Super-Resolution Microscopy of Chromatin
Abstract
1. Introduction
2. Challenges in Microscopy of Chromatin
2.1. Limited Imaging Resolution
2.2. Limited Contrast
2.2.1. High Sensitivity Based on Bright and Stable Emitters
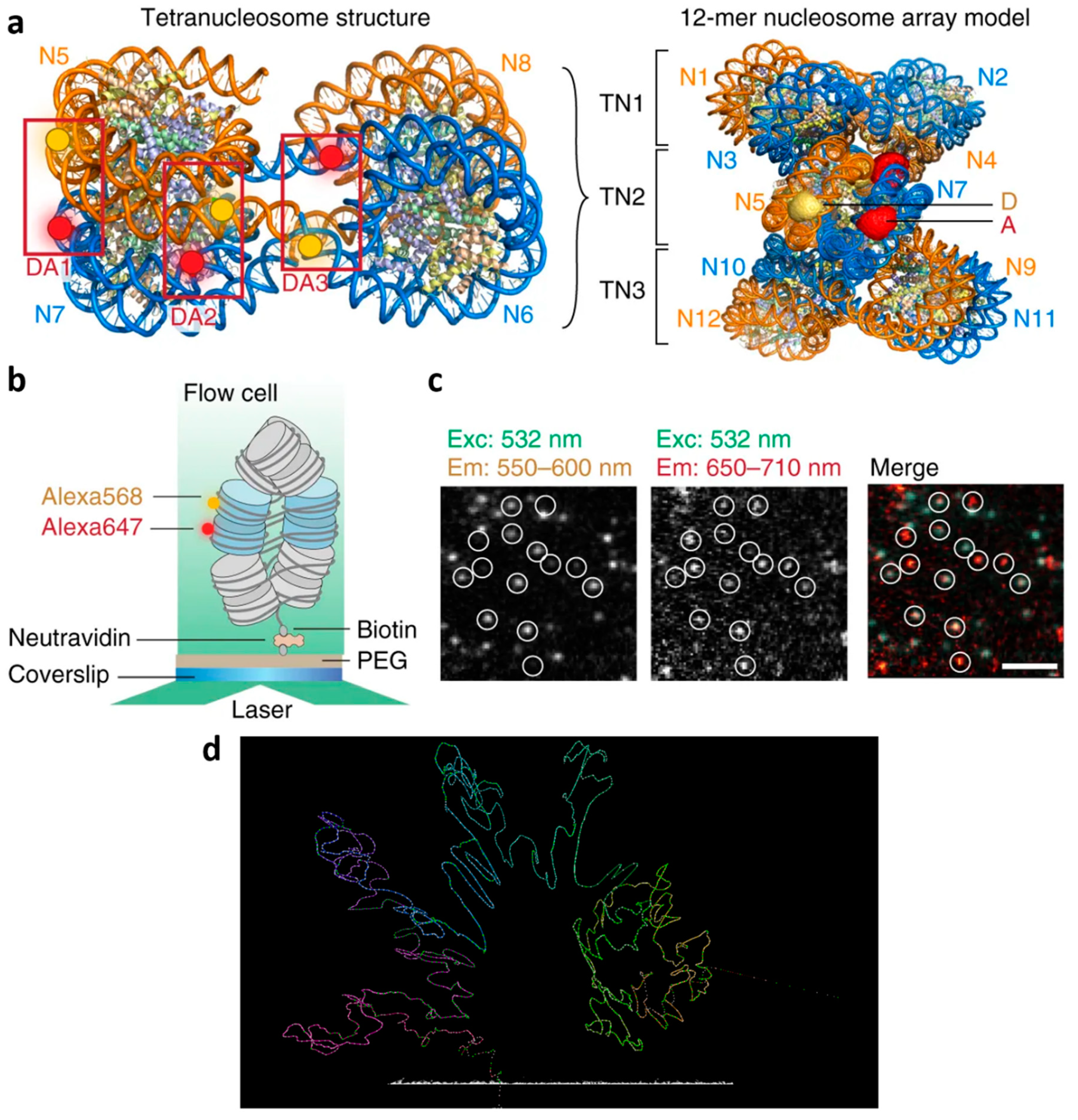
2.2.2. High Specificity Based on Stable Binding of the Emitters to Their Target

2.3. Limited Temporal Resolution
2.3.1. Fast and Sensitive Detection
2.3.2. Fast Reconstruction Software
2.4. Structural Integrity
2.4.1. Labeling Artifacts Such as Sterical and/or Functional Hindrance
2.4.2. Harsh Chemical Treatments
2.4.3. Mechanical and Chemical Effects of Cell Separation: Cells Outside Their Natural Environment
2.4.4. Swelling and Clearing
3. Techniques
3.1. Structured Illumination Microscopy (SIM)
3.2. Stimulated Emission Depletion Microscopy (STED)
3.3. Single Molecule Localization Microscopy (SMLM)
3.4. Smart Illumination Schemes
3.5. Statistical Analyses
3.6. Deep Learning
3.7. Single-Molecule Dynamics
3.8. Correlative Microscopy
3.9. Specific Considerations Needed for Imaging Chromatin
4. Conclusions
Funding
Conflicts of Interest
References
- Ulitzur, N.; Harel, A.; Goldberg, M.; Feinstein, N.; Gruenbaum, Y. Nuclear membrane vesicle targeting to chromatin in a Drosophila embryo cell-free system. Mol. Biol. Cell 1997, 8, 1439–1448. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hüve, J.; Wesselmann, R.; Kahms, M.; Peters, R. 4Pi microscopy of the nuclear pore complex. Biophys. J. 2008, 95, 877–885. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reymann, J.; Baddeley, D.; Gunkel, M.; Lemmer, P.; Stadter, W.; Jegou, T.; Rippe, K.; Cremer, C.; Birk, U. High-precision structural analysis of subnuclear complexes in fixed and live cells via spatially modulated illumination (SMI) microscopy. Chromosome Res. 2008, 16, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Peterman, E.J.G.; Sosa, H.; Moerner, W.E. Single-Molecule Fluorescence Spectroscopy and Microscopy of Biomolecular Motors. Annu. Rev. Phys. Chem. 2004, 55, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Failla, A.V.; Spori, U.; Cremer, C.; Pombo, A. Measuring the size of biological nanostructures with spatially modulated illumination microscopy. Mol. Biol. Cell 2004, 15, 2449–2455. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, S.; Su, J.-H.; Beliveau, B.J.; Bintu, B.; Moffitt, J.R.; Wu, C.; Zhuang, X. Spatial organization of chromatin domains and compartments in single chromosomes. Science 2016, 353, 598–602. [Google Scholar] [CrossRef]
- Wiehle, L.; Thorn, G.J.; Raddatz, G.; Clarkson, C.T.; Rippe, K.; Lyko, F.; Breiling, A.; Teif, V.B. DNA (de)methylation in embryonic stem cells controls CTCF-dependent chromatin boundaries. Genome Res. 2019, 29, 750–761. [Google Scholar] [CrossRef]
- Nuebler, J.; Fudenberg, G.; Imakaev, M.; Abdennur, N.; Mirny, L.A. Chromatin organization by an interplay of loop extrusion and compartmental segregation. Proc. Natl. Acad. Sci. USA 2018, 115, E6697–E6706. [Google Scholar] [CrossRef]
- Cremer, M.; Cremer, T. Nuclear compartmentalization, dynamics, and function of regulatory DNA sequences. Genes Chromosomes Cancer 2019, 58, 427–436. [Google Scholar] [CrossRef]
- Erdel, F.; Rippe, K. Formation of Chromatin Subcompartments by Phase Separation. Biophys. J. 2018, 114, 2262–2270. [Google Scholar] [CrossRef]
- Odenheimer, J.; Heermann, D.W.; Kreth, G. Brownian dynamics simulations reveal regulatory properties of higher-order chromatin structures. Eur. Biophys. J. 2009, 38, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Cremer, T.; Cremer, M.; Hübner, B.; Strickfaden, H.; Smeets, D.; Popken, J.; Sterr, M.; Markaki, Y.; Rippe, K.; Cremer, C. The 4D nucleome: Evidence for a dynamic nuclear landscape based on co-aligned active and inactive nuclear compartments. FEBS Lett. 2015, 589, 2931–2943. [Google Scholar] [CrossRef]
- Dekker, J.; Belmont, A.S.; Guttman, M.; Leshyk, V.O.; Lis, J.T.; Lomvardas, S.; Mirny, L.A.; O’Shea, C.C.; Park, P.J.; Ren, B.; et al. The 4D nucleome project. Nature 2017, 549, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Shopland, L.S.; Lynch, C.R.; Peterson, K.A.; Thornton, K.; Kepper, N.; von Hase, J.; Stein, S.; Vincent, S.; Molloy, K.R.; Kreth, G.; et al. Folding and organization of a contiguous chromosome region according to the gene distribution pattern in primary genomic sequence. J. Cell Biol. 2006, 174, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Görisch, S.M.; Wachsmuth, M.; Tóth, K.F.; Lichter, P.; Rippe, K. Histone acetylation increases chromatin accessibility. J. Cell. Sci. 2005, 118, 5825–5834. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, R.; Hibino, K.; Ashwin, S.S.; Babokhov, M.; Fujishiro, S.; Imai, R.; Nozaki, T.; Tamura, S.; Tani, T.; Kimura, H.; et al. Single nucleosome imaging reveals loose genome chromatin networks via active RNA polymerase II. J. Cell Biol. 2019, 218, 1511–1530. [Google Scholar] [CrossRef] [PubMed]
- Bustin, M.; Misteli, T. Nongenetic functions of the genome. Science 2016, 352, aad6933. [Google Scholar] [CrossRef] [PubMed]
- Spöri, U.; Failla, A.V.; Cremer, C. Superresolution size determination in fluorescence microscopy: A comparison between spatially modulated illumination and confocal laser scanning microscopy. J. Appl. Phys. 2004, 95, 8436–8443. [Google Scholar] [CrossRef]
- Cremer, C.; Birk, U. Perspectives in super-resolved fluorescence microscopy: What comes next? Front. Phys. 2016, 4, 11. [Google Scholar] [CrossRef][Green Version]
- Francia, G.T. di Resolving Power and Information. J. Opt. Soc. Am. 1955, 45, 497–501. [Google Scholar] [CrossRef]
- Ou, H.D.; Phan, S.; Deerinck, T.J.; Thor, A.; Ellisman, M.H.; O’Shea, C.C. ChromEMT: Visualizing 3D chromatin structure and compaction in interphase and mitotic cells. Science 2017, 357. [Google Scholar] [CrossRef] [PubMed]
- Mahamid, J.; Pfeffer, S.; Schaffer, M.; Villa, E.; Danev, R.; Cuellar, L.K.; Förster, F.; Hyman, A.A.; Plitzko, J.M.; Baumeister, W. Visualizing the molecular sociology at the HeLa cell nuclear periphery. Science 2016, 351, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Birk, U.J. Super-Resolution Microscopy: A Practical Guide; 1st ed.; Wiley VCH: Weinheim, Germany, 2017; ISBN 3-527-34133–34131. [Google Scholar]
- Boettiger, A.N.; Bintu, B.; Moffitt, J.R.; Wang, S.; Beliveau, B.J.; Fudenberg, G.; Imakaev, M.; Mirny, L.A.; Wu, C.; Zhuang, X. Super-resolution imaging reveals distinct chromatin folding for different epigenetic states. Nature 2016, 529, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.A.; Manzo, C.; García-Parajo, M.F.; Lakadamyali, M.; Cosma, M.P. Chromatin fibers are formed by heterogeneous groups of nucleosomes in vivo. Cell 2015, 160, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Mathée, H.; Baddeley, D.; Wotzlaw, C.; Fandrey, J.; Cremer, C.; Birk, U. Nanostructure of specific chromatin regions and nuclear complexes. Histochem. Cell Biol. 2006, 125, 75–82. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kirmes, I.; Szczurek, A.; Prakash, K.; Charapitsa, I.; Heiser, C.; Musheev, M.; Schock, F.; Fornalczyk, K.; Ma, D.; Birk, U.; et al. A transient ischemic environment induces reversible compaction of chromatin. Genome Biol. 2015, 16, 246. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ma, H.; Jin, J.; Uttam, S.; Fu, R.; Huang, Y.; Liu, Y. Super-resolution imaging of higher-order chromatin structures at different epigenomic states in single mammalian cells. Cell Rep. 2018, 24, 873–882. [Google Scholar] [CrossRef]
- Prieto, E.I.; Maeshima, K. Dynamic chromatin organization in the cell. Essays Biochem. 2019, 63, 133–145. [Google Scholar] [CrossRef]
- Toh, K.C.; Ramdas, N.M.; Shivashankar, G.V. Actin cytoskeleton differentially alters the dynamics of lamin A, HP1α and H2B core histone proteins to remodel chromatin condensation state in living cells. Integr Biol. 2015, 7, 1309–1317. [Google Scholar] [CrossRef]
- Heuvelman, G.; Erdel, F.; Wachsmuth, M.; Rippe, K. Analysis of protein mobilities and interactions in living cells by multifocal fluorescence fluctuation microscopy. Eur. Biophys. J. 2009, 38, 813–828. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Talwar, S.; Mazumder, A.; Shivashankar, G.V. Spatio-temporal plasticity in chromatin organization in mouse cell differentiation and during Drosophila embryogenesis. Biophys. J. 2009, 96, 3832–3839. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mora-Bermúdez, F.; Ellenberg, J. Measuring structural dynamics of chromosomes in living cells by fluorescence microscopy. Methods 2007, 41, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Salic, A.; Mitchison, T.J. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 2415–2420. [Google Scholar] [CrossRef] [PubMed]
- Raulf, A.; Spahn, C.K.; Zessin, P.J.M.; Finan, K.; Bernhardt, S.; Heckel, A.; Heilemann, M. Click chemistry facilitates direct labelling and super-resolution imaging of nucleic acids and proteins†Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra01027bClick here for additional data file. RSC Adv. 2014, 4, 30462–30466. [Google Scholar] [CrossRef] [PubMed]
- Beliveau, B.J.; Boettiger, A.N.; Nir, G.; Bintu, B.; Yin, P.; Zhuang, X.; Wu, C.-T. In Situ Super-Resolution Imaging of Genomic DNA with OligoSTORM and OligoDNA-PAINT. Methods Mol. Biol. 2017, 1663, 231–252. [Google Scholar] [PubMed]
- Szczurek, A.T.; Prakash, K.; Lee, H.-K.; Żurek-Biesiada, D.J.; Best, G.; Hagmann, M.; Dobrucki, J.W.; Cremer, C.; Birk, U. Single molecule localization microscopy of the distribution of chromatin using Hoechst and DAPI fluorescent probes. Nucleus 2014, 5, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Szczurek, A.; Klewes, L.; Xing, J.; Gourram, A.; Birk, U.; Knecht, H.; Dobrucki, J.W.; Mai, S.; Cremer, C. Imaging chromatin nanostructure with binding-activated localization microscopy based on DNA structure fluctuations. Nucleic Acids Res. 2017, 45, e56. [Google Scholar] [CrossRef]
- Chen, B.; Guan, J.; Huang, B. Imaging Specific Genomic DNA in Living Cells. Annu. Rev. Biophys. 2016, 45, 1–23. [Google Scholar] [CrossRef]
- Weiss, L.E.; Naor, T.; Shechtman, Y. Observing DNA in live cells. Biochem. Soc. Trans. 2018, 46, 729–740. [Google Scholar] [CrossRef]
- Ricci, M.A.; Cosma, M.P.; Lakadamyali, M. Super resolution imaging of chromatin in pluripotency, differentiation, and reprogramming. Curr. Opin. Genet. Dev. 2017, 46, 186–193. [Google Scholar] [CrossRef]
- Boutorine, A.S.; Novopashina, D.S.; Krasheninina, O.A.; Nozeret, K.; Venyaminova, A.G. Fluorescent probes for nucleic Acid visualization in fixed and live cells. Molecules 2013, 18, 15357–15397. [Google Scholar] [CrossRef] [PubMed]
- Sander, J.D.; Joung, J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014, 32, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Lauterbach, M.A. Finding, defining and breaking the diffraction barrier in microscopy–a historical perspective. Opt. Nanoscopy 2012, 1, 8. [Google Scholar] [CrossRef]
- Li, D.; Shao, L.; Chen, B.-C.; Zhang, X.; Zhang, M.; Moses, B.; Milkie, D.E.; Beach, J.R.; Hammer, J.A.; Pasham, M. Extended-resolution structured illumination imaging of endocytic and cytoskeletal dynamics. Science 2015, 349, aab3500. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Betzig, E. Response to comment on “Extended-resolution structured illumination imaging of endocytic and cytoskeletal dynamics.”. Science 2016, 352, 527. [Google Scholar] [CrossRef] [PubMed]
- Confocal Microscopy List | Mailing List Archive. Available online: http://confocal-microscopy-list.588098.n2.nabble.com/ (accessed on 17 June 2019).
- SPIM - OpenSPIM. Available online: https://openspim.org/ (accessed on 17 June 2019).
- Holden, S.; Sage, D. Super-resolution fight club. Nat. Photon. 2016, 10, 152–153. [Google Scholar] [CrossRef]
- Sage, D.; Pham, T.-A.; Babcock, H.; Lukes, T.; Pengo, T.; Chao, J.; Velmurugan, R.; Herbert, A.; Agrawal, A.; Colabrese, S.; et al. Super-resolution fight club: assessment of 2D and 3D single-molecule localization microscopy software. Nat. Methods 2019, 16, 387. [Google Scholar] [CrossRef]
- Baddeley, D.; Chagin, V.O.; Schermelleh, L.; Martin, S.; Pombo, A.; Carlton, P.M.; Gahl, A.; Domaing, P.; Birk, U.; Leonhardt, H.; et al. Measurement of replication structures at the nanometer scale using super-resolution light microscopy. Nucleic Acids Res. 2010, 38, e8. [Google Scholar] [CrossRef]
- Wassie, A.T.; Zhao, Y.; Boyden, E.S. Expansion microscopy: principles and uses in biological research. Nat. Methods 2019, 16, 33–41. [Google Scholar] [CrossRef]
- Flors, C.; Earnshaw, W.C. Super-resolution fluorescence microscopy as a tool to study the nanoscale organization of chromosomes. Curr. Opin. Chem. Biol. 2011, 15, 838–844. [Google Scholar] [CrossRef]
- Heintzmann, R.; Cremer, C.G. Laterally modulated excitation microscopy: improvement of resolution by using a diffraction grating. In Proceedings of the Optical Biopsies and Microscopic Techniques III; International Society for Optics and Photonics: Bellingham, WA, USA, 1999; Vol. 3568, pp. 185–196. [Google Scholar]
- Gustafsson, M.G. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 2000, 198, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Betzig, E.; Patterson, G.H.; Sougrat, R.; Lindwasser, O.W.; Olenych, S.; Bonifacino, J.S.; Davidson, M.W.; Lippincott-Schwartz, J.; Hess, H.F. Imaging intracellular fluorescent proteins at nanometer resolution. Science 2006, 313, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.J.; Bates, M.; Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 2006, 3, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Hell, S.W.; Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 1994, 19, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Markaki, Y.; Smeets, D.; Fiedler, S.; Schmid, V.J.; Schermelleh, L.; Cremer, T.; Cremer, M. The potential of 3D-FISH and super-resolution structured illumination microscopy for studies of 3D nuclear architecture: 3D structured illumination microscopy of defined chromosomal structures visualized by 3D (immuno)-FISH opens new perspectives for studies of nuclear architecture. Bioessays 2012, 34, 412–426. [Google Scholar] [PubMed]
- Hansen, A.S.; Pustova, I.; Cattoglio, C.; Tjian, R.; Darzacq, X. CTCF and cohesin regulate chromatin loop stability with distinct dynamics. eLife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.R.; Sossick, A.J.; Boulton, S.J.; Jackson, S.P. BRCA1-associated exclusion of 53BP1 from DNA damage sites underlies temporal control of DNA repair. J. Cell. Sci. 2012, 125, 3529–3534. [Google Scholar] [CrossRef] [PubMed]
- Lukinavičius, G.; Blaukopf, C.; Pershagen, E.; Schena, A.; Reymond, L.; Derivery, E.; Gonzalez-Gaitan, M.; D’Este, E.; Hell, S.W.; Wolfram Gerlich, D.; et al. SiR-Hoechst is a far-red DNA stain for live-cell nanoscopy. Nat. Commun. 2015, 6, 8497. [Google Scholar] [CrossRef]
- Mitchell-Jordan, S.; Chen, H.; Franklin, S.; Stefani, E.; Bentolila, L.A.; Vondriska, T.M. Features of endogenous cardiomyocyte chromatin revealed by super-resolution STED microscopy. J. Mol. Cell. Cardiol. 2012, 53, 552–558. [Google Scholar] [CrossRef]
- Persson, F.; Bingen, P.; Staudt, T.; Engelhardt, J.; Tegenfeldt, J.O.; Hell, S.W. Fluorescence nanoscopy of single DNA molecules by using stimulated emission depletion (STED). Angew. Chem. Int. Ed. Engl. 2011, 50, 5581–5583. [Google Scholar] [CrossRef]
- Nozaki, T.; Imai, R.; Tanbo, M.; Nagashima, R.; Tamura, S.; Tani, T.; Joti, Y.; Tomita, M.; Hibino, K.; Kanemaki, M.T.; et al. Dynamic Organization of Chromatin Domains Revealed by Super-Resolution Live-Cell Imaging. Mol. Cell 2017, 67, 282–293.e7. [Google Scholar] [CrossRef] [PubMed]
- Prakash, K.; Fournier, D.; Redl, S.; Best, G.; Borsos, M.; Tiwari, V.K.; Tachibana-Konwalski, K.; Ketting, R.F.; Parekh, S.H.; Cremer, C. Superresolution imaging reveals structurally distinct periodic patterns of chromatin along pachytene chromosomes. Proc. Natl. Acad. Sci. USA 2015, 112, 14635–14640. [Google Scholar] [CrossRef] [PubMed]
- Huisken, J.; Swoger, J.; Del Bene, F.; Wittbrodt, J.; Stelzer, E.H.K. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science 2004, 305, 1007–1009. [Google Scholar] [CrossRef] [PubMed]
- Siedentopf, H.; Zsigmondy, R. Uber sichtbarmachung und grö\s senbestimmung ultramikoskopischer teilchen, mit besonderer anwendung auf goldrubingläser. Ann. Phys. 1902, 315, 1–39. [Google Scholar] [CrossRef]
- Birk, U.; Hase, J.V.; Cremer, C. Super-resolution microscopy with very large working distance by means of distributed aperture illumination. Sci. Rep. 2017, 7, 3685. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-L.; Upadhyayula, S.; Milkie, D.E.; Singh, V.; Wang, K.; Swinburne, I.A.; Mosaliganti, K.R.; Collins, Z.M.; Hiscock, T.W.; Shea, J.; et al. Observing the cell in its native state: Imaging subcellular dynamics in multicellular organisms. Science 2018, 360. [Google Scholar] [CrossRef]
- Sinkó, J.; Szabó, G.; Erdélyi, M. Ray tracing analysis of inclined illumination techniques. Opt. Express 2014, 22, 18940–18948. [Google Scholar] [CrossRef]
- Galland, R.; Grenci, G.; Aravind, A.; Viasnoff, V.; Studer, V.; Sibarita, J.-B. 3D high- and super-resolution imaging using single-objective SPIM. Nat. Methods 2015, 12, 641–644. [Google Scholar] [CrossRef]
- Cella Zanacchi, F.; Lavagnino, Z.; Perrone Donnorso, M.; Del Bue, A.; Furia, L.; Faretta, M.; Diaspro, A. Live-cell 3D super-resolution imaging in thick biological samples. Nat. Methods 2011, 8, 1047–1049. [Google Scholar] [CrossRef]
- Chen, B.-C.; Legant, W.R.; Wang, K.; Shao, L.; Milkie, D.E.; Davidson, M.W.; Janetopoulos, C.; Wu, X.S.; Hammer, J.A.; Liu, Z.; et al. Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science 2014, 346, 1257998. [Google Scholar] [CrossRef]
- Mathée, H.; Baddeley, D.; Wotzlaw, C.; Cremer, C.G.; Birk, U.J. Spatially modulated illumination microscopy using one objective lens. OE 2007, 46, 083603. [Google Scholar] [CrossRef]
- Baddeley, D.; Weiland, Y.; Batram, C.; Birk, U.; Cremer, C. Model based precision structural measurements on barely resolved objects. J. Microsc. 2010, 237, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.; Rosten, E.; Monypenny, J.; Jovanovic-Talisman, T.; Burnette, D.T.; Lippincott-Schwartz, J.; Jones, G.E.; Heintzmann, R. Bayesian localisation microscopy reveals nanoscale podosome dynamics. Nat. Methods 2011, 9, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, N.; Culley, S.; Ashdown, G.; Owen, D.M.; Pereira, P.M.; Henriques, R. Fast live-cell conventional fluorophore nanoscopy with ImageJ through super-resolution radial fluctuations. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Dertinger, T.; Colyer, R.; Iyer, G.; Weiss, S.; Enderlein, J. Fast, background-free, 3D super-resolution optical fluctuation imaging (SOFI). Proc. Natl. Acad. Sci. USA 2009, 106, 22287–22292. [Google Scholar] [CrossRef] [PubMed]
- Nehme, E.; Weiss, L.E.; Michaeli, T.; Shechtman, Y. Deep-STORM: super-resolution single-molecule microscopy by deep learning. Optica 2018, 5, 458–464. [Google Scholar] [CrossRef]
- Wang, H.; Rivenson, Y.; Jin, Y.; Wei, Z.; Gao, R.; Günaydın, H.; Bentolila, L.A.; Kural, C.; Ozcan, A. Deep learning enables cross-modality super-resolution in fluorescence microscopy. N. Methods 2019, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- Ruprecht, V.; Axmann, M.; Wieser, S.; Schutz, G.J. What Can We Learn from Single Molecule Trajectories? Available online: https://www.ingentaconnect.com/content/ben/cpps/2011/00000012/00000008/art00005 (accessed on 17 June 2019).
- Kilic, S.; Felekyan, S.; Doroshenko, O.; Boichenko, I.; Dimura, M.; Vardanyan, H.; Bryan, L.C.; Arya, G.; Seidel, C.A.M.; Fierz, B. Single-molecule FRET reveals multiscale chromatin dynamics modulated by HP1α. Nat. Commun. 2018, 9, 235. [Google Scholar] [CrossRef]
- Moser, F.; Pražák, V.; Mordhorst, V.; Andrade, D.M.; Baker, L.A.; Hagen, C.; Grünewald, K.; Kaufmann, R. Cryo-SOFI enabling low-dose super-resolution correlative light and electron cryo-microscopy. Proc. Natl. Acad. Sci. USA 2019, 116, 4804–4809. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Bestvater, F.; Schaufler, W.; Heintzmann, R.; Cremer, C. Patterned illumination single molecule localization microscopy (piSMLM): user defined blinking regions of interest. Opt. Express 2018, 26, 30009–30020. [Google Scholar] [CrossRef]
- Birk, U.; Szczurek, A.; Cremer, C. Super-resolved linear fluorescence localization microscopy using photostable fluorophores: A virtual microscopy study. Opt. Commun. 2017, 404, 42–50. [Google Scholar] [CrossRef]
- Szydlowski, N.A.; Go, J.S.; Hu, Y.S. Chromatin imaging and new technologies for imaging the nucleome. Wiley Interdiscip. Rev. Syst. Biol. Med. 2019, 11, e1442. [Google Scholar] [CrossRef] [PubMed]
- Cremer, C.; Szczurek, A.; Schock, F.; Gourram, A.; Birk, U. Super-resolution microscopy approaches to nuclear nanostructure imaging. Methods 2017, 123, 11–32. [Google Scholar] [CrossRef]
- Sharonov, A.; Hochstrasser, R.M. Wide-field subdiffraction imaging by accumulated binding of diffusing probes. Proc. Natl. Acad. Sci. USA 2006, 103, 18911–18916. [Google Scholar] [CrossRef] [PubMed]
- Nieves, D.J.; Gaus, K.; Baker, M.A.B. DNA-Based Super-Resolution Microscopy: DNA-PAINT. Genes 2018, 9, 621. [Google Scholar] [CrossRef]
- Todd, S.; Todd, P.; McGowan, S.J.; Hughes, J.R.; Kakui, Y.; Leymarie, F.F.; Latham, W.; Taylor, S. CSynth: A Dynamic Modelling and Visualisation Tool for 3D Chromatin Structure. bioRxiv 2019, 499806. [Google Scholar]
- Creative Commons—Attribution 4.0 International — CC BY 4.0. Available online: https://creativecommons.org/licenses/by/4.0/ (accessed on 17 June 2019).
- Szczurek, A.; Birk, U.; Knecht, H.; Dobrucki, J.; Mai, S.; Cremer, C. Super-resolution binding activated localization microscopy through reversible change of DNA conformation. Nucleus 2018, 9, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Cremer, C.; Szczurek, A.; Strickfaden, H.; Birk, U.; Cremer, M.; Cremer, T. Der Zellkern - eine Stadt in der Zelle, Teil 2: Chromatin-Nanoarchitektur und Genregulation. Biol. Unserer Zeit 2018, 48, 45–53. [Google Scholar] [CrossRef]
- Hipp, L.; Beer, J.; Kuchler, O.; Reisser, M.; Sinske, D.; Michaelis, J.; Gebhardt, J.C.M.; Knöll, B. Single-molecule imaging of the transcription factor SRF reveals prolonged chromatin-binding kinetics upon cell stimulation. Proc. Natl. Acad. Sci. USA 2019, 116, 880–889. [Google Scholar] [CrossRef]
- Chen, X.; Shen, Y.; Draper, W.; Buenrostro, J.D.; Litzenburger, U.; Cho, S.W.; Satpathy, A.T.; Carter, A.C.; Ghosh, R.P.; East-Seletsky, A.; et al. ATAC-see reveals the accessible genome by transposase-mediated imaging and sequencing. Nat. Methods 2016, 13, 1013–1020. [Google Scholar] [CrossRef]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birk, U.J. Super-Resolution Microscopy of Chromatin. Genes 2019, 10, 493. https://doi.org/10.3390/genes10070493
Birk UJ. Super-Resolution Microscopy of Chromatin. Genes. 2019; 10(7):493. https://doi.org/10.3390/genes10070493
Chicago/Turabian StyleBirk, Udo J. 2019. "Super-Resolution Microscopy of Chromatin" Genes 10, no. 7: 493. https://doi.org/10.3390/genes10070493
APA StyleBirk, U. J. (2019). Super-Resolution Microscopy of Chromatin. Genes, 10(7), 493. https://doi.org/10.3390/genes10070493




