1. Introduction
Spermatozoa are unique cells because of their mission to transmit genes from one generation to the next. After ejaculation, mammalian sperm must swim a long distance and overcome several obstacles in the female reproductive tract, and very few sperm succeed in entering the oviduct (the fertilization site) [
1]. Many sperm that reach the oviduct are held in a sperm reservoir by binding to oviductal epithelial cells until the time of ovulation [
2]. This attachment of sperm to oviduct cell membranes has a beneficial effect on the maintenance of sperm viability in the oviduct [
3]. Generally, only a single sperm fertilizes an oocyte, despite billions of sperm ejaculated by natural mating. Considerable research has focused upon sperm competition or the difference between successful and unsuccessful sperm regarding fertilization. However, the reason why so many sperm cells exist in the female reproductive tract remains undetermined.
Spermatozoa are also recognized as heterogeneous cells. Diversity can be found in several sperm characteristics such as DNA content, epigenetic state, membrane composition, morphology, and motility [
4,
5]. In assisted reproductive technologies or the field of animal reproduction, selection of sperm with high quality is important for obtaining a stable and high fertilization rate during in vitro fertilization. Many researchers have investigated sperm selection using several different methods [
6]. Overall, special attention has been devoted to sperm competition and selection, but not to sperm cooperation. Due to this focus, mammalian sperm are often considered to function as individual cells, despite the fact that a huge number of sperm are ejaculated at once. However, it is noteworthy that occasional physical interactions of two or more sperm such as sperm pairs, sperm rosettes, sperm rouleaux, and sperm trains have been reported in both in vitro and in vivo studies [
7].
In many mammalian and non-mammalian species, it has been observed that many sperm become agglutinated with other sperm via the head, termed head-to-head agglutination [
7,
8]. Head-to-head agglutination of mammalian sperm may be induced by the addition of serum or egg yolk to culture medium, and also by exposing sperm to female reproductive tract fluids or to medium used for in vitro fertilization [
7,
9,
10,
11,
12]. These facts suggest that sperm agglutination may play a role in the mammalian fertilization process. Indeed, it has been shown that sperm agglutination enhances sperm motility in some rodents [
8,
13,
14]. Interestingly, in promiscuous deer mice, sperm preferentially agglutinated to sperm from the same male, not from a different male, and increased their swimming velocities as a group, suggesting sperm cooperation provides a fertilization advantage in the competitive environment of promiscuous mice [
13].
In the bull, however, the biological significance of sperm agglutination remains largely unclear, despite head-to-head agglutination of bovine sperm being first reported in 1950s [
15]. One study indicated that bull agglutinated sperm were more likely to maintain intact acrosomes compared with single sperm, suggesting head-to-head agglutination provides a protective role for the bovine sperm acrosome [
11]. However, another study showed that the proportion of bovine sperm with intact acrosomes and plasma membranes was similar between sperm incubated in sperm-agglutinating versus non-agglutinating medium, challenging the potential protective role of sperm agglutination [
12]. It is also unknown whether sperm agglutination has other roles in other sperm organelles or functions in the bull. These contradictory and limited studies on bovine sperm agglutination led us to further investigate the characteristics of agglutinated sperm to determine a possible role of sperm agglutination in the fertilization process, and to address the long-standing question of why so many sperm become agglutinated. The identification of the characteristics and function of sperm agglutination may lead to an understanding of the fertilization strategy of sperm cooperation, and provide the first description of a functional sperm group in bulls.
Thus, the objective of this study was to examine the relationship between bovine sperm agglutination and fundamental sperm characteristics such as viability and motility. To achieve this purpose, we investigated temporal changes to the rate of head-to-head agglutination, and compared sperm viability and motility rates between agglutinated and unagglutinated sperm. Additionally, we evaluated the ability of sperm to agglutinate using sperm combined from different males or different species, to determine whether bovine sperm cooperated with sperm from a single male but not multiple males, like sperm from the promiscuous deer mouse [
13]. Finally, we examined the effect of heparin, which is involved in the sperm–oviduct binding process [
16], upon sperm agglutination and viability to determine the molecular mechanism of sperm–sperm binding.
2. Materials and Methods
2.1. Ethics Statement
All animal care and experiments in this study were conducted in compliance with the Regulations for Animal Experiments and Related Activities at Tohoku University. The study was approved by the Tohoku University Institutional Animal Care and Use Committee (2019-003-02).
2.2. Sperm Preparation
Bovine sperm samples were prepared as reported previously [
17]. Briefly, this study used four semen straws from different mature Japanese Black cattle bulls. The four bulls were of known fertility and each semen sample contained at least 50% progressively motile sperm after thawing. Each frozen semen sample was thawed in a water bath at 38.5 °C for 15 s and then transferred to 15 mL plastic tubes. The samples were suspended in 3 mL of bovine serum albumin-free bovine gamete medium 1 (BGM-1) [
18] or IVF100 medium (Cat. No. IFP9630, Research Institute for the Functional Peptides Co., Yamagata, Japan). The albumin-free BGM-1 was designed for sperm culture and does not contain any sperm capacitation inducers nor sperm agglutination factors, while IVF100 medium was designed for in vitro fertilization and contains agglutination inducers such as bovine serum albumin and capacitation inducers such as heparin. Therefore, we defined albumin-free BGM-1 as non-capacitation medium, and IVF100 medium as a capacitation medium. After centrifugation at 430×
g for 5 min at room temperature, the supernatant was discarded and the sediment was resuspended in non-capacitation or capacitation medium at 1 × 10
7 cells/mL. Each sperm suspension was used for all of the experiments.
Ejaculated boar sperm was collected from three different mature boars at 1, 4, and 5 years of age, which were of known fertility. Each semen sample contained at least 50% progressively motile sperm after washing. Each sample was suspended in 2 mL of IVF100 medium, and centrifuged at 430× g for 5 min at room temperature. After the supernatant was discarded, the sediment was resuspended in IVF100 medium at 1 × 107 cells/mL. Each boar sperm suspension was used for all experiments.
2.3. Sperm Agglutination Assay
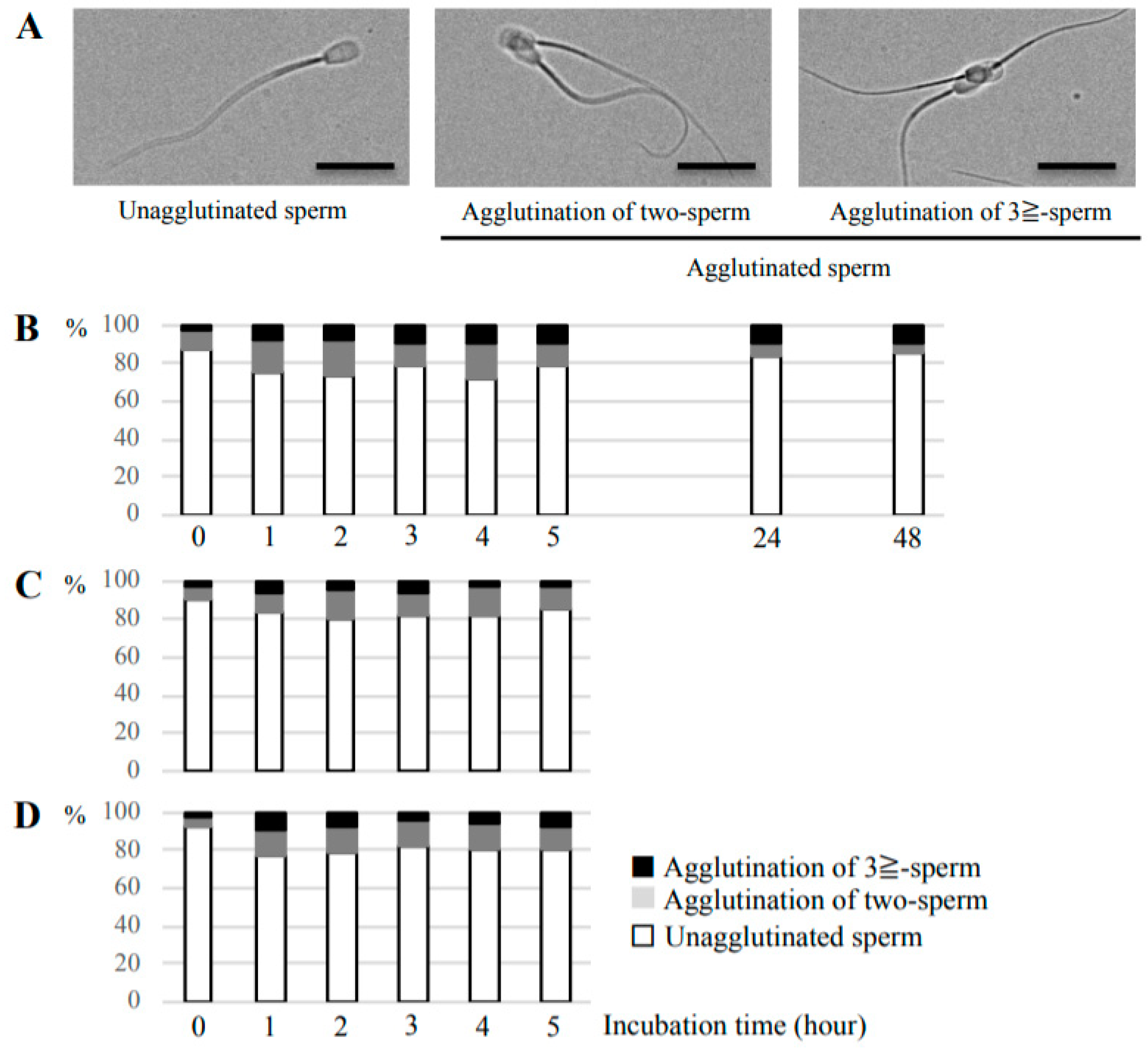
Sperm suspensions derived from one bull alone or two different bulls were used in this experiment. The suspensions were incubated for 1, 2, 3, 4, 5, 24, or 48 h in non-capacitation or capacitation medium at 38.5 °C with 5% CO2. A small amount of each suspension was added to a glass slides, covered with glass coverslips, then observed under an inverted fluorescence microscope (BZ-X710, KEYENCE, Osaka, Japan). We obtained images from random fields (size: 1085 × 1500 μm), and counted the number of unagglutinated sperm, head-to-head agglutination of two sperm, and head-to-head agglutination of three or more sperm. Note that we counted the number of agglutinations but not the sperm number constituting an agglutination. At least 100 single sperm cells or sperm agglutinations were counted, and the percentage of each of the above three groups at each timepoint were calculated.
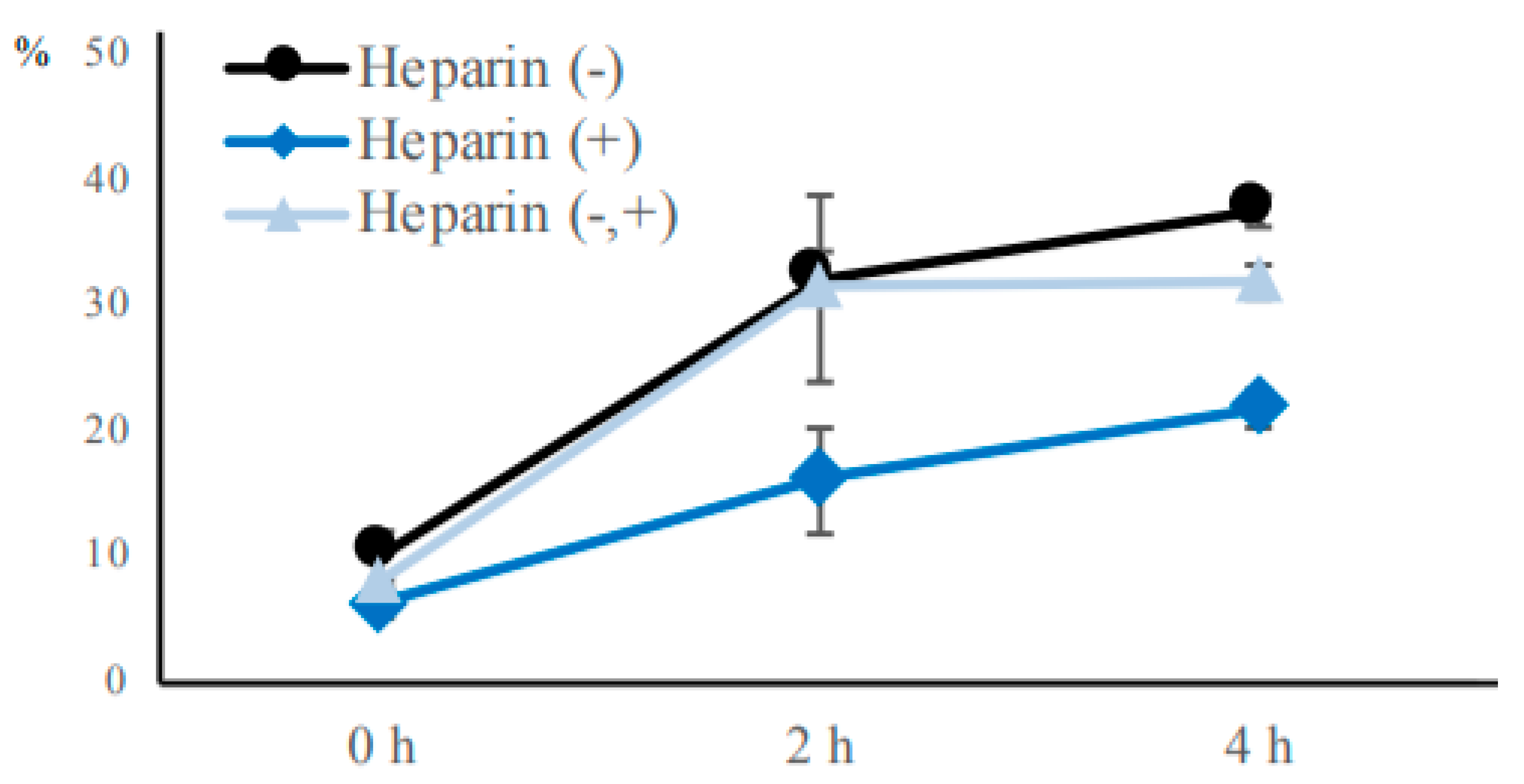
To examine the effect of heparin on bovine sperm agglutination, we added a final concentration of 100 μg/mL heparin sodium (Nacalai Tesque, Kyoto, Japan) to sperm suspensions before incubation or after two hours of incubation. Sperm samples were then incubated in non-capacitation medium supplemented with or without heparin for 0, 2, or 4 h. After incubation, the sperm agglutination rate was determined as above-mentioned.
2.4. Evaluation of Head-to-Head Agglutination of Two Sperm
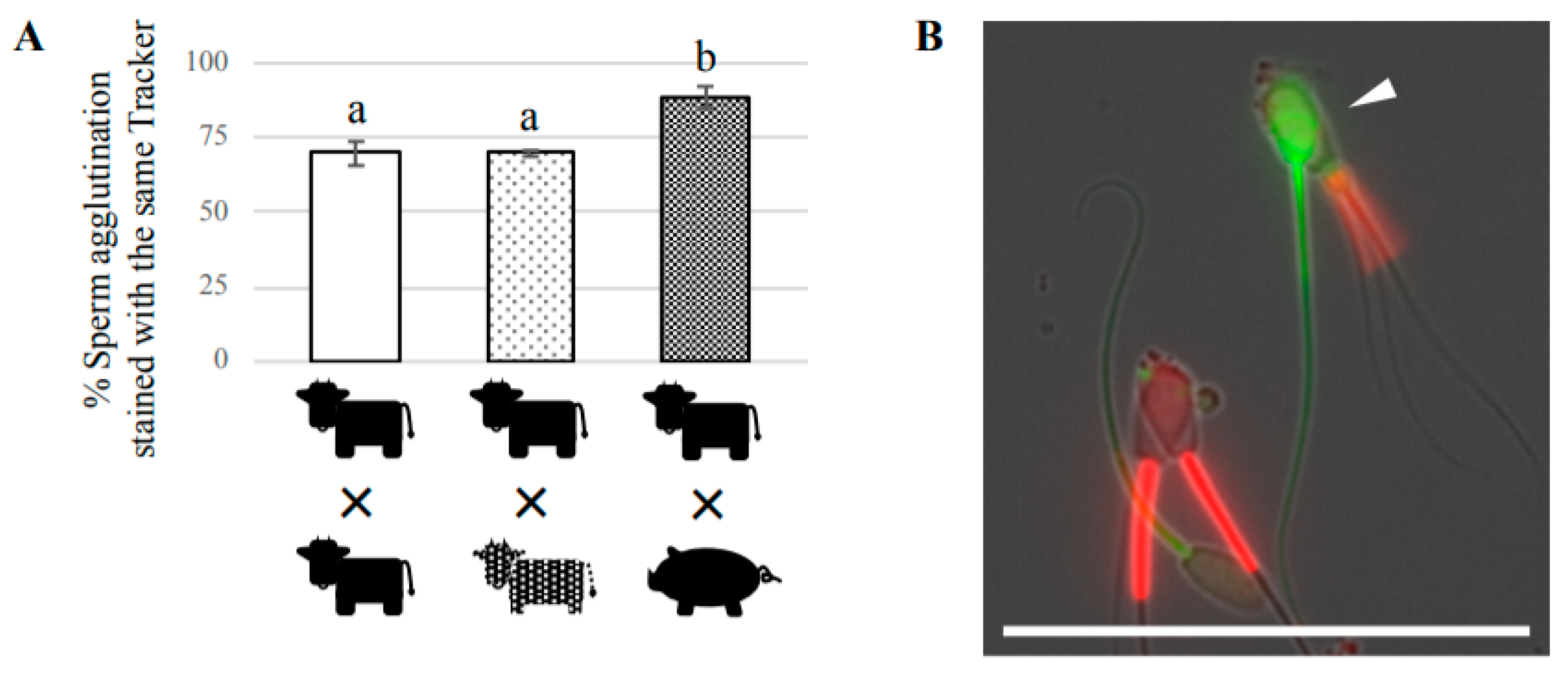
To determine whether two sperm agglutinated preferentially from the same bull or randomly from different bulls, two sperm suspensions (each from a different bull) were separately stained with a 25 μM CellTrackerTM Green CMFDA Dye or 50 nM MitoTracker Red FM (Thermo Fisher Scientific) in capacitation medium for 20 min at 38.5 °C with 5% CO2. After staining, each suspension was centrifuged at 400× g for 5 min at room temperature, then the supernatant was discarded. The sediment was resuspended in the capacitation medium and centrifuged again. After the supernatant was discarded, the sediment was resuspended in the capacitation medium at 1 × 107 cells/mL. The same amount of both sperm suspensions stained with different trackers was mixed together, and incubated for 1 h at 38.5 °C with 5% CO2, then observed under a fluorescence microscope (BZ-X710, KEYENCE). We counted the total number of two-sperm agglutinations after coincubation for 60 min, and subtracted the number of two-sperm agglutinations after the preincubation for 20 min with either CellTracker or MitoTracker from these numbers. At least 100 two-sperm agglutinations were evaluated to determine the percentage of two-sperm agglutination stained with each tracker. We also performed the same experiment using boar sperm to examine the possibility of cross-species agglutination between bovine and porcine sperm.
2.5. Comparison of Agglutinated and Unagglutinated Sperm Viability
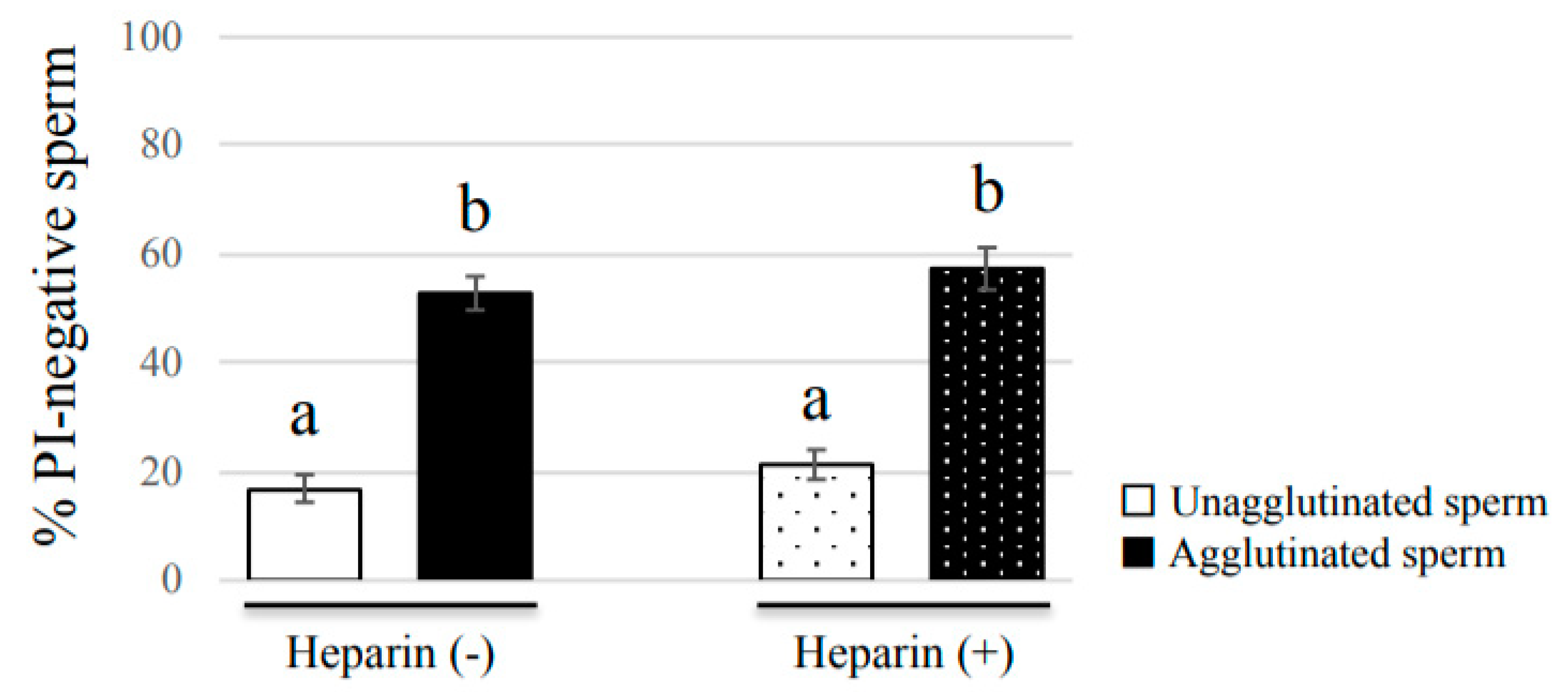
Sperm viability was evaluated by SYBR14/PI staining using a commercially available kit (LIVE/DEAD Sperm Viability Kit, Thermo Fisher Scientific, Waltham, MA, USA) [
19]. Briefly, sperm suspension was stained with 100 nM SYBR14 for 10 min, then with 12 μM propidium iodide (PI). After staining, the sample was incubated for 3, 5, or 24 h in the non-capacitation or capacitation medium at 38.5 °C with 5% CO
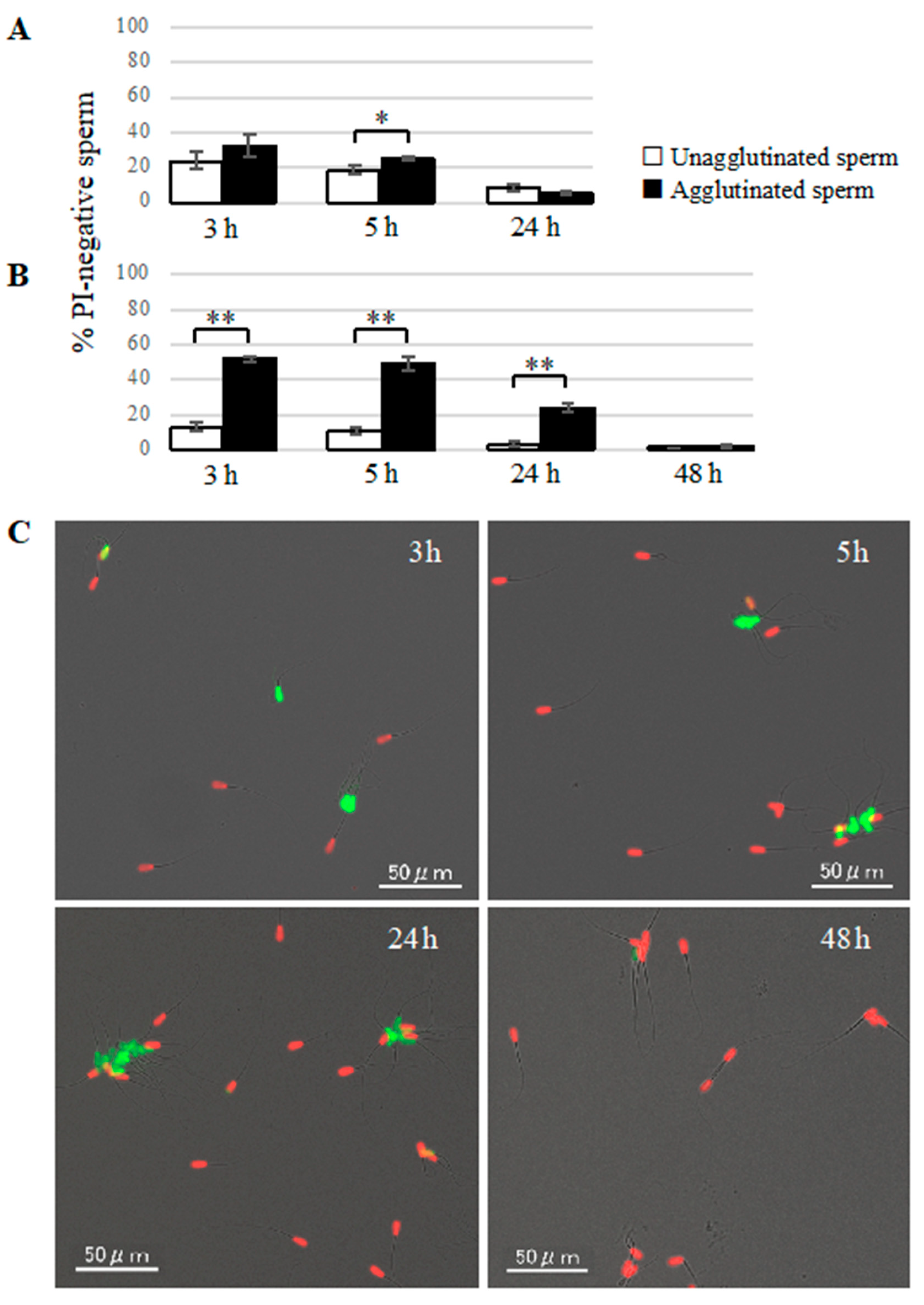
2. Suspension samples were mounted on glass slides and covered with glass coverslips, then observed under a fluorescence microscope (BZ-X710, KEYENCE). At least 100 agglutinated sperm and 100 unagglutinated sperm were divided into live sperm (SYBR-positive and PI-negative) and dead sperm (SYBR-negative and PI-positive) to compare the viability between agglutinated sperm and unagglutinated sperm.
To examine the effect of heparin on bovine sperm viability, we added a final concentration of 100 μg/mL heparin sodium to sperm samples before incubation. The samples were then stained with SYBR/PI, and incubated in non-capacitation medium supplemented with or without heparin for 2 h. After incubation, samples were observed to calculate the PI-negative rate for each group, as above-mentioned.
2.6. Comparison of Motility between Agglutinated and Unagglutinated Sperm
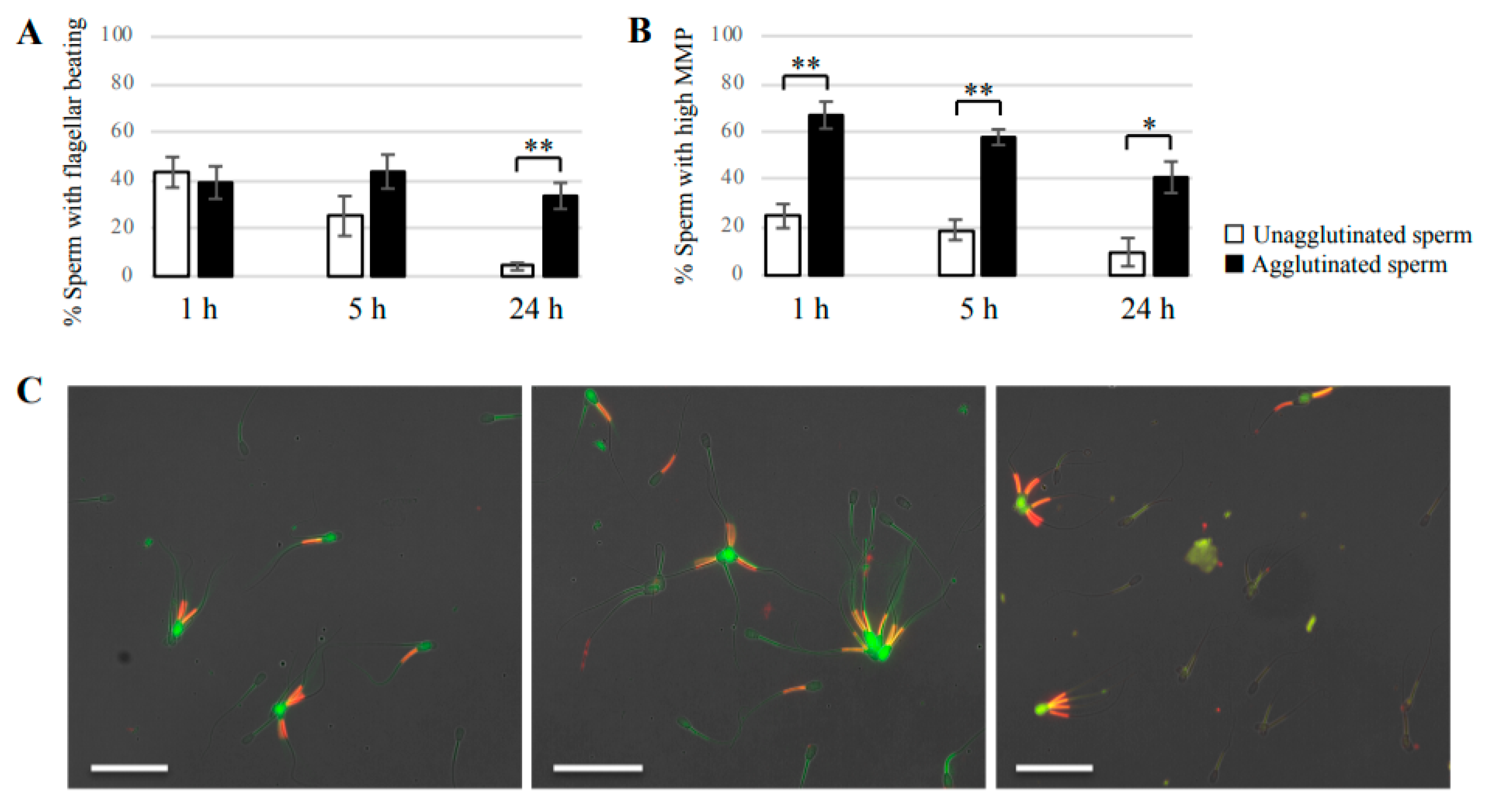
Sperm suspension was incubated for 3, 5, or 24 h in non-capacitation medium at 38.5 °C with 5% CO2. After incubation, the sample was mounted on glass slides and covered with glass coverslips, and then observed using an inverted microscope (BZ-X710, KEYENCE). To calculate the percentage of motile sperm, at least 100 agglutinated sperm or 100 unagglutinated sperm were observed to identify sperm with beating flagella or no beating flagella.
2.7. Mitochondrial Membrane Potential of Agglutinated and Unagglutinated Sperm
To compare the quality of mitochondrial function between agglutinated and unagglutinated sperm, the mitochondrial membrane potential (MMP) of sperm was evaluated by 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) staining using a commercially available kit as reported previously (MitoProbe
TM JC-1 Assay Kit, Thermo Fisher Scientific) [
20]. Briefly, sperm suspension was incubated in non-capacitation medium for 0, 5 or 24 h at 38.5 °C with 5% CO
2. After incubation, sperm samples were incubated with 2 μM JC-1 for 30 min at 38.5 °C with 5% CO
2 in the dark. Samples were then mounted on glass slides and covered with glass coverslips. Images were obtained under a fluorescence microscope. At a high MMP, JC-1 forms J-aggregates inside mitochondria and emits orange/red fluorescence, whereas in a low MMP state, JC-1 remains as a monomer and emits green fluorescence. At least 100 unagglutinated sperm and 100 agglutinated sperm were counted to determine the presence of sperm with high MMP and low MMP.
2.8. Statistical Analysis
All experiments were repeated at least three times. Data are presented as mean ± standard error (SE). Statistical analyses were carried out using the two-tailed Student’s t-test for a single comparison and the Tukey–Kramer test for multiple comparisons. Values of p < 0.05 were considered to indicate significant differences (* p < 0.05, ** p < 0.01).
4. Discussion
Despite head-to-head agglutination of bovine sperm being first reported in 1950s [
15], there has been little investigation of this unique sperm–sperm interaction. Here, we investigated why many bull sperm agglutinate with each other, and propose the possible role of mammalian sperm agglutination in the fertilization process.
This study found temporal changes in the degree of head-to-head agglutination of bull sperm in in vitro culture. We revealed that agglutination occurred in the capacitation medium, and also in the non-capacitation medium containing minerals, sodium lactate, and sodium pyruvate, but lacking known agglutination inducers such as serum. Although several sperm agglutination inducers have been reported in many species including cattle [
7,
9,
10,
11,
12], our results indicated that bovine sperm intrinsically agglutinate for a specific purpose. To determine this purpose, we examined the relationship between sperm agglutination and fundamental functional characteristics such as sperm motility and viability.
Our study showed that agglutinated sperm exhibited increased motility, viability (more PI-negative cells), and elevated intact mitochondrial function compared with unagglutinated sperm. Furthermore, heparin inhibited sperm agglutination but did not affect the viability of unagglutinated sperm, suggesting that sperm agglutination dictated sperm viability. These findings indicate that agglutinated sperm have a longer lifespan in culture, representing a potential role for sperm head-to-head agglutination in the fertilization process. Sperm with a longer lifespan will have a greater chance to fertilize an oocyte, so sperm agglutination may enhance successful fertilization. Interestingly, sperm viability was also maintained by sperm binding to oviductal epithelial cells, the sperm–oviduct interaction [
2]. Thus, sperm may be able to survive for longer periods if they physically bind to other cells. In addition to sperm viability, the sperm acrosome, an organelle located on the sperm head where it serves an important role in fertilization, was reported to be preserved by both sperm agglutination and sperm binding to the oviduct [
11,
26]. Furthermore, we often observed agglutinated sperm attached to the surface of glass slides (
Video S4). Observations in previous in vitro studies also suggested that some sperm bound to the oviduct was agglutinated [
26,
27,
28]. Considering these findings, we propose that these sperm–sperm and sperm–oviduct interactions are functionally similar, and may coincide in the oviduct.
It is well-known that the BSP proteins play a crucial role in attaching sperm to the oviduct in cows [
16], and heparin, which specifically binds to BSP proteins on sperm, facilitates the detachment of sperm from the oviduct [
23,
24,
25]. Our results also indicated that the head-to-head agglutination of bovine sperm was suppressed by heparin. Considering the BSP proteins are located in the sperm head [
29], we propose that heparin interacts with the BSP proteins on the surface of unagglutinated sperm heads, preventing them from attaching to each other. Thus, the heparin–BSP protein interactions are involved in bovine sperm agglutination as well as sperm attachment to the oviduct. Therefore, sperm–sperm and sperm–oviduct interactions may involve similar features and regulatory molecules. Although the presence of heparin or heparin-like glycosaminoglycans has been reported in the oviduct, ooplasm, and follicular fluid [
30,
31,
32], our results suggest that heparin was not involved in releasing sperm from agglutination. There is limited information about the process and associated factors that facilitate the detachment of sperm from each other, but it was reported that hamster sperm agglutination breaks apart when sperm motility becomes hyperactivated [
7]. Interestingly, hyperactivation was also reported to be involved in releasing sperm from the oviduct [
16]. Thus, we propose that sperm maintains viability by binding to other sperm or the oviduct, and are then released by hyperactivated motility or unknown factors to increase the possibility of successful fertilization and prevent polyspermy.
We found that agglutination composed of two bovine sperm accounted for the major portion of head-to-head agglutination, although we also observed agglutination ranging from two to over ten sperm. The agglutination of two sperm, termed sperm pairs, was first described in bull in this study, and has been also well-described in marsupials such as opossums [
7,
33]. Therefore, sperm pairs may be prevalent in mammals. By focusing on two-sperm agglutination, we also examined the ability of agglutinated bovine sperm to form pairs with sperm from a different bull, or with boar sperm. Our results showed that bovine sperm do not preferentially agglutinate with sperm from the same male, but do preferentially agglutinate with sperm from the same species. This finding contrasts with a previous study of promiscuous deer mice showing that sperm from the same male agglutinated to enhance swimming velocity, and cooperated with each other in a competitive environment [
13]. It is possible that cows are not normally inseminated by several different males in a short period of time, so bovine sperm do not have selective pressure to distinguish between sperm from different males, unlike promiscuous animals. In contrast, bovine sperm are likely to have the ability to distinguish between sperm from different species. However, it should be noted that we observed some two-sperm agglutinations composed of bovine and boar sperm with beating flagella, suggesting that there may be a weak interaction between bovine and boar sperm. These findings indicate that most of the mechanisms and/or molecular factors of sperm agglutination are species-specific in mammals, although some similarity is present between bull and boar. To the best of our knowledge, this is the first description of inter-species sperm agglutination in mammals
In summary, we have demonstrated that agglutinated bovine sperm maintains viability and motility for a longer time in culture, although they do not preferentially agglutinate with sperm from the same male. We propose that head-to-head agglutination is an important sperm–sperm interaction that may enhance the fertilizing ability of sperm. Our study has revealed functional characteristics and the possible biological significance for this unique sperm–sperm interaction. This may represent the first evidence of sperm cooperation in the bull, reflecting a process also in other mammals including rodents and marsupials [
7]. The extended function of agglutinated sperm may explain why so many sperm enter the female reproductive tract. Further study, notably in vivo investigations, are required to examine whether the role of sperm agglutination proposed in this study can be applied to the in vivo fertilization process in mammals.