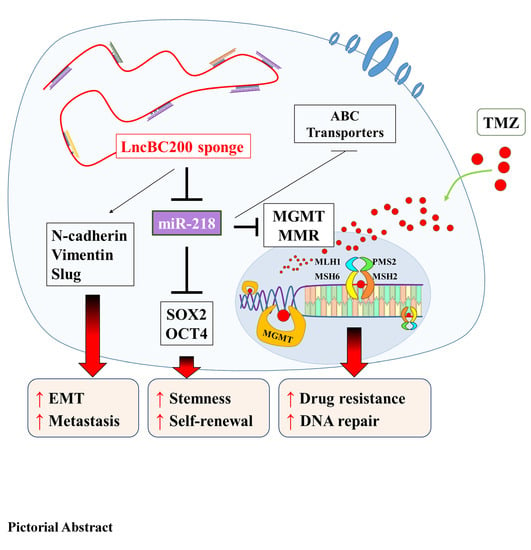
Targeting BC200/miR218-5p Signaling Axis for Overcoming Temozolomide Resistance and Suppressing Glioma Stemness
Abstract
Share and Cite
Su, Y.-K.; Lin, J.W.; Shih, J.-W.; Chuang, H.-Y.; Fong, I.-H.; Yeh, C.-T.; Lin, C.-M. Targeting BC200/miR218-5p Signaling Axis for Overcoming Temozolomide Resistance and Suppressing Glioma Stemness. Cells 2020, 9, 1859. https://doi.org/10.3390/cells9081859
Su Y-K, Lin JW, Shih J-W, Chuang H-Y, Fong I-H, Yeh C-T, Lin C-M. Targeting BC200/miR218-5p Signaling Axis for Overcoming Temozolomide Resistance and Suppressing Glioma Stemness. Cells. 2020; 9(8):1859. https://doi.org/10.3390/cells9081859
Chicago/Turabian StyleSu, Yu-Kai, Jia Wei Lin, Jing-Wen Shih, Hao-Yu Chuang, Iat-Hang Fong, Chi-Tai Yeh, and Chien-Min Lin. 2020. "Targeting BC200/miR218-5p Signaling Axis for Overcoming Temozolomide Resistance and Suppressing Glioma Stemness" Cells 9, no. 8: 1859. https://doi.org/10.3390/cells9081859
APA StyleSu, Y.-K., Lin, J. W., Shih, J.-W., Chuang, H.-Y., Fong, I.-H., Yeh, C.-T., & Lin, C.-M. (2020). Targeting BC200/miR218-5p Signaling Axis for Overcoming Temozolomide Resistance and Suppressing Glioma Stemness. Cells, 9(8), 1859. https://doi.org/10.3390/cells9081859