Ligation of MHC Class II Induces PKC-Dependent Clathrin-Mediated Endocytosis of MHC Class II
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mice
2.3. Preparation of BMDCs
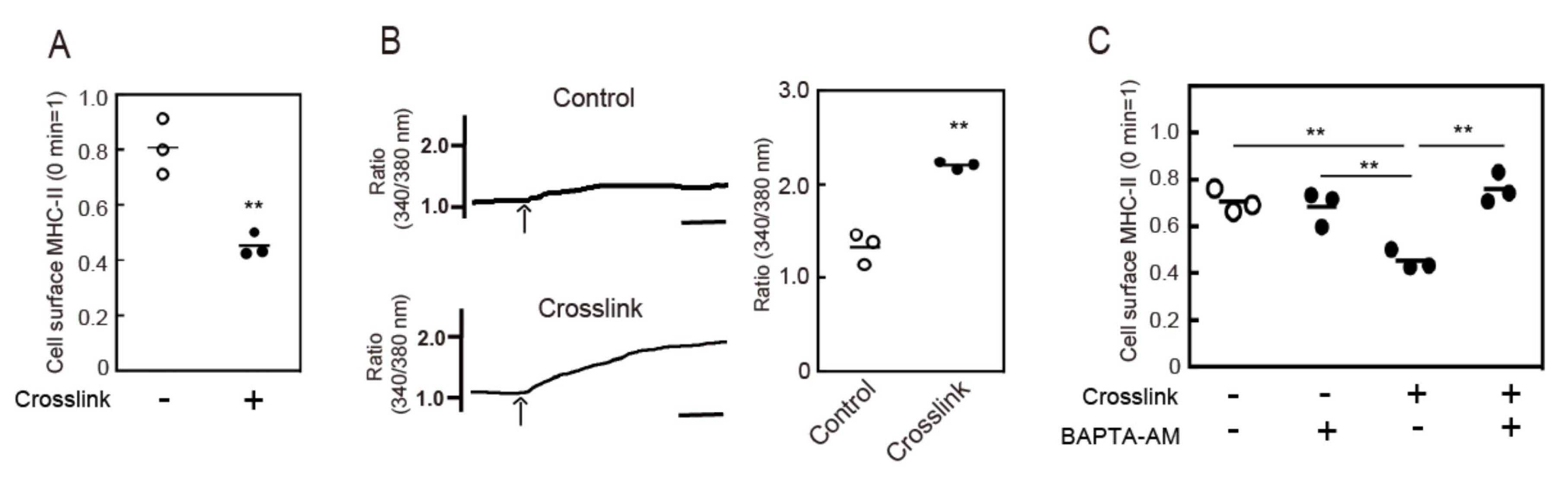
2.4. Crosslinking of Cell Surface MHC-II
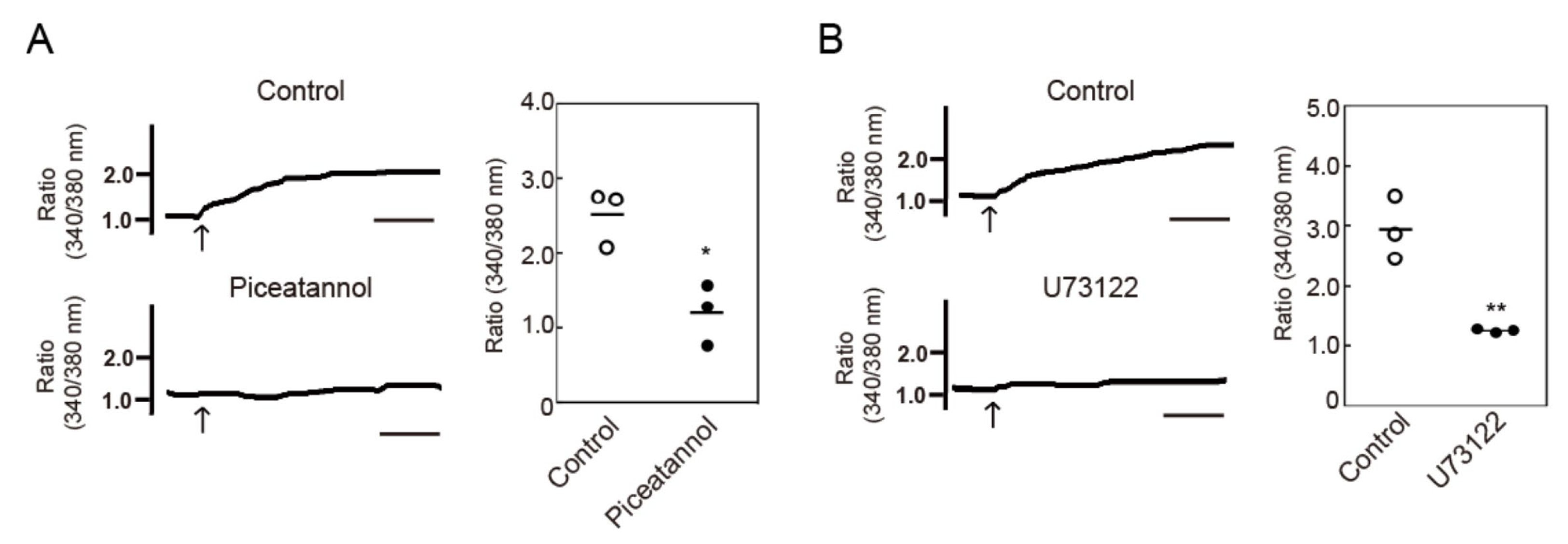
2.5. Measurement of Cytosolic Ca2+ Concentrations
2.6. Immunofluorescence Microscopy
2.7. Statistical Analysis
3. Results
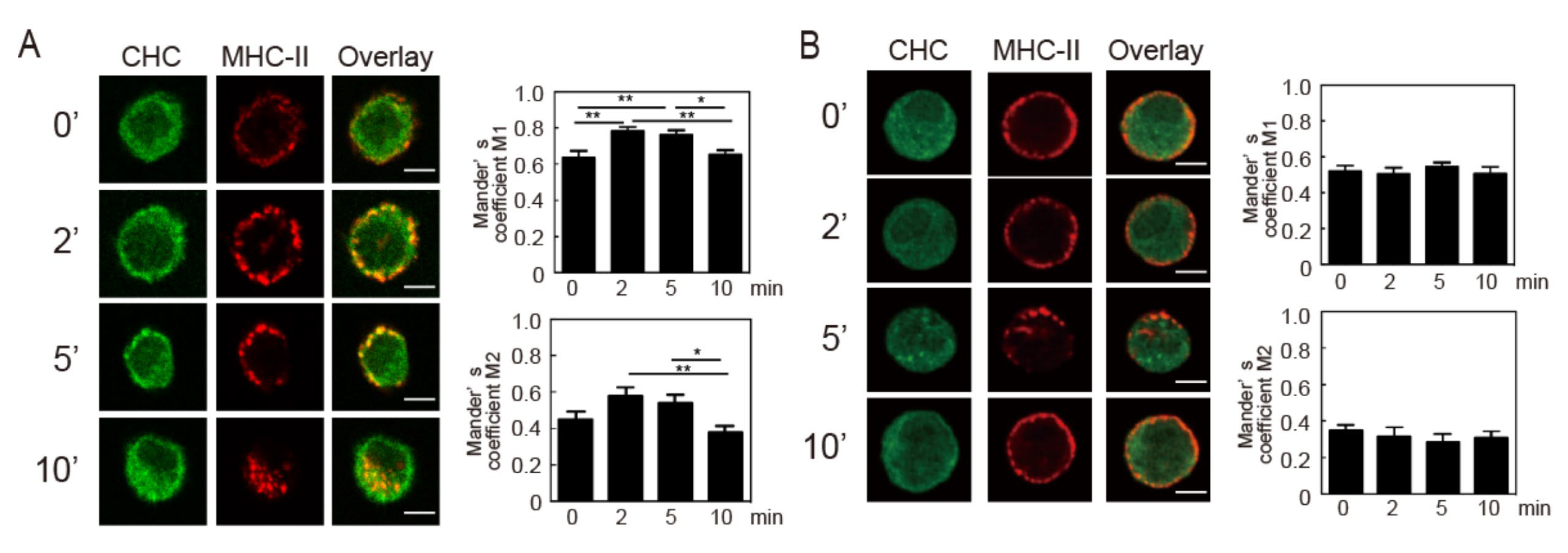
3.1. Crosslinking-Induced Intracellular Ca2+ Mobilization is Required for Endocytosis of MHC-II
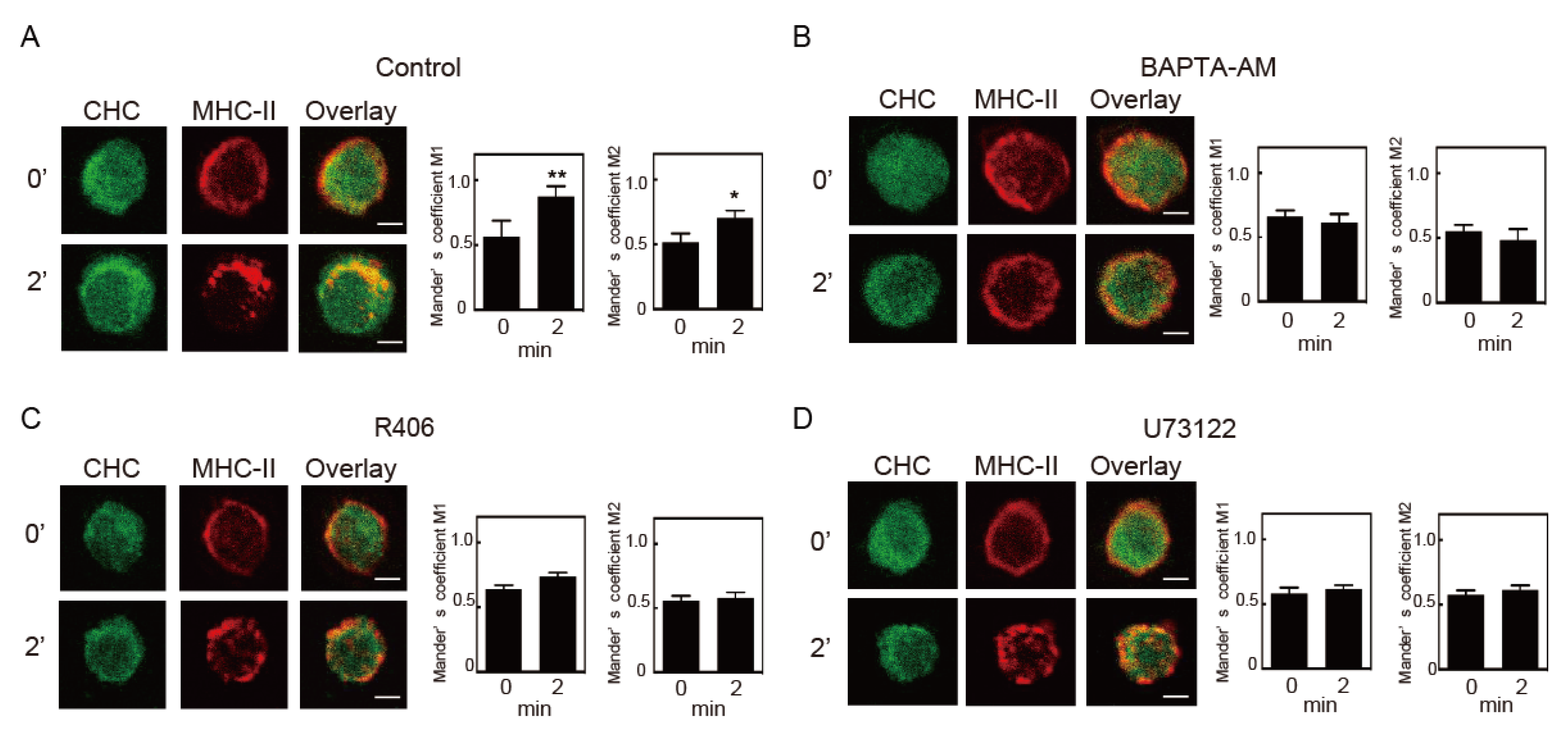
3.2. Intracellular Ca2+ Mobilization Induces Endocytosis of MHC-II
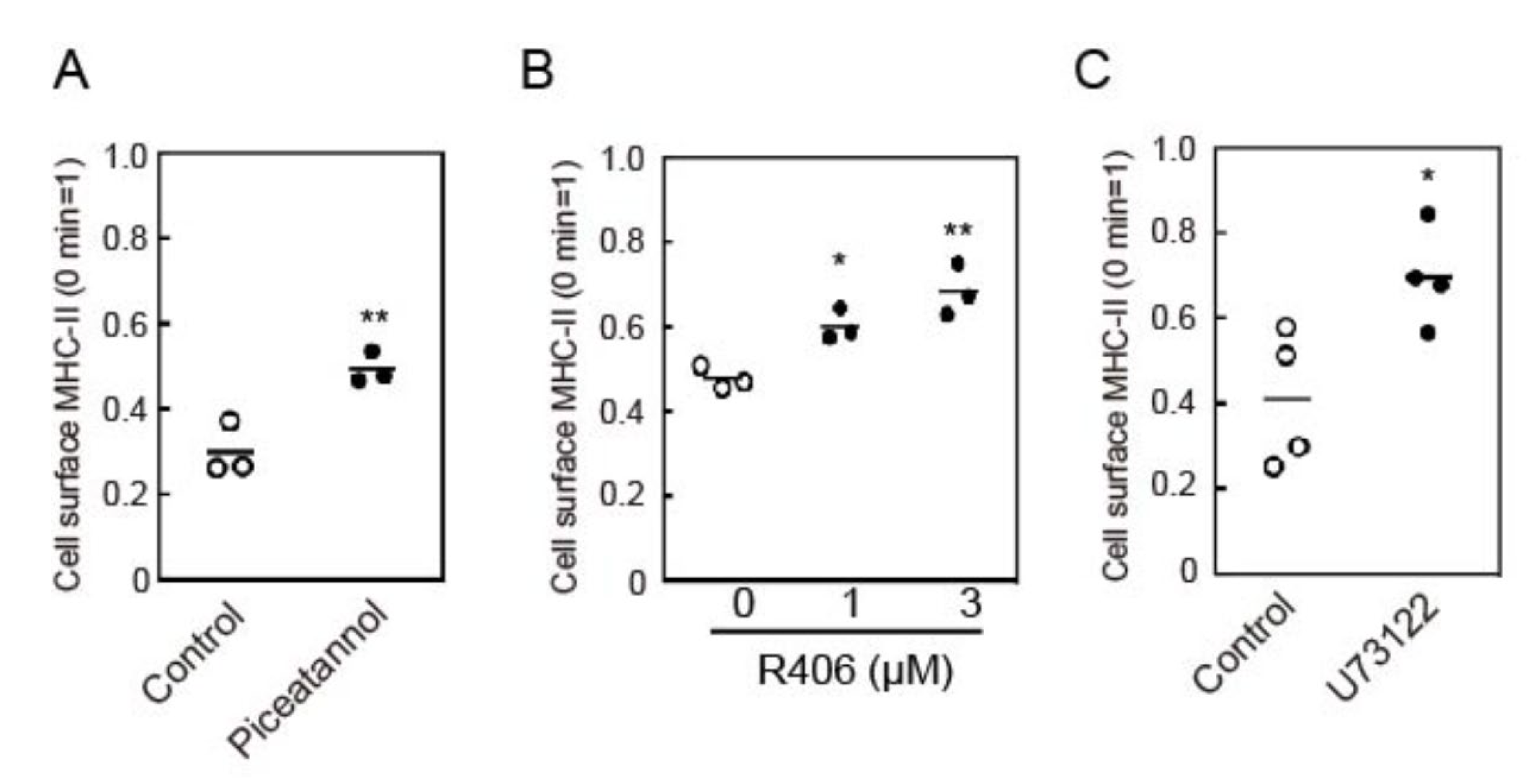
3.3. Crosslinking-Induced Endocytosis of MHC-II is Syk and Phospholipase C (PLC)-Dependent
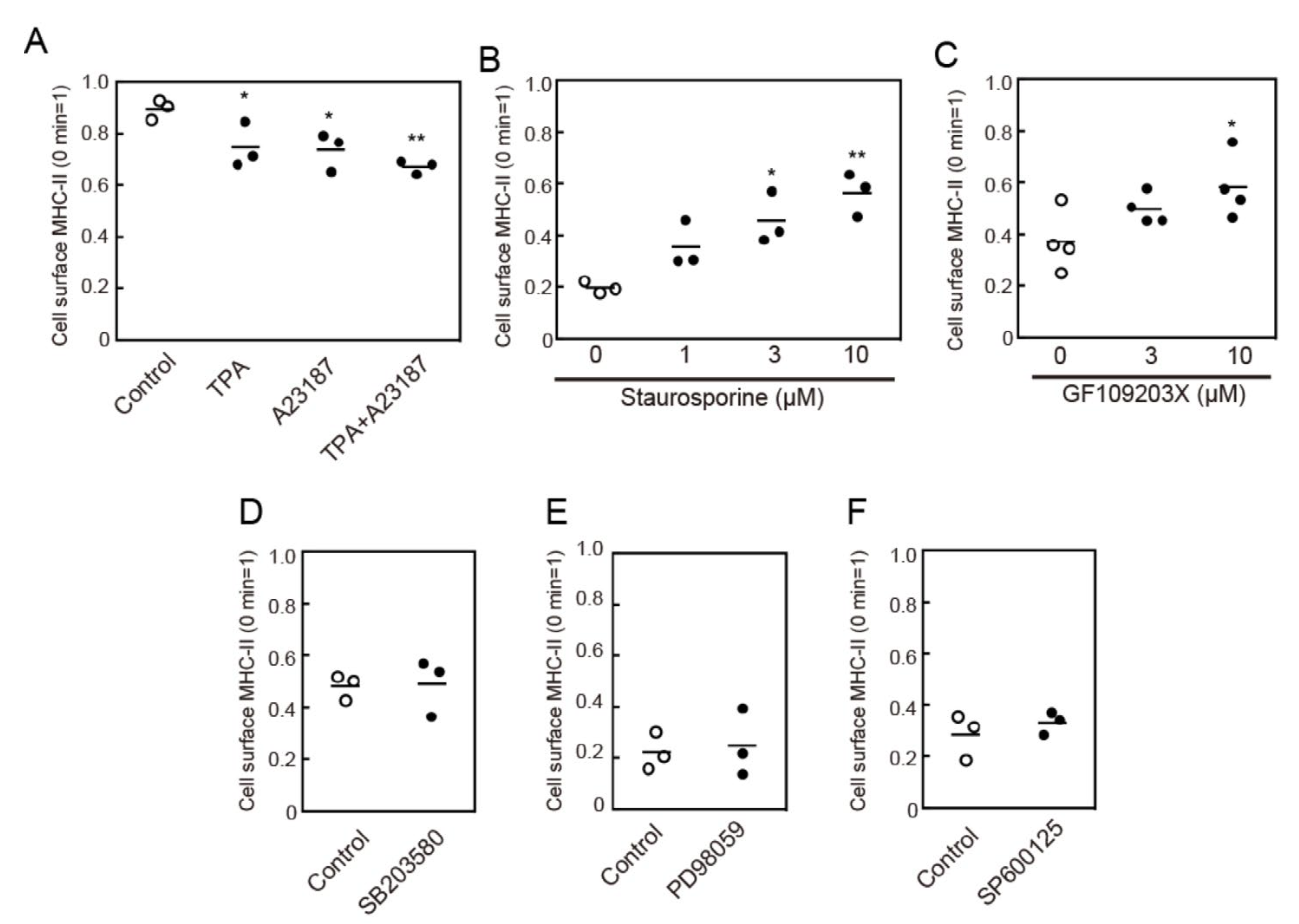
3.4. Protein Kinase C (PKC) Activation is Involved in MHC-II Endocytosis
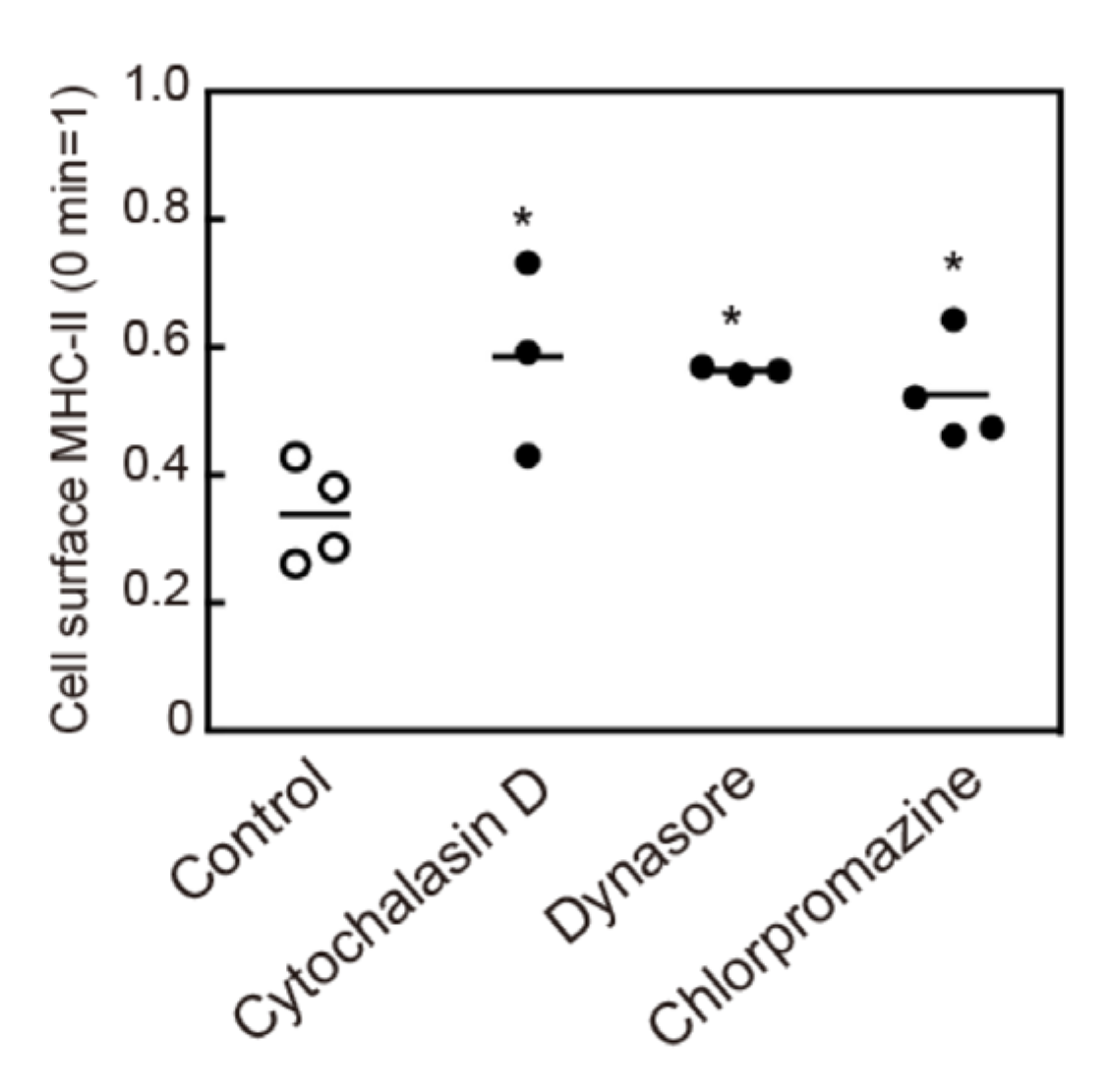
3.5. Crosslinking-Induced Endocytosis of MHC-II is a Clathrin-Dependent Response
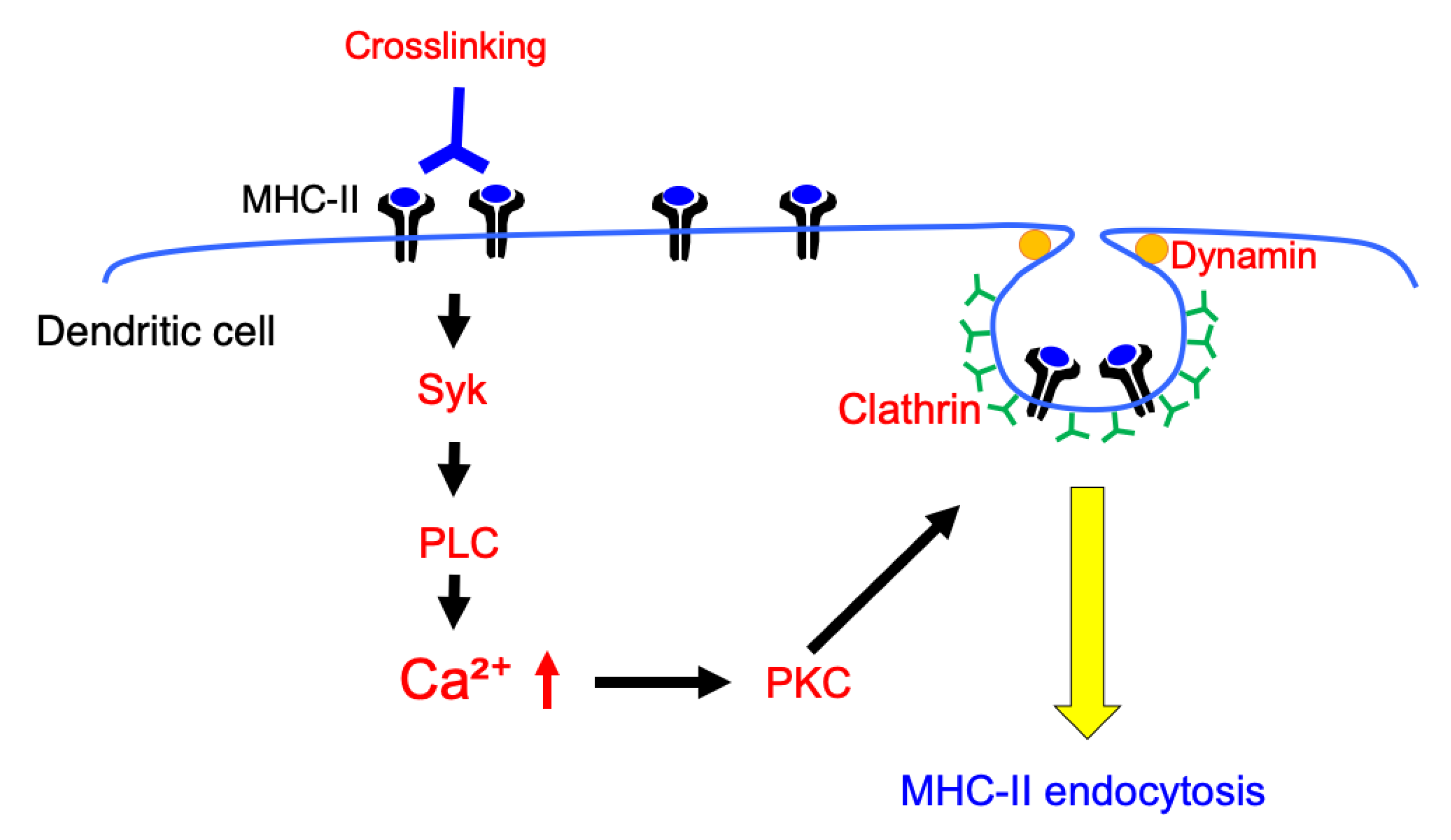
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mellman, I.; Steinman, R.M. Dendritic cells: Specialized and regulated antigen processing machines. Cell 2001, 106, 255–258. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, E.S.; Mellman, I. Cell biology of antigen processing in vitro and in vivo. Annu. Rev. Immunol. 2005, 23, 975–1028. [Google Scholar] [CrossRef] [PubMed]
- Roche, P.A.; Furuta, K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 2015, 15, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Inaba, K.; Turley, S.; Iyoda, T.; Yamaide, F.; Shimoyama, S.; Reis e Sousa, C.; Germain, R.N.; Mellman, I.; Steinman, R.M. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J. Exp. Med. 2000, 191, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Al-Daccak, R.; Mooney, N.; Charron, D. MHC class II signaling in antigen-presenting cells. Curr. Opin. Immunol. 2004, 16, 108–113. [Google Scholar] [CrossRef]
- Liang, B.; Workman, C.; Lee, J.; Chew, C.; Dale, B.M.; Colonna, L.; Flores, M.; Li, N.; Schweighoffer, E.; Greenberg, S.; et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J. Immunol. 2008, 180, 5916–5926. [Google Scholar] [CrossRef]
- Furuta, K.; Ishido, S.; Roche, P.A. Encounter with antigen-specific primed CD4 T cells promotes MHC class II degradation in dendritic cells. Proc. Natl. Acad. Sci. USA 2012, 109, 19380–19385. [Google Scholar] [CrossRef]
- Wolfe, B.L.; Trejo, J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic 2007, 8, 462–470. [Google Scholar] [CrossRef]
- Doherty, G.J.; McMahon, H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef]
- Mayor, S.; Pagano, R.E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Walseng, E.; Bakke, O.; Roche, P.A. Major histocompatibility complex class II-peptide complexes internalize using a clathrin- and dynamin-independent endocytosis pathway. J. Biol. Chem. 2008, 283, 14717–14727. [Google Scholar] [CrossRef] [PubMed]
- Inaba, K.; Inaba, M.; Romani, N.; Aya, H.; Deguchi, M.; Ikehara, S.; Muramatsu, S.; Steinman, R.M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992, 176, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Furuta, K.; Walseng, E.; Roche, P.A. Internalizing MHC class II-peptide complexes are ubiquitinated in early endosomes and targeted for lysosomal degradation. Proc. Natl. Acad. Sci. USA 2013, 110, 20188–20193. [Google Scholar] [CrossRef]
- Manders, E.M.M.; Verbeek, F.J.; Aten, J.A. Measurement of co-localization of objects in dual-colour confocal images. J. Microsc. 1992, 169, 375–382. [Google Scholar] [CrossRef]
- Nashar, T.O.; Drake, J.R. Dynamics of MHC class II-activating signals in murine resting B cells. J. Immunol. 2006, 176, 827–838. [Google Scholar] [CrossRef]
- Jin, L.; Stolpa, J.C.; Young, R.M.; Pugh-Bernard, A.E.; Refaeli, Y.; Cambier, J.C. MHC class II structural requirements for the association with Igalpha/beta, and signaling of calcium mobilization and cell death. Immunol. Lett. 2008, 116, 184–194. [Google Scholar] [CrossRef]
- Mooney, N.A.; Grillot-Courvalin, C.; Hivroz, C.; Ju, L.Y.; Charron, D. Early biochemical events after MHC class II-mediated signaling on human B lymphocytes. J. Immunol. 1990, 145, 2070–2076. [Google Scholar]
- Xu, S.; Huo, J.; Lee, K.G.; Kurosaki, T.; Lam, K.P. Phospholipase Cgamma2 is critical for Dectin-1-mediated Ca2+ flux and cytokine production in dendritic cells. J. Biol. Chem. 2009, 284, 7038–7046. [Google Scholar] [CrossRef]
- Vergarajauregui, S.; San Miguel, A.; Puertollano, R. Activation of p38 mitogen-activated protein kinase promotes epidermal growth factor receptor internalization. Traffic 2006, 7, 686–698. [Google Scholar] [CrossRef]
- Ivanov, A.I. Pharmacological inhibition of endocytic pathways: Is it specific enough to be useful? Methods Mol. Biol. 2008, 440, 15–33. [Google Scholar] [PubMed]
- Wang, L.H.; Rothberg, K.G.; Anderson, R.G. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 1993, 123, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, M.B.; Lanier, L.L.; Nakamura, M.C. Role of ITAM-containing adapter proteins and their receptors in the immune system and bone. Immunol. Rev. 2005, 208, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Draber, P.; Vonkova, I.; Stepanek, O.; Hrdinka, M.; Kucova, M.; Skopcova, T.; Otahal, P.; Angelisova, P.; Horejsi, V.; Yeung, M.; et al. SCIMP, a transmembrane adaptor protein involved in major histocompatibility complex class II signaling. Mol. Cell. Biol. 2011, 31, 4550–4562. [Google Scholar] [CrossRef]
- Jin, L.; Waterman, P.M.; Jonscher, K.R.; Short, C.M.; Reisdorph, N.A.; Cambier, J.C. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol. Cell. Biol. 2008, 28, 5014–5026. [Google Scholar] [CrossRef]
- Leveille, C.; Castaigne, J.G.; Charron, D.; Al-Daccak, R. MHC class II isotype-specific signaling complex on human B cells. Eur. J. Immunol. 2002, 32, 2282–2291. [Google Scholar] [CrossRef]
- Guo, W.; Castaigne, J.G.; Mooney, N.; Charron, D.; Al-Daccak, R. Signaling through HLA-DR induces PKC beta-dependent B cell death outside rafts. Eur. J. Immunol. 2003, 33, 928–938. [Google Scholar] [CrossRef]
- Bertho, N.; Blancheteau, V.M.; Setterblad, N.; Laupeze, B.; Lord, J.M.; Drenou, B.; Amiot, L.; Charron, D.J.; Fauchet, R.; Mooney, N. MHC class II-mediated apoptosis of mature dendritic cells proceeds by activation of the protein kinase C-delta isoenzyme. Int. Immunol. 2002, 14, 935–942. [Google Scholar] [CrossRef]
- Lokshin, A.E.; Kalinski, P.; Sassi, R.R.; Mailliard, R.B.; Muller-Berghaus, J.; Storkus, W.J.; Peng, X.; Marrangoni, A.M.; Edwards, R.P.; Gorelik, E. Differential regulation of maturation and apoptosis of human monocyte-derived dendritic cells mediated by MHC class II. Int. Immunol. 2002, 14, 1027–1037. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, N.; Zheng, M.; Kim, K.M. Clathrin-mediated endocytosis is responsible for the lysosomal degradation of dopamine D3 receptor. Biochem. Biophys. Res. Commun. 2016, 476, 245–251. [Google Scholar] [CrossRef]
- Bonefeld, C.M.; Rasmussen, A.B.; Lauritsen, J.P.; von Essen, M.; Odum, N.; Andersen, P.S.; Geisler, C. TCR comodulation of nonengaged TCR takes place by a protein kinase C and CD3 gamma di-leucine-based motif-dependent mechanism. J. Immunol. 2003, 171, 3003–3009. [Google Scholar] [CrossRef] [PubMed]
- Monjas, A.; Alcover, A.; Alarcon, B. Engaged and bystander T cell receptors are down-modulated by different endocytotic pathways. J. Biol. Chem. 2004, 279, 55376–55384. [Google Scholar] [CrossRef] [PubMed]
- Von Essen, M.; Nielsen, M.W.; Bonefeld, C.M.; Boding, L.; Larsen, J.M.; Leitges, M.; Baier, G.; Odum, N.; Geisler, C. Protein kinase C (PKC) alpha and PKC theta are the major PKC isotypes involved in TCR down-regulation. J. Immunol. 2006, 176, 7502–7510. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Arenas, E.; Calleja, E.; Martinez-Martin, N.; Gharbi, S.I.; Navajas, R.; Garcia-Medel, N.; Penela, P.; Alcami, A.; Mayor, F., Jr.; Albar, J.P.; et al. Beta-Arrestin-1 mediates the TCR-triggered re-routing of distal receptors to the immunological synapse by a PKC-mediated mechanism. EMBO J. 2014, 33, 559–577. [Google Scholar] [CrossRef]
- Vina-Vilaseca, A.; Bender-Sigel, J.; Sorkina, T.; Closs, E.I.; Sorkin, A. Protein kinase C-dependent ubiquitination and clathrin-mediated endocytosis of the cationic amino acid transporter CAT-1. J. Biol. Chem. 2011, 286, 8697–8706. [Google Scholar] [CrossRef]
- Garcia-Tardon, N.; Gonzalez-Gonzalez, I.M.; Martinez-Villarreal, J.; Fernandez-Sanchez, E.; Gimenez, C.; Zafra, F. Protein kinase C (PKC)-promoted endocytosis of glutamate transporter GLT-1 requires ubiquitin ligase Nedd4-2-dependent ubiquitination but not phosphorylation. J. Biol. Chem. 2012, 287, 19177–19187. [Google Scholar] [CrossRef]
- Lajoie, P.; Nabi, I.R. Regulation of raft-dependent endocytosis. J. Cell. Mol. Med. 2007, 11, 644–653. [Google Scholar] [CrossRef]
- Stoddart, A.; Dykstra, M.L.; Brown, B.K.; Song, W.; Pierce, S.K.; Brodsky, F.M. Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity 2002, 17, 451–462. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masaki, K.; Hiraki, Y.; Onishi, H.; Satoh, Y.; Roche, P.A.; Tanaka, S.; Furuta, K. Ligation of MHC Class II Induces PKC-Dependent Clathrin-Mediated Endocytosis of MHC Class II. Cells 2020, 9, 1810. https://doi.org/10.3390/cells9081810
Masaki K, Hiraki Y, Onishi H, Satoh Y, Roche PA, Tanaka S, Furuta K. Ligation of MHC Class II Induces PKC-Dependent Clathrin-Mediated Endocytosis of MHC Class II. Cells. 2020; 9(8):1810. https://doi.org/10.3390/cells9081810
Chicago/Turabian StyleMasaki, Kento, Yuhji Hiraki, Hiroka Onishi, Yuka Satoh, Paul A. Roche, Satoshi Tanaka, and Kazuyuki Furuta. 2020. "Ligation of MHC Class II Induces PKC-Dependent Clathrin-Mediated Endocytosis of MHC Class II" Cells 9, no. 8: 1810. https://doi.org/10.3390/cells9081810
APA StyleMasaki, K., Hiraki, Y., Onishi, H., Satoh, Y., Roche, P. A., Tanaka, S., & Furuta, K. (2020). Ligation of MHC Class II Induces PKC-Dependent Clathrin-Mediated Endocytosis of MHC Class II. Cells, 9(8), 1810. https://doi.org/10.3390/cells9081810





