Insights into HP1a-Chromatin Interactions
Abstract
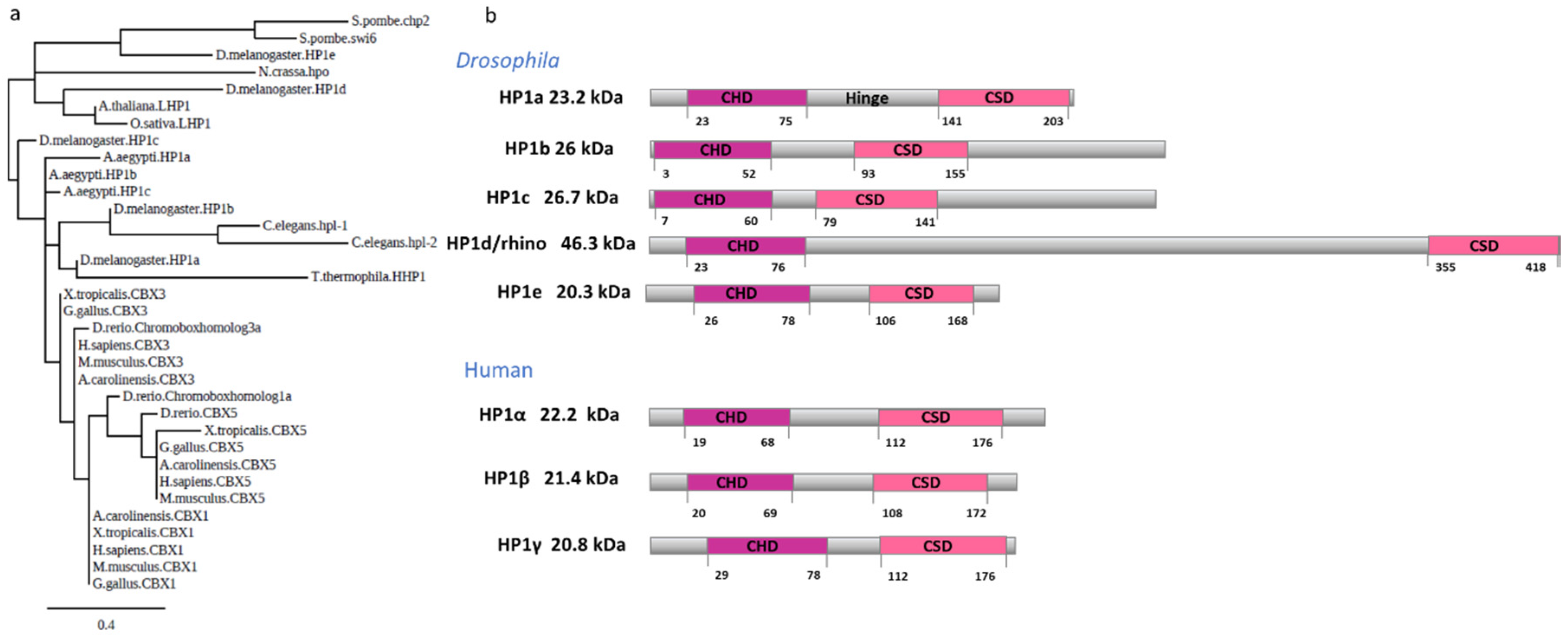
1. Introduction
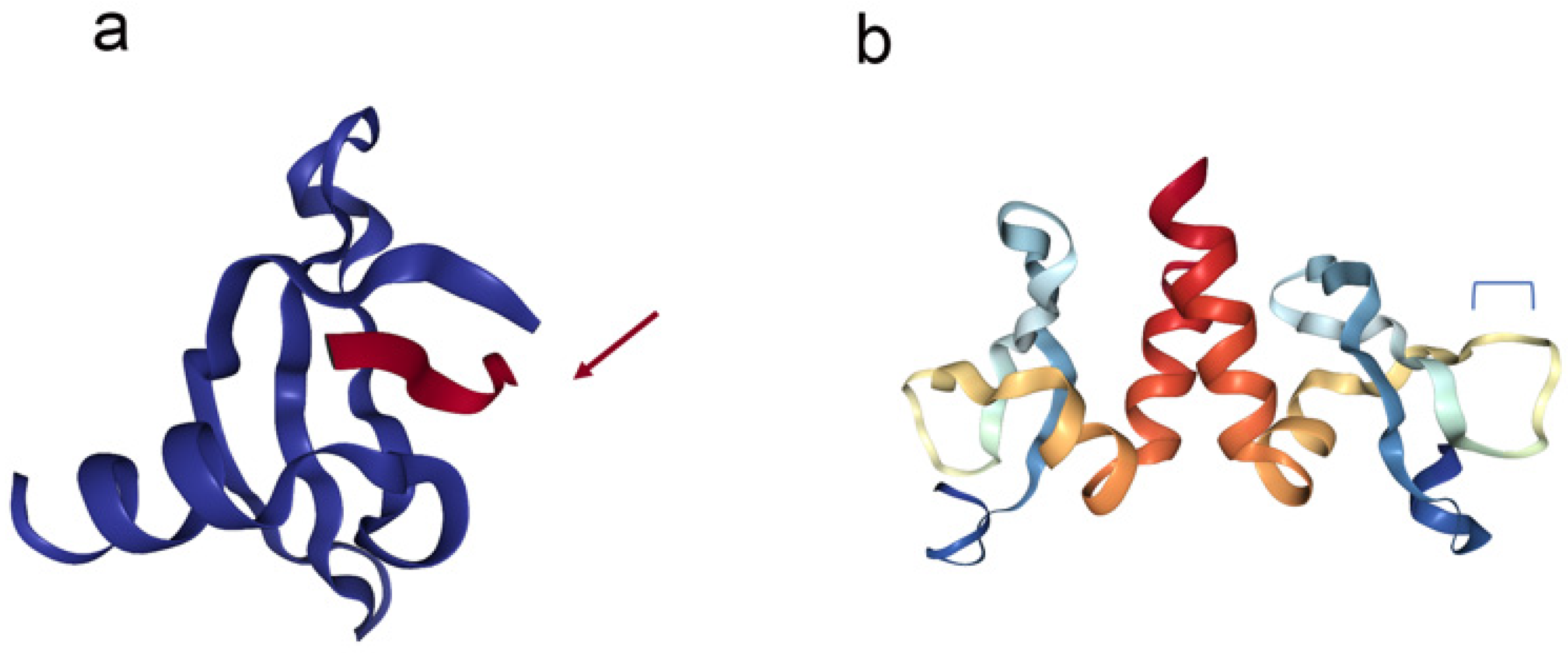
2. Functions of Conserved HP1a Domains
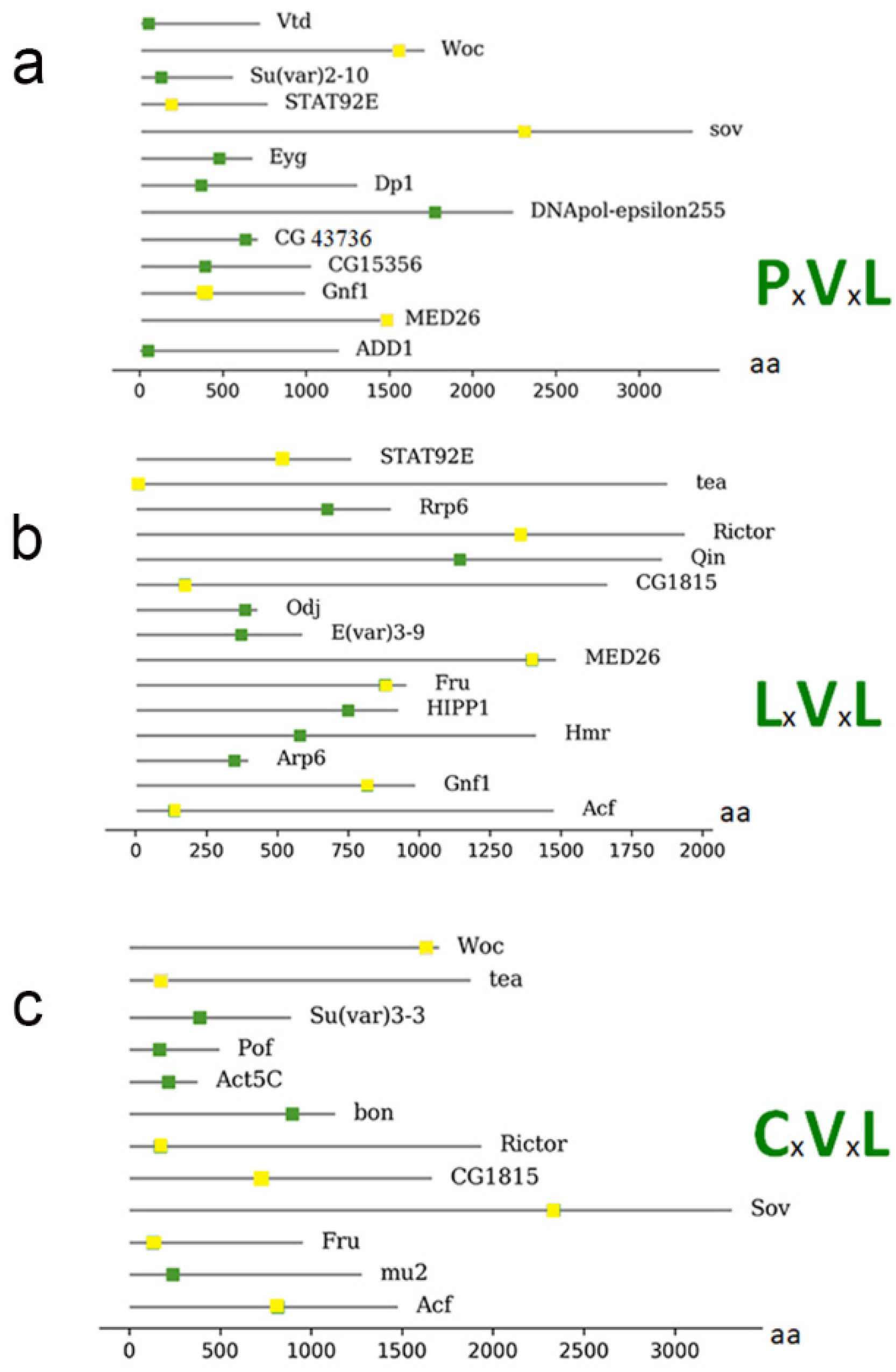
3. HP1a Conserved Domains Direct Specific Protein Interactions
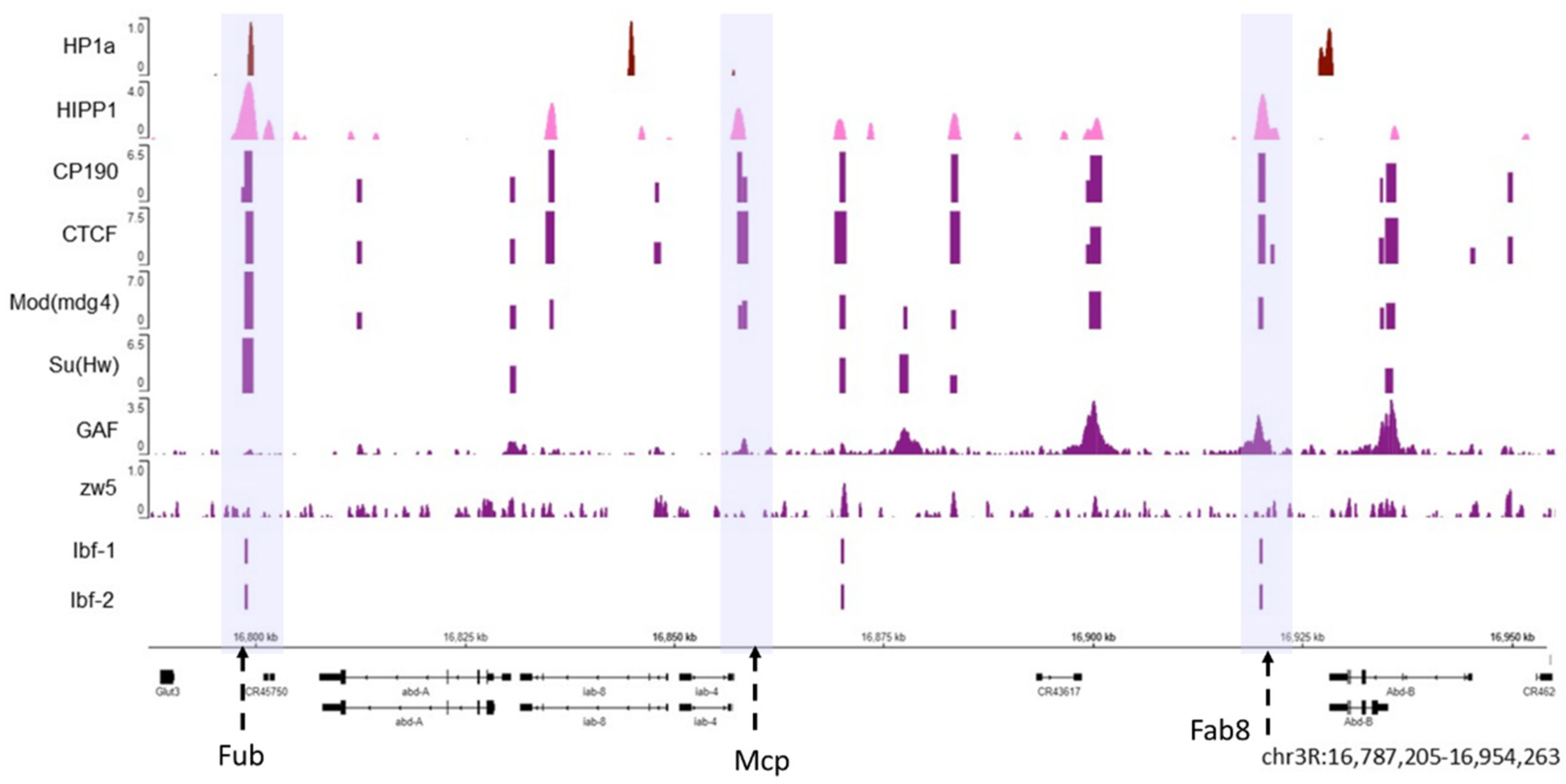
4. HP1a Interaction with Insulator and Architectural Proteins
5. HP1a Interaction Partners in Silenced Chromatin
6. HP1a Interaction Partners in Euchromatin
7. Future Directions
8. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Felsenfeld, G.; Groudine, M. Controlling the double helix. Nature 2003, 42, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Luger, K.; Dechassa, M.L.; Tremethick, D.J. New insights into nucleosome and chromatin structure: An ordered state or a disordered affair? Nat. Rev. Mol. Cell Biol. 2012, 13, 436–447. [Google Scholar] [CrossRef]
- Brueckner, L.; van Arensbergen, J.; Akhtar, W.; Pagie, L.; van Steensel, B. High-throughput assessment of context-dependent effects of chromatin proteins. Epigenetics Chromatin. 2016, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Luger, K.; Mader, A.W.; Richmond, R.K.; Sargent, D.F.; Richmond, T.J. Crystal structure of the nucleosome resolution core particle at 2.8 A. Nature 1997, 389, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Bayona-Feliu, A.; Casas-Lamesa, A.; Reina, O.; Bernués, J.; Azorín, F. Linker histone H1 prevents R-loop accumulation and genome instability in heterochromatin. Nat. Commun. 2017, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Richards, E.J.; Elgin, S.C.R. Epigenetic codes for heterochromatin formation and silencing: Rounding up the usual suspects. Cell 2002, 108, 489–500. [Google Scholar] [CrossRef]
- Piacentini, L.; Fanti, L.; Negri, R.; Del Vescovo, V.; Fatica, A.; Altieri, F.; Pimpinelli, S. Heterochromatin Protein 1 (HP1a) positively regulates euchromatic gene expression through RNA transcript association and interaction with hnRNPs in Drosophila. PLoS Genet. 2009, 5, e1000670. [Google Scholar] [CrossRef]
- Passarge, E. Emil Heitz and the concept of heterochromatin: Longitudinal chromosome differentiation was recognized fifty years ago. Am. J. Hum. Genet. 1979, 31, 106–115. [Google Scholar]
- Dillon, N. Heterochromatin structure and function. Biol. Cell 2004, 96, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Britten, R.J.; Kohne, D.E. Repeated sequences in DNA. Science 1968, 161, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Fodor, B.D.; Shukeir, N.; Reuter, G.; Jenuwein, T. Mammalian Su (var) genes in chromatin control. Annu. Rev. Cell Dev. Biol. 2010, 26, 471–501. [Google Scholar] [CrossRef] [PubMed]
- Guthmann, M.; Burton, A.; Torres-Padilla, M. Expression and phase separation potential of heterochromatin proteins during early mouse development. EMBO Rep. 2019, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nishibuchi, G.; Déjardin, J. The molecular basis of the organization of repetitive DNA-containing constitutive heterochromatin in mammals. Chromosom. Res. 2017, 25, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Fanti, L.; Pimpinelli, S. HP1: A functionally multifaceted protein. Curr. Opin. Genet. Dev. 2008, 18, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Pindyurin, A.V.; Ilyin, A.A.; Ivankin, A.V.; Tselebrovsky, M.V.; Nenasheva, V.V.; Mikhaleva, E.A.; Pagie, L.; van Steensel, B.; Shevelyov, Y.Y. The large fraction of heterochromatin in Drosophila neurons is bound by both B-type lamin and HP1a. Epigenetics Chromatin. 2018, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Riddle, N.C.; Minoda, A.; Kharchenko, P.V.; Alekseyenko, A.A.; Schwartz, Y.B.; Tolstorukov, M.Y.; Gorchakov, A.A.; Jaffe, J.D.; Kennedy, C.; Linder-Basso, D.; et al. Plasticity in patterns of histone modifications and chromosomal proteins in Drosophila heterochromatin. Genome Res. 2011, 21, 147–163. [Google Scholar] [CrossRef]
- Marshall, O.J.; Brand, A.H. Chromatin state changes during neural development revealed by in vivo cell-type specific profiling. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Grewal, S. Heterochromatin and epigenetic control of gene expression. Science 2003, 301, 798–802. [Google Scholar] [CrossRef]
- Wang, X.; Moazed, D. DNA sequence-dependent epigenetic inheritance of gene silencing and histone H3K9 methylation. Science 2017, 91, 88–91. [Google Scholar] [CrossRef]
- Riddle, N.C.; Jung, Y.L.; Gu, T.; Alekseyenko, A.A.; Asker, D.; Gui, H.; Kharchenko, P.V.; Minoda, A.; Plachetka, A.; Schwartz, Y.B.; et al. Enrichment of HP1a on drosophila chromosome 4 genes creates an alternate chromatin structure critical for regulation in this heterochromatic domain. PLoS Genet. 2012, 8, e1002954. [Google Scholar] [CrossRef]
- Muller, H.J.; Altenburg, E. The frequency of translocations produced by X-rays in Drosophila. Genetics 1930, 15, 283–311. [Google Scholar] [PubMed]
- James, T.C.; Elgin, S.C. Identification of a nonhistone chromosomal protein associated with heterochromatin in Drosophila melanogaster and its gene. Mol. Cell. Biol. 1986, 6, 3862–3872. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, D.A.R.; Mottus, R.C.; Grigliatti, T.A. Genes which suppress position-effect variegation in Drosophila melanogaster are clustered. MGG Mol. Gen. Genet. 1983, 191, 326–333. [Google Scholar] [CrossRef]
- Lorentz, A.; Ostermann, K.; Fleck, O.; Schmidt, H. Switching gene swi6, involved in repression of silent mating-type loci in fission yeast, encodes a homologue of chromatin-associated proteins from Drosophila and mammals. Gene 1994, 143, 139–143. [Google Scholar] [CrossRef]
- Thon, G.; Verhein-hansen, J. Four chromo-domain proteins of schizosaccharomyces pombe differentially repress transcription at various chromosomal locations. Genetics 2000, 155, 551–568. [Google Scholar] [PubMed]
- Meehan, R.R.; Kao, C.F.; Pennings, S. HP1 binding to native chromatin in vitro is determined by the hinge region and not by the chromodomain. EMBO J. 2003, 22, 3164–3174. [Google Scholar] [CrossRef] [PubMed]
- Istomina, N.E.; Shushanov, S.S.; Springhetti, E.M.; Karpov, V.L.; Krasheninnikov, I.A.; Stevens, K.; Zaret, K.S.; Singh, P.B.; Grigoryev, S.A. Insulation of the chicken beta-globin chromosomal domain from a chromatin-condensing protein, MENT. Mol. Cell. Biol. 2003, 23, 6455–6468. [Google Scholar] [CrossRef]
- Wreggett, K.A.; Hill, F.; James, P.S.; Hutchings, A.; Butcher, G.W.; Singh, P.B. A mammalian homologue of Drosophila heterochromatin protein 1 (HP1) is a component of constitutive heterochromatin. Cytogenet. Cell Genet. 1994, 66, 99–103. [Google Scholar] [CrossRef]
- Cowell, I.G.; Aucott, R.; Mahadevaiah, S.K.; Burgoyne, P.S.; Huskisson, N.; Bongiorni, S.; Prantera, G.; Fanti, L.; Pimpinelli, S.; Wu, R.; et al. Heterochromatin, HP1 and methylation at lysine 9 of histone H3 in animals. Chromosoma 2002, 111, 22–36. [Google Scholar] [CrossRef]
- Pak, D.T.; Pflumm, M.; Chesnokov, I.; Huang, D.W.; Kellum, R.; Marr, J.; Romanowski, P.; Botchan, M.R. Association of the origin recognition complex with heterochromatin and HP1 in higher eukaryotes. Cell 1997, 91, 311–323. [Google Scholar] [CrossRef]
- Polioudaki, H.; Kourmouli, N.; Drosou, V.; Bakou, A.; Theodoropoulos, P.A.; Singh, P.B.; Giannakouros, T.; Georgatos, S.D. Histones H3/H4 form a tight complex with the inner nuclear membrane protein LBR and heterochromatin protein 1. EMBO Rep. 2001, 2, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Raffa, G.D.; Ciapponi, L.; Cenci, G.; Gatti, M. Terminin: A protein complex that mediates epigenetic maintenance of Drosophila telomeres. Nucleus 2011, 2, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Brasher, S.V.; Smith, B.O.; Fogh, R.H.; Nietlispach, D.; Thiru, A.; Nielsen, P.R.; Broadhurst, R.W.; Ball, L.J.; Murzina, N.V.; Laue, E.D. The structure of mouse HP1 suggests a unique mode of single peptide recognition by the shadow chromo domain dimer. EMBO J. 2000, 19, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Hendzel, M.J.; Wei, Y.; Mancini, M.A.; Van Hooser, A.; Ranalli, T.; Brinkley, B.R.; Bazett-Jones, D.P.; Allis, C.D. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 1997, 106, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Hirota, T.; Lipp, J.J.; Toh, B.H.; Peters, J.M. Histone H3 serine 10 phosphorylation by Aurora B causes HP1 dissociation from heterochromatin. Nature 2005, 438, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.A.; Khorasanizadeh, S. Structure of HP1 chromodomain bound to a lysine 9-methylated histone H3 tail. Science 2002, 295, 2080–2083. [Google Scholar] [CrossRef]
- Daujat, S.; Zeissler, U.; Waldmann, T.; Happel, N.; Schneider, R. HP1 binds specifically to Lys26-methylated histone H1.4, whereas simultaneous Ser27 phosphorylation blocks HP1 binding. J. Biol. Chem. 2005, 280, 38090–38095. [Google Scholar] [CrossRef]
- Eissenberg, J.C.; James, T.C.; Foster-Hartnett, D.M.; Hartnett, T.; Ngan, V.; Elgin, S.C. Mutation in a heterochromatin-specific chromosomal protein is associated with suppression of position-effect variegation in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1990, 87, 9923–9927. [Google Scholar] [CrossRef]
- Penke, T.J.R.; McKay, D.J.; Strahl, B.D.; Matera, A.G.; Duronio, R.J. Direct interrogation of the role of H3K9 in metazoan heterochromatin function. Genes Dev. 2016, 30, 1866–1880. [Google Scholar] [CrossRef]
- Penke, T.; McKay, D.J.; Strahl, B.D.; Matera, A.G.; Duronio, R.J. Functional redundancy of variant and canonical histone H3 lysine 9 modification in drosophila. Genetics 2018, 208, 229–244. [Google Scholar] [CrossRef]
- Fanti, L.; Giovinazzo, G.; Berloco, M.; Pimpinelli, S. The heterochromatin protein 1 prevents telomere fusions in Drosophila. Mol. Cell 1998, 2, 527–538. [Google Scholar] [CrossRef]
- Mendez, D.L.; Kim, D.; Chruszcz, M.; Stephens, G.E.; Minor, W.; Khorasanizadeh, S.; Elgin, S.C. The HP1a disordered C terminus and chromo shadow domain cooperate to select target peptide partners. ChemBioChem 2011, 12, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.S.; Bradley, A.R.; Valasatava, Y.; Duarte, J.M.; Prlic, A.; Rose, P.W. NGL viewer: Web-based molecular graphics for large complexes. Bioinformatics 2018, 34, 3755–3758. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Workman, J.L. HP1c casts light on dark matter. Cell Cycle 2011, 10, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Azzaz, A.M.; Vitalini, M.W.; Thomas, A.S.; Price, J.P.; Blacketer, M.J.; Cryderman, D.E.; Zirbel, L.N.; Woodcock, C.L.; Elcock, A.H.; Wallrath, L.L.; et al. Human heterochromatin protein 1α promotes nucleosome associations that drive chromatin condensation. J. Biol. Chem. 2014, 289, 6850–6861. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.J.; Schneider, R.; Bauer, U.M.; Bannister, A.J.; Morrison, A.; O’Carroll, D.; Firestein, R.; Cleary, M.; Jenuwein, T.; Herrera, R.E.; et al. Rb targets histone H3 methylation and HP1 to promoters. Nature 2001, 412, 561–565. [Google Scholar] [CrossRef]
- Smothers, J.F.; Henikoff, S. The hinge and chromo shadow domain impart distinct targeting of HP1-like proteins. Mol. Cell. Biol. 2001, 21, 2555–2569. [Google Scholar] [CrossRef]
- Cowieson, N.P.; Partridge, J.F.; Allshire, R.C.; McLaughlin, P.J. Dimerisation of a chromo shadow domain and distinctions from the chromodomain as revealed by structural analysis. Curr. Biol. 2000, 10, 517–525. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sonoda, M. Self-interaction of heterochromatin protein 1 is required for direct binding to histone methyltransferase, SUV39H1. Biochem. Biophys. Res. Commun. 2003, 301, 287–292. [Google Scholar] [CrossRef]
- Lechner, M.S.; Begg, G.E.; Speicher, D.W.; Rauscher, F.J. Molecular determinants for targeting heterochromatin protein 1-mediated gene silencing: Direct chromoshadow domain-KAP-1 corepressor interaction is essential. Mol. Cell. Biol. 2000, 20, 6449–6465. [Google Scholar] [CrossRef]
- Jacobs, S.A.; Taverna, S.D.; Zhang, Y.; Briggs, S.D.; Li, J.; Eissenberg, J.C.; Allis, C.D.; Khorasanizadeh, S. Specificity of the HP1 chromo domain for the methylated N-terminus of histone H3. EMBO J. 2001, 20, 5232–5241. [Google Scholar] [CrossRef] [PubMed]
- Verni, F.; Cenci, G. The drosophila histone variant H2A.V works in concert with HP1 to promote kinetochore-driven microtubule formation. Cell Cycle 2015, 14, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Ryu, H.W.; Kim, G.W.; Kwon, S.H. Comparison of three heterochromatin protein 1 homologs in Drosophila. J. Cell Sci. 2019, 132, jcs222729. [Google Scholar] [CrossRef] [PubMed]
- Kourmouli, N.; Theodoropoulos, P.A.; Dialynas, G.; Bakou, A.; Politou, A.S.; Cowell, I.G.; Singh, P.B.; Georgatos, S.D. Dynamic associations of heterochromatin protein 1 with the nuclear envelope. EMBO J. 2000, 19, 6558–6568. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.L.; Oulad-Abdelghani, M.; Ortiz, J.A.; Remboutsika, E.; Chambon, P.; Losson, R. Heterochromatin formation in mammalian cells: Interaction between histones and HP1 Proteins. Mol. Cell 2001, 7, 729–739. [Google Scholar] [CrossRef]
- Lachner, M.; O’Carroll, D.; Rea, S.; Mechtler, K.; Jenuwein, T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 2001, 410, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Bannister, A.J.; Zegerman, P.; Partridge, J.F.; Miska, E.A.; Thomas, J.O.; Allshire, R.C.; Kouzarides, T. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 2001, 410, 120–124. [Google Scholar] [CrossRef]
- Rohr, O.; Lecestre, D.; Chasserot-Golaz, S.; Marban, C.; Avram, D.; Aunis, D.; Leid, M.; Schaeffer, E. Recruitment of tat to heterochromatin protein HP1 via interaction with CTIP2 inhibits human immunodeficiency virus type 1 replication in microglial cells. J. Virol. 2003, 77, 5415–5427. [Google Scholar] [CrossRef]
- Marban, C.; Redel, L.; Suzanne, S.; Van Lint, C.; Lecestre, D.; Chasserot-Golaz, S.; Leid, M.; Aunis, D.; Schaeffer, E.; Rohr, O. COUP-TF interacting protein 2 represses the initial phase of HIV-1 gene transcription in human microglial cells. Nucleic Acids Res. 2005, 33, 2318–2331. [Google Scholar] [CrossRef]
- Smallwood, A.; Estève, P.O.; Pradhan, S.; Carey, M. Functional cooperation between HP1 and DNMT1 mediates gene silencing. Genes Dev. 2007, 21, 1169–1178. [Google Scholar] [CrossRef]
- Schwendemann, A.; Matkovic, T.; Linke, C.; Klebes, A.; Hofmann, A.; Korge, G. Hip, an HP1-interacting protein, is a haplo- and triplo-suppressor of position effect variegation. Proc. Natl. Acad. Sci. USA 2008, 105, 204–209. [Google Scholar] [CrossRef]
- Hines, K.A.; Cryderman, D.E.; Flannery, K.M.; Yang, H.; Vitalini, M.W.; Hazelrigg, T.; Mizzen, C.A.; Wallrath, L.L. Domains of heterochromatin protein 1 required for drosophila melanogaster heterochromatin spreading. Genetics 2009, 182, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Linder, B.; Gerlach, N.; Jäckle, H. The Drosophila homolog of the human AF10 is an HP1-interacting suppressor of position effect variegation. EMBO Rep. 2001, 2, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Cléard, F.; Delattre, M.; Spierer, P. SU(VAR)3-7, a Drosophila heterochromatin-associated protein and companion of HP1 in the genomic silencing of position-effect variegation. EMBO J. 1997, 16, 5280–5288. [Google Scholar] [CrossRef] [PubMed]
- Delattre, M.; Spierer, A.; Tonka, C.H.; Spierer, P. The genomic silencing of position-effect variegation in Drosophila melanogaster: Interaction between the heterochromatin-associated proteins Su(var)3-7 and HP1. J. Cell Sci. 2000, 113, 4253–4261. [Google Scholar] [PubMed]
- Yin, H.; Lin, H. An epigenetic activation role of Piwi and a Piwi-associated piRNA in Drosophila melanogaster. Nature 2007, 450, 304–308. [Google Scholar] [CrossRef]
- Brower-Toland, B.; Findley, S.D.; Jiang, L.; Liu, L.; Yin, H.; Dus, M.; Zhou, P.; Elgin, S.C.; Lin, H. Drosophila PIWI associates with chromatin and interacts directly with HP1a. Genes Dev. 2007, 21, 2300–2311. [Google Scholar] [CrossRef] [PubMed]
- Alekseyenko, A.A.; Gorchakov, A.A.; Zee, B.M.; Fuchs, S.M.; Kharchenko, P.V.; Kuroda, M.I. Heterochromatin-associated interactions of Drosophila HP1a with dADD1, HIPP1, and repetitive RNAs. Genes Dev. 2014, 28, 1445–1460. [Google Scholar] [CrossRef]
- Lin, C.H.; Li, B.; Swanson, S.; Zhang, Y.; Florens, L.; Washburn, M.P.; Abmayr, S.M.; Workman, J.L. Heterochromatin Protein 1a stimulates histone H3 Lysine 36 demethylation by the drosophila KDM4A demethylase. Mol. Cell 2008, 32, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Swenson, J.M.; Colmenares, S.U.; Strom, A.R.; Costes, S.V.; Karpen, G.H. The composition and organization of Drosophila heterochromatin are heterogeneous and dynamic. eLife 2016, 5, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Florens, L.; Swanson, S.K.; Washburn, M.P.; Abmayr, S.M.; Workman, J.L. Heterochromatin protein 1 (HP1) connects the FACT histone chaperone complex to the phosphorylated CTD of RNA polymerase II. Genes Dev. 2010, 24, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- Schotta, G.; Ebert, A.; Krauss, V.; Fischer, A.; Hoffmann, J.; Rea, S.; Jenuwein, T.; Dorn, R.; Reuter, G. Central role of Drosophila SU(VAR)3-9 in histone H3-K9 methylation and heterochromatic gene silencing. EMBO J. 2002, 21, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Sato, K.; Koganezawa, M.; Ote, M.; Matsumoto, K.; Hama, C.; Yamamoto, D. Fruitless recruits two antagonistic chromatin factors to establish single-neuron sexual dimorphism. Cell 2012, 149, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, C.D.; Stephens, G.E.; Thompson, B.A.; Funches, L.; Bernat, J.A.; Craig, C.A.; Elgin, S.C. Heterochromatin protein 2 (HP2), a partner of HP1 in Drosophila heterochromatin. Proc. Natl. Acad. Sci. USA 2002, 99, 14332–14337. [Google Scholar] [CrossRef] [PubMed]
- Mendez, D.L.; Mandt, R.E.; Elgin, S.C. Heterochromatin protein 1a (HP1a) partner specificity is determined by critical amino acids in the chromo shadow domain and C-terminal extension. J. Biol. Chem. 2013, 288, 22315–22323. [Google Scholar] [CrossRef] [PubMed]
- Bassett, A.R.; Cooper, S.E.; Ragab, A.; Travers, A.A. The chromatin remodelling factor dATRX is involved in heterochromatin formation. PLoS ONE 2008, 3, e2099. [Google Scholar] [CrossRef]
- Emelyanov, A.V.; Konev, A.Y.; Vershilova, E.; Fyodorov, D.V. Protein complex of Drosophila ATRX/XNP and HP1a is required for the formation of pericentric beta-heterochromatin in vivo. J. Biol. Chem. 2010, 285, 15027–15037. [Google Scholar] [CrossRef]
- López-Falcón, B.; Meyer-Nava, S.; Hernández-Rodríguez, B.; Campos, A.; Montero, D.; Rudiño, E.; Vázquez, M.; Zurita, M.; Valadez-Graham, V. Characterization of the Drosophila group ortholog to the amino-terminus of the alpha-thalassemia and mental retardation X-linked (ATRX) vertebrate protein. PLoS ONE 2014, 9, e113182. [Google Scholar] [CrossRef]
- Joppich, C.; Scholz, S.; Korge, G.; Schwendemann, A. Umbrea, a chromo shadow domain protein in Drosophila melanogaster heterochromatin, interacts with Hip, HP1 and HOAP. Chromosom. Res. 2009, 17, 19–36. [Google Scholar] [CrossRef][Green Version]
- Yang, F.; Quan, Z.; Huang, H.; He, M.; Liu, X.; Cai, T.; Xi, R. Ovaries absent links dLsd1 to HP1a for local H3K4 demethylation required for heterochromatic gene silencing. eLife 2019, 8, 1–21. [Google Scholar] [CrossRef]
- Le Douarin, B.; Nielsen, A.L.; Garnier, J.M.; Ichinose, H.; Jeanmougin, F.; Losson, R.; Chambon, P. A possible involvement of TIF1 alpha and TIF1 beta in the epigenetic control of transcription by nuclear receptors. EMBO J. 1996, 15, 6701–6715. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.L.; Ortiz, J.A.; You, J.; Oulad-Abdelghani, M.; Khechumian, R.; Gansmuller, A.; Chambon, P.; Losson, R. Interaction with members of the heterochromatin protein 1 (HP1) family and histone deacetylation are differentially involved in transcriptional silencing by members of the TIF1 family. EMBO J. 1999, 18, 6385–6395. [Google Scholar] [CrossRef] [PubMed]
- Murzina, N.; Verreault, A.; Laue, E.; Stillman, B. Heterochromatin dynamics in mouse cells: Interaction between chromatin assembly factor 1 and HP1 proteins. Mol. Cell 1999, 4, 529–540. [Google Scholar] [CrossRef]
- Agarwal, N.; Hardt, T.; Brero, A.; Nowak, D.; Rothbauer, U.; Becker, A.; Leonhardt, H.; Cardoso, M.C. MeCP2 interacts with HP1 and modulates its heterochromatin association during myogenic differentiation. Nucleic Acids Res. 2007, 35, 5402–5408. [Google Scholar] [CrossRef]
- Lechner, M.S.; Schultz, D.C.; Negorev, D.; Maul, G.G.; Rauscher, F.J. The mammalian heterochromatin protein 1 binds diverse nuclear proteins through a common motif that targets the chromoshadow domain. Biochem. Biophys. Res. Commun. 2005, 331, 929–937. [Google Scholar] [CrossRef]
- Seeler, J.S.; Marchio, A.; Sitterlin, D.; Transy, C.; Dejean, A. Interaction of SP100 with HP1 proteins: A link between the promyelocytic leukemia-associated nuclear bodies and the chromatin compartment. Proc. Natl. Acad. Sci. USA 1998, 95, 7316–7321. [Google Scholar] [CrossRef]
- McDowell, T.L.; Gibbons, R.J.; Sutherland, H.; O’Rourke, D.M.; Bickmore, W.A.; Pombo, A.; Turley, H.; Gatter, K.; Picketts, D.J.; Buckle, V.; et al. Localization of a putative transcriptional regulator (ATRX) at pericentromeric heterochromatin and the short arms of acrocentric chromosomes. Proc. Natl. Acad. Sci. USA 1999, 96, 13983–13988. [Google Scholar] [CrossRef]
- Song, K.; Jung, Y.; Jung, D.; Lee, I. Human Ku70 Interacts with Heterochromatin Protein 1a. J. Biol. Chem. 2001, 276, 8321–8327. [Google Scholar] [CrossRef]
- Vassallo, M.F.; Tanese, N. Isoform-specific interaction of HP1 with human TAFII130. Proc. Natl. Acad. Sci. USA 2002, 99, 5919–5924. [Google Scholar] [CrossRef]
- Scholzen, T.; Endl, E.; Wohlenberg, C.; van der Sar, S.; Cowell, I.G.; Gerdes, J.; Singh, P.B. The Ki-67 protein interacts with members of the heterochromatin protein 1 (HP1) family: A potential role in the regulation of higher-order chromatin structure. J. Pathol. 2002, 196, 135–144. [Google Scholar] [CrossRef]
- Nielsen, P.R.; Nietlispach, D.; Mott, H.R.; Callaghan, J.; Bannister, A.; Kouzarides, T.; Murzin, A.G.; Murzina, N.V.; Laue, E.D. Structure of the HP1 chromodomain bound to histone H3 methylated at lysine 9. Nature 2002, 416, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, R.S.; Nagao, K.; Masuda, H.T.; Iwasaki, O.; Hirota, T.; Nozaki, N.; Kimura, H.; Obuse, C. Human POGZ modulates dissociation of HP1α from mitotic chromosome arms through Aurora, B. activation. Nat. Cell Biol. 2010, 12, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Worman, H.J. Interaction between an integral protein of the nuclear envelope inner membrane and human. J. Biol. Chem. 1996, 271, 14653–14656. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Chaudhary, J.; Dong, H.; Kim, S.; Brautigam, C.A.; Yu, H. Mitotic centromeric targeting of HP1 and its binding to Sgo1 are dispensable for sister-chromatid cohesion in human cells. Mol. Biol. Cell 2011, 22, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Nishikawa, H.; Fukuda, T.; Vittal, V.; Asano, M.; Miyoshi, Y.; Klevit, R.E.; Ohta, T. Interaction of BARD1 and HP1 is required for BRCA1 retention at sites of DNA damage. Cancer Res. 2015, 75, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Borgel, J.; Tyl, M.; Schiller, K.; Pusztai, Z.; Dooley, C.M.; Deng, W.; Wooding, C.; White, R.J.; Warnecke, T.; Leonhardt, H.; et al. KDM2A integrates DNA and histone modification signals through a CXXC/PHD module and direct interaction with HP1. Nucleic Acids Res. 2017, 45, 1114–1129. [Google Scholar] [CrossRef]
- Akram, S.; Yang, F.; Li, J.; Adams, G.; Liu, Y.; Zhuang, X.; Chu, L.; Liu, X.; Emmett, N.; Thompson, W.; et al. LRIF1 interacts with HP1α to coordinate accurate chromosome segregation during mitosis. J. Mol. Cell Biol. 2018, 10, 527–538. [Google Scholar] [CrossRef]
- Yi, Q.; Chen, Q.; Liang, C.; Yan, H.; Zhang, Z.; Xiang, X.; Zhang, M.; Qi, F.; Zhou, L.; Wang, F. HP1 links centromeric heterochromatin to centromere cohesion in mammals. EMBO Rep. 2018, 19, e45484. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Shin, Y.; Lee, S.; Kim, M.Y.; Punj, V.; Shin, H.I.; Kim, K.; Koh, J.M.; Jeong, D.; An, W. MacroH2A1.2 inhibits prostate cancer-induced osteoclastogenesis through cooperation with HP1α and H1.2. Oncogene 2018, 37, 5749–5765. [Google Scholar] [CrossRef]
- Zhang, C.L.; McKinsey, T.A.; Olson, E.N. Association of class II histone deacetylases with heterochromatin protein 1: Potential role for histone methylation in control of muscle differentiation. Mol. Cell. Biol. 2002, 22, 7302–7312. [Google Scholar] [CrossRef]
- Vo, N.; Suong, D.N.A.; Yoshino, N.; Yoshida, H.; Cotterill, S.; Yamaguchi, M. Novel roles of HP1a and Mcm10 in DNA replication, genome maintenance and photoreceptor cell differentiation. Nucleic Acids Res. 2017, 45, 1233–1254. [Google Scholar] [CrossRef] [PubMed]
- Apger, J.; Reubens, M.; Henderson, L.; Gouge, C.A.; Ilic, N.; Zhou, H.H.; Christensen, T.W. Multiple functions for drosophila Mcm10 suggested through analysis of two mcm10 mutant alleles. Genetics 2010, 185, 1151–1165. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roelens, B.; Clémot, M.; Leroux-Coyau, M.; Klapholz, B.; Dostatni, N. Maintenance of heterochromatin by the large subunit of the CAF-1 replication-coupled histone chaperone requires its interaction with HP1a through a conserved motif. Genetics 2017, 205, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Cenci, G.; Ciapponi, L.; Marzullo, M.; Raffa, G.D.; Morciano, P.; Raimondo, D.; Burla, R.; Saggio, I.; Gatti, M. The Analysis of Pendolino (peo) mutants reveals differences in the fusigenic potential among drosophila telomeres. PLoS Genet. 2015, 11, e1005260. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vedelek, B.; Blastyák, A.; Boros, I.M. Cross-species interaction between rapidly evolving telomere-specific drosophila proteins. PLoS ONE 2015, 10, e0=142771. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gao, G.; Walser, J.C.; Beaucher, M.L.; Morciano, P.; Wesolowska, N.; Chen, J.; Rong, Y.S. HipHop interacts with HOAP and HP1 to protect Drosophila telomeres in a sequence-independent manner. EMBO J. 2010, 29, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Raffa, G.D.; Siriaco, G.; Cugusi, S.; Ciapponi, L.; Cenci, G.; Wojcik, E.; Gatti, M. The Drosophila modigliani (moi) gene encodes a HOAP-interacting protein required for telomere protection. Proc. Natl. Acad. Sci. USA 2009, 106, 7–12. [Google Scholar] [CrossRef]
- Quénet, D.; Gasser, V.; Fouillen, L.; Cammas, F.; Sanglier-Cianferani, S.; Losson, R.; Dantzer, F. The histone subcode: Poly(ADP-ribose) polymerase-1 (Parp-1) and Parp-2 control cell differentiation by regulating the transcriptional intermediary factor TIF1 and the heterochromatin protein HP1. FASEB J. 2008, 22, 3853–3865. [Google Scholar] [CrossRef]
- Lin, C.; Li, C.; Huang, P.; Lee, F.S. A developmentally regulated ARF-like 5 protein (ARL5), localized to nuclei and nucleoli, interacts with heterochromatin protein 1. J. Cell Sci. 2002, 115, 4433–4445. [Google Scholar] [CrossRef]
- Ainsztein, A.M.; Kandels-Lewis, S.E.; Mackay, A.M.; Earnshaw, W.C. INCENP centromere and spindle targeting: Identification of essential conserved motifs and involvement of heterochromatin protein HP1. J. Cell Biol. 1998, 143, 1763–1774. [Google Scholar] [CrossRef]
- Ye, Q.; Callebaut, I.; Pezhman, A.; Courvalin, J.-C.; Worman, H.J. Domain-specific interactions of human HP1-type chromosomain proteins and inner nuclear membrane protein LBR. J. Biol. Chem. 1997, 271, 14983–14989. [Google Scholar] [CrossRef] [PubMed]
- Lomberk, G.; Wallrath, L.L.; Urrutia, R. The heterochromatin protein 1 family. Genome Biol. 2006, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.; Adaixo, R.; Stunnenberg, R.; Woolcock, K.J.; Hiller, S.; Bühler, M. HP1 Swi6 mediates the recognition and destruction of heterochromatic RNA transcripts. Mol. Cell 2012, 47, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ma, A.; Chow, C.M.; Horsley, D.; Brown, N.R.; Cowell, I.G.; Singh, P.B. Conservation of heterochromatin protein 1 function. Mol. Cell. Biol. 2000, 20, 6970–6983. [Google Scholar] [CrossRef] [PubMed]
- Shareef, M.M.; King, C.; Damaj, M.; Badagu, R.; Huang, D.W.; Kellum, R. Drosophila Heterochromatin Protein 1 (HP1)/Origin Recognition Complex (ORC) Protein is associated with HP1 and ORC and functions in heterochromatin- induced silencing. Mol. Biol. Cell 2001, 12, 1671–1685. [Google Scholar] [CrossRef] [PubMed]
- Badugu, R.K.; Yoo, Y.; Singh, P.B.; Kellum, R. Mutations in the heterochromatin protein 1 (HP1) hinge domain affect HP1 protein interactions and chromosomal distribution. Chromosoma 2005, 113, 370–384. [Google Scholar] [CrossRef]
- Zhao, T.; Heyduk, T.; Eissenberg, J.C. Phosphorylation site mutations in heterochromatin Protein 1 (HP1) reduce or eliminate silencing activity. J. Biol. Chem. 2001, 276, 9512–9518. [Google Scholar] [CrossRef]
- Gaudin, V.; Libault, M.; Pouteau, S.; Juul, T.; Zhao, G.; Lefebvre, D.; Grandjean, O. Mutations in LIKE HETEROCHROMATIN PROTEIN 1 affect flowering time and plant architecture in Arabidopsis. Development 2001, 128, 4847–4858. [Google Scholar]
- Bawa-Khalfe, T.; Lu, L.S.; Zuo, Y.; Huang, C.; Dere, R.; Lin, F.M.; Yeh, E.T. Differential expression of SUMO-specific protease 7 variants regulates epithelial-mesenchymal transition. Proc. Natl. Acad. Sci. USA 2012, 109, 17466–17471. [Google Scholar] [CrossRef]
- Machida, S.; Takizawa, Y.; Ishimaru, M.; Sugita, Y.; Sekine, S.; Nakayama, J.I.; Wolf, M.; Kurumizaka, H. Structural basis of heterochromatin formation by human HP1. Mol. Cell 2018, 69, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Saunders, W.S.; Chue, C.; Goebl, M.; Craig, C.; Clark, R.F.; Powers, J.A.; Eissenberg, J.C.; Elgin, S.C.; Rothfield, N.F.; Earnshaw, W.C. Molecular cloning of a human homologue of Drosophila heterochromatin protein HP1 using anti-centromere autoantibodies with anti-chromo specificity. J. Cell Sci. 1993, 104, 573–582. [Google Scholar] [PubMed]
- Vermaak, D.; Henikoff, S.; Malik, H.S. Positive selection drives the evolution of rhino, a member of the heterochromatin protein 1 family in drosophila. PLoS Genet. 2005, 1, e10009. [Google Scholar] [CrossRef] [PubMed]
- Klattenhoff, C.; Xi, H.; Li, C.; Lee, S.; Xu, J.; Khurana, J.S.; Zhang, F.; Schultz, N.; Koppetsch, B.S.; Nowosielska, A.; et al. The Drosophila HP1 homolog rhino is required for transposon silencing and piRNA production by dual-strand clusters. Cell 2009, 138, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Fanti, L.; Berloco, M.; Piacentini, L.; Pimpinelli, S. Chromosomal distribution of heterochromatin protein 1 (HP1) in drosophila: A cytological map of euchromatic HP1 binding sites. Genetica 2003, 117, 135–147. [Google Scholar] [CrossRef]
- Font-Burgada, J.; Rossell, D.; Auer, H.; Azorín, F. Drosophila HP1c isoform interacts with the zinc-finger proteins WOC and Relative-of-WOC to regulate gene expression. Genes Dev. 2008, 22, 3007–3023. [Google Scholar] [CrossRef] [PubMed]
- Vermaak, D.; Malik, H.S. Multiple roles for heterochromatin protein 1 genes in drosophila. Annu. Rev. Genet. 2009, 43, 467–492. [Google Scholar] [CrossRef]
- Li, Y.; Kirschmann, D.A.; Wallrath, L.L. Does heterochromatin protein 1 always follow code? Proc. Natl. Acad. Sci. USA 2002, 99, 16462–16469. [Google Scholar] [CrossRef]
- Larson, A.G.; Elnatan, D.; Keenen, M.M.; Trnka, M.J.; Johnston, J.B.; Burlingame, A.L.; Agard, D.A.; Redding, S.; Narlikar, G.J. Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature 2017, 547, 236–240. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, D.; Sun, F. Drosophila melanogaster heterochromatin protein HP1b plays important roles in transcriptional activation and development. Chromosoma 2011, 120, 97–108. [Google Scholar] [CrossRef]
- Abel, J.; Eskeland, R.; Raffa, G.D.; Kremmer, E. Imhof. Drosophila HP1c is regulated by an auto-regulatory feedback loop through its binding partner Woc. PLoS ONE 2009, 4, e5089. [Google Scholar] [CrossRef]
- Frankel, S.; Sigel, E.A.; Craig, C.; Elgin, S.C.R.; Mooseker, M.S.; Tsakonas, S.A. An actin-related protein in Drosophila colocalizes with heterochromatin protein 1 in pericentric heterochromatin. J. Cell Sci. 1997, 110, 1999–2012. [Google Scholar] [PubMed]
- Kato, M.; Sasaki, M.; Mizuno, S.; Harata, M. Novel actin-related proteins in vertebrates: Similarities of structure and expression pattern to Arp6 localized on Drosophila heterochromatin. Gene 2001, 268, 133–140. [Google Scholar] [CrossRef]
- Stephens, G.E.; Xiao, H.; Lankenau, D.H.; Wu, C.; Elgin, S.C.R. Heterochromatin protein 2 interacts with Nap-1 and NURF: A link between heterochromatin-induced gene silencing and the chromatin remodeling machinery in Drosophila. Biochemistry 2006, 45, 14990–14999. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, T.; Yonezawa, M.; Lein, S.; Heidrich, K.; Kubicek, S.; Schäfer, C.; Phalke, S.; Walther, M.; Schmidt, A.; Jenuwein, T.; et al. Heterochromatin formation in drosophila is initiated through active removal of H3K4. Mol. Cell. 2007, 2, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.M.; Stenberg, P.; Pettersson, F.; Larsson, J. POF and HP1 bind expressed exons, suggesting a balancing mechanism for gene regulation. PLoS Genet. 2007, 3, e30209. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, T.-Y.; Lee, C.-H.; Chan, L.-W.; Shen, C.-K.J. Epigenetic regulation of the Drosophila chromosome 4 by the histone H3K9 methyltransferase dSETDB1. Proc. Natl. Acad. Sci. USA 2007, 104, 12691–12696. [Google Scholar] [CrossRef]
- Przewloka, M.R.; Zhang, W.; Costa, P.; Archambault, V.; D’Avino, P.P.; Lilley, K.S.; Laue, E.D.; McAinsh, A.D.; Glover, D.M. Molecular analysis of core kinetochore composition and assembly in drosophila melanogaster. PLoS ONE 2007, 2, e478. [Google Scholar] [CrossRef]
- Guruharsha, K.G.; Rual, J.F.; Zhai, B.; Mintseris, J.; Vaidya, P.; Vaidya, N.; Beekman, C.; Wong, C.; Rhee, D.Y.; Cenaj, O.; et al. A protein complex network of drosophila melanogaster. Cell 2011, 147, 690–703. [Google Scholar] [CrossRef]
- Gracheva, E.; Dus, M.; Elgin, S.C.R. Drosophila RISC component VIG and its homolog Vig2 impact heterochromatin formation. PLoS ONE 2009, 4, e6182. [Google Scholar] [CrossRef][Green Version]
- Raffa, G.D.; Raimondo, D.; Sorino, C.; Cugusi, S.; Cenci, G.; Cacchione, S.; Gatti, M.; Ciapponi, L. Verrocchio, a Drosophila OB fold-containing protein, is a component of the terminin telomere-capping complex. Genes Dev. 2010, 24, 1596–1601. [Google Scholar] [CrossRef]
- Seong, K.; Li, D.; Shimizu, H.; Nakamura, R.; Ishii, S. Inheritance of stress-induced, ATF-2-dependent epigenetic change. Cell 2011, 145, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Kai, T. The tudor domain protein Kumo is required to assemble the nuage and to generate germline piRNAs in Drosophila. EMBO J. 2011, 31, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Dronamraju, R.; Mason, J.M. MU2 and HP1a regulate the recognition of double strand breaks in Drosophila melanogaster. PLoS ONE 2011, 6, e25439. [Google Scholar] [CrossRef] [PubMed]
- Chiolo, I.; Minoda, A.; Colmenares, S.U.; Polyzos, A.; Costes, S.V.; Karpen, G.H. Double-strand breaks in heterochromatin move outside of a dynamic HP1a domain to complete recombinational repair. Cell 2011, 144, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Qurashi, A.; Poidevin, M.; Nelson, D.L.; Li, H.; Jin, P. Retrotransposon activation contributes to fragile X premutation rCGG-mediated neurodegeneration. Hum. Mol. Genet. 2012, 21, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Salvany, L.; Requena, D.; Azpiazu, N. Functional association between eyegone and HP1a mediates wingless transcriptional repression during development. Mol. Cell. Biol. 2012, 32, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Kavi, H.; Emelyanov, A.V.; Fyodorov, D.V.; Skoultchi, A.I. Independent biological and biochemical functions for individual structural domains of drosophila linker histone H1. J. Biol. Chem. 2016, 291, 15143–15155. [Google Scholar] [CrossRef]
- Lu, X.; Wontakal, S.N.; Kavi, H.; Kim, B.J.; Guzzardo, P.M.; Emelyanov, A.V.; Xu, N.; Hannon, G.J.; Zavadil, J.; Fyodorov, D.V.; et al. Drosophila H1 regulates the genetic activity od heterochromatin by recruitment of su(var)3-9. Science 2013, 340, 78–81. [Google Scholar] [CrossRef]
- Thomae, A.W.; Schade, G.O.; Padeken, J.; Borath, M.; Vetter, I.; Kremmer, E.; Heun, P.; Imhof, A. Article A pair of centromeric proteins mediates reproductive isolation in drosophila species. Dev. Cell 2013, 27, 412–424. [Google Scholar] [CrossRef]
- Satyaki, P.R.; Cuykendall, T.N.; Wei, K.H.; Brideau, N.J.; Kwak, H.; Aruna, S.; Ferree, P.M.; Ji, S.; Barbash, D.A. The Hmr and Lhr hybrid incompatibility genes suppress a broad range of heterochromatic repeats. PLoS Genet. 2014, 10, e1004240. [Google Scholar] [CrossRef][Green Version]
- Brideau, N.J.; Barbash, D.A. Functional conservation of the Drosophila hybrid incompatibility gene Lhr. BMC E Biol. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Emelyanov, A.V.; Fyodorov, D.V.; Skoultchi, A.I. Drosophila linker histone H1 coordinates STAT-dependent organization of heterochromatin and suppresses tumorigenesis caused by hyperactive JAK-STAT signaling. Epigenetics Chromatin. 2014, 7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shi, S.; Larson, K.; Guo, D.; Lim, S.J.; Dutta, P.; Yan, S.J.; Li, W.X. Drosophila STAT is required for directly maintaining HP1 localization and heterochromatin stability. Nat. Cell Biol. 2008, 10, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Marr, S.K.; Lis, J.T.; Treisman, J.E.; Ii, T.M. Viability and is found at both active genes and pericentric heterochromatin in drosophila melanogaster. Mol. Cell. Biol. 2014, 34, 2710–2720. [Google Scholar] [CrossRef] [PubMed]
- Messina, G.; Damia, E.; Fanti, L.; Atterrato, M.T.; Celauro, E.; Mariotti, F.R.; Accardo, M.C.; Walther, M.; Vernì, F.; Picchioni, D.; et al. Yeti, an essential Drosophila melanogaster gene, encodes a protein required for chromatin organization. J. Cell Sci. 2014, 127, 2577–2588. [Google Scholar] [CrossRef] [PubMed]
- van Bemmel, J.G.; Filion, G.J.; Rosado, A.; Talhout, W.; de Haas, M.; van Welsem, T.; van Leeuwen, F.; van Steensel, B. Resource a network model of the molecular organization of chromatin in drosophila. Mol. Cell 2013, 49, 759–771. [Google Scholar] [CrossRef]
- Shokri, L.; Inukai, S.; Hafner, A.; Weinand, K.; Hens, K.; Vedenko, A.; Gisselbrecht, S.S.; Dainese, R.; Bischof, J.; Furger, E.; et al. A comprehensive drosophila melanogaster transcription factor interactome. Cell Rep. 2019, 27, 955–970.e7. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D. HP1a/KDM4A is involved in the autoregulatory loop of the oncogene gene c-Jun. Epigenetics 2015, 10, 453–459. [Google Scholar] [CrossRef][Green Version]
- Eberle, A.B.; Jordán-Pla, A.; Gañez-Zapater, A.; Hessle, V.; Silberberg, G.; von Euler, A.; Silverstein, R.A.; Visa, N. An interaction between RRP6 and SU(VAR)3-9 Targets RRP6 to heterochromatin and contributes to heterochromatin maintenance in drosophila melanogaster. PLoS Genet. 2015, 11, e1005523. [Google Scholar] [CrossRef]
- Cabrera, J.R.; Olcese, U.; Horabin, J.I. A balancing act: Heterochromatin protein 1a and the polycomb group coordinate their levels to silence chromatin in Drosophila. Epigenetics Chromatin. 2015, 8, 1–21. [Google Scholar] [CrossRef]
- Cipressa, F.; Morciano, P.; Bosso, G.; Mannini, L.; Galati, A.; Raffa, G.D.; Cacchione, S.; Musio, A.; Cenci, G. A role for Separase in telomere protection. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Vinayagam, A.; Kulkarni, M.M.; Sopko, R.; Sun, X.; Hu, Y.; Nand, A.; Villalta, C.; Moghimi, A.; Yang, X.; Mohr, S.E.; et al. An integrative analysis of the InR/PI3K/Akt network identifies the dynamic response to insulin signaling. Cell Rep. 2016, 16, 3062–3074. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Tahara, E.; Kanke, M.; Kuwata, K.; Nishiyama, T. Drosophila dalmatian combines sororin and shugoshin roles in establishment and protection of cohesion. EMBO J. 2017, 36, 1513–1527. [Google Scholar] [CrossRef] [PubMed]
- Bischof, J.; Duffraisse, M.; Furger, E.; Ajuria, L.; Giraud, G.; Vanderperre, S.; Paul, R.; Björklund, M.; Ahr, D.; Ahmed, A.W.; et al. Generation of a versatile BiFC ORFeome library for analyzing protein—Protein interactions in live Drosophila. eLife 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Jankovics, F.; Bence, M.; Sinka, R.; Faragó, A.; Bodai, L.; Pettkó-Szandtner, A.; Ibrahim, K.; Takács, Z.; Szarka-Kovács, A.B.; Erdélyi, M. Drosophila small ovary gene is required for transposon silencing and heterochromatin organization, and ensures germline stem cell maintenance and differentiation. Development 2018, 145, 23. [Google Scholar] [CrossRef]
- Thiru, A.; Nietlispach, D.; Mott, H.R.; Okuwaki, M.; Lyon, D.; Nielsen, P.R.; Hirshberg, M.; Verreault, A.; Murzina, N.V.; Laue, E.D. Structural basis of HP1/PXVXL motif peptide interactions and HP1 localisation to heterochromatin. EMBO J. 2004, 23, 489–499. [Google Scholar] [CrossRef]
- Lomberk, G.; Bensi, D.; Fernandez-Zapico, M.E.; Urrutia, R. Evidence for the existence of an HP1-mediated subcode within the histone code. Nat. Cell Biol. 2006, 8, 407–415. [Google Scholar] [CrossRef]
- Stephens, G.E.; Slawson, E.E.; Craig, C.A.; Elgin, S.C. Interaction of Heterochromatin Protein 2 with HP1 Defines a Novel HP1-Binding. Biochemistry 2005, 44, 13394–13403. [Google Scholar] [CrossRef]
- Eustermann, S.; Yang, J.C.; Law, M.J.; Amos, R.; Chapman, L.M.; Jelinska, C.; Garrick, D.; Clynes, D.; Gibbons, R.J.; Rhodes, D.; et al. Combinatorial readout of histone H3 modifications specifies localization of ATRX to heterochromatin. Nat. Struct. Mol. Biol. 2011, 18, 777–782. [Google Scholar] [CrossRef]
- van Bortle, K.; Corces, V.G. Nuclear organization and genome function. Annu. Rev. Cell Dev. Biol. 2012, 28, 163–187. [Google Scholar] [CrossRef]
- Beishline, K.; Vladimirova, O.; Tutton, S.; Wang, Z.; Deng, Z.; Lieberman, P.M. CTCF driven TERRA transcription facilitates completion of telomere DNA replication. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lis, J.T.; Simon, J.A.; Sutton, C.A. New heat shock puffs and β-galactosidase activity resulting from transformation of Drosophila with an hsp70-lacZ hybrid gene. Cell 1983, 35, 403–410. [Google Scholar] [CrossRef]
- Kellum, R.; Schedl, P. A position-effect assay for boundaries of higher order chromosomal domains. Cell 1991, 64, 941–950. [Google Scholar] [CrossRef]
- Udvardy, A.; Maine, E.; Schedl, P. The 87A7 chromomere. Identification of novel chromatin structures flanking the heat shock locus that may define the boundaries of higher order domains. J. Mol. Biol. 1985, 185, 341–358. [Google Scholar] [CrossRef]
- Li, Q.; Starnatoyannopoulos, G. Hypersensitive site 5 of the human B Locus control region functions as a chromatin insulator. Blood 1994, 84, 1399–1401. [Google Scholar] [CrossRef]
- Filippova, G.N.; Fagerlie, S.; Klenova, E.M.; Myers, C.; Dehner, Y.; Goodwin, G.; Neiman, P.E.; Collins, S.J.; Lobanenkov, V.V. An exceptionally conserved transcriptional repressor, CTCF, employs different combinations of zinc fingers to bind diverged promoter sequences of avian and mammalian c-myc oncogenes. Mol. Cell. Biol. 1996, 16, 2802–2813. [Google Scholar] [CrossRef]
- Felsenfeld, G.; Bell, A.C. Methylation of a CTCF-dependent boundary controls imprinted expressionof the Igf2 gene. Nature 2000, 405, 482–485. [Google Scholar]
- Rao, S.S.; Huntley, M.H.; Durand, N.C.; Stamenova, E.K.; Bochkov, I.D.; Robinson, J.T.; Sanborn, A.L.; Machol, I.; Omer, A.D.; Lander, E.S.; et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 2014, 159, 1665–1680. [Google Scholar] [CrossRef]
- Dixon, J.R.; Selvaraj, S.; Yue, F.; Kim, A.; Li, Y.; Shen, Y.; Hu, M.; Liu, J.S.; Ren, B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 2012, 485, 376–380. [Google Scholar] [CrossRef]
- Brackley, C.A.; Johnson, J.; Michieletto, D.; Morozov, A.; Nicodemi, M.; Cook, P.; Marenduzzo, D. Nonequilibrium chromosome looping via molecular slip links. Phys. Rev. Lett. 2017, 119, 1–5. [Google Scholar] [CrossRef]
- Maksimenko, O.; Bartkuhn, M.; Stakhov, V.; Herold, M.; Zolotarev, N.; Jox, T.; Buxa, M.K.; Kirsch, R.; Bonchuk, A.; Fedotova, A.; et al. Two new insulator proteins, Pita and ZIPIC, target CP190 to chromatin. Genome Res. 2015, 25, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Van Bortle, K.; Nichols, M.H.; Li, L.; Ong, C.T.; Takenaka, N.; Qin, Z.S.; Corces, V.G. Insulator function and topological domain border strength scale with architectural protein occupancy. Genome Biol. 2014, 15, R82. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ramos, E.; Corces, V.G. The BEAF-32 insulator coordinates genome organization and function during the evolution of Drosophila species. Genome Res. 2012, 22, 2199–2207. [Google Scholar] [CrossRef] [PubMed]
- Roseman, R.R.; Pirrotta, V.; Geyer, P.K. The su(Hw) protein insulates expression of the drosophila melanogaster white gene from chromosomal position-effects. EMBO J. 1993, 12, 435–442. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, W.A.; Menon, D.; Bartlett, N.J.; Sperry, G.E.; Rasheva, V.; Meller, V.; Lloyd, V.K. The drosophila homolog of the mammalian imprint regulator, CTCF, maintains the maternal genomic imprint in drosophila melanogaster. BMC Biol. 2010, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, S.; Levine, M. GAGA mediates the enhancer blocking activity of the eve promoter in the drosophila embryo. Genes Dev. 1998, 12, 3325–3330. [Google Scholar] [CrossRef]
- Gaszner, M.; Vazquez, J.; Schedl, P. The Zw5 protein, a component of the scs chromatin domain boundary, is able to block enhancer-promoter interaction. Genes Dev. 1999, 13, 2098–2107. [Google Scholar] [CrossRef]
- Aoki, T.; Sarkeshik, A.; Yates, J.; Schedl, P. Elba, a novel developmentally regulated chromatin boundary factor is a hetero-tripartite DNA binding complex. eLife 2012, 1, 1–24. [Google Scholar] [CrossRef]
- Bag, I.; Dale, R.K.; Palmer, C.; Lei, E.P. The zinc-finger protein CLAMP promotes gypsy chromatin insulator function in Drosophila. J. Cell Sci. 2019, 132, 6. [Google Scholar] [CrossRef]
- Cuartero, S.; Fresán, U.; Reina, O.; Planet, E.; Espinàs, M.L. Ibf1 and Ibf2 are novel CP190-interacting proteins required for insulator function. EMBO J. 2014, 33, 637–647. [Google Scholar] [CrossRef]
- Gerasimova, T.I.; Gdula, D.A.; Gerasimov, D.V.; Simonova, O.; Corces, V.G. A drosophila protein that imparts directionality on a chromatin insulator is an enhancer of position-effect variegation. Cell 1995, 82, 587–597. [Google Scholar] [CrossRef]
- Savitsky, M.; Kim, M.; Kravchuk, O.; Schwartz, Y.B. Distinct roles of chromatin insulator proteins in control of the Drosophila bithorax complex. Genetics 2016, 202, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Bartkuhn, M.; Straub, T.; Herold, M.; Herrmann, M.; Rathke, C.; Saumweber, H.; Gilfillan, G.D.; Becker, P.B.; Renkawitz, R. Active promoters and insulators are marked by the centrosomal protein 190. EMBO J. 2009, 28, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Pherson, M.; Misulovin, Z.; Gause, M.; Dorsett, D. Cohesin occupancy and composition at enhancers and promoters are linked to DNA replication origin proximity in Drosophila. Genome Res. 2019, 29, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Lahn, B.T.; Tang, Z.L.; Zhou, J.; Barndt, R.J.; Parvinen, M.; Allis, C.D.; Page, D.C. Previously uncharacterized histone acetyltransferases implicated in mammalian spermatogenesis. Proc. Natl. Acad. Sci. USA 2002, 99, 8707–8712. [Google Scholar] [CrossRef] [PubMed]
- Caron, C.; Pivot-Pajot, C.; van Grunsven, L.A.; Col, E.; Lestrat, C.; Rousseaux, S.; Khochbin, S. Cdyl: A new transcriptional co-repressor. EMBO Rep. 2003, 4, 877–882. [Google Scholar] [CrossRef]
- Liu, S.; Yu, H.; Liu, Y.; Liu, X.; Zhang, Y.; Bu, C.; Yuan, S.; Chen, Z.; Xie, G.; Li, W.; et al. Chromodomain protein CDYL acts as a Crotonyl-CoA hydratase to regulate histone crotonylation and spermatogenesis. Mol. Cell 2017, 67, 853–866. [Google Scholar] [CrossRef]
- Glenn, S.E.; Geyer, P.K. Investigation of the developmental requirements of drosophila HP1 and insulator protein partner, HIPP1. G3 Genes Genomes Genet. 2019, 9, 345–357. [Google Scholar] [CrossRef]
- Stow, E.C.; An, R.; Schoborg, T.A.; Davenport, N.M.; Simmons, J.R. A drosophila insulator interacting protein suppresses enhancer-blocking function and modulates replication timing. bioRxiv 2019. [Google Scholar] [CrossRef]
- Melnikova, L.; Molodina, V.; Erokhin, M.; Georgiev, P.; Golovnin, A. HIPP1 stabilizes the interaction between CP190 and Su(Hw) in the drosophila insulator complex. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Parelho, V.; Hadjur, S.; Spivakov, M.; Leleu, M.; Sauer, S.; Gregson, H.C.; Jarmuz, A.; Canzonetta, C.; Webster, Z.; Nesterova, T.; et al. Cohesins functionally associate with CTCF on mammalian chromosome arms. Cell 2008, 132, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Rubio, E.D.; Reiss, D.J.; Welcsh, P.L.; Disteche, C.M.; Filippova, G.N.; Baliga, N.S.; Aebersold, R.; Ranish, J.A.; Krumm, A. CTCF physically links cohesin to chromatin. Proc. Natl. Acad. Sci. USA. 2008, 105, 8309–8314. [Google Scholar] [CrossRef] [PubMed]
- Holohan, E.E.; Kwong, C.; Adryan, B.; Bartkuhn, M.; Herold, M.; Renkawitz, R.; Russell, S.; White, R. CTCF genomic binding sites in Drosophila and the organisation of the bithorax complex. PLoS Genet. 2007, 3, e30112. [Google Scholar] [CrossRef]
- Misulovin, Z.; Schwartz, Y.B.; Li, X.Y.; Kahn, T.G.; Gause, M.; MacArthur, S.; Fay, J.C.; Eisen, M.B.; Pirrotta, V.; Biggin, M.D.; et al. Association of cohesin and Nipped-B with transcriptionally active regions of the drosophila melanogaster genome. Chromosoma 2008, 117, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Karch, F.; Galloni, M.; Sipos, L.; Gausz, J.; Gyurkovics, H.; Sched, P. Mcp and Fab-7: Molecular analysis of putative boundaries of cis-regulatory domains in the bithorax complex of drosophila melanogaster. Nucleic Acids Res. 1994, 22, 3138–3146. [Google Scholar] [CrossRef]
- Cavalli, G.; Paro, R. The Drosophila Fab-7 chromosomal element conveys epigenetic inheritance during mitosis and meiosis. Cell 1998, 93, 505–518. [Google Scholar] [CrossRef]
- Pérez-Lluch, S.; Cuartero, S.; Azorín, F.; Espinàs, M.L. Characterization of new regulatory elements within the drosophila bithorax complex. Nucleic Acids Res. 2008, 36, 6926–6933. [Google Scholar] [CrossRef]
- Moon, H.; Filippova, G.; Loukinov, D.; Pugacheva, E.; Chen, Q.; Smith, S.T.; Munhall, A.; Grewe, B.; Bartkuhn, M.; Arnold, R.; et al. CTCF is conserved from Drosophila to humans and confers enhancer blocking of the Fab-8 insulator. EMBO Rep. 2005, 6, 165–170. [Google Scholar] [CrossRef]
- Mohan, M.; Bartkuhn, M.; Herold, M.; Philippen, A.; Heinl, N.; Bardenhagen, I.; Leers, J.; White, A.R.; Renkawitz-Pohl, R.; Saumweber, H.; et al. The drosophila insulator proteins CTCF and CP190 link enhancer blocking to body patterning. EMBO J. 2007, 26, 4203–4214. [Google Scholar] [CrossRef]
- Muller, M.; Hagstrom, K.; Gyurkovics, H.; Pirrotta, V.; Schedl, P. The Mcp element from the Drosophila melanogaster bithorax complex mediates long-distance regulatory interactions. Genetics 1999, 153, 1333–1356. [Google Scholar]
- Sipos, L.; Gyurkovics, H. Long-distance interactions between enhancers and promoters: The case of the Abd-B domain of the Drosophila bithorax complex. FEBS J. 2005, 272, 3253–3259. [Google Scholar] [CrossRef] [PubMed]
- Kyrchanova, O.; Toshchakov, S.; Podstreshnaya, Y.; Parshikov, A.; Georgiev, P. Functional interaction between the Fab-7 and Fab-8 boundaries and the upstream promoter region in the drosophila Abd-B gene. Mol. Cell. Biol. 2008, 28, 4188–4195. [Google Scholar] [CrossRef] [PubMed]
- Postika, N.; Metzler, M.; Affolter, M.; Müller, M.; Schedl, P.; Georgiev, P.; Kyrchanova, O. Boundaries mediate long-distance interactions between enhancers and promoters in the drosophila bithorax complex. PLoS Genet. 2018, 14, e1007702. [Google Scholar] [CrossRef] [PubMed]
- Heger, P.; Marin, B.; Bartkuhn, M.; Schierenberg, E.; Wiehe, T. The chromatin insulator CTCF and the emergence of metazoan diversity. Proc. Natl. Acad. Sci. USA 2012, 109, 17507–17512. [Google Scholar] [CrossRef] [PubMed]
- Heger, P.; George, R.; Wiehe, T. Successive gain of insulator proteins in arthropod evolution. Evolution 2013, 67, 2945–2956. [Google Scholar] [CrossRef] [PubMed]
- Seller, C.A.; Cho, C.-Y.; O’Farrell, P.H. Rapid embryonic cell cycles defer the establishment of heterochromatin by Eggless/SetDB1 in drosophila. Genes Dev. 2019, 33, 403–417. [Google Scholar] [CrossRef]
- Armstrong, R.L.; Duronio, R.J. Phasing in heterochromatin during development. Genes Dev. 2019, 33, 379–381. [Google Scholar] [CrossRef]
- Fuks, F.; Hurd, P.J.; Deplus, R.; Kouzarides, T. The DNA methyltransferases associate with HP1 and the SUV39H1 histone methyltransferase. Nucleic Acids Res. 2003, 31, 2305–2312. [Google Scholar] [CrossRef]
- Muramatsu, D.; Kimura, H.; Kotoshiba, K.; Tachibana, M.; Shinkai, Y. Pericentric H3K9me3 Formation by HP1 Interaction-defective Histone Methyltransferase Suv39h1. Cell Struct. Funct. 2016, 41, 145–152. [Google Scholar] [CrossRef][Green Version]
- Eissenberg, J.C.; Elgin, S.C. The HP1 protein family: Getting a grip on chromation. Curr. Opin. Genet. Dev. 2000, 10, 204. [Google Scholar] [CrossRef]
- Eissenberg, J.C.; Elgin, S.C.R. HP1a: A structural chromosomal protein regulating transcription. Trends Genet. 2014, 30, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.K.; Eissenberg, J.C.; Worman, H.J. Transcriptional repression of euchromatic genes by Drosophila heterochromatin protein 1 and histone modifiers. Proc. Natl. Acad. Sci. USA 2001, 98, 11423–11427. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.B.; Georgatos, S.D. HP1: Facts, open questions, and speculation. J. Struct. Biol. 2002, 140, 10–16. [Google Scholar] [CrossRef]
- Reuter, G.; Giarre, M.; Farah, J.; Gausz, J.; Spierer, A.; Spierer, P. Dependence of position-effect variegation in Drosophila on dose of a gene encoding an unusual zinc-finger protein. Nature 1990, 6263, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Tschiersch, B.; Hofmann, A.; Krauss, V.; Dorn, R.; Korge, G.; Reuter, G. The protein encoded by the Drosophila position-effect variegation suppressor gene Su(var)3-9 combines domains of antagonistic regulators of homeotic gene complexes. EMBO J. 1994, 13, 3822–3831. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, R.A.; Smith, C.D.; Carlson, J.W.; Carvalho, A.B.; Halpern, A.; Kaminker, J.S.; Kennedy, C.; Mungall, C.; Sullivan, B.A.; Sutton, G.; et al. Heterochromatic sequences in a drosophila whole-genome shotgun assembly. Genome Biol. 2002, 3, 1–16. [Google Scholar] [CrossRef]
- Hoskins, R.A.; Carlson, J.W.; Kennedy, C.; Acevedo, D.; Evans-Holm, M.; Frise, E.; Wan, K.H.; Park, S.; Mendez-Lago, M.; Rossi, F.; et al. Sequence finishing and mapping of drosophila melanogaster heterochromatin. Science 2007, 316, 1625–1628. [Google Scholar] [CrossRef]
- Ciavatta, D.; Rogers, S.; Magnuson, T. Drosophila CTCF Is Required for Fab-8 enhancer blocking activity in S2 Cells. J. Mol. Biol. 2007, 373, 233–239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bonchuk, A.; Maksimenko, O.; Kyrchanova, O.; Ivlieva, T.; Mogila, V.; Deshpande, G.; Wolle, D.; Schedl, P.; Georgiev, P. Functional role of dimerization and CP190 interacting domains of CTCF protein in drosophila melanogaster. BMC Biol. 2015, 13, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Brower-Toland, B.; Riddle, N.C.; Jiang, H.; Huisinga, K.L.; Elgin, S.C.R. Multiple SET methyltransferases are required to maintain normal heterochromatin domains in the genome of drosophila melanogaster. Genetics 2009, 181, 1303–1319. [Google Scholar] [CrossRef] [PubMed]
- Chavez, J.; Murillo-Maldonado, J.M.; Bahena, V.; Cruz, A.K.; Castañeda-Sortibrán, A.; Rodriguez-Arnaiz, R.; Zurita, M.; Valadez-Graham, V. dAdd1 and dXNP prevent genome instability by maintaining HP1a localization at Drosophila telomeres. Chromosoma 2017, 126, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Nava, S.; Torres, A.; Zurita, M.; Valadez-Graham, V. Molecular effects of dADD1 misexpression in chromatin organization and transcription. BMC Mol. Cell Biol. 2020, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Strom, A.R.; Emelyanov, A.V.; Mir, M.; Fyodorov, D.V.; Darzacq, X.; Karpen, G.H. Phase separation drives heterochromatin domain formation. Nature 2017, 547, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Kinoshita, K. PrDOS: Prediction of disordered protein regions from amino acid sequence. Nucleic Acids Res. 2007, 35, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Frydrychova, R.C.; Biessmann, H.; Mason, J.M. Regulation of telomere length in Drosophila. Cytogenet. Genome Res. 2009, 122, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, R.J.; Picketts, D.J.; Villard, L.; Higgs, D.R. Mutations in a putative global transcriptional regulator cause X-linked mental retardation with α-thalassemia (ATR-X syndrome). Cell 1995, 80, 837–845. [Google Scholar] [CrossRef]
- Sadic, D.; Schmidt, K.; Groh, S.; Kondofersky, I.; Ellwart, J.; Fuchs, C.; Theis, F.J.; Schotta, G. Atrx promotes heterochromatin formation at retrotransposons. EMBO Rep. 2015, 16, 836–850. [Google Scholar] [CrossRef]
- Valle-García, D.; Qadeer, Z.A.; McHugh, D.S.; Ghiraldini, F.G.; Chowdhury, A.H.; Hasson, D.; Dyer, M.A.; Recillas-Targa, F.; Bernstein, E. ATRX binds to atypical chromatin domains at the 3′ exons of zinc finger genes to preserve H3K9me3 enrichment. Epigenetics 2016, 11, 398–414. [Google Scholar] [CrossRef]
- de Wit, E.; Greil, F.; van Steensel, B. High-resolution mapping reveals links of HP1 with active and inactive chromatin components. PLoS Genet. 2007, 3, e30038. [Google Scholar] [CrossRef]
- Ilyin, A.A.; Stolyarenko, A.D.; Klenov, M.S.; Shevelyov, Y.Y. Various modes of HP1a interactions with the euchromatic chromosome arms in Drosophila ovarian somatic cells. Chromosoma 2020. [Google Scholar] [CrossRef]
- Li, Y.; Danzer, J.R.; Alvarez, P.; Belmont, A.S.; Wallrath, L.L. Effects of tethering HP1 to euchromatic regions of the Drosophila genome. Development 2003, 130, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, L.; Fanti, L.; Berloco, M.; Perrini, B.; Pimpinelli, S. Heterochromatin protein 1 (HP1) is associated with induced gene expression in Drosophila euchromatin. J. Cell Biol. 2003, 161, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Petesch, S.J.; Lis, J.T. Rapid, transcription-independent loss of nucleosomes over a large chromatin domain at Hsp70 Loci. Cell 2008, 134, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, I.J.; Orsi, G.A.; Hughes, K.T.; Loppin, B.; Ahmad, K. Nucleosome-depleted chromatin gaps recruit assembly factors for the H3.3 histone variant. Proc. Natl. Acad. Sci. USA 2012, 109, 19721–19726. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.Y.; Emtage, P.C.; Duyf, B.J.; Hilliker, A.J.; Eissenberg, J.C. heterochromatin protein 1 is required for the normal expression of two heterochromatin genes in drosophila. Genetics 2000, 155, 699–708. [Google Scholar] [PubMed]
- Danzer, J.R.; Wallrath, L.L. Mechanisms of HP1-mediated gene silencing in Drosophila. Development 2004, 131, 3571–3580. [Google Scholar] [CrossRef]
- de Lucia, F.; Ni, J.Q.; Vaillant, C.; Sun, F.L. HP1 modulates the transcription of cell-cycle regulators in drosophila melanogaster. Nucleic Acids Res. 2005, 33, 2852–2858. [Google Scholar] [CrossRef]
- Minc, E.; Courvalin, J.; Buendia, B. HP1Ɣ associates associates with euchromatin and heterochromatin in mammalian nuclei and chromosomes. Cytogenet Cell Genet. 2005, 284, 279–284. [Google Scholar]
- Vakoc, C.R.; Mandat, S.A.; Olenchock, B.A.; Blobel, G.A. Histone H3 lysine 9 methylation and HP1γ are associated with transcription elongation through mammalian chromatin. Mol. Cell 2005, 19, 381–391. [Google Scholar] [CrossRef]
- Lee, Y.C.G.; Ogiyama, Y.; Martins, N.M.C.; Beliveau, B.J.; Acevedo, D.; Wu, C.-T.; Cavalli, G.; Karpen, G.H. Pericentromeric heterochromatin is hierarchically organized and spatially contacts H3K9me2 islands in euchromatin. PLoS Genet. 2020, 16, e1008673. [Google Scholar]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef] [PubMed]
- de Castro, E.; Sigrist, C.J.A.; Gattiker, A.; Bulliard, V.; Langendijk-Genevaux, P.S.; Gasteiger, E.; Bairoch, A.; Hulo, N. ScanProsite: Detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006, 34, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Contrino, S.; Smith, R.N.; Butano, D.; Carr, A.; Hu, F.; Lyne, R.; Rutherford, K.; Kalderimis, A.; Sullivan, J.; Carbon, S.; et al. modMine: Flexible access to modENCODE data. Nucleic Acids Res. 2012, 40, D1082–D1088. [Google Scholar] [CrossRef]
- Ong, C.T.; van Bortle, K.; Ramos, E.; Corces, V.G. XPoly(ADP-ribosyl)ation regulates insulator function and intrachromosomal interactions in drosophila. Cell 2013, 155, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Thorvaldsdóttir, H.; Robinson, J.T.; Mesirov, J.P. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 2012, 14, 178–192. [Google Scholar] [CrossRef]
- Thurmond, J.; Goodman, J.L.; Strelets, V.B.; Attrill, H.; Gramates, L.S.; Marygold, S.J.; Mathews, B.B.; Millburn, G.; Antonazzo, G.; Trovisco, V.; et al. FlyBase 2.0: The next generation. Nucleic Acids Res. 2018, 47, D759–D765. [Google Scholar] [CrossRef]
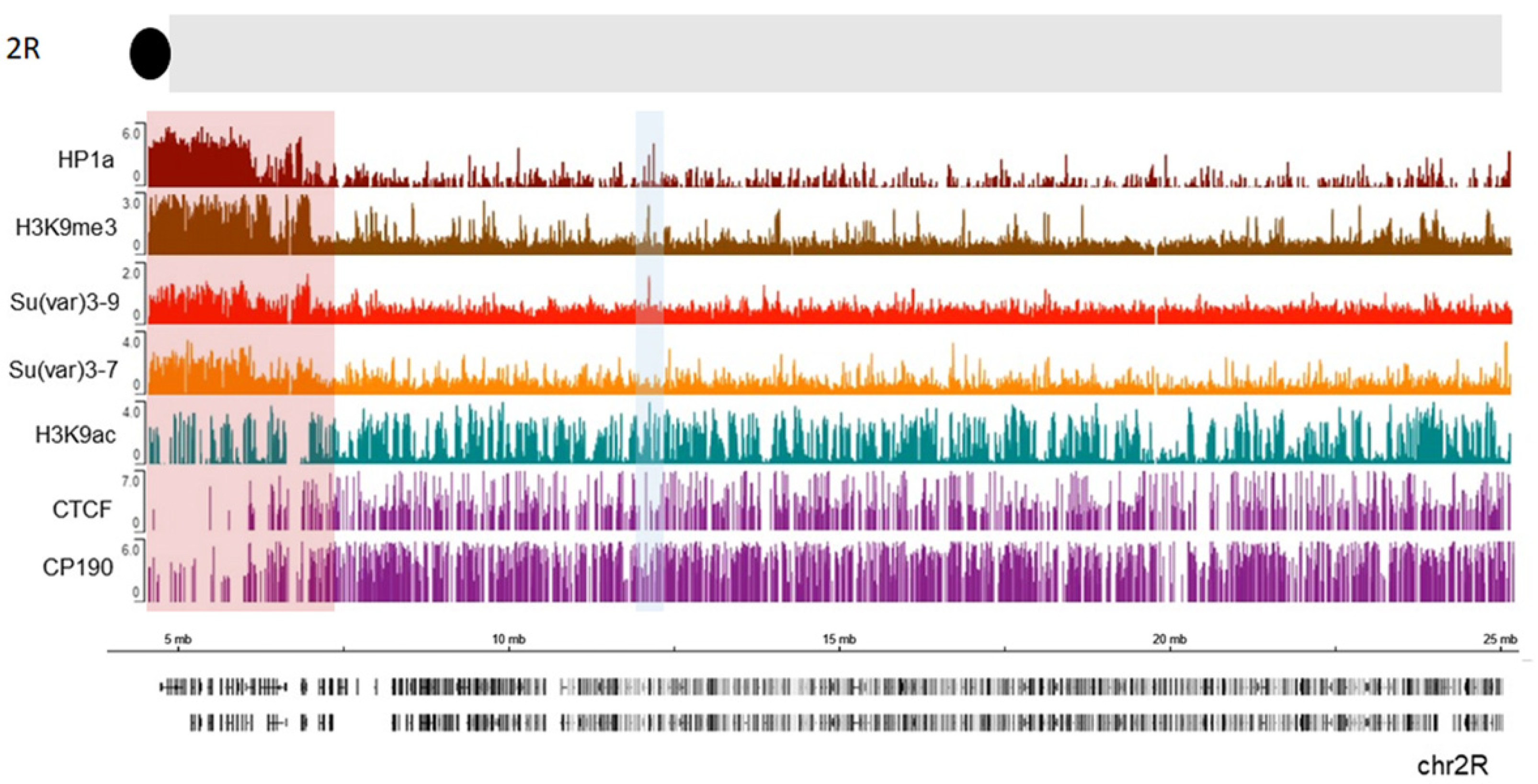
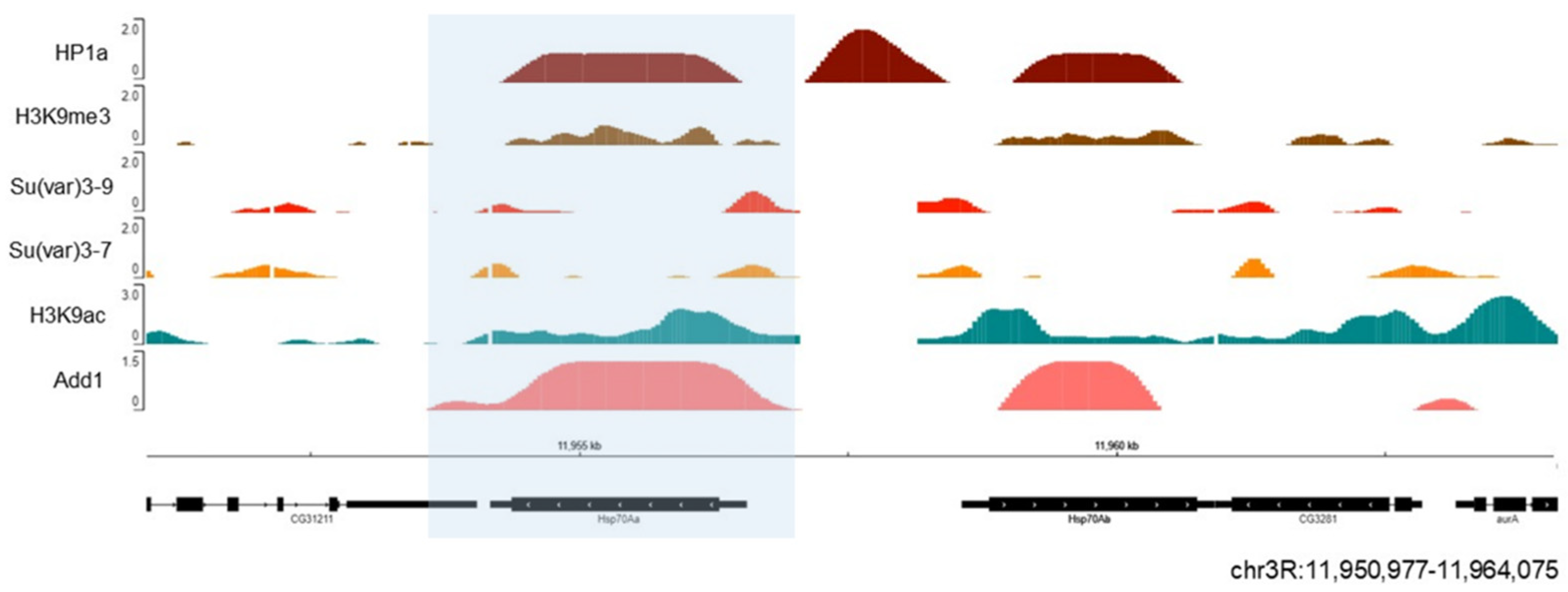
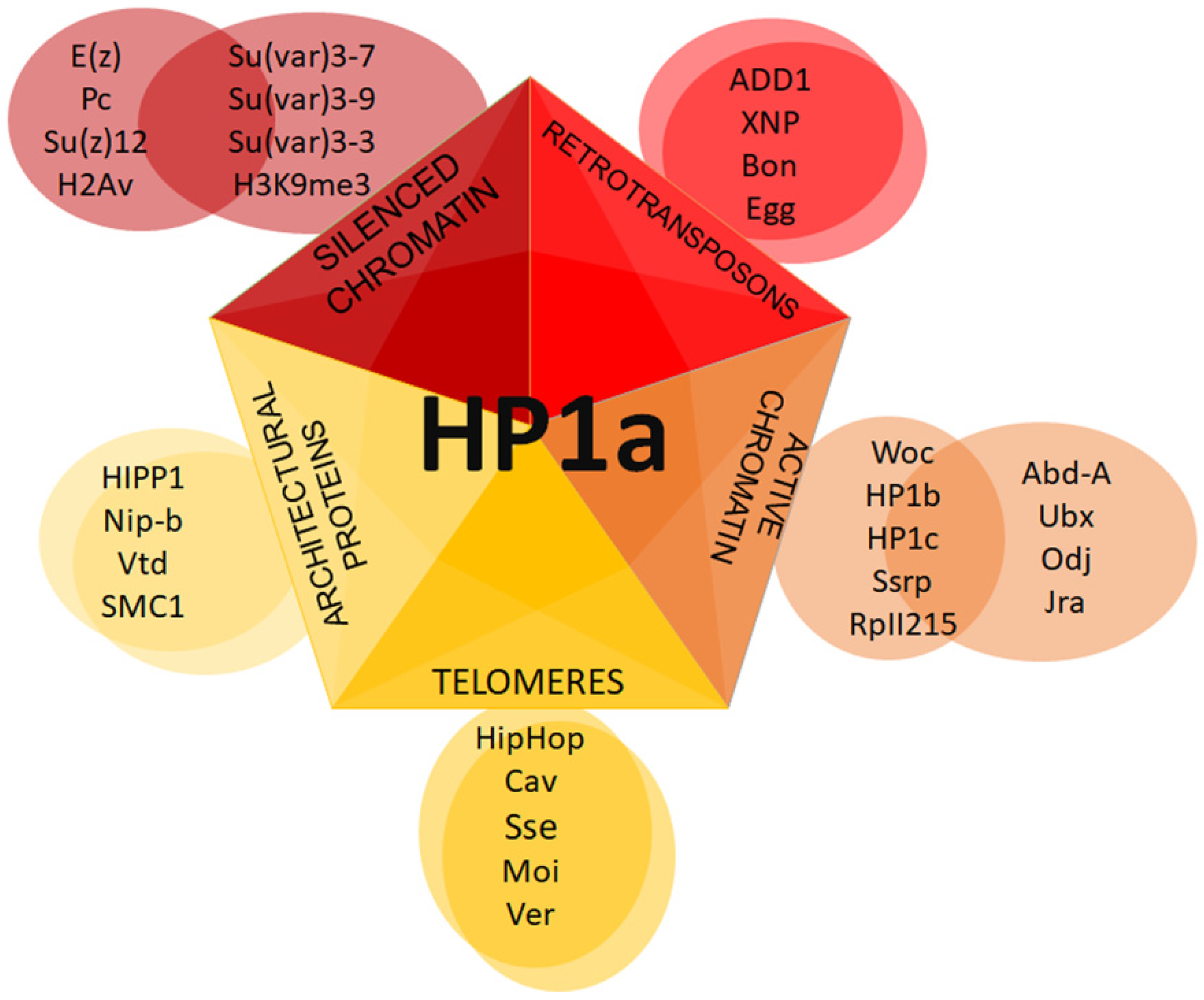
| Protein or Cellular Component | Organism | Methodology | References | |
|---|---|---|---|---|
| CHD | ||||
| Methylated H3K9 | Drosophila | IF, FAITC, NMR | [51] | |
| H2Av | Drosophila | IF, tagIP, rPD, | [52] | |
| RpII215 | Drosophila | IP, WB, rPD | [7,53] | |
| Nuclear envelope | Mouse | IF, BA | [54] | |
| H3 | Mouse | IP, FW, rPD | [31,55] | |
| H1 | Mouse | rPD, FW | [55] | |
| Methylated H3K9 | Mouse | rPD | [56] | |
| Methylated H3K9 | Human | rPD, SPRA | [57] | |
| CTIP2 | Human | rPD, IP | [58,59] | |
| Methylated H1.4K26 | Human | BD, IP, rPD, IF | [31,37] | |
| DNMT1 | Human | rPD | [60] | |
| CSD | ||||
| Hip/HP4 | Drosophila | Y2H, tagIP, rPD, IF, tag-WB | [61,62] | |
| AF10/Alh | Drosophila | transPD | [42,63] | |
| Su(var)3-7 | Drosophila | Y2H, IP, IF, WB | [64,65] | |
| PIWI | Drosophila | Y2H, IP, IF, NMR, Y2H | [42,66,67] | |
| Kdm4A | Drosophila | transIP, tag-WB, MW, WB, fingerprinting, MS | [68,69,70] | |
| Ssrp | Drosophila | transPD, WB, IP, tandem affinity technology, tagIP, tag-WB | [53,71] | |
| Su(var)3-9 | Drosophila | transPD, At, ATC, WB, Y2H, IP, tag-WB, tagIP, fingerprinting, IF, MS | [53,68,70,72,73] | |
| Su(var)2-HP2 | Drosophila | IP, At, Y2H, NMR, FAITC, PP, tagIP, fingerprinting, co-sedimentation, molecular weight, molecular sieving, MS | [42,68,70,74,75] | |
| XNP/dATRX | Drosophila | transIP, transPD, MS, IF, WB | [68,76,77,78] | |
| HP6 | Drosophila | IP, WB, transPD, tag-WB | [79] | |
| egg | Drosophila | transIP, fingerprinting, rPD | [53,68] | |
| G9a | Drosophila | IP, WB, rPD | [53] | |
| ova | Drosophila | IP, Y2H | [80] | |
| HP1-BP84 | Mouse | Y2H | [81] | |
| TIF1α | Mouse | Y2H, rPD | [50,81,82] | |
| CAF-1 p150 | Mouse | Y2H, rPD, IF, GFC, NMR | [33,83] | |
| mSNF2β | Mouse | Y2H | [50] | |
| KAP1/TIFβ | Mouse | IP, rPD, IF, SPRA, GFC | [50,55,83] | |
| H4 | Mouse | In vitro cross-linking | [31] | |
| MeCP2 | Mouse | tagIP | [84] | |
| KAP1/TIFβ | Human | Y2H, IP, rPD, IF, GFC | [83,85] | |
| SP100 | Human | Y2H, rPD, transPD, IF | [86] | |
| Polycomb | Human | IP, rPD, IF | [33] | |
| ATRX | Human | Y2H, IF, rPD | [85,87] | |
| CAF-1 p150 | Human | rPD | [50] | |
| Ku70 | Human | Y2H, IP, rPD | [88] | |
| TAFII130 | Human | Y2H, exPD, transPD | [89] | |
| Ki-67 | Human | Y2H, exPD, IF, ChIP | [90] | |
| BRG1 | Human | IP, rPD, TransPD, IF | [91] | |
| SUV39H1 | Human | rPD, Y2H | [49] | |
| NIPBL/hScc2 | Human | rPD | [85,92] | |
| HP1-BP74 | Human | rPD | [85] | |
| LBR (Lamin B receptor) | Human | rPD, Y2H, IP | [85,93] | |
| Sgo1 | Human | Y2H, MS. IP | [92,94] | |
| POGZ | Human | Y2H, MS | [92] | |
| BARD1 | Human | tragIP, transPD | [95] | |
| KDM2A | Human | IP, transPD, IF | [96] | |
| LRIF1 | Human | IP, transPD | [97] | |
| Haspin | Human | tragIP, rPD | [98] | |
| MacroH2A1.2 | Human | IP, transPD | [99] | |
| Hinge | ||||
| HP-BP74 H1-like | Mouse | Y2H, FW, rPD | [55,81] | |
| MITR, HDAC4/5 | Mouse | IP, rPD | [100] | |
| Combination of Domains | ||||
| ORC1-6 | Drosophila | tagIP | CHD, CSD | [30] |
| Mcm10 | Drosophila | proximity ligation assay, IF, IP, WB, transPD, tag-WB, Y2H | CHD, CSD | [101,102] |
| SuUR | Drosophila | Y2H, rPD, transPD, WB, IP, MS, tagIP, fingerprinting | Hin + CSD | [68,93] |
| Caf1-180 | Drosophila | transPD, tag-WB, WB, transIP, fingerprinting, IP | Hin + CSD | [68,103] |
| Cav/HOAP | Drosophila | tagIP, IF, IP, exPD | Hin + CSD | [79,104,105,106,107] |
| Parp-2 | Mouse | rPD | Hin + CSD | [108] |
| TIf1β | Mouse | rPD | Hin + CSD | [108] |
| ARFL5 | Human | Y2H, rPD | CHD + CSD | [109] |
| INCENP | Human | Y2H, tranPD | Hin + CSD | [94,110] |
| Protein or Cellular Component | Methodology | Reference |
|---|---|---|
| Arp6 | IF | [131,132] |
| E(bx) | WB | [133] |
| Nap1 | WB | [133] |
| Su(var) 3-3 | IP, WB, transIP, fingerprinting | [68,134] |
| POF | IF | [135,136] |
| Ndc80 | transIP, fingerprinting | [137] |
| HP5 | MS, IP, WB, | [68,70,138] |
| Pep | IP, WB | [7] |
| moi | transPD, tag-WB | [107] |
| ACF | transPD | [130] |
| Dp1 | IP, WB, | [7] |
| vig | IP, WB | [139] |
| vig2 | IP, WB, rPD | [139] |
| Hmt4-20 | IF | [62] |
| dre4 | tandem affinity purification, multidimensional protein identification technology, WB | [71] |
| ver | transPD, tag-WB | [140] |
| HP1c | transPD, WB | [53,71] |
| Atg8a | PA | [138] |
| CG11474 | PA | [138] |
| Atf-2 | IP, WB | [141] |
| qin | transIP, WB, IP, tag-WB | [142] |
| mu2 | transIP, WB, Y2H, IP, tag-WB, transPD | [143] |
| CG15356 | FAITC, PP | [42] |
| jnj | transIP, WB | [144] |
| SMC5 | transIP, WB | [144] |
| Hrb87F | transIP, WB | [7,145] |
| Hrb98DE | transIP, WB | [145] |
| bon | IP, WB | [73] |
| fru | IP, WB | [73] |
| eyg | IP, WB, transIP | [146] |
| Hers | cosedimentation, WB, IP | [73] |
| woc | FAITC, PP | [75] |
| H1 | rPD, WB, tagIP | [147,148] |
| Su(var)2-10 | IP, At, Y2H, NMR, FAITC, PP, transIP, fingerprinting, cosedimentation, molecular weight, molecular sieving | [42,68,74,75] |
| Lhr | Y2H, transIP, WB, IP, fingerprinting, tag-WB | [68,149,150,151] |
| Hmr | IP, WB, tag-WB, transIP, fingerprinting | [68,149,150] |
| STAT92E | IP, rPD, IF, transPD, tag-WB | [152,153] |
| MED26 | IP, WB, ATC | [154] |
| MED17 | IP, WB | [154] |
| Incenp | transIP, fingerprinting | [68] |
| borr | transIP, fingerprinting | [68] |
| HIPP1 | transIP, WB, fingerprinting | [68] |
| CAP | transIP, fingerprinting | [68] |
| SMC1 | transIP, fingerprinting | [68] |
| Yeti | transPD, tag-WB | [155] |
| Mau2 | transIP, fingerprinting | [68] |
| Nipped-B | transIP, fingerprinting | [68] |
| vtd | transIP, fingerprinting | [68] |
| Odj | transIP, fingerprinting, Y2H, MS | [68,70,156,157] |
| vers | transIP, fingerprinting | [68] |
| HP1b | transIP, fingerprinting, rPD | [53,62,68] |
| dADD1 | transIP, tag-WB, fingerprinting, WB, MS | [68,70,78] |
| tea | transIP, fingerprinting | [68] |
| sle | transIP, fingerprinting | [68] |
| CG43736 | transIP, fingerprinting | [68] |
| E(var)3-9 | transIP, fingerprinting | [68] |
| CG1815 | transIP, fingerprinting | [68] |
| NSD | transIP, fingerprinting | [68] |
| CG7692 | transIP, fingerprinting, MS | [68,70] |
| CG1737 | transIP, fingerprinting | [68] |
| CG30403 | transIP, fingerprinting | [68] |
| Jra | IP, WB, transIP, MS | [158] |
| Rrp6 | coimmunoprecipitation, tag-WB, transIP, WB | [159] |
| Pc | IP, WB | [160] |
| Su(z)12 | IP, WB | [160] |
| E(z) | IP, WB | [160] |
| HipHop | transPD, WB, IP, chromatography technology, molecular sieving, MW | [105,106] |
| CG8108 | transIP, fingerprinting, MS | [68,70] |
| Sse | transIP, tag-WB, transPD, WB | [161] |
| Hsc70-3 | MS | [70] |
| βTub56D | MS | [70] |
| Chd64 | MS | [70] |
| Hsp83 | MS | [70] |
| Act5C | MS | [70] |
| rictor | transIP, fingerprinting | [162] |
| Tsr | MS | [70] |
| dmt | Y2H, transIP, MS | [163] |
| DNApol-ɛ255 | proximity ligation assay, fluorescence microscopy | [101] |
| Gnf1 | IP, WB, proximity ligation assay, fluorescence microscopy | [101] |
| Ubx | IF, BiFC | [164] |
| abd-A | IF, BiFC | [164] |
| sov | transIP, fingerprinting | [68,165] |
| H3 | exPD, FAITC, FW, NMR, PP | [45,51,53,71,125] |
| bbx | Y2H | [157] |
| tj | Y2H | [157] |
| Protein | PxVxL | CxVxL | LxVxL |
|---|---|---|---|
| CTCF | |||
| Su(Hw) | |||
| BEAF-32 | |||
| pita | |||
| ZIPIC | |||
| Ibf1 | |||
| Ibf2 | |||
| Mod(mdg4) | |||
| CP190 | |||
| Cap-H2 | X | ||
| Elba1 | |||
| Elba2 | |||
| Elba3 | X | ||
| Shep | |||
| Zw5 | X | ||
| Clamp | |||
| GAF | X | ||
| Nip-b | |||
| Vtd | X | ||
| SA | X | ||
| Smc1 | |||
| Smc2 | X | ||
| Smc3 | |||
| HIPP1 | X |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer-Nava, S.; Nieto-Caballero, V.E.; Zurita, M.; Valadez-Graham, V. Insights into HP1a-Chromatin Interactions. Cells 2020, 9, 1866. https://doi.org/10.3390/cells9081866
Meyer-Nava S, Nieto-Caballero VE, Zurita M, Valadez-Graham V. Insights into HP1a-Chromatin Interactions. Cells. 2020; 9(8):1866. https://doi.org/10.3390/cells9081866
Chicago/Turabian StyleMeyer-Nava, Silvia, Victor E. Nieto-Caballero, Mario Zurita, and Viviana Valadez-Graham. 2020. "Insights into HP1a-Chromatin Interactions" Cells 9, no. 8: 1866. https://doi.org/10.3390/cells9081866
APA StyleMeyer-Nava, S., Nieto-Caballero, V. E., Zurita, M., & Valadez-Graham, V. (2020). Insights into HP1a-Chromatin Interactions. Cells, 9(8), 1866. https://doi.org/10.3390/cells9081866





