Abstract
The aminoacyl-tRNA synthetases (aaRSs) are well established as the translators of the genetic code, because their products, the aminoacyl-tRNAs, read codons to translate messenger RNAs into proteins. Consequently, deleterious errors by the aaRSs can be transferred into the proteome via misacylated tRNAs. Nevertheless, many microorganisms use an indirect pathway to produce Asn-tRNAAsn via Asp-tRNAAsn. This intermediate is produced by a non-discriminating aspartyl-tRNA synthetase (ND-AspRS) that has retained its ability to also generate Asp-tRNAAsp. Here we report the discovery that ND-AspRS and its discriminating counterpart, AspRS, are also capable of specifically producing Glu-tRNAGlu, without producing misacylated tRNAs like Glu-tRNAAsn, Glu-tRNAAsp, or Asp-tRNAGlu, thus maintaining the fidelity of the genetic code. Consequently, bacterial AspRSs have glutamyl-tRNA synthetase-like activity that does not contaminate the proteome via amino acid misincorporation.
1. Introduction
The fidelity of protein translation depends on the accurate pairing of cognate tRNAs to their cognate amino acids. The ligation of the amino acid to its tRNA is catalyzed by a highly specific group of enzymes known as the aminoacyl-tRNA synthetases (aaRSs) [1,2,3]. Under normal conditions, these enzymes maintain high accuracy and specificity in selecting their cognate amino acid and tRNA substrates. In fact, decades of research have been dedicated to demonstrating that the aaRSs are exquisitely specific for their cognate substrates and are key players in defining the accuracy of the proteome [2,4,5,6]. An increasing body of work, however, is emerging that demonstrates that some aaRSs can alter their activity or relax their selectivity in response to stress, even promoting errors in translation [7,8,9,10,11,12].
Aspartyl-tRNA synthetase (AspRS) is an exception to the rule of one aaRS per amino acid/tRNA pair. This enzyme is found in two general forms, discriminating and non-discriminating, based on divergent tRNA specificities. Discriminating AspRS, the canonical enzyme, is found in eukaryotes, and some bacteria and archaea, and catalyzes the aspartylation of tRNAAsp to produce Asp-tRNAAsp (Figure 1A) [13]. The non-discriminating form, ND-AspRS, is found in many bacteria and archaea and some organelles; this enzyme cannot differentiate between tRNAAsp and tRNAAsn and aminoacylates both with aspartate to generate Asp-tRNAAsp and the misacylated Asp-tRNAAsn, respectively (Figure 1B) [14,15,16,17]. To maintain the fidelity of the genetic code, Asp-tRNAAsn is then converted to Asn-tRNAAsn by a glutamine-dependent amidotransferase (AdT) [14,15,18,19].
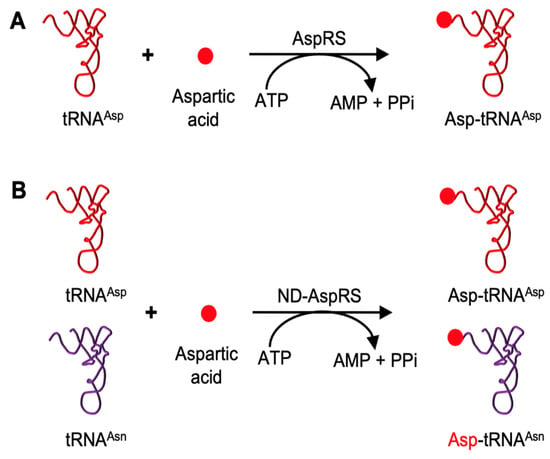
Figure 1.
Canonical roles of AspRS and ND-AspRS. (A) AspRS aminoacylates tRNAAsp with aspartic acid to produce Asp-tRNAAsp. (B) ND-AspRS catalyzes the aspartylation of both tRNAAsp and tRNAAsn to produce Asp-tRNAAsp and the misacylated Asp-tRNAAsn.
The requirement for a discriminating AspRS versus an ND-AspRS differs from organism to organism; however, ND-AspRS is always accompanied by AdT [15,18,19,20]. ND-AspRS and AdT are typically found in organisms that lack asparaginyl-tRNA synthetase (AsnRS) and/or asparagine synthetase (AsnS). For example, Pseudomonas aeruginosa and Helicobacter pylori (H. pylori or Hp) lack both AsnRS and AsnS and consequently require ND-AspRS and AdT to produce both Asn-tRNAAsn and asparagine [21,22]. Mycobacterium tuberculosis lacks AsnRS but has AsnS, so it only uses indirect tRNA aminoacylation to produce Asn-tRNAAsn [12]. In contrast, Staphylococcal aureus (S. aureus or Sa) has a functioning AsnRS but lacks AsnS; thus, indirect aminoacylation is still required as the sole biosynthetic route to asparagine [23]. Some bacteria like Thermus thermophilus and Deinococcus radiodurans encode for more than one copy of AspRS [24,25]. In these cases, the longer, bacterial-type AspRS is discriminating and catalyzes the synthesis of Asp-tRNAAsp. The second AspRS (AspRS2) is non-discriminating and produces Asp-tRNAAsn along with Asp-tRNAAsp.
While ND-AspRS clearly demonstrates relaxed tRNA substrate specificity, it remains fine-tuned as it only aspartylates tRNAAsp and tRNAAsn, only in organisms that have AdT, and it presumably discriminates against other tRNAs as potential substrates. In this way, the fidelity of the genetic code is maintained. Evidence of further relaxation in substrate specificity for either a discriminating AspRS or an ND-AspRS has not been reported to our knowledge. Here we demonstrate that these enzymes are capable of specifically producing Glu-tRNAGlu while maintaining their canonical roles: The production of Asp-tRNAAsp (AspRS and ND-AspRS) and Asp-tRNAAsn (ND-AspRS only). To the best of our knowledge, this is the first example of an aaRS capable of producing a non-cognate, but correctly aminoacylated tRNA.
2. Materials and Methods
2.1. Materials
Potassium dihydrogen phosphate (KH2PO4), sodium dihydrogen phosphate (NaH2PO4), L-aspartic acid (catalog #A93100, batch #12002LC), L-glutamic acid (catalog #G1251, lot #SLBK8671V), magnesium chloride, and oligonucleotides were purchased from Sigma-Aldrich (St. Louis, MO, USA). The radiolabeled reagents aspartic acid, L-[2,3-3H] (catalog #ART 0211, lot #180606), glutamic acid, L-[2,3,4-3H] (catalog #ART 0132, lot #180710) and adenosine triphosphate (ATP) [γ-32P] were purchased from American Radiolabeled Chemicals (St. Louis, MO, USA). Ampicillin, chloramphenicol, kanamycin, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), ethylenediaminetetraacetic acid (EDTA), isopropyl β-d-thiogalactopyranoside (IPTG), phenylmethanesulfonyl fluoride (PMSF), and tris(hydroxymethyl) aminomethane (Tris) were from GoldBio Biotechnology, Inc., (St. Louis, MO, USA). Potassium chloride (KCl), sodium chloride (NaCl), trichloroacetic acid (TCA), and ammonium acetate (NH4OAc) were purchased from Fisher Scientific (Hampton, NH, USA).
2.2. Overexpression and Purification of aaRSs
Hp ND-AspRS [22], the Hp tRNAGln-specific glutamyl-tRNA synthetase (GluRS2) [26], Mycobacterium smegmatis (M. smegmatis or Ms) ND-AspRS (vector provided by Dr. Babak Javid), and Escherichia coli (E. coli or Ec) AspRS and GluRS (vectors provided by Dr. Takuya Ueda [27]) were each overexpressed in Ec Bl21 (DE3) RIL in Luria-Bertani medium (LB, 1 L) inoculated with a saturated overnight culture grown from a single colony. The cultures were incubated at 37 °C, 200 rpm. IPTG (1 mM) was used to induce overexpression in each case. For Hp ND-AspRS and GluRS2 overexpression, the medium was supplemented with ampicillin (100 μg/mL), chloramphenicol (100 μg/mL), and glucose (0.5%). IPTG was added when the OD600 reached 0.8–1.0. Cells were collected after a 1 h induction period. For Ms ND-AspRS, the only difference was that kanamycin (25 μg/mL) was added instead of ampicillin. The two discriminating Ec enzymes were grown in the same medium as the two Hp enzymes. IPTG induction was initiated when the OD600 reached between 0.4 and 0.6 and continued for 4 h. Cells were harvested by centrifugation and frozen at −80 °C until ready for use.
The Sa ND-AspRS (vector provided by Dr. Kelly Sheppard) was overexpressed and purified as previously described [23]. In all other cases, cell pellets were suspended in lysis buffer (50 mM NaH2PO4, pH 7.4, 300 mM NaCl, 10 mM imidazole) and the cells were lysed with lysozyme (2 mg/mL) and sonication. Saturated PMSF (15 μL/mL) was added every 20 min to reduce proteolysis. Typically, a cell extract was added to a polyprep column that contained high-density cobalt agarose beads (~2 mL, Gold Biotechnology, Inc.) pre-washed with lysis buffer. The lysate was incubated with the resin by rotating at 4 °C for 1 h. The His6-tagged aaRSs were eluted according to the manufacturer’s protocol.
The eluents were dialyzed against dialysis buffer (20 mM KH2PO4, pH 7.2, 0.5 mM Na2EDTA, 5 mM β-mercaptoethanol) at 4 °C for 1–2 h and then against fresh dialysis buffer overnight. The aaRSs were adsorbed onto a HiTrapTM DEAE FF column (GE Healthcare Life Sciences, Pittsburgh, PA, USA). The proteins were eluted with a stepwise gradient of 20–300 mM KH2PO4, pH 7.2, supplemented with 0.5 mM Na2EDTA, 5 mM β-mercaptoethanol. The proteins were concentrated with 30 kDa molecular weight spin filters (EMD Millipore, Burlington, MA, USA). After this two-column purification procedure, all enzymes were judged to have been purified to near homogeneity by SDS-PAGE (Figure S1). Final protein concentrations were determined by UV-Vis spectroscopy at 280 nm, using extinction coefficients calculated using the ExPASy ProtParam tool [28]. The aaRSs were immediately used in aminoacylation assays. All experiments were conducted using biological replicates in triplicate.
2.3. In Vivo Transcription and Purification of tRNAs
Hp tRNAAsn [22] and tRNAGln [26] were overexpressed in Ec MV1184 and purified as previously described [29]. After deacylation, the tRNAs were electroeluted in a denaturing urea gel for further purification. The tRNA band was excised from the gel, crushed, and incubated overnight at 37 °C in crush and soak buffer (0.5 mM NH4OAc, pH 5.2, 1 mM Na2EDTA). The eluted tRNA sample was isopropanol precipitated. The resultant pellet was dissolved in water, folded, and quantified by aminoacylation assay, as previously described [29]. These purifications yield tRNA that is enriched with the overexpressed tRNA isoacceptor but also contains total Ec tRNA. Separately, total Ec tRNA was isolated from a saturated Ec MV1184 culture that had been grown at 37 °C in LB medium supplemented with glucose (0.5%) and purified as described [29]. The concentration of total Ec tRNA was calculated by UV-Vis spectroscopy at 260 nm assuming 1 OD = 1.6 μM.
2.4. Initial Rate Aminoacylation Assays
Each tRNA was pre-folded as previously described [29]. Aminoacylation assays were conducted in buffer containing 100 mM Na-HEPES, pH 7.5, 30 mM KCl, 12 mM MgCl2, 2 mM ATP, 0.1 mg/mL BSA, 20 μM amino acid, 50 μCi/mL 3H-labeled amino acid, and 10 μM Hp tRNAAsn or tRNAGln as noted. Assays were conducted at 37 °C and were initiated with the addition of 200 nM Hp ND-AspRS or GluRS2. Aliquots were removed and quenched on Whatman filter pads containing TCA. The pads were washed 3 times for 15 min with chilled 5% TCA. Pads were dried and counted in 3 mL ECOLITE (+) scintillation fluid (MP Biomedicals, Solon, OH, USA). Aminoacylation assays with total Ec tRNA were carried out in the same buffer containing 50–100 μM total tRNA. Hp ND-AspRS (100 nM or 500 nM) or Hp GluRS2 (100 nM or 500 nM) were used in these assays as noted.
2.5. Extended Aminoacylation Assays (90 Min)
Hp ND-AspRS and GluRS2 used in these assays were only purified by cobalt affinity purification. The aminoacylation assays were conducted at 37 °C in buffer containing 20 mM HEPES-OH, pH 7.5, 4 mM MgCl2, 2 mM ATP, 100 μM amino acid, and 25 μCi/mL 3H-labeled amino acid. All aaRSs were added to a final concentration of 1 μM and overexpressed tRNA isoacceptor was added to a final concentration of 10 μM.
2.6. Acid Gel Electrophoresis and Northern Blot Analysis
Aminoacylation reactions were conducted in the same buffer used for initial rate aminoacylation assays only without radiolabeled amino acid for 90 min at 37 °C with 500 nM aaRS. NaOAc (0.3 mM, pH 4.5) was added to each reaction and they were quenched with phenol/chloroform (1:1 v/v, pH 4.5). The tRNAs were ethanol precipitated and the resultant pellets were dissolved in buffer containing 10 mM NaOAc, pH 4.5, 1 mM Na2EDTA. Acid gel electrophoresis was used to separate deacylated tRNAs from their aminoacylated counterparts. The tRNA samples were quantified by UV-Vis spectroscopy at 260 nm, and 0.05 OD260 units were loaded per lane for overexpressed tRNAs. This amount was increased to 0.25 OD260 units for total Ec tRNA. Isoacceptor-specific oligonucleotides were used in Northern blots to visualize each tRNA (Table S1). The tRNA specific DNA probes were prepared as previously described [26]. The acid gel and Northern blot analyses were performed as previously described [26,30], with the exception that a non-specific DNA oligonucleotide (0.1 μM) was added during the first three washing steps after probe hybridization.
3. Results
3.1. H. pylori ND-AspRS Appears to Aminoacylate H. pylori tRNAGln with Aspartate and Glutamate
ND-AspRS has relaxed tRNA specificity and aspartylates both tRNAAsp and tRNAAsn to produce Asp-tRNAAsp and Asp-tRNAAsn, respectively [14,15]. We have previously demonstrated that the Hp ND-AspRS has this dual tRNA specificity, as expected [22]. Given the nature of the genetic code and decades of work characterizing the aaRSs, ND-AspRS should not demonstrate broader substrate specificity for non-cognate tRNAs or amino acids. We examined Hp ND-AspRS and GluRS2, a tRNAGln-specific GluRS [26,31], for possible cross-reactivity with overexpressed tRNAAsn and tRNAGln with both aspartate and glutamate (Figure 2). We performed these assays with longer time points to look at plateau levels of aminoacylation and to detect any low levels of aminoacylation. As expected, ND-AspRS produced Asp-tRNAAsn and did not produce Glu-tRNAAsn (Figure 2A,B, respectively). Unexpectedly, this enzyme showed aminoacylation activity in both assays with tRNAGln (Figure 2C,D), suggesting that it was producing the non-cognate product Hp Asp-tRNAGln and Glu-tRNAGln. However, because these tRNAs were overexpressed in vivo, a practice necessary to introduce required post-transcriptional modifications like queuosine [32] and 2-thiouridine [33], the tRNAs used in these experiments were contaminated with total Ec tRNA. Thus, the possibility also exists that Hp ND-AspRS is aminoacylating one or more Ec tRNAs instead. For comparison, GluRS2 showed only its expected activity, producing Glu-tRNAGln (Figure 2D), but not Asp-tRNAAsn, Glu-tRNAAsn, or Asp-tRNAGln (Figure 2A–C). We conducted these same, longer aminoacylation assays using the Ms ND-AspRS and ND-GluRS (Figure S2): We observed the same behavior with glutamate, indicating that this phenomenon is found in bacteria beyond H. pylori.
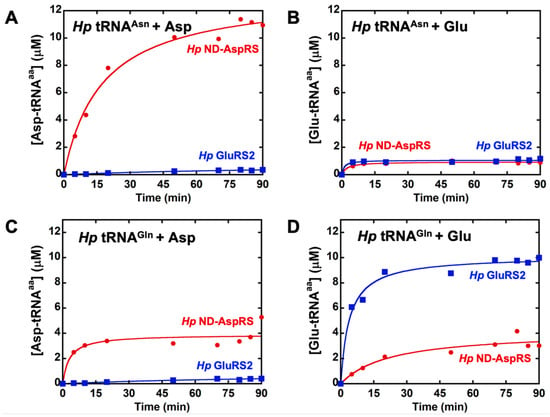
Figure 2.
H. pylori ND-AspRS appears to attach aspartate and glutamate to tRNAGln. Hp ND-AspRS (●, 1 μM) and GluRS2 (■, 1 μM) were tested in cross-aminoacylation assays using Hp tRNAAsn and tRNAGln with aspartate and glutamate. The tRNA isoacceptor concentration in each assay was 10 μM; each tRNA was contaminated with total Ec tRNA. (A) Hp tRNAAsn aminoacylated with aspartate, (B) Hp tRNAAsn aminoacylated with glutamate, (C) Hp tRNAGln aminoacylated with aspartate, and (D) Hp tRNAGln aminoacylated with glutamate.
We repeated the assays shown in Figure 2 with shorter time points and lower enzyme concentrations to look at initial rates (Figure S3). Under these more standard conditions, the ability of ND-AspRS to ligate glutamate onto either Hp tRNAGln or a contaminating Ec tRNA was masked (Figure S3D), offering an explanation for why this activity has remained undiscovered. Robust aspartylation by Hp ND-AspRS, presumably of contaminating Ec tRNAAsp and/or tRNAAsn, was observed as anticipated (Figure S3C).
Our use of extended time point assays (Figure 2) revealed an unexpected activity for ND-AspRS that was invisible under initial rate conditions (Figure S3). To our knowledge, this is the first evidence of an ND-AspRS utilizing glutamate instead of aspartate as its amino acid substrate. This activity makes some sense, however, given the close structural similarities between aspartate and glutamate. Nevertheless, the results presented in Figure 2 and Figures S2 and S3, still contained ambiguity with respect to the identity of the relevant tRNA substrate since each tRNA was overexpressed and purified with contaminating Ec tRNAs.
3.2. H. pylori ND-AspRS Aminoacylates E. coli tRNAGlu with Glutamate to Produce Glu-tRNAGlu
To more precisely examine the unexpected aminoacylation reaction(s) catalyzed by Hp ND-AspRS, we turned to acid gel electrophoresis and Northern blotting techniques [30]. We continued to use Hp GluRS2 as a control because of its high specificity for Glu-tRNAGln production [26,31]. The advantages of this approach are two-fold. First, the acidic pH of these gels enables the separation of aminoacyl-tRNAs from their deacylated counterparts due to the extra charge provided by the protonated amino terminus of the amino acid (30). Second, oligonucleotides can be designed for Northern blot visualizations that are specific for a desired tRNA isoacceptor. Thus, this approach allows us to unequivocally identify the tRNA(s) being aminoacylated in a given experiment, clearly resolving the ambiguity of the results presented above. Since Hp ND-AspRS uses glutamate as a substrate (Figure 2D), we hypothesized that it may be adding glutamate onto Hp tRNAGln or Ec tRNAGlu. Consequently, we used this methodology to examine Hp ND-AspRS-catalyzed aminoacylation of Hp tRNAGln and Ec tRNAGlu with aspartate versus glutamate as its amino acid substrates. Northern blots were conducted with overexpressed Hp tRNAGln contaminated with total Ec tRNA as a source of Ec tRNAGlu. In both cases, oligonucleotides were designed to visualize only the tRNAs of interest: Hp tRNAGln and Ec tRNAGlu.
Hp ND-AspRS does not aminoacylate Hp tRNAGln with either aspartate or glutamate (Figure 3, blot 1, lanes 2 and 3), suggesting that the activities observed above (Figure 2C,D) are the result of aminoacylation of one or more Ec tRNAs (compared to the production of Glu-tRNAGln by Hp GluRS2 in Figure 3, blot 1, lane 4.) In contrast, Hp ND-AspRS aminoacylates Ec tRNAGlu with glutamate to produce Glu-tRNAGlu (Figure 3, blot 2, lane 3), offering direct evidence that this ND-AspRS has non-canonical aminoacylation activity that goes beyond its ability to aspartylate tRNAAsp and tRNAAsn. We considered the possibility that E. coli glutamyl-tRNA synthetase (Ec GluRS) had inadvertently co-purified with Hp ND-AspRS, however, no evidence of this contamination was observed by SDS-PAGE (Figure S1). Remarkably, Hp ND-AspRS remains accurate by pairing glutamate with tRNAGlu to produce correctly acylated Glu-tRNAGlu but not the misacylated Asp-tRNAGlu (Figure 3, blot 2, lanes 3 and 2 respectively). In other words, this enzyme is exhibiting activity consistent with a canonical GluRS, in that it is ligating glutamate specifically to tRNAGlu.
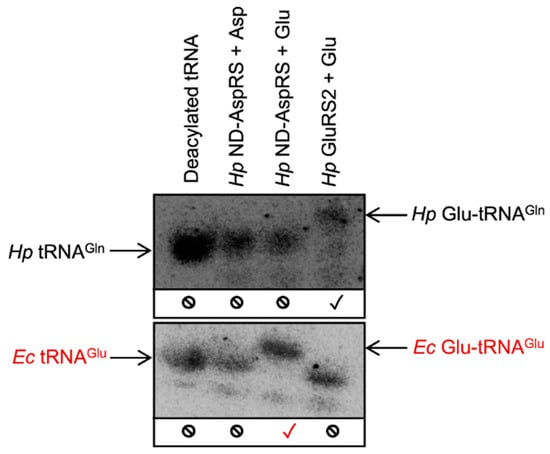
Figure 3.
H. pylori ND-AspRS aminoacylates E. coli tRNAGlu with glutamate producing Glu-tRNAGlu. Hp tRNAGln, contaminated with total Ec tRNA, was aminoacylated with either Hp ND-AspRS or GluRS2 and with aspartate versus glutamate. Aminoacylated versus deacylated tRNAs were separated in an acid gel. Specific tRNAs and aminoacyl-tRNAs were imaged using 32P-labeled oligonucleotides in Northern blots. The blots were visualized with a Hp tRNAGln-specific primer (blot 1) and an Ec tRNAGlu-specific primer (blot 2). Expected tRNA aminoacylation activity is indicated with a black check mark (✓); unexpected aminoacylation activity is indicated in red (✓); the absence of aminoacylation activity with a given tRNA is indicated with a no symbol (⦸).
Next, we compared the initial rates and extents of glutamylation of total Ec tRNA by Hp GluRS2, an enzyme that naturally uses glutamate as its substrate [26,31], and Hp ND-AspRS, which unexpectedly also uses glutamate (Figure 4 and Figure S4). Here, to ensure that we could visualize this novel activity, we increased the concentration of each enzyme when looking at aminoacylation of a non-cognate tRNA. We also verified that Ms ND-AspRS has this ability to glutamylate total Ec tRNA (Figure S5). In these experiments, the ability of ND-AspRS to produce Glu-tRNAGlu is clearly apparent.
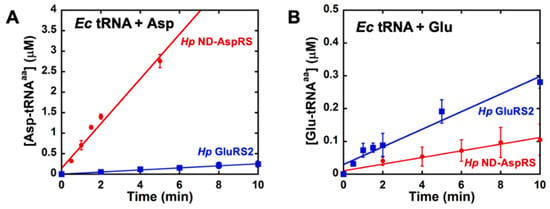
Figure 4.
H. pylori ND-AspRS aminoacylates E. coli tRNA with glutamate. Hp ND-AspRS was tested for its activity to aminoacylate Ec tRNA (50–100 μM) with aspartate and glutamate. Hp GluRS2 was also assayed for comparison. (A) Aminoacylation of Ec tRNA with aspartate by Hp ND-AspRS (●, 100 nM) versus GluRS2 (■, 500 nM). (B) Aminoacylation of Ec tRNA with glutamate by Hp ND-AspRS (●, 500 nM) versus GluRS2 (■, 100 nM). Error bars represent standard deviation from biological replicates in triplicate.
3.3. Glu-tRNAGlu Production Is Common among Bacterial Non-Discriminating and Discriminating AspRSs
All experiments described so far with Hp and Ms ND-AspRS demonstrate that these enzymes can attach glutamate to Ec tRNA and Hp ND-AspRS specifically produces Ec Glu-tRNAGlu. Given that these are inter-species pairings in both cases, we decided that it was important to examine the Ec discriminating AspRS for this activity with its homologous Ec tRNA. We also included the ND-AspRS from S. aureus to gain additional perspective into the ubiquity of this unexpected activity. Acid gels and Northern blot analyses using oligonucleotide probes that are specific for four different Ec tRNAs (tRNAGlu, tRNAGln, tRNAAsp, and tRNAAsn) were conducted with all four enzymes: The discriminating AspRS from Ec and the ND-AspRSs from Hp, Ms, and Sa (Figure 5). In many of the Northern blots shown below, the deacylated and the aminoacylated tRNAs appear as two bands, presumably due to differences in post-transcriptional modifications of the tRNAs, as previously reported [26].
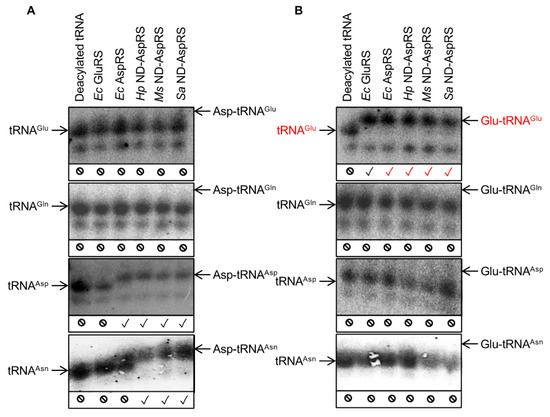
Figure 5.
Some bacterial AspRSs aminoacylate E. coli tRNAGlu with glutamate producing Glu-tRNAGlu. Total Ec tRNA was aminoacylated with (A) aspartate and (B) glutamate by Ec GluRS, Ec AspRS, Hp ND-AspRS, Ms ND-AspRS, and Sa ND-AspRS. The aminoacylated versus deacylated tRNAs were separated in acid gels. 32P-labeled oligonucleotides specific for Ec tRNAGlu, tRNAGln, tRNAAsp, and tRNAAsn were used for each blot as indicated. Expected tRNA aminoacylation activities are indicated with a black check mark (✓); unexpected activities are indicated in red (✓); the absence of aminoacylation activity with a given tRNA is indicated with a no symbol (⦸).
Figure 5 shows the Northern blot results obtained from total Ec tRNA aminoacylated with aspartate (A) and glutamate (B) by different bacterial aaRSs: Ec GluRS was used as a control. The results of panel A reveal that each AspRS attaches aspartate to tRNAAsp and the three ND-AspRSs also attach aspartate to tRNAAsn, as expected (Figure 5A, blots 3 and 4). Furthermore, none of the enzymes tested were capable of attaching significant amounts of aspartate to either non-cognate tRNA substrates tRNAGlu or tRNAGln (Figure 5A, blots 1 and 2).
The most interesting and critical results are revealed in blot 1 in Figure 5B and are highlighted with red checkmarks. This blot was probed with a 32P-labeled oligonucleotide specific for Ec tRNAGlu and Glu-tRNAGlu formation with Ec GluRS was used as a positive control. A clear shift is observed between deacylated tRNAGlu and Glu-tRNAGlu produced by Ec GluRS (Figure 5B, blot 1, lanes 1 and 2). What is remarkable is that all four AspRSs showed this same shift, clearly indicative of ubiquitous Glu-tRNAGlu production. The remaining blots in Figure 5B demonstrate that this glutamylation activity is specific for tRNAGlu as none of the enzymes tested were capable of adding glutamate onto Ec tRNAGln, tRNAAsp, or tRNAAsn. These data demonstrate that Ec AspRS and the ND-AspRSs from Hp, Ms, and Sa aminoacylate tRNAGlu with glutamate to produce Glu-tRNAGlu. These reactions represent a new, non-canonical activity for AspRS that can be viewed as that of a discriminating GluRS.
4. Discussion
In this work, we demonstrate that bacterial discriminating and non-discriminating AspRSs have quantifiable GluRS activity as they are capable of specifically generating Glu-tRNAGlu. Using total Ec tRNA, this activity was observed with four different AspRSs: The Ec discriminating AspRS and three non-discriminating AspRSs from Hp, Ms, and Sa. All four enzymes have canonical AspRS activity and produce Asp-tRNAAsp (Figure 5A, blot 3). The three ND-AspRSs also produce misacylated Asp-tRNAAsn (Figure 5A, blot 4); this intermediate would be converted to Asn-tRNAAsn in vivo by AdT. All four enzymes were also capable of specifically producing Glu-tRNAGlu (Figure 5B, blot 1) without adding glutamate to other related tRNAs.
We considered the possibility of the co-purification of contaminating Ec GluRS as an alternative explanation for this activity. However, each AspRS was purified in two steps: cobalt affinity and DEAE chromatography. Ec GluRS elutes from our DEAE column at a lower KH2PO4 concentration (~50–100 mM) than the tested AspRSs (~200–300 mM), such that any GluRS that survived the affinity purification step would have been removed by DEAE chromatography. Furthermore, no evidence of Ec GluRS contamination was visible by using SDS-PAGE gel (Figure S1). Thus, this two-step purification strategy eliminates the possibility of contaminated Ec GluRS in the AspRS samples, confirming that this activity is due to AspRS alone.
This GluRS-like activity of AspRSs is remarkable and unexpected because it is accurate and yet outside the normal purview of an AspRS. It truly is GluRS activity as each AspRS specifically attaches glutamate only to tRNAGlu producing Glu-tRNAGlu. The fact that Ec AspRS demonstrates this activity is particularly important because of the homologous nature of this result: Ec AspRS produces Ec Glu-tRNAGlu. All other results herein arose from heterologous pairings of an ND-AspRS with Ec tRNA. Our results with Ec AspRS demonstrate that this activity is not simply a result of cross-species recognition of a non-cognate tRNA. It is also remarkable that no evidence of misacylated Asp-tRNAGlu, Glu-tRNAAsp, or Glu-tRNAAsn was observed, given the ability of these AspRSs to produce Glu-tRNAGlu. This observation is important because it demonstrates that this GluRS-like activity does not threaten the fidelity of the genetic code. Nevertheless, it poses an unexpected problem with molecular recognition. How does AspRS know to specifically attach aspartate to tRNAAsp (and tRNAAsn) and glutamate to tRNAGlu without cross misacylation of these tRNAs? Our results suggest that communication between the amino acid and tRNA binding sites must occur to achieve this specificity. Further research is needed to understand this apparent tRNA-induced conformational change.
AaRSs almost always recognize discrete molecular features in their tRNA substrates, termed identity elements. These determinants can be positive and favor recognition or negative and disfavor interactions. For Ec tRNAAsp, the GUC anticodon, the G73 discriminator base, the G2:C71 base pair in the acceptor stem, C38 in the anticodon loop, and G10 in the D arm of the tRNA serve as the positive identity elements for Ec AspRS [34,35,36]. In contrast, Ec GluRS recognizes the G1:C72 and U2:A71 base pairs in the acceptor stem, A37 in the anticodon loop, U11:A24 base pair in D arm, and the U13:G22-A46 tertiary base pair in Ec tRNAGlu. Further, the lack of a nucleotide at position 47 and the modified uridine (s2U) in the UUC anticodon of tRNAGlu are also known identity determinants [33,34,37,38,39]. Ec tRNAGlu does contain several tRNAAsp identity elements: The G73 discriminator base, G10 in the D arm, and C38 in the anticodon loop. But are these shared elements enough for recognition of Ec tRNAGlu by Ec AspRS? Or is an alternative, expanded identity set recognized in such a way as to favor glutamylation of Ec tRNAGlu? To answer these questions, the identity elements in Ec tRNAGlu that allow recognition by Ec AspRS would have to be specifically evaluated.
In conclusion, we have demonstrated that bacterial AspRSs have GluRS activity and are capable of producing Glu-tRNAGlu, at least in vitro. The relevance of this reaction in vivo is very difficult to demonstrate as it is unlikely that this activity is robust enough to enable deletion of the native Ec GluRS, especially in the presence of the cognate tRNAAsp substrate. It is possible that this activity exists as a backup in case the cognate GluRS becomes damaged, for example. It is also possible that it is a remnant of early AspRS evolution. The early genetic code was likely sloppy with ancestral aaRSs capable of aminoacylating multiple tRNAs with different amino acids [5]. Evidence also suggests that Class I and II enzymes emerged in pairs [40,41], with early AspRSs and GluRSs perhaps as a co-evolving pair that recognized and acylated the same early tRNA or tRNA-like substrates. These enzymes would have recognized opposite faces of the tRNA, facilitating this co-evolution and protecting the tRNA from hydrolysis [41]. Given that the background GluRS activity exhibited by AspRS does not put the genetic code in jeopardy, it would not have been selected against as the genetic code evolved to be more selective and specific. The results presented here also suggest the possibility that other aaRSs retain similar background activities, a hypothesis that awaits further testing.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/10/4/262/s1, Methodology for the overexpression and purification of Ms ND-GluRS, in vitro transcription of tRNAs, and extending tRNA aminoacylation assays. Figure S1. SDS-PAGE gels of purified aaRSs. Figure S2. Extended M. smegmatis tRNAAsn and tRNAGln aminoacylation assays with M. smegmatis ND-AspRS and ND-GluRS with aspartate versus glutamate. Figure S3. H. pylori ND-AspRS shows unexpected aminoacylation activity with overexpressed H. pylori tRNAGln. Figure S4. Extended total E. coli tRNA aminoacylation assays with H. pylori ND-AspRS and GluRS2 with aspartate versus glutamate. Figure S5. Extended total E. coli tRNA aminoacylation assays with M. smegmatis ND-AspRS and ND-GluRS with aspartate versus glutamate. Table S1: The tRNA specific oligonucleotide sequences used in northern blot analysis
Author Contributions
Conceptualization, U.M.R. and T.L.H.; Methodology, U.M.R. and T.L.H.; Writing-Original Draft Preparation, U.M.R. and T.L.H.; Writing-Review & Editing, U.M.R. and T.L.H.; Visualization, U.M.R. and T.L.H.; Supervision, T.L.H.; Project Administration, T.L.H.; Funding Acquisition, T.L.H.
Funding
This research was supported by Wayne State University and private donors.
Acknowledgments
The authors thank Babak Javid, Takuya Ueda, and Kelly Sheppard for their kind gifts of plasmids. They also thank Whitney Wood for helpful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ribas de Pouplana, L.; Schimmel, P. A view into the origin of life: aminoacyl-tRNA synthetases. Cell. Mol. Life Sci. 2000, 57, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Ibba, M.; Soll, D. Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 2000, 69, 617–650. [Google Scholar] [CrossRef]
- Giege, R.; Springer, M. Aminoacyl-tRNA synthetases in the bacterial world. EcoSal Plus 2016, 7. [Google Scholar] [CrossRef]
- Schimmel, P.; Giege, R.; Moras, D.; Yokoyama, S. An operational RNA code for amino acids and possible relationship to genetic code. Proc. Natl. Acad. Sci. USA 1993, 90, 8763–8768. [Google Scholar] [CrossRef]
- Schimmel, P.; Ribas de Pouplana, L. Transfer RNA: From minihelix to genetic code. Cell 1995, 81, 983–986. [Google Scholar] [CrossRef]
- Kubyshkin, V.; Acevedo-Rocha, C.G.; Budisa, N. On universal coding events in protein biogenesis. Biosystems 2018, 164, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Mohler, K.; Ibba, M. Translational fidelity and mistranslation in the cellular response to stress. Nat. Microbiol. 2017, 2, 17117. [Google Scholar] [CrossRef]
- Ribas de Pouplana, L.; Santos, M.A.; Zhu, J.H.; Farabaugh, P.J.; Javid, B. Protein mistranslation: Friend or foe? Trends Biochem. Sci. 2014, 39, 355–362. [Google Scholar] [CrossRef]
- Reynolds, N.M.; Lazazzera, B.A.; Ibba, M. Cellular mechanisms that control mistranslation. Nat. Rev. Microbiol. 2010, 8, 849–856. [Google Scholar] [CrossRef]
- Wiltrout, E.; Goodenbour, J.M.; Frechin, M.; Pan, T. Misacylation of tRNA with methionine in Saccharomyces cerevisiae. Nucleic Acids Res. 2012, 40, 10494–10506. [Google Scholar] [CrossRef]
- Schwartz, M.H.; Pan, T. Determining the fidelity of tRNA aminoacylation via microarrays. Methods 2017, 113, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Javid, B.; Sorrentino, F.; Toosky, M.; Zheng, W.; Pinkham, J.T.; Jain, N.; Pan, M.; Deighan, P.; Rubin, E.J. Mycobacterial mistranslation is necessary and sufficient for rifampicin phenotypic resistance. Proc. Natl. Acad. Sci. USA 2014, 111, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Woese, C.R.; Olsen, G.J.; Ibba, M.; Soll, D. Aminoacyl-tRNA synthetases, the genetic code, and the evolutionary process. MMBR 2000, 64, 202–236. [Google Scholar] [CrossRef] [PubMed]
- Curnow, A.W.; Ibba, M.; Soll, D. tRNA-dependent asparagine formation. Nature 1996, 382, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.D.; Kern, D. Thermus thermophilus: A link in evolution of the tRNA-dependent amino acid amidation pathways. Proc. Natl. Acad. Sci. USA 1998, 95, 12832–12837. [Google Scholar] [CrossRef]
- Ibba, M.; Soll, D. Aminoacyl-tRNAs: Setting the limits of the genetic code. Genes Dev. 2004, 18, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Schon, A.; Kannangara, C.G.; Gough, S.; Soll, D. Protein biosynthesis in organelles requires misaminoacylation of tRNA. Nature 1988, 331, 187–190. [Google Scholar] [CrossRef]
- Curnow, A.W.; Hong, K.; Yuan, R.; Kim, S.; Martins, O.; Winkler, W.; Henkin, T.M.; Soll, D. Glu-tRNAGln amidotransferase: A novel heterotrimeric enzyme required for correct decoding of glutamine codons during translation. Proc. Natl. Acad. Sci. USA 1997, 94, 11819–11826. [Google Scholar] [CrossRef]
- Rathnayake, U.M.; Wood, W.N.; Hendrickson, T.L. Indirect tRNA aminoacylation during accurate translation and phenotypic mistranslation. Curr. Opin. Chem. Biol. 2017, 41, 114–122. [Google Scholar] [CrossRef]
- Tumbula, D.L.; Becker, H.D.; Chang, W.Z.; Soll, D. Domain-specific recruitment of amide amino acids for protein synthesis. Nature 2000, 407, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Akochy, P.M.; Bernard, D.; Roy, P.H.; Lapointe, J. Direct glutaminyl-tRNA biosynthesis and indirect asparaginyl-tRNA biosynthesis in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2004, 186, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Chuawong, P.; Hendrickson, T.L. The nondiscriminating aspartyl-tRNA synthetase from Helicobacter pylori: Anticodon-binding domain mutations that impact tRNA specificity and heterologous toxicity. Biochemistry 2006, 45, 8079–8087. [Google Scholar] [CrossRef]
- Mladenova, S.R.; Stein, K.R.; Bartlett, L.; Sheppard, K. Relaxed tRNA specificity of the Staphylococcus aureus aspartyl-tRNA synthetase enables RNA-dependent asparagine biosynthesis. FEBS Lett. 2014, 588, 1808–1812. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.D.; Reinbolt, J.; Kreutzer, R.; Giege, R.; Kern, D. Existence of two distinct aspartyl-tRNA synthetases in Thermus thermophilus. Structural and biochemical properties of the two enzymes. Biochemistry 1997, 36, 8785–8797. [Google Scholar] [CrossRef]
- Min, B.; Pelaschier, J.T.; Graham, D.E.; Tumbula-Hansen, D.; Soll, D. Transfer RNA-dependent amino acid biosynthesis: an essential route to asparagine formation. Proc. Natl. Acad. Sci. USA 2002, 99, 2678–2683. [Google Scholar] [CrossRef]
- Skouloubris, S.; Ribas de Pouplana, L.; De Reuse, H.; Hendrickson, T.L. A noncognate aminoacyl-tRNA synthetase that may resolve a missing link in protein evolution. Proc. Natl. Acad. Sci. USA 2003, 100, 11297–11302. [Google Scholar] [CrossRef]
- Shimizu, Y.; Inoue, A.; Tomari, Y.; Suzuki, T.; Yokogawa, T.; Nishikawa, K.; Ueda, T. Cell-free translation reconstituted with purified components. Nat. Biotechnol. 2001, 19, 751–755. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar]
- Zhao, L.; Rathnayake, U.M.; Dewage, S.W.; Wood, W.N.; Veltri, A.J.; Cisneros, G.A.; Hendrickson, T.L. Characterization of tunnel mutants reveals a catalytic step in ammonia delivery by an aminoacyl-tRNA amidotransferase. FEBS Lett. 2016, 590, 3122–3132. [Google Scholar] [CrossRef]
- Varshney, U.; Lee, C.P.; RajBhandary, U.L. Direct analysis of aminoacylation levels of tRNAs in vivo. Application to studying recognition of Escherichia coli initiator tRNA mutants by glutaminyl-tRNA synthetase. J. Biol. Chem. 1991, 266, 24712–24718. [Google Scholar]
- Salazar, J.C.; Ahel, I.; Orellana, O.; Tumbula-Hansen, D.; Krieger, R.; Daniels, L.; Soll, D. Coevolution of an aminoacyl-tRNA synthetase with its tRNA substrates. Proc. Natl. Acad. Sci. USA 2003, 100, 13863–13868. [Google Scholar] [CrossRef]
- Kasai, H.; Oashi, Z.; Harada, F.; Nishimura, S.; Oppenheimer, N.J.; Crain, P.F.; Liehr, J.G.; von Minden, D.L.; McCloskey, J.A. Structure of the modified nucleoside Q isolated from Escherichia coli transfer ribonucleic acid. 7-(4,5-cis-Dihydroxy-1-cyclopenten-3-ylaminomethyl)-7-deazaguanosine. Biochemistry 1975, 14, 4198–4208. [Google Scholar] [CrossRef]
- Sylvers, L.A.; Rogers, K.C.; Shimizu, M.; Ohtsuka, E.; Soll, D. A 2-thiouridine derivative in tRNAGlu is a positive determinant for aminoacylation by Escherichia coli glutamyl-tRNA synthetase. Biochemistry 1993, 32, 3836–3841. [Google Scholar] [CrossRef]
- Giege, R.; Sissler, M.; Florentz, C. Universal rules and idiosyncratic features in tRNA identity. Nucleic Acids Res. 1998, 26, 5017–5035. [Google Scholar] [CrossRef]
- Hasegawa, T.; Himeno, H.; Ishikura, H.; Shimizu, M. Discriminator base of tRNAAsp is involved in amino acid acceptor activity. Biochem. Biophys. Res. Commun. 1989, 163, 1534–1538. [Google Scholar] [CrossRef]
- Nameki, N.; Tamura, K.; Himeno, H.; Asahara, H.; Hasegawa, T.; Shimizu, M. Escherichia coli tRNAAsp recognition mechanism differing from that of the yeast system. Biochem. Biophys. Res. Commun. 1992, 189, 856–862. [Google Scholar] [CrossRef]
- Gregory, S.T.; Dahlberg, A.E. Effects of mutations at position 36 of tRNAGlu on missense and nonsense suppression in Escherichia coli. FEBS Lett. 1995, 361, 25–28. [Google Scholar] [CrossRef]
- Sekine, S.; Nureki, O.; Sakamoto, K.; Niimi, T.; Tateno, M.; Go, M.; Kohno, T.; Brisson, A.; Lapointe, J.; Yokoyama, S. Major identity determinants in the “augmented D helix” of tRNAGlu from Escherichia coli. J. Mol. Biol. 1996, 256, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Sekine, S.; Nureki, O.; Tateno, M.; Yokoyama, S. The identity determinants required for the discrimination between tRNAGlu and tRNAAsp by glutamyl-tRNA synthetase from Escherichia coli. Eur. J. Biochem. 1999, 261, 354–360. [Google Scholar] [CrossRef]
- Rodin, S.N.; Ohno, S. Four primordial modes of tRNA-synthetase recognition, determined by the (G,C) operational code. Proc. Natl. Acad. Sci. USA 1997, 94, 5183–5188. [Google Scholar] [CrossRef]
- Ribas de Pouplana, L.; Schimmel, P. Two classes of tRNA synthetases suggested by sterically compatible dockings on tRNA acceptor stem. Cell 2001, 104, 191–193. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).