0.00 | 2.1 × 107 (0–0) | 0.997 | 1.6077 × 1010 (0–0) | 1.000 |
| AG | 0.15 | 0.15 | 0.98 (0.55–1.72) | 0.933 | 0.95 (0.52–1.73) | 0.860 |
| GG | 0.85 | 0.85 | 0.99 (0.56–1.40) | 0.970 | 1.00 (0.55–1.83) | 0.994 |
| A | 0.07 | 0.08 | 1.04 (0.61–1.79) | 0.881 | 1.05 (0.59–1.85) | 0.878 |
| G | 0.42 | 0.42 | 0.96 (0.56–1.65) | 0.881 | 0.96 (0.54–1.69) | 0.878 |
| rs3088051 149/216 (Control/AMD cases) | ||||||
| CC | 0.07 | 0.05 | 0.71 (0.30–1.73) | 0.455 | 0.69 (0.26–1.84) | 0.462 |
| CT | 0.36 | 0.38 | 1.10 (0.72–0.69) | 0.665 | 1.13 (0.71–1.80) | 0.599 |
| TT | 0.58 | 0.57 | 0.98 (0.65–1.50) | 0.941 | 0.96 (0.61–1.50) | 0.855 |
| C | 0.21 | 0.22 | 0.96 (0.68–1.35) | 0.822 | 0.98 (0.68–1.41) | 0.903 |
| T | 0.29 | 0.29 | 1.04 (0.74–1.46) | 0.822 | 1.02 (0.71–1.48) | 0.903 |
| rs73105013 156/210 (Control/AMD cases) | ||||||
| CT | 0.21 | 0.18 | 0.79 (0.47–1.32) | 0.366 | 0.73 (0.42–1.23) | 0.252 |
| TT | 0.76 | 0.82 | 1.53 (0.92–2.53) | 0.102 | 1.65 (0.96–2.83) | 0.069 |
| C | 0.12 | 0.09 | 0.597 (0.376–0.949) 0.601 (0.376–0.960) 2 | 0.029 0.033 2 | 0.561 (0.344–0.919) 2 0.565 (0.337–0.947)0.695 | 0.021 0.030 |
| T | 0.38 | 0.41 | 1.674 (1.054–2.660) 1.686 (1.052–2.703) 2 | 0.029 0.030 2 | 1.779 (1.089–2.910) 2 1.776 (1.078–2.927)0.993 | 0.021 0.024 |
| rs10277 136/212 (Control/AMD cases) | ||||||
| CC | 0.46 | 0.35 | 0.657 (0.426–1.015) 0.656 (0.429–1.004) 2 | 0.059 0.052 2 | 0.658 (0.414–1.047) | 0.077 |
| CT | 0.41 | 0.49 | 1.34 (0.87–2.06) | 0.182 | 1.28 (0.80–2.03) | 0.297 |
| TT | 0.13 | 0.16 | 1.27 (0.68–2.38) | 0.450 | 1.41 (0.72–2.76) | 0.316 |
| C | 0.44 | 0.42 | 0.75 (0.54–1.03) | 0.071 | 0.74 (0.53–1.03) | 0.074 |
| T | 0.06 | 0.08 | 1.34 (0.97–1.36) | 0.078 | 1.36 (0.97–1.91) | 0.074 |
| rs10902469 161/217 (Control/AMD cases) | ||||||
| CG | 0.15 | 0.15 | 1.34 (0.97–1.86) | 0.934 | 0.95 (0.52–1.73) | 0.086 |
| GG | 0.85 | 0.85 | 0.99 (0.56–1.74) | 0.970 | 1.00 (0.55–1.83) | 0.994 |
| C | 0.08 | 0.08 | 1.04 (0.61–1.79) | 0.881 | 1.05 (0.59–1.85) | 0.888 |
| G | 0.42 | 0.42 | 0.96 (0.56–1.65) | 0.881 | 0.95 (0.54–1.69) | 0.880 |
| Polymorphism | Genotype | R | p |
|---|---|---|---|
| rs2295080 | GG | 0.137 | 0.040 1 |
| GT | 0.136 | 0.041 1 | |
| TT | 0.088 | 0.377 | |
| rs11121704 | CC | 0.089 | 0.362 |
| CT | 0.099 | 0.313 | |
| TT | 0.089 | 0.367 | |
| rs1057079 | CC | 0.100 | 0.309 |
| CT | 0.106 | 0.281 | |
| TT | 0.131 | 0.049 1 | |
| rs1064261 | GG | 0.093 | 0.344 |
| GA | 0.129 | 0.053 | |
| AA | 0.111 | 0.257 | |
| rs573775 | AA | 0.083 | 0.398 |
| AG | 0.105 | 0.287 | |
| GG | 0.107 | 0.274 | |
| rs11246867 | AA | 0.106 | 0.114 |
| AG | 0.133 | 0.045 1 | |
| GG | 0.106 | 0.278 | |
| rs3088051 | CC | 0.148 | 0.026 1 |
| CT | 0.104 | 0.290 | |
| TT | 0.083 | 0.398 | |
| rs73105013 | CC | ||
| CT | 0.105 | 0.281 | |
| TT | 0.105 | 0.281 | |
| rs10277 | CC | 0.131 | 0.049 1 |
| CT | 0.086 | 0.380 | |
| TT | 0.087 | 0.194 | |
| rs10902469 | CC | ||
| CG | 0.084 | 0.391 | |
| GG | 0.084 | 0.391 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paterno, J.J.; Koskela, A.; Hyttinen, J.M.T.; Vattulainen, E.; Synowiec, E.; Tuuminen, R.; Watala, C.; Blasiak, J.; Kaarniranta, K. Autophagy Genes for Wet Age-Related Macular Degeneration in a Finnish Case-Control Study. Genes 2020, 11, 1318. https://doi.org/10.3390/genes11111318
Paterno JJ, Koskela A, Hyttinen JMT, Vattulainen E, Synowiec E, Tuuminen R, Watala C, Blasiak J, Kaarniranta K. Autophagy Genes for Wet Age-Related Macular Degeneration in a Finnish Case-Control Study. Genes. 2020; 11(11):1318. https://doi.org/10.3390/genes11111318
Chicago/Turabian StylePaterno, Jussi J., Ali Koskela, Juha M.T. Hyttinen, Elina Vattulainen, Ewelina Synowiec, Raimo Tuuminen, Cezary Watala, Janusz Blasiak, and Kai Kaarniranta. 2020. "Autophagy Genes for Wet Age-Related Macular Degeneration in a Finnish Case-Control Study" Genes 11, no. 11: 1318. https://doi.org/10.3390/genes11111318
APA StylePaterno, J. J., Koskela, A., Hyttinen, J. M. T., Vattulainen, E., Synowiec, E., Tuuminen, R., Watala, C., Blasiak, J., & Kaarniranta, K. (2020). Autophagy Genes for Wet Age-Related Macular Degeneration in a Finnish Case-Control Study. Genes, 11(11), 1318. https://doi.org/10.3390/genes11111318








