Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases
Abstract
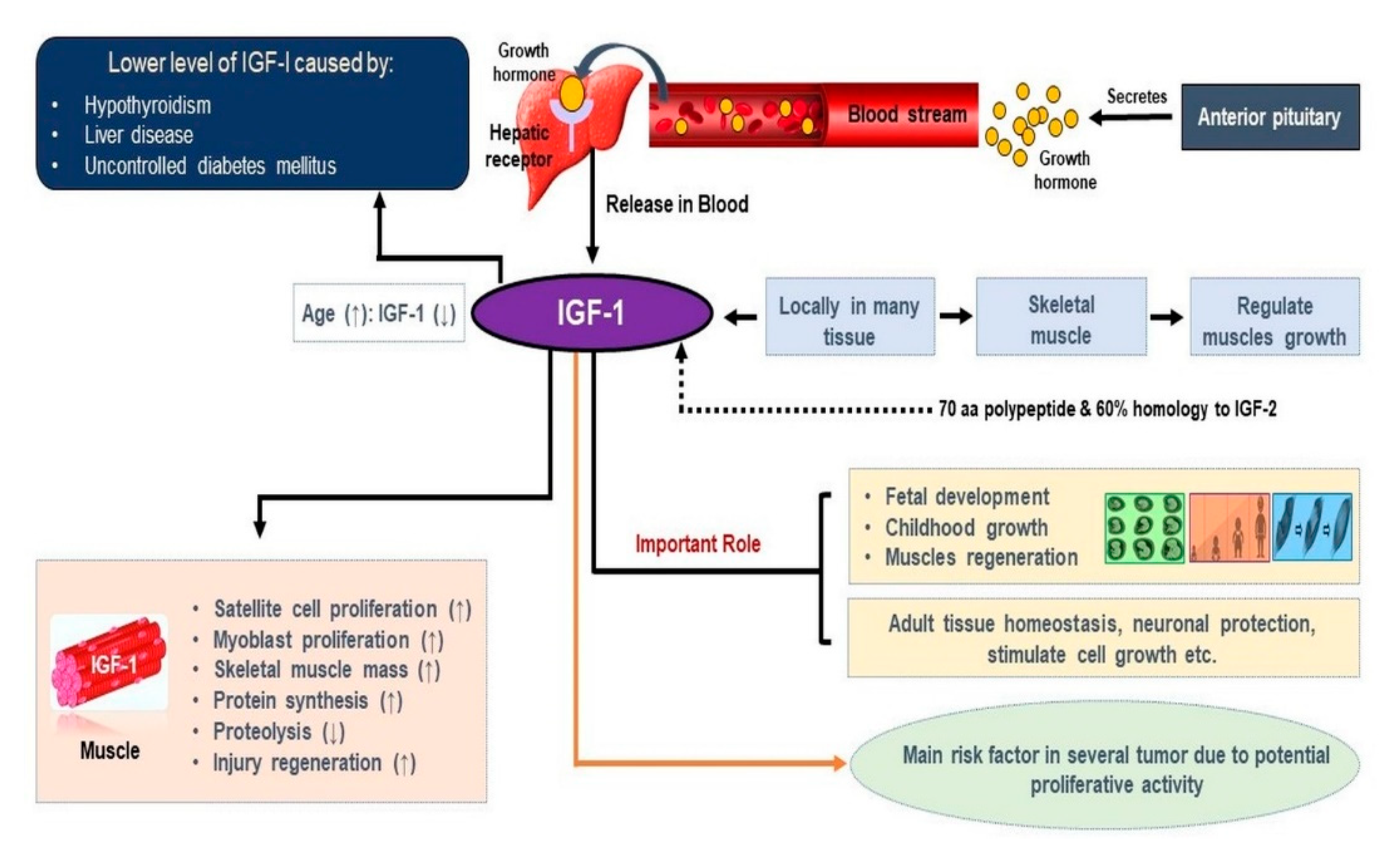
1. Introduction
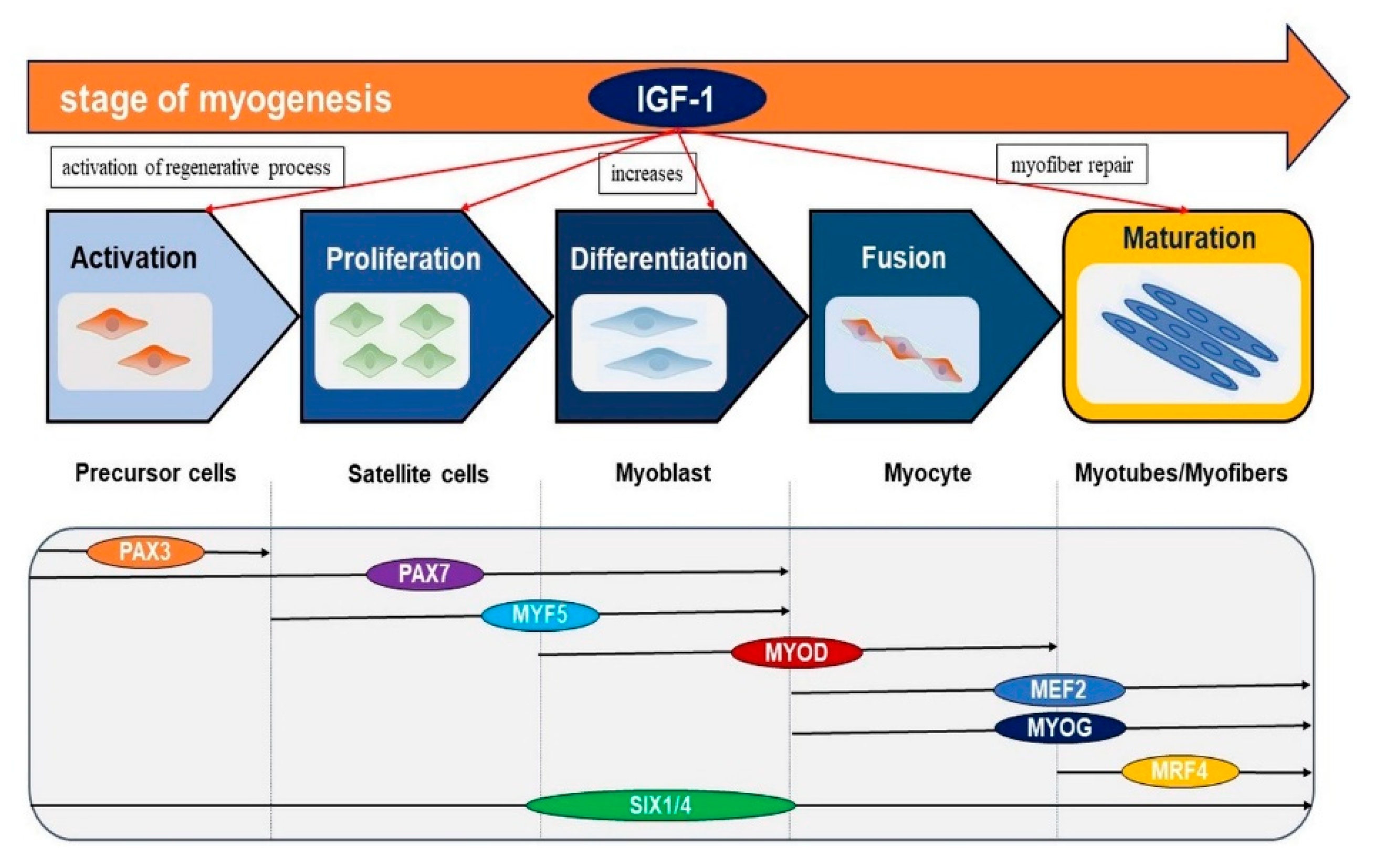
2. Role of IGF-1 in Skeletal Muscle
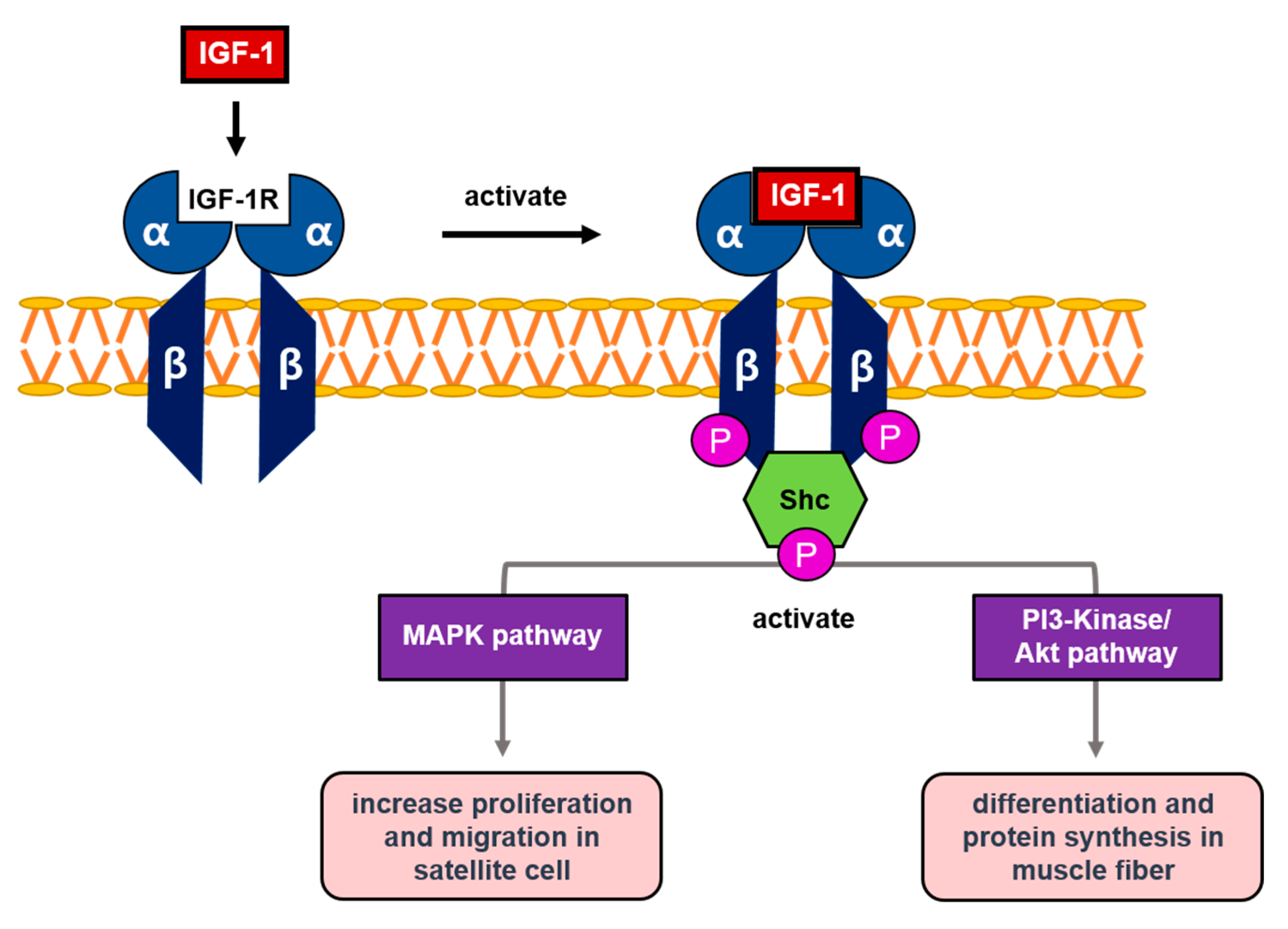
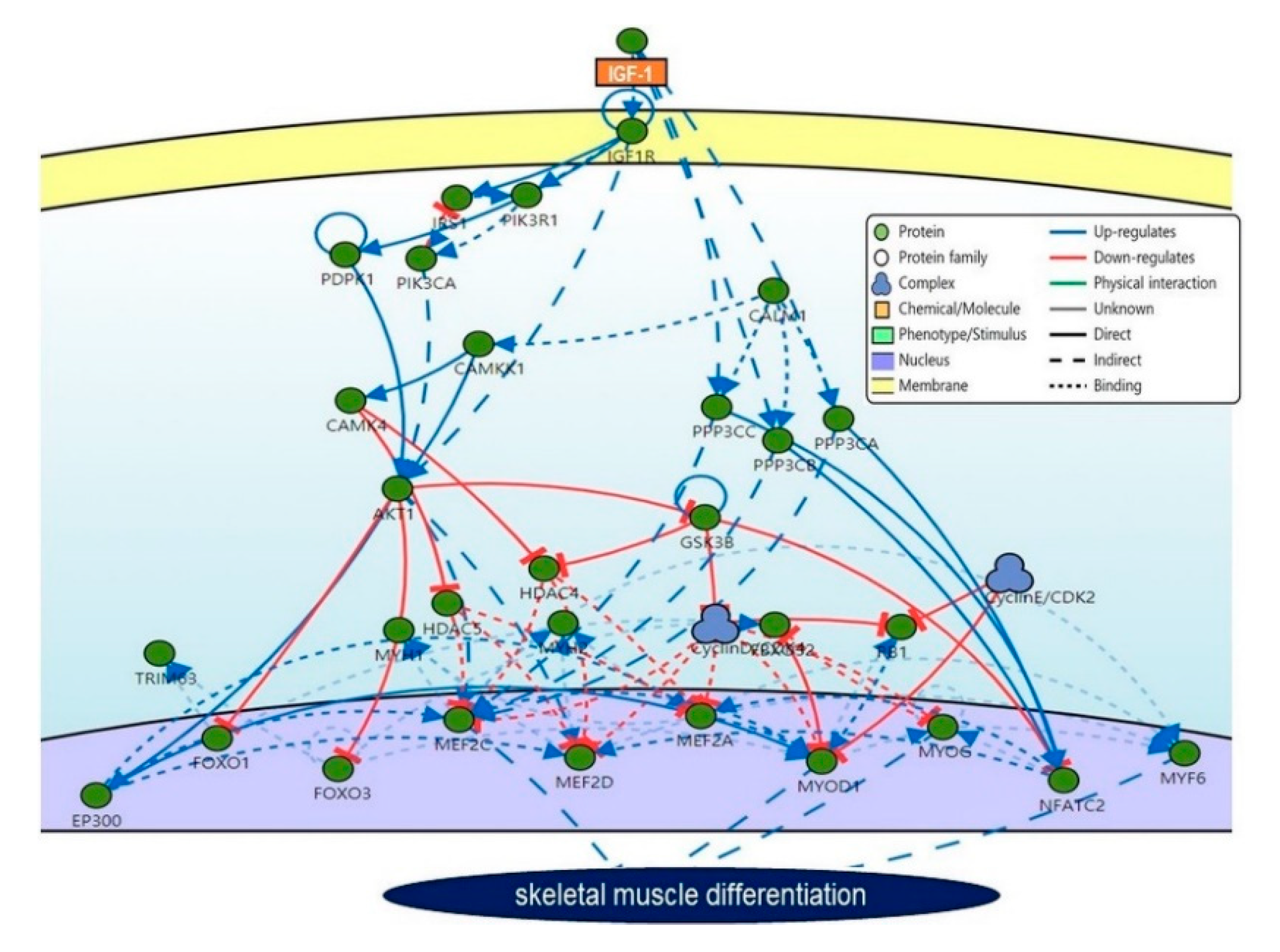
3. Mechanism of IGF-1 in Skeletal Muscle
4. Relationship Between IGF-1 and Myostatin
5. Role of IGF-1 in Different Diseases
5.1. Role of IGF-1 in Duchenne Muscular Dystrophy
5.2. Role of IGF-1 in Muscle Atrophy
5.3. Role of IGF-1 in Cancer
5.4. Role of IGF-1 in Neurodegeneration
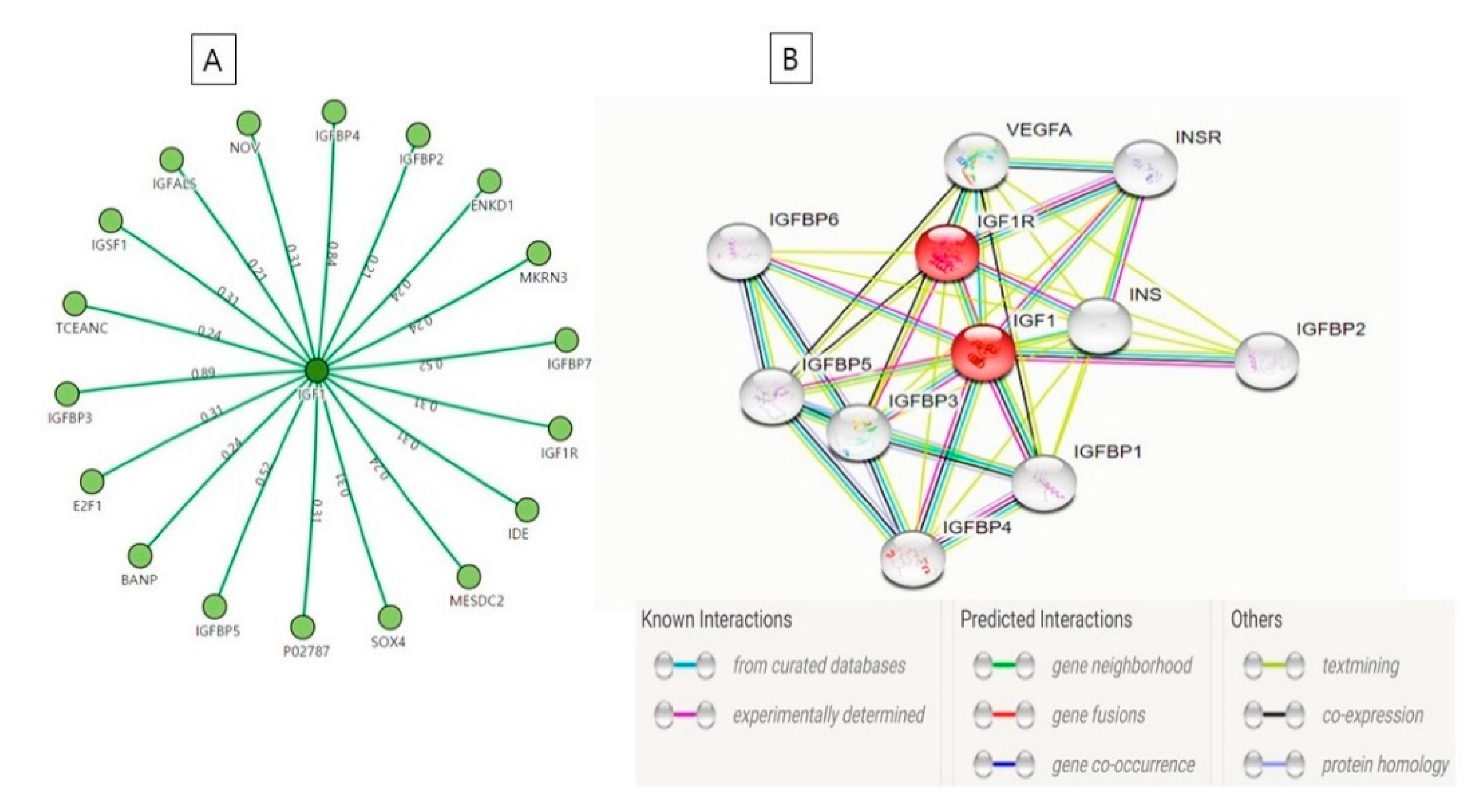
6. Interaction Between IGF-1 and IGF-1R
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SM | skeletal muscle |
| GFs | growth factors |
| ECM | extracellular matrix |
| MSC | muscle satellite cell |
| IGF-1 | insulin-like growth factor-1 |
| IGF-1R | insulin-like growth factor type 1 receptor |
| TGF-β | transforming growth factor beta |
| GH | growth hormone |
| MSTN | myostatin |
| DMD | Duchenne muscular dystrophy |
| MA | muscle atrophy |
| IGFBP | IGF binding proteins |
References
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Ahmad, K.; Shaikh, S.; Jan, A.T.; Seo, M.G.; Lee, E.J.; Choi, I. Dermatopontin in skeletal muscle extracellular matrix regulates myogenesis. Cells 2019, 8, 332. [Google Scholar] [CrossRef] [PubMed]
- Langlois, S.; Cowan, K.N. Regulation of skeletal muscle myoblast differentiation and proliferation by pannexins. Adv. Exp. Med. Biol. 2017, 925, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Pokharel, S.; Jan, A.T.; Huh, S.; Galope, R.; Lim, J.H.; Lee, D.M.; Choi, S.W.; Nahm, S.S.; Kim, Y.W.; et al. Transthyretin: A transporter protein essential for proliferation of myoblast in the myogenic program. Int. J. Mol. Sci. 2017, 18, 115. [Google Scholar] [CrossRef]
- Halper, J. Growth factors as active participants in carcinogenesis: A perspective. Vet. Pathol. 2010, 47, 77–97. [Google Scholar] [CrossRef]
- Vlasova-St Louis, I.; Bohjanen, P.R. Post-transcriptional regulation of cytokine and growth factor signaling in cancer. Cytokine Growth Factor Rev. 2017, 33, 83–93. [Google Scholar] [CrossRef]
- Chen, F.M.; Zhang, M.; Wu, Z.F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials 2010, 31, 6279–6308. [Google Scholar] [CrossRef]
- Macri, L.; Clark, R.A. Tissue engineering for cutaneous wounds: Selecting the proper time and space for growth factors, cells and the extracellular matrix. Skin Pharmacol. Physiol. 2009, 22, 83–93. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic wound healing: A review of current management and treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Lee, E.J.; Jan, A.T.; Baig, M.H.; Ashraf, J.M.; Nahm, S.-S.; Kim, Y.-W.; Park, S.-Y.; Choi, I. Fibromodulin: A master regulator of myostatin controlling progression of satellite cells through a myogenic program. FASEB J. 2016, 30, 2708–2719. [Google Scholar] [CrossRef]
- Lee, E.J.; Nam, J.H.; Choi, I. Fibromodulin modulates myoblast differentiation by controlling calcium channel. Biochem. Biophys. Res. Commun. 2018, 503, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Jan, A.T.; Baig, M.H.; Ahmad, K.; Malik, A.; Rabbani, G.; Kim, T.; Lee, I.K.; Lee, Y.H.; Park, S.Y.; et al. Fibromodulin and regulation of the intricate balance between myoblast differentiation to myocytes or adipocyte-like cells. FASEB J. 2018, 32, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Jan, A.T.; Baig, M.H.; Lee, E.J.; Choi, I. Matrix gla protein: An extracellular matrix protein regulates myostatin expression in the muscle developmental program. Life Sci. 2017, 172, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Schreml, S.; Szeimies, R.M.; Prantl, L.; Landthaler, M.; Babilas, P. Wound healing in the 21st century. J. Am. Acad. Dermatol. 2010, 63, 866–881. [Google Scholar] [CrossRef]
- Posnett, J.; Gottrup, F.; Lundgren, H.; Saal, G. The resource impact of wounds on health-care providers in Europe. J. Wound Care 2009, 18, 154–161. [Google Scholar] [CrossRef]
- Menke, N.B.; Ward, K.R.; Witten, T.M.; Bonchev, D.G.; Diegelmann, R.F. Impaired wound healing. Clin. Dermatol. 2007, 25, 19–25. [Google Scholar] [CrossRef]
- Robson, M.C.; Mustoe, T.A.; Hunt, T.K. The future of recombinant growth factors in wound healing. Am. J. Surg. 1998, 176, 80S–82S. [Google Scholar] [CrossRef]
- Park, J.W.; Hwang, S.R.; Yoon, I.S. Advanced growth factor delivery systems in wound management and skin regeneration. Molecules 2017, 22, 1259. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Kim, S.H.; Kiick, K.L. Heparin-mimetic sulfated peptides with modulated affinities for heparin-binding peptides and growth factors. Peptides 2007, 28, 2125–2136. [Google Scholar] [CrossRef]
- Nimni, M.E. Polypeptide growth factors: Targeted delivery systems. Biomaterials 1997, 18, 1201–1225. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Lukyanov, A.N. Peptide and protein drug delivery to and into tumors: Challenges and solutions. Drug Discov. Today 2003, 8, 259–266. [Google Scholar] [CrossRef]
- Edelman, E.R.; Nugent, M.A.; Smith, L.T.; Karnovsky, M.J. Basic fibroblast growth factor enhances the coupling of intimal hyperplasia and proliferation of vasa vasorum in injured rat arteries. J. Clin. Invest. 1992, 89, 465–473. [Google Scholar] [CrossRef][Green Version]
- Gospodarowicz, D.; Cheng, J. Heparin protects basic and acidic FGF from inactivation. J. Cell. Physiol. 1986, 128, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Roghani, M.; Mansukhani, A.; Dell’Era, P.; Bellosta, P.; Basilico, C.; Rifkin, D.B.; Moscatelli, D. Heparin increases the affinity of basic fibroblast growth factor for its receptor but is not required for binding. J. Biol. Chem. 1994, 269, 3976–3984. [Google Scholar] [PubMed]
- Liao, I.C.; Wan, A.C.; Yim, E.K.; Leong, K.W. Controlled release from fibers of polyelectrolyte complexes. J. Control. Release 2005, 104, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.; Yu, V.M.; Lowe, J.B., 3rd; Brenner, M.J.; Hunter, D.A.; Mackinnon, S.E.; Sakiyama-Elbert, S.E. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp. Neurol. 2003, 184, 295–303. [Google Scholar] [CrossRef]
- Martin, A.I.; Priego, T.; Lopez-Calderon, A. Hormones and muscle atrophy. Adv. Exp. Med. Biol. 2018, 1088, 207–233. [Google Scholar] [CrossRef]
- Le Roith, D. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N. Engl. J. Med. 1997, 336, 633–640. [Google Scholar] [CrossRef]
- Zhang, X.; Yee, D. Tyrosine kinase signalling in breast cancer: Insulin-like growth factors and their receptors in breast cancer. Breast Cancer Res. 2000, 2, 170–175. [Google Scholar] [CrossRef]
- Daughaday, W.H.; Rotwein, P. Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr. Rev. 1989, 10, 68–91. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Liu, J.P.; Robertson, E.J.; Efstratiadis, A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 1993, 75, 73–82. [Google Scholar] [CrossRef]
- Sell, C.; Dumenil, G.; Deveaud, C.; Miura, M.; Coppola, D.; DeAngelis, T.; Rubin, R.; Efstratiadis, A.; Baserga, R. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol. Cell. Biol. 1994, 14, 3604–3612. [Google Scholar] [CrossRef]
- Vitale, G.; Pellegrino, G.; Vollery, M.; Hofland, L.J. ROLE of IGF-1 system in the modulation of longevity: Controversies and new insights from a centenarians’ perspective. Front. Endocrinol. 2019, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Song, J.L.; Delafontaine, P.; Godard, M.P. The therapeutic potential of IGF-I in skeletal muscle repair. Trends Endocrinol. Metab. 2013, 24, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto-Uezumi, M.; Uezumi, A.; Tsuchida, K.; Fukada, S.; Yamamoto, H.; Yamamoto, N.; Shiomi, K.; Hashimoto, N. Pro-insulin-like growth factor-ii ameliorates age-related inefficient regenerative response by orchestrating self-reinforcement mechanism of muscle regeneration. Stem Cells 2015, 33, 2456–2468. [Google Scholar] [CrossRef] [PubMed]
- Annibalini, G.; Bielli, P.; De Santi, M.; Agostini, D.; Guescini, M.; Sisti, D.; Contarelli, S.; Brandi, G.; Villarini, A.; Stocchi, V.; et al. MIR retroposon exonization promotes evolutionary variability and generates species-specific expression of IGF-1 splice variants. Biochim. Biophys. Acta 2016, 1859, 757–768. [Google Scholar] [CrossRef]
- Matheny, R.W., Jr.; Nindl, B.C. Loss of IGF-IEa or IGF-IEb impairs myogenic differentiation. Endocrinology 2011, 152, 1923–1934. [Google Scholar] [CrossRef]
- Cheema, U.; Brown, R.; Mudera, V.; Yang, S.Y.; McGrouther, G.; Goldspink, G. Mechanical signals and IGF-I gene splicing in vitro in relation to development of skeletal muscle. J. Cell. Physiol. 2005, 202, 67–75. [Google Scholar] [CrossRef]
- Sandona, D.; Desaphy, J.F.; Camerino, G.M.; Bianchini, E.; Ciciliot, S.; Danieli-Betto, D.; Dobrowolny, G.; Furlan, S.; Germinario, E.; Goto, K.; et al. Adaptation of mouse skeletal muscle to long-term microgravity in the MDS mission. PLoS ONE 2012, 7, e33232. [Google Scholar] [CrossRef]
- Kineman, R.D.; Del Rio-Moreno, M.; Sarmento-Cabral, A. 40 YEARS of IGF1: Understanding the tissue-specific roles of IGF1/IGF1R in regulating metabolism using the Cre/loxP system. J. Mol. Endocrinol. 2018, 61, T187–T198. [Google Scholar] [CrossRef] [PubMed]
- De Santi, M.; Annibalini, G.; Barbieri, E.; Villarini, A.; Vallorani, L.; Contarelli, S.; Berrino, F.; Stocchi, V.; Brandi, G. Human IGF1 pro-forms induce breast cancer cell proliferation via the IGF1 receptor. Cell. Oncol. 2016, 39, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, C.; Mohan, S.; Sjogren, K.; Tivesten, A.; Isgaard, J.; Isaksson, O.; Jansson, J.O.; Svensson, J. The role of liver-derived insulin-like growth factor-I. Endocr. Rev. 2009, 30, 494–535. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, S.; Xu, J.; Zhuang, L.; Xing, K.; Zhang, M.; Zhu, X.; Wang, L.; Gao, P.; Xi, Q.; et al. Dietary protein-induced hepatic IGF-1 secretion mediated by PPARgamma activation. PLoS ONE 2017, 12, e0173174. [Google Scholar] [CrossRef]
- Savage, M.O. Insulin-like growth factors, nutrition and growth. World Rev. Nutr. Diet. 2013, 106, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Kjobsted, R.; Hingst, J.R.; Fentz, J.; Foretz, M.; Sanz, M.N.; Pehmoller, C.; Shum, M.; Marette, A.; Mounier, R.; Treebak, J.T.; et al. AMPK in skeletal muscle function and metabolism. FASEB J. 2018, 32, 1741–1777. [Google Scholar] [CrossRef]
- Ascenzi, F.; Barberi, L.; Dobrowolny, G.; Villa Nova Bacurau, A.; Nicoletti, C.; Rizzuto, E.; Rosenthal, N.; Scicchitano, B.M.; Musaro, A. Effects of IGF-1 isoforms on muscle growth and sarcopenia. Aging Cell 2019, 18, e12954. [Google Scholar] [CrossRef]
- Firth, S.M.; Baxter, R.C. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 2002, 23, 824–854. [Google Scholar] [CrossRef]
- Williams, R.M.; McDonald, A.; O’Savage, M.; Dunger, D.B. Mecasermin rinfabate: rhIGF-I/rhIGFBP-3 complex: iPLEX. Expert Opin. Drug. Metab. Toxicol. 2008, 4, 311–324. [Google Scholar] [CrossRef]
- Yu, M.; Wang, H.; Xu, Y.; Yu, D.; Li, D.; Liu, X.; Du, W. Insulin-like growth factor-1 (IGF-1) promotes myoblast proliferation and skeletal muscle growth of embryonic chickens via the PI3K/Akt signalling pathway. Cell Biol. Int. 2015, 39, 910–922. [Google Scholar] [CrossRef]
- Clemmons, D.R. Role of IGF-I in skeletal muscle mass maintenance. Trends Endocrino.l Metab. 2009, 20, 349–356. [Google Scholar] [CrossRef]
- Nindl, B.C.; Santtila, M.; Vaara, J.; Hakkinen, K.; Kyrolainen, H. Circulating IGF-I is associated with fitness and health outcomes in a population of 846 young healthy men. Growth Horm. IGF Res. 2011, 21, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Alzhanov, D.T.; McInerney, S.F.; Rotwein, P. Long range interactions regulate Igf2 gene transcription during skeletal muscle differentiation. J. Biol. Chem. 2010, 285, 38969–38977. [Google Scholar] [CrossRef] [PubMed]
- Gardner, S.; Alzhanov, D.; Knollman, P.; Kuninger, D.; Rotwein, P. TGF-beta inhibits muscle differentiation by blocking autocrine signaling pathways initiated by IGF-II. Mol. Endocrinol. 2011, 25, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Laron, Z. Insulin-like growth factor 1 (IGF-1): A growth hormone. Mol. Pathol. 2001, 54, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.L.; Philp, A.; MacKenzie, M.G.; Baar, K. A limited role for PI(3,4,5)P3 regulation in controlling skeletal muscle mass in response to resistance exercise. PLoS ONE 2010, 5, e11624. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Yin, L.; Lin, X.; Lu, J.; Wang, X. Effects of cyclic mechanical stretch on the proliferation of L6 myoblasts and its mechanisms: PI3K/Akt and MAPK signal pathways regulated by IGF-1 receptor. Int. J. Mol. Sci. 2018, 19, 1649. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Barberi, L.; Bijlsma, A.Y.; Blaauw, B.; Dyar, K.A.; Milan, G.; Mammucari, C.; Meskers, C.G.; Pallafacchina, G.; Paoli, A.; et al. Signalling pathways regulating muscle mass in ageing skeletal muscle: The role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology 2013, 14, 303–323. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, S. Amphioxus IGF-like peptide induces mouse muscle cell development via binding to IGF receptors and activating MAPK and PI3K/Akt signaling pathways. Mol. Cell. Endocrinol. 2011, 343, 45–54. [Google Scholar] [CrossRef]
- Peng, X.D.; Xu, P.Z.; Chen, M.L.; Hahn-Windgassen, A.; Skeen, J.; Jacobs, J.; Sundararajan, D.; Chen, W.S.; Crawford, S.E.; Coleman, K.G.; et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003, 17, 1352–1365. [Google Scholar] [CrossRef]
- Borselli, C.; Storrie, H.; Benesch-Lee, F.; Shvartsman, D.; Cezar, C.; Lichtman, J.W.; Vandenburgh, H.H.; Mooney, D.J. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc. Natl. Acad. Sci. USA 2010, 107, 3287–3292. [Google Scholar] [CrossRef] [PubMed]
- Barton-Davis, E.R.; Shoturma, D.I.; Sweeney, H.L. Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol. Scand. 1999, 167, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Edwall, D.; Schalling, M.; Jennische, E.; Norstedt, G. Induction of insulin-like growth factor I messenger ribonucleic acid during regeneration of rat skeletal muscle. Endocrinology 1989, 124, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Alnaqeeb, M.; Simpson, H.; Goldspink, G. Cloning and characterization of an IGF-1 isoform expressed in skeletal muscle subjected to stretch. J. Muscle Res. Cell Motil. 1996, 17, 487–495. [Google Scholar] [CrossRef]
- Hennebry, A.; Oldham, J.; Shavlakadze, T.; Grounds, M.D.; Sheard, P.; Fiorotto, M.L.; Falconer, S.; Smith, H.K.; Berry, C.; Jeanplong, F.; et al. IGF1 stimulates greater muscle hypertrophy in the absence of myostatin in male mice. J. Endocrinol. 2017, 234, 187–200. [Google Scholar] [CrossRef]
- Czaja, W.; Nakamura, Y.K.; Li, N.; Eldridge, J.A.; DeAvila, D.M.; Thompson, T.B.; Rodgers, B.D. Myostatin regulates pituitary development and hepatic IGF1. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E1036–E1049. [Google Scholar] [CrossRef]
- Barbe, C.; Kalista, S.; Loumaye, A.; Ritvos, O.; Lause, P.; Ferracin, B.; Thissen, J.P. Role of IGF-I in follistatin-induced skeletal muscle hypertrophy. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E557–E567. [Google Scholar] [CrossRef]
- Liu, J.P.; Baker, J.; Perkins, A.S.; Robertson, E.J.; Efstratiadis, A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 1993, 75, 59–72. [Google Scholar] [CrossRef]
- Lipina, C.; Kendall, H.; McPherron, A.C.; Taylor, P.M.; Hundal, H.S. Mechanisms involved in the enhancement of mammalian target of rapamycin signalling and hypertrophy in skeletal muscle of myostatin-deficient mice. FEBS Lett. 2010, 584, 2403–2408. [Google Scholar] [CrossRef]
- Morissette, M.R.; Cook, S.A.; Buranasombati, C.; Rosenberg, M.A.; Rosenzweig, A. Myostatin inhibits IGF-I-induced myotube hypertrophy through Akt. Am. J. Physiol. Cell Physiol. 2009, 297, C1124–C1132. [Google Scholar] [CrossRef]
- Rommel, C.; Bodine, S.C.; Clarke, B.A.; Rossman, R.; Nunez, L.; Stitt, T.N.; Yancopoulos, G.D.; Glass, D.J. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 2001, 3, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Suthar, R.; Sankhyan, N. Duchenne muscular dystrophy: A practice update. Indian J. Pediatr. 2018, 85, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Shaikh, S.; Ahmad, S.S.; Lee, E.J.; Choi, I. Cross-talk between extracellular matrix and skeletal muscle: Implications for myopathies. Front. Pharmacol. 2020, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, S. The biological function of insulin-like growth factor-I in myogenesis and its therapeutic effect on muscular dystrophy. Acta Myol. 2005, 24, 115–118. [Google Scholar] [PubMed]
- Patel, K.; Macharia, R.; Amthor, H. Molecular mechanisms involving IGF-1 and myostatin to induce muscle hypertrophy as a therapeutic strategy for Duchenne muscular dystrophy. Acta Myol. 2005, 24, 230–241. [Google Scholar] [PubMed]
- Fang, X.B.; Song, Z.B.; Xie, M.S.; Liu, Y.M.; Zhang, W.X. Synergistic effect of glucocorticoids and IGF-1 on myogenic differentiation through the Akt/GSK-3beta pathway in C2C12 myoblasts. Int. J. Neurosci. 2020, 130, 1–11. [Google Scholar] [CrossRef]
- Lynch, G.S.; Cuffe, S.A.; Plant, D.R.; Gregorevic, P. IGF-I treatment improves the functional properties of fast- and slow-twitch skeletal muscles from dystrophic mice. Neuromuscul. Disord. 2001, 11, 260–268. [Google Scholar] [CrossRef]
- Cao, R.Y.; Li, J.; Dai, Q.; Li, Q.; Yang, J. Muscle Atrophy: Present and Future. Adv. Exp. Med. Biol. 2018, 1088, 605–624. [Google Scholar] [CrossRef]
- Fink, J.; Schoenfeld, B.J.; Nakazato, K. The role of hormones in muscle hypertrophy. Phys. Sportsmed. 2018, 46, 129–134. [Google Scholar] [CrossRef]
- Accorsi, A.; Kumar, A.; Rhee, Y.; Miller, A.; Girgenrath, M. IGF-1/GH axis enhances losartan treatment in Lama2-related muscular dystrophy. Hum. Mol. Genet. 2016, 25, 4624–4634. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, X.; Shen, B. Molecular Imaging of IGF-1R in Cancer. Mol. Imaging 2017, 16, 1–7. [Google Scholar] [CrossRef]
- Pickard, A.; McCance, D.J. IGF-Binding Protein 2—Oncogene or Tumor Suppressor? Front. Endocrinol. 2015, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Sun, S.; Zhou, X.; Guo, W.; Zhang, L. IGF-binding protein 2 is a candidate target of therapeutic potential in cancer. Tumour Biol. 2016, 37, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Thant, A.A.; Wu, Y.; Vadgama, J.V. Cancer invasion and angiogenesis by IGF-1-induced MMP-2 and VEGF in human Breast cancer cells. Proc. Am. Assoc. Cancer Res. 2005, 46, 695. [Google Scholar]
- Baig, M.H.; Adil, M.; Khan, R.; Dhadi, S.; Ahmad, K.; Rabbani, G.; Bashir, T.; Imran, M.A.; Husain, F.M.; Lee, E.J.; et al. Enzyme targeting strategies for prevention and treatment of cancer: Implications for cancer therapy. Semin. Cancer Biol. 2019, 56, 1–11. [Google Scholar] [CrossRef]
- Philippou, A.; Christopoulos, P.F.; Koutsilieris, D.M. Clinical studies in humans targeting the various components of the IGF system show lack of efficacy in the treatment of cancer. Mutat. Res. Rev. Mutat. Res. 2017, 772, 105–122. [Google Scholar] [CrossRef]
- Major, J.M.; Laughlin, G.A.; Kritz-Silverstein, D.; Wingard, D.L.; Barrett-Connor, E. Insulin-like growth factor-I and cancer mortality in older men. J. Clin. Endocrinol. Metab. 2010, 95, 1054–1059. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Khan, H.; Danish Rizvi, S.M.; Ansari, S.A.; Ullah, R.; Rastrelli, L.; Mahmood, H.M.; Siddiqui, M.H. Computational study of natural compounds for the clearance of amyloid-betaeta: A potential therapeutic management strategy for alzheimer’s disease. Molecules 2019, 24, 3233. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Kamal, M.A. Current updates on the regulation of beta-secretase movement as a potential restorative focus for management of alzheimer’s disease. Protein Pept. Lett. 2019, 26, 579–587. [Google Scholar] [CrossRef]
- Waldthaler, J.; Timmermann, L. Update on diagnostics and therapy of idiopathic Parkinson’s disease. Fortschr. Neurol. Psychiatr. 2019, 87, 445–461. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Waheed, T.; Rozeen, S.; Mahmood, S.; Kamal, M.A. Therapeutic study of phytochemicals against cancer and alzheimer’s disease management. Curr. Drug Metab. 2019, 20, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.C.; Jucker, M. Neurodegenerative diseases: Expanding the prion concept. Annu. Rev. Neurosci. 2015, 38, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Morel, G.R.; Leon, M.L.; Uriarte, M.; Reggiani, P.C.; Goya, R.G. Therapeutic potential of IGF-I on hippocampal neurogenesis and function during aging. Neurogenesis 2017, 4, e1259709. [Google Scholar] [CrossRef]
- Rosenbloom, A.L.; Rivkees, S.A. Off-label use of recombinant igf-I to promote growth: Is it appropriate? J. Clin. Endocrinol. Metab. 2010, 95, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.E.; Locatelli, V.; Rizzi, L. Neurotrophic and neuroregenerative effects of GH/IGF1. Int. J. Mol. Sci. 2017, 18, 2441. [Google Scholar] [CrossRef]
- Park, J.; Yan, G.; Kwon, K.C.; Liu, M.; Gonnella, P.A.; Yang, S.; Daniell, H. Oral delivery of novel human IGF-1 bioencapsulated in lettuce cells promotes musculoskeletal cell proliferation, differentiation and diabetic fracture healing. Biomaterials 2020, 233, 119591. [Google Scholar] [CrossRef]
- Sukhanov, S.; Higashi, Y.; Shai, S.Y.; Snarski, P.; Danchuk, S.; D’Ambra, V.; Tabony, M.; Woods, T.C.; Hou, X.; Li, Z.; et al. SM22alpha (Smooth Muscle protein 22-alpha) promoter-driven IGF1R (Insulin-like Growth Factor 1 Receptor) deficiency promotes atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2306–2317. [Google Scholar] [CrossRef]
- Shapiro, L.; Elsangeedy, E.; Lee, H.; Atala, A.; Yoo, J.J.; Lee, S.J.; Ju, Y.M. In vitro evaluation of functionalized decellularized muscle scaffold for in situ skeletal muscle regeneration. Biomed. Mater. 2019, 14, 045015. [Google Scholar] [CrossRef]
- Manzella, L.; Massimino, M.; Stella, S.; Tirro, E.; Pennisi, M.S.; Martorana, F.; Motta, G.; Vitale, S.R.; Puma, A.; Romano, C.; et al. Activation of the IGF axis in thyroid cancer: Implications for tumorigenesis and treatment. Int. J. Mol. Sci. 2019, 20, 3258. [Google Scholar] [CrossRef]
- Maffezzoni, F.; Formenti, A.M. Acromegaly and bone. Minerva Endocrinol. 2018, 43, 168–182. [Google Scholar] [CrossRef]
- Van Nieuwpoort, I.C.; Vlot, M.C.; Schaap, L.A.; Lips, P.; Drent, M.L. The relationship between serum IGF-1, handgrip strength, physical performance and falls in elderly men and women. Eur. J. Endocrinol. 2018, 179, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Procaccini, C.; Santopaolo, M.; Faicchia, D.; Colamatteo, A.; Formisano, L.; de Candia, P.; Galgani, M.; De Rosa, V.; Matarese, G. Role of metabolism in neurodegenerative disorders. Metabolism 2016, 65, 1376–1390. [Google Scholar] [CrossRef] [PubMed]
- Kopczak, A.; Stalla, G.K.; Uhr, M.; Lucae, S.; Hennings, J.; Ising, M.; Holsboer, F.; Kloiber, S. IGF-I in major depression and antidepressant treatment response. Eur. Neuropsychopharmacol. 2015, 25, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Licata, L.; Lo Surdo, P.; Iannuccelli, M.; Palma, A.; Micarelli, E.; Perfetto, L.; Peluso, D.; Calderone, A.; Castagnoli, L.; Cesareni, G. SIGNOR 2.0, the SIGnaling network open resource 2.0: 2019 update. Nucleic Acid. Res. 2020, 48, D504–D510. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acid. Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef]
- Buckingham, M.; Relaix, F. PAX3 and PAX7 as upstream regulators of myogenesis. Semin. Cell Dev. Biol. 2015, 44, 115–125. [Google Scholar] [CrossRef]
- Forcina, L.; Miano, C.; Scicchitano, B.M.; Musaro, A. Signals from the niche: Insights into the role of IGF-1 and IL-6 in modulating skeletal muscle fibrosis. Cells 2019, 8, 232. [Google Scholar] [CrossRef]
- Ding, H.; Wu, T. Insulin-like growth factor binding proteins in autoimmune diseases. Front. Endocrinol. 2018, 9, 499. [Google Scholar] [CrossRef] [PubMed]
- Boisclair, Y.R.; Rhoads, R.P.; Ueki, I.; Wang, J.; Ooi, G.T. The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: An important but forgotten component of the circulating IGF system. J. Endocrinol. 2001, 170, 63–70. [Google Scholar] [CrossRef]
- Tzeng, H.E.; Chen, J.C.; Tsai, C.H.; Kuo, C.C.; Hsu, H.C.; Hwang, W.L.; Fong, Y.C.; Tang, C.H. CCN3 increases cell motility and MMP-13 expression in human chondrosarcoma through integrin-dependent pathway. J. Cell. Physiol. 2011, 226, 3181–3189. [Google Scholar] [CrossRef]
- Bidlingmaier, M.; Strasburger, C.J. Growth hormone. Handb. Exp. Pharmacol. 2010, 195, 187–200. [Google Scholar] [CrossRef]
- Jones, B.H.; Hauschild, V.D.; Canham-Chervak, M. Musculoskeletal training injury prevention in the U.S. Army: Evolution of the science and the public health approach. J. Sci. Med. Sport 2018, 21, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Martins, K.J.; Gehrig, S.M.; Naim, T.; Saenger, S.; Baum, D.; Metzger, F.; Lynch, G.S. Intramuscular administration of PEGylated IGF-I improves skeletal muscle regeneration after myotoxic injury. Growth Horm. IGF Res. 2013, 23, 128–133. [Google Scholar] [CrossRef] [PubMed]
| S. No. | Role of IGF-1 | Year | References |
|---|---|---|---|
| 1. | IGF-1 helps in the growth and regeneration of SM and bones. Its signaling in the smooth muscle cell and in fibroblast is a critical factor of normal vascular wall growth and atheroprotection. | 2020 | [96,97] |
| 2. | IGF-1 helps in the activation of IGF-1R and muscle tissue recovery. Shapiro et al. indicate that the IGFBP-3/IGF1 conjugated framework has the potential to be utilized for in-situ muscle tissue recovery. | 2019 | [98,99] |
| 3. | IGF-1 have pleiotropic consequences on the skeleton during the life expectancy by prompting the bone development and resorption. Lower IGF-1 levels are related to lower handgrip strength and more terrible physical execution. | 2018 | [100,101] |
| 4. | GH/IGF-1 treatment had various impacts on patients with traumatic brain injury, proving a high recuperation of neurons and clinical results. | 2017 | [95] |
| 5. | IGF-1 appear in the regulation of neuronal harm, toxic insults, and a few other neurodegenerative procedures. | 2016 | [102] |
| 6. | According to Kopczak et al., the signaling of IGF-1 could play a role in the pathophysiology of depression. | 2015 | [103] |
| S. No. | Name | Function |
|---|---|---|
| 1. | IGF-1R | Cell growth and survival control |
| 2. | IGFBP3 and IGFBP4 | Enhance the capability of IGF-1 to promote cell growth |
| 3. | Probable E3 ubiquitin-protein ligase makorin-3 (MKRN3) | Catalyze the covalent interactions of ubiquitin moieties onto substrate proteins |
| 4. | IGFBP-complex acid-labile subunit (IGFALS) | Regulation of the circulation of IGFs and receptor-ligand binding [110] |
| 5. | Protein BANP | Cell cycle arrest |
| 6. | Transcription factor E2F1 | Mediate cell proliferation |
| 7 | Transcription factor SOX-4 | High-affinity binding to the T-cell enhancer motif 5’-AACAAAG-3’ motif |
| 8. | IGFBP7 | Stimulates cell adhesion |
| 9. | IGFBP5 | Change the interaction of IGFs with their cell surface receptors. |
| 10. | Immunoglobulin superfamily member 1 (IGSF1) | Essential to mediate a specific antagonistic effect of inhibin B on activin-stimulated transcription |
| 11. | Insulin-degrading enzyme | Cellular breakdown of insulin |
| 12. | IGFBP1, IGFBP3, IGFBP5 | Stimulate IGF actions |
| 13. | LDLR chaperone MESD (low-density lipoprotein receptors) | Help in embryonic polarity and mesoderm induction |
| 14. | Protein NOV homolog (IGFBP9) | Binds with integrins or other membrane receptors e.g., NOTCH1 [111] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, S.S.; Ahmad, K.; Lee, E.J.; Lee, Y.-H.; Choi, I. Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases. Cells 2020, 9, 1773. https://doi.org/10.3390/cells9081773
Ahmad SS, Ahmad K, Lee EJ, Lee Y-H, Choi I. Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases. Cells. 2020; 9(8):1773. https://doi.org/10.3390/cells9081773
Chicago/Turabian StyleAhmad, Syed Sayeed, Khurshid Ahmad, Eun Ju Lee, Yong-Ho Lee, and Inho Choi. 2020. "Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases" Cells 9, no. 8: 1773. https://doi.org/10.3390/cells9081773
APA StyleAhmad, S. S., Ahmad, K., Lee, E. J., Lee, Y.-H., & Choi, I. (2020). Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases. Cells, 9(8), 1773. https://doi.org/10.3390/cells9081773






