Unravelling the Complex Duplication History of Deuterostome Glycerol Transporters
Abstract
1. Introduction
2. Material and Methods
2.1. Coding Sequence Assembly, Phylogenetic and Syntenic Analyses
2.2. Experimental Animals and Tissue Samples
2.3. Cloning of Salmon Aquaglyceroporins
2.4. Water and Glycerol Uptake Assays in Xenopus laevis Oocytes
2.5. Tissue Expression Profiles of Salmon Aquaglyceroporins
3. Results
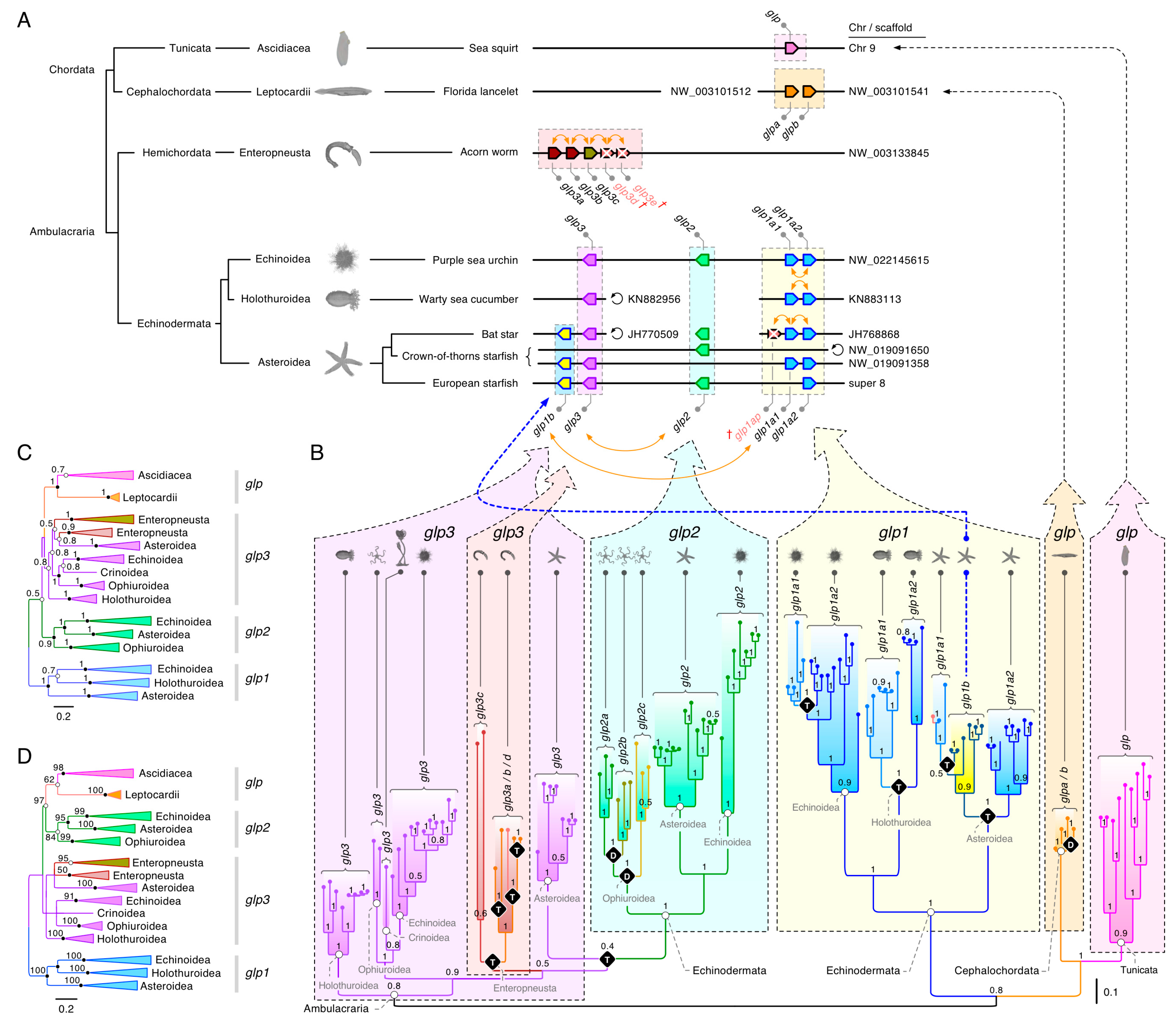
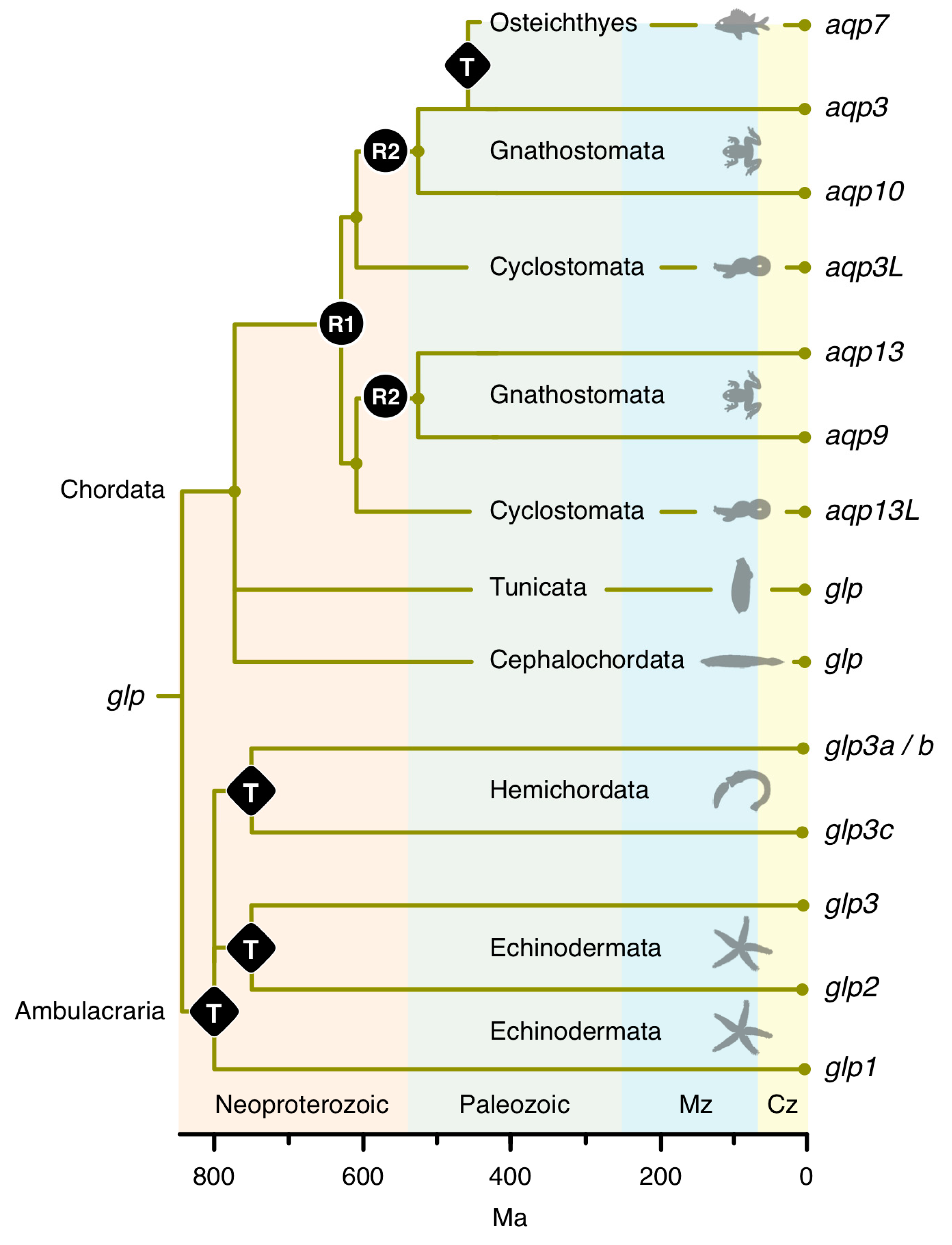
3.1. The Polyphyly of Deuterostome Glps
3.2. Common and Uncommon Duplications in Echinoderms and Hemichordates
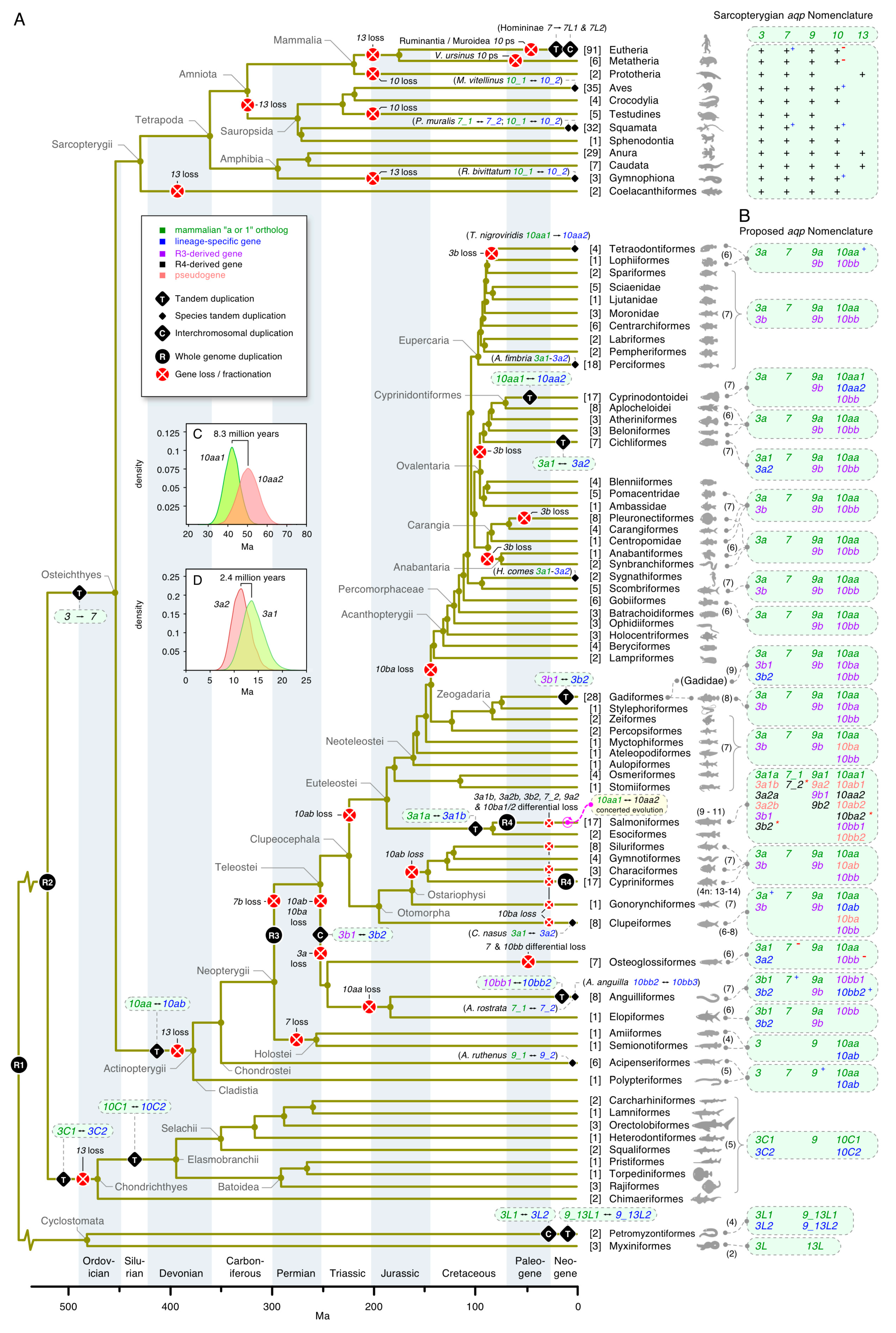
3.3. Unexpected Duplications in Jawed Vertebrates
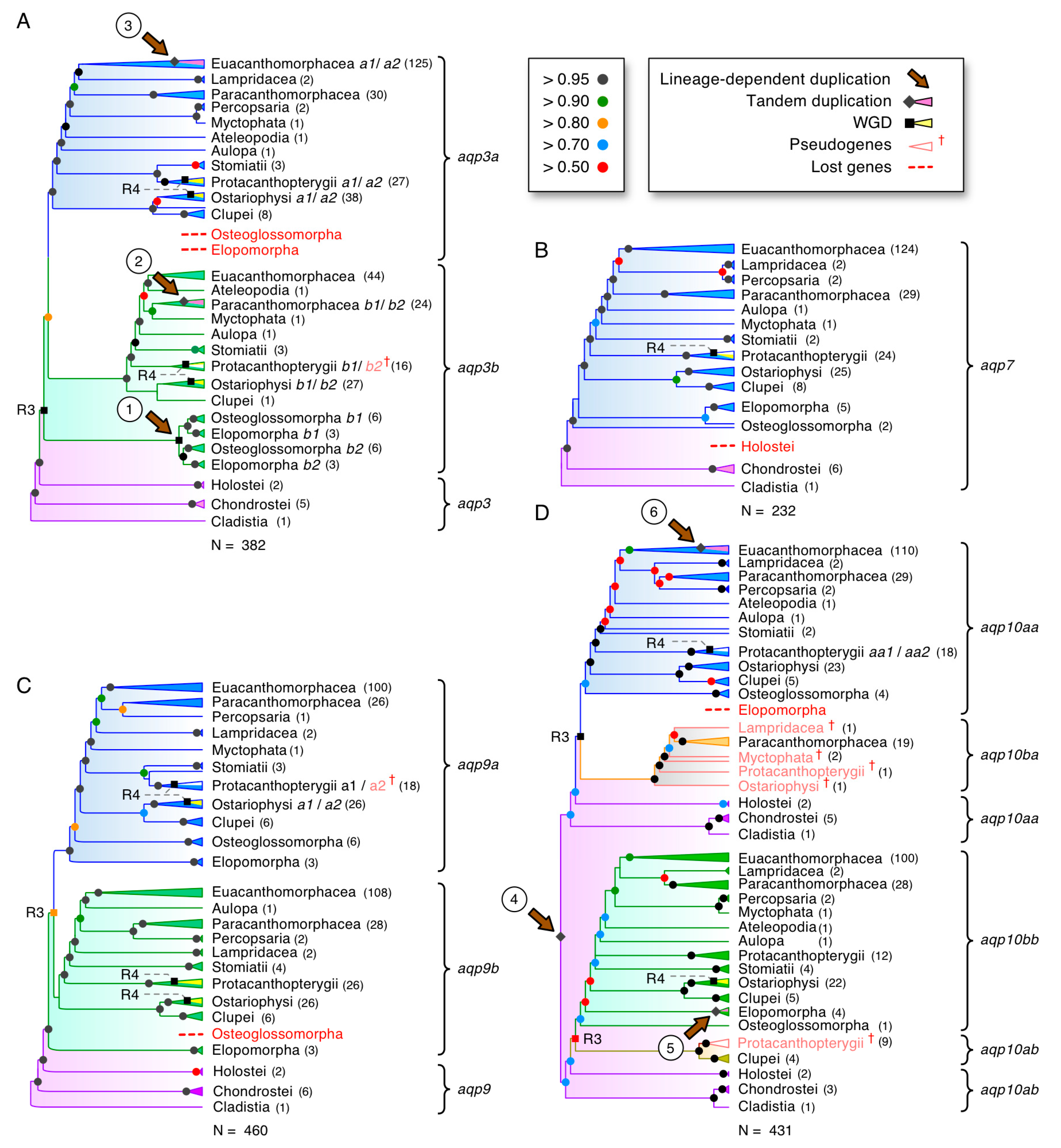
3.4. Dependent and Independent Duplicates in Teleosts
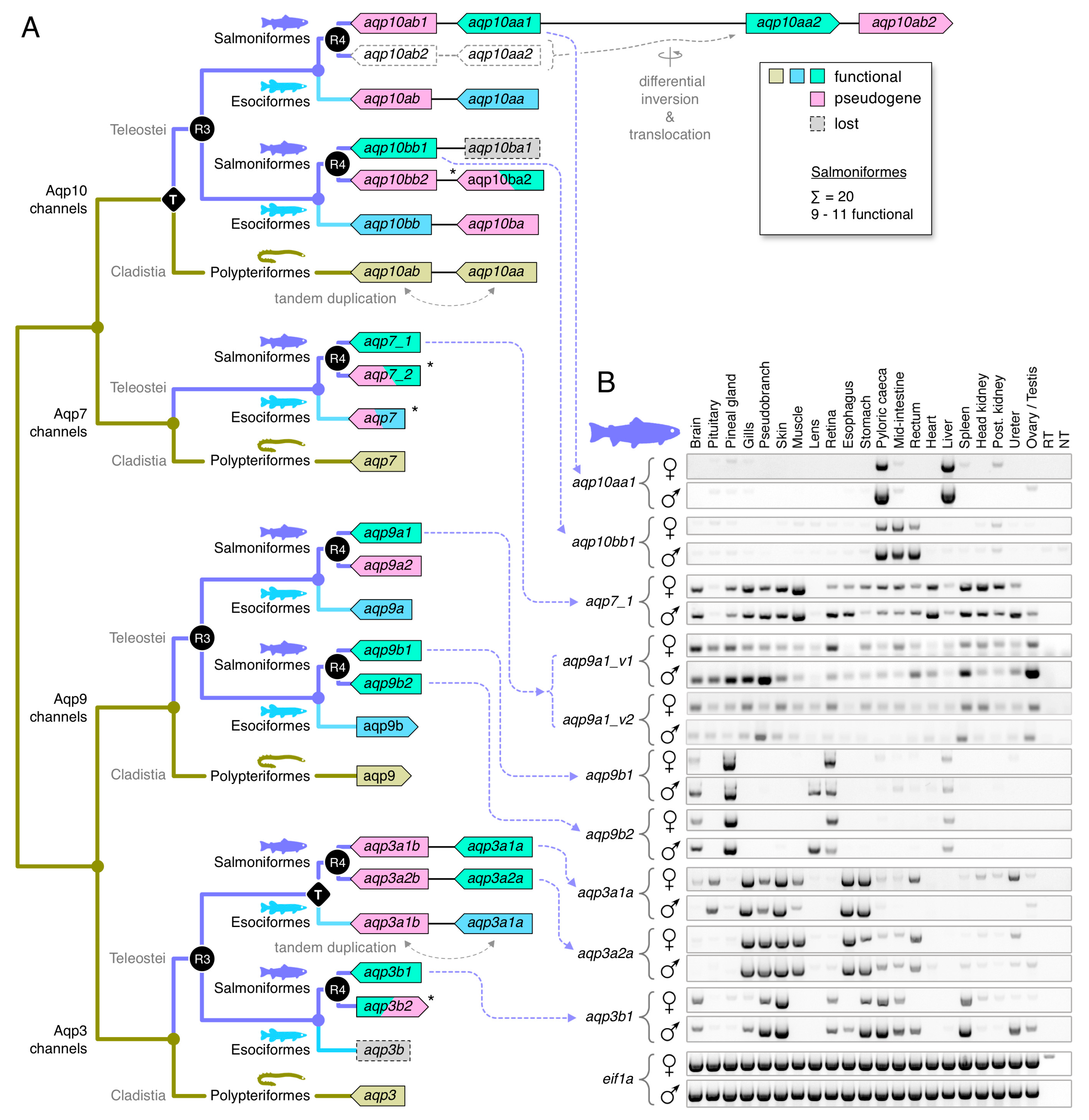
3.5. Glp Evolution in Paleotetraploid Teleosts
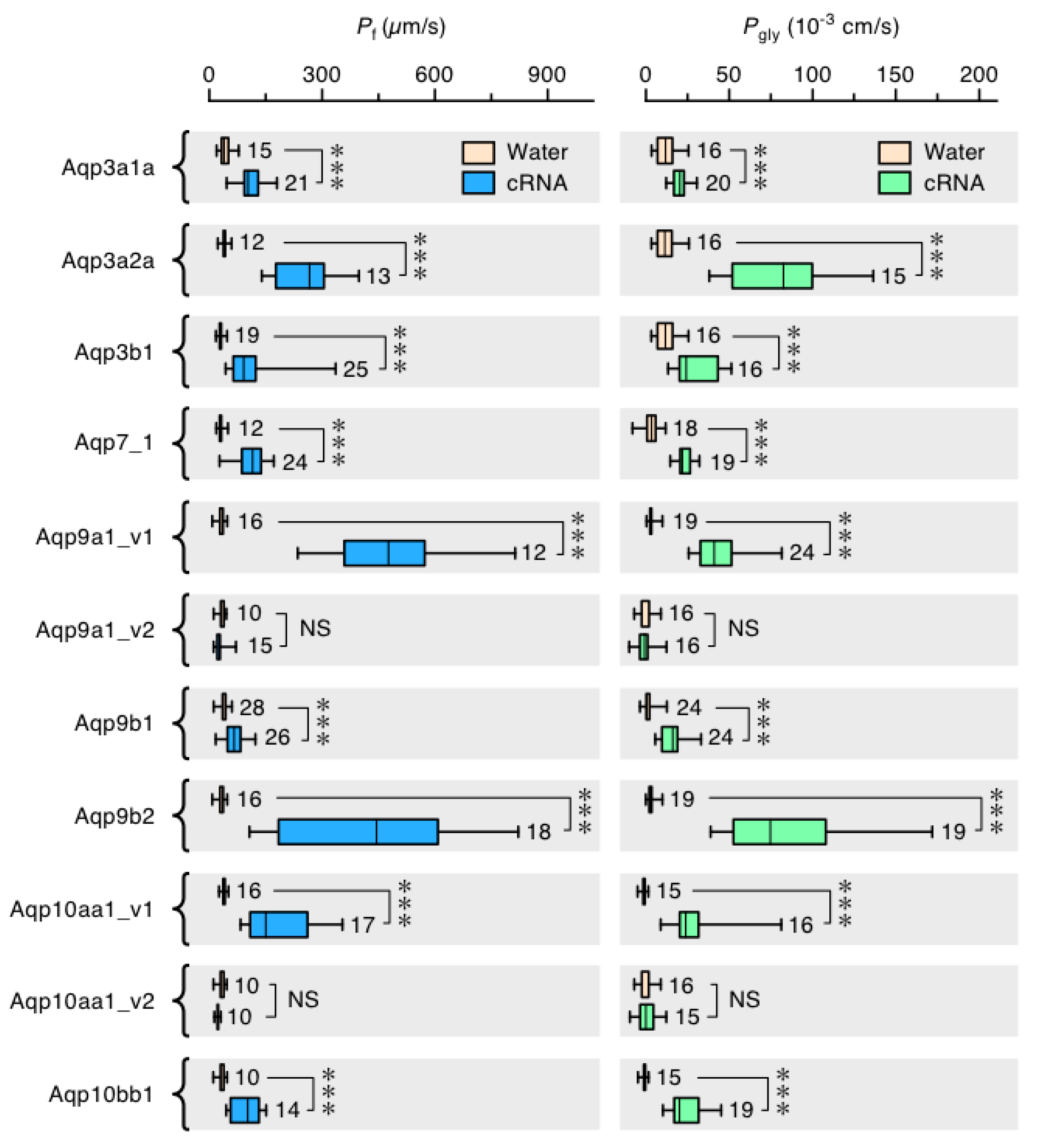
3.6. Functional Glp Repertoires in Paleotetraploid Salmonids
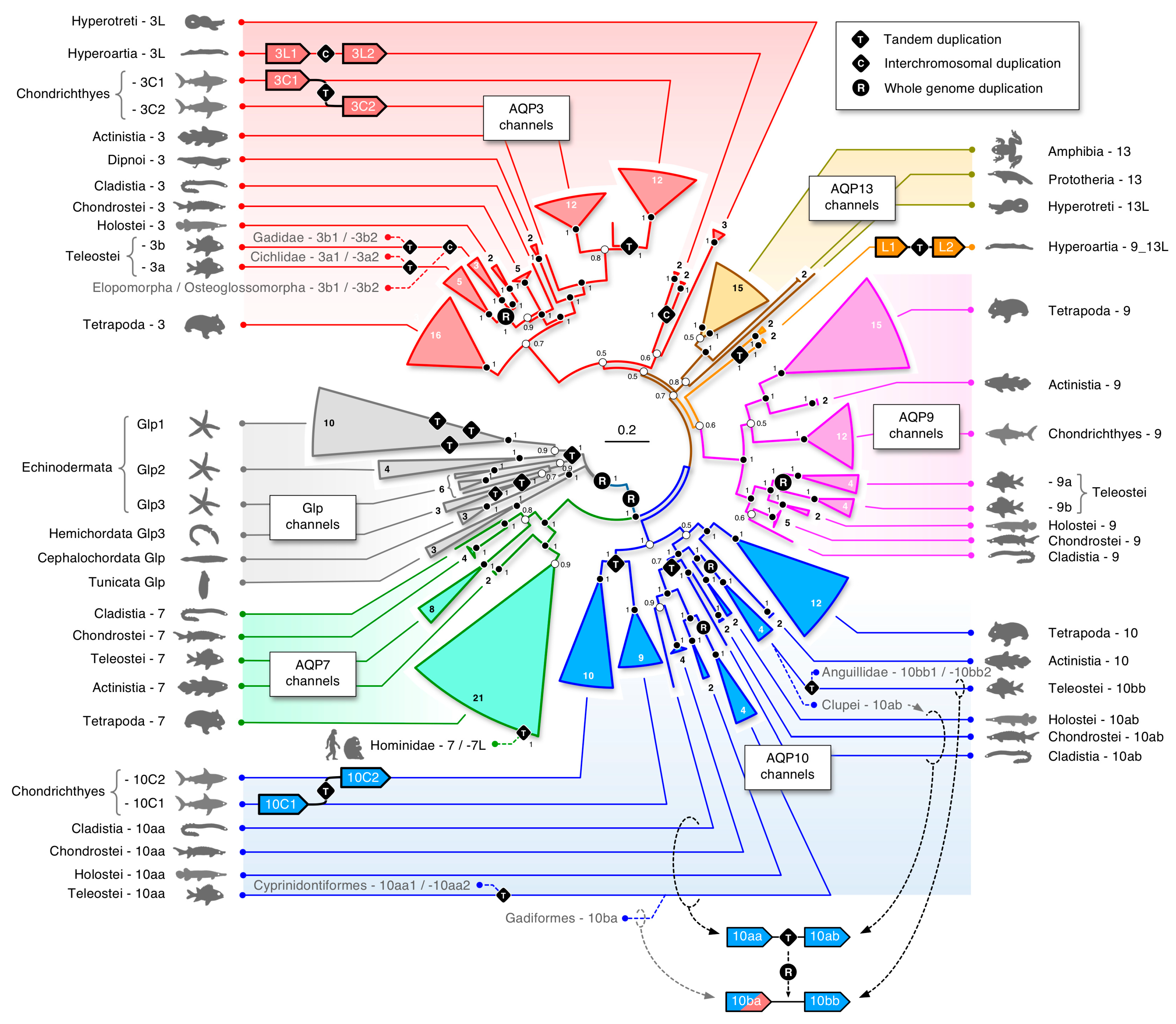
3.7. A Timeline of Vertebrate Glp Evolution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robergs, R.A.; Griffin, S.E. Glycerol. Sports Med. 1998, 26, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Heuvel, J.V.D.; Soucaille, P.; Qu, Y.; Zeng, A. Comparative Genomic Analysis of dha Regulon and Related Genes for Anaerobic Glycerol Metabolism in Bacteria. Biotechnol. Prog. 2003, 19, 263–272. [Google Scholar] [CrossRef]
- Sømme, L. Effects of Glycerol on Cold-Hardiness in Insects. Can. J. Zool. 1964, 42, 87–101. [Google Scholar] [CrossRef]
- Wegmann, K.; Ben-Amotz, A.; Avron, M. Effect of Temperature on Glycerol Retention in the Halotolerant Algae Dunaliella and Asteromonas. Plant Physiol. 1980, 66, 1196–1197. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.H.; Chudek, J.A.; Foster, R.; Gadd, G.M. Osmotic significance of glycerol accumulation in exponentially growing yeasts. Appl. Environ. Microbiol. 1987, 53, 2119–2123. [Google Scholar] [CrossRef]
- Tamás, M.J.; Luyten, K.; Sutherland, F.C.W.; Hernández, A.; Albertyn, J.; Valadi, H.; Li, H.; Prior, B.A.; Kilian, S.G.; Ramos, J.; et al. Fps1p controls the accumulation and release of the compatible solute glycerol in yeast osmoregulation. Mol. Microbiol. 1999, 31, 1087–1104. [Google Scholar] [CrossRef]
- Driedzic, W. Rainbow smelt: The unusual case of cryoprotection by sustained glycerol production in an aquatic animal. Syst. Environ. Physiol. 2015, 185, 487–499. [Google Scholar] [CrossRef]
- Toxopeus, J.; Sinclair, B.J. Mechanisms underlying insect freeze tolerance. Biol. Rev. 2018, 93, 1891–1914. [Google Scholar] [CrossRef]
- Romano, A.H. Microbial sugar transport systems and their importance in biotechnology. Trends Biotechnol. 1986, 4, 207–213. [Google Scholar] [CrossRef]
- Park, J.; Saier, J.M. Phylogenetic Characterization of the MIP Family of Transmembrane Channel Proteins. J. Membr. Biol. 1996, 153, 171–180. [Google Scholar] [CrossRef]
- Zardoya, R. Phylogeny and evolution of the major intrinsic protein family. Biol. Cell 2005, 97, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Abascal, F.; Irisarri, I.; Zardoya, R. Diversity and evolution of membrane intrinsic proteins. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1468–1481. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.N.; Cerda, J. Evolution and Functional Diversity of Aquaporins. Biol. Bull. 2015, 229, 6–23. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.N.; Cerdà, J. Aquaporin. In Encyclopedia of Signaling Molecules, 2nd ed.; Choi, S., Ed.; Springer: New York, NY, USA, 2018; pp. 1–18. [Google Scholar]
- De Maré, S.W.; Venskutonytė, R.; Eltschkner, S.; De Groot, B.L.; Lindkvist-Petersson, K. Structural Basis for Glycerol Efflux and Selectivity of Human Aquaporin 7. Structure 2019, 28, 215–222.e3. [Google Scholar] [CrossRef]
- Sweet, G.; Gandor, C.; Voegele, R.; Wittekindt, N.; Beuerle, J.; Truniger, V.; Lin, E.C.; Boos, W. Glycerol facilitator of Escherichia coli: Cloning of glpF and identification of the glpF product. J. Bacteriol. 1990, 172, 424–430. [Google Scholar] [CrossRef]
- Pao, G.M.; Wu, L.-F.; Johnson, K.D.; Chrispeels, M.J.; Sandal, N.N.; Höfte, H.; Sweet, G.; Saier, M.H. Evolution of the MIP family of integral membrane transport proteins. Mol. Microbiol. 1991, 5, 33–37. [Google Scholar] [CrossRef]
- Agre, P.; Sasaki, S.; Chrispeels, M.J. Aquaporins: A family of water channel proteins. Am. J. Physiol. 1993, 265, F461. [Google Scholar] [CrossRef]
- Maurel, C.; Reizer, J.; Schroeder, J.I.; Chrispeels, M.J.; Saier, M.H. Functional characterization of the Escherichia coli glycerol facilitator, GlpF, in Xenopus oocytes. J. Biol. Chem. 1994, 269, 11869–11872. [Google Scholar]
- Kozono, D.; Ding, X.D.; Iwasaki, I.; Meng, X.Y.; Kamagata, Y.; Agre, P.; Kitagawa, Y. Functional expression and characterization of an archaeal aquaporin—AqpM from Methanothermobacter marburgensis. J. Biol. Chem. 2003, 278, 10649–10656. [Google Scholar] [CrossRef]
- Holm, L.M.; Klaerke, D.A.; Zeuthen, T. Aquaporin 6 is permeable to glycerol and urea. Pflügers Arch. Eur. J. Physiol. 2004, 448, 181–186. [Google Scholar] [CrossRef]
- Lee, J.K.; Kozono, D.; Remis, J.; Kitagawa, Y.; Agre, P.; Stroud, R.M. Structural basis for conductance by the archaeal aquaporin AqpM at 1.68 A. Proc. Natl. Acad. Sci. USA 2005, 102, 18932–18937. [Google Scholar] [CrossRef] [PubMed]
- Engelund, M.B.; Chauvigné, F.; Christensen, B.M.; Finn, R.N.; Cerda, J.; Madsen, S.S. Differential expression and novel permeability properties of three aquaporin 8 paralogs from seawater-challenged Atlantic salmon smolts. J. Exp. Biol. 2013, 216, 3873–3885. [Google Scholar] [CrossRef]
- Madeira, A.; Fernández-Veledo, S.; Camps, M.; Zorzano, A.; De Moura, T.M.F.; Mallafré, V.C.; Vendrell, J.; Soveral, G. Human aquaporin-11 is a water and glycerol channel and localizes in the vicinity of lipid droplets in human adipocytes. Obesity 2014, 22, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.N.; Chauvigné, F.; Stavang, J.A.; Belles, X.; Cerda, J. Insect glycerol transporters evolved by functional co-option and gene replacement. Nat. Commun. 2015, 6, 7814. [Google Scholar] [CrossRef] [PubMed]
- Van Ekert, E.; Chauvigné, F.; Finn, R.N.; Mathew, L.G.; Hull, J.J.; Cerdà, J.; Fabrick, J.A. Molecular and functional characterization of Bemisia tabaci aquaporins reveals the water channel diversity of hemipteran insects. Insect Biochem. Mol. Biol. 2016, 77, 39–51. [Google Scholar] [CrossRef]
- Chauvigné, F.; Yilmaz, O.; Ferré, A.; Fjelldal, P.G.; Finn, R.N.; Cerda, J. The vertebrate Aqp14 water channel is a neuropeptide-regulated polytransporter. Commun. Biol. 2019, 2, 1–13. [Google Scholar] [CrossRef]
- Zardoya, R.; Ding, X.; Kitagawa, Y.; Chrispeels, M.J. Origin of plant glycerol transporters by horizontal gene transfer and functional recruitment. Proc. Natl. Acad. Sci. USA 2002, 99, 14893–14896. [Google Scholar] [CrossRef]
- Pommerrenig, B.; Diehn, T.A.; Bernhardt, N.; Bienert, M.D.; Mitani-Ueno, N.; Fuge, J.; Bieber, A.; Spitzer, C.; Bräutigam, A.; Ma, J.F.; et al. Functional evolution of nodulin 26-like intrinsic proteins: From bacterial arsenic detoxification to plant nutrient transport. New Phytol. 2019, 225, 1383–1396. [Google Scholar] [CrossRef]
- Ishibashi, K.; Sasaki, S.; Fushimi, K.; Uchida, S.; Kuwahara, M.; Saito, H.; Furukawa, T.; Nakajima, K.; Yamaguchi, Y.; Gojobori, T. Molecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc. Natl. Acad. Sci. USA 1994, 91, 6269–6273. [Google Scholar] [CrossRef]
- Ishibashi, K.; Kuwahara, M.; Gu, Y.; Kageyama, Y.; Tohsaka, A.; Suzuki, F.; Marumo, F.; Sasaki, S. Cloning and functional expression of a new water channel abundantly expressed in the testis permeable to water, glycerol, and urea. J. Biol. Chem. 1997, 272, 20782–20786. [Google Scholar] [CrossRef]
- Ishibashi, K.; Kuwahara, M.; Gu, Y.; Tanaka, Y.; Marumo, F.; Sasaki, S. Cloning and Functional Expression of a New Aquaporin (AQP9) Abundantly Expressed in the Peripheral Leukocytes Permeable to Water and Urea, but Not to Glycerol. Biochem. Biophys. Res. Commun. 1998, 244, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Morinaga, T.; Kuwahara, M.; Sasaki, S.; Imai, M. Cloning and identification of a new member of water channel (AQP10) as an aquaglyceroporin. Biochim. Biophys. Acta Gene Struct. Expr. 2002, 1576, 335–340. [Google Scholar] [CrossRef]
- Virkki, L.V.; Cooper, G.J.; Boron, W.F. Cloning and functional expression of an MIP (AQP0) homolog from killifish (Fundulus heteroclitus) lens. Am. J. Physiol. Integr. Comp. Physiol. 2001, 281, R1994–R2003. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.N.; Chauvigné, F.; Hlidberg, J.B.; Cutler, C.P.; Cerda, J. The Lineage-Specific Evolution of Aquaporin Gene Clusters Facilitated Tetrapod Terrestrial Adaptation. PLoS ONE 2014, 9, e113686. [Google Scholar] [CrossRef]
- Tingaud-Sequeira, A.; Calusinska, M.; Chauvigné, F.; Lozano, J.; Finn, R.N.; Cerdà, J. The zebrafish genome encodes the largest vertebrate repertoire of functional aquaporins with dual parology and substrate specificities similar to tetrapods. BMC Evol. Biol. 2010, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Stavang, J.A.; Chauvigné, F.; Kongshaug, H.; Cerda, J.; Nilsen, F.; Finn, R.N. Phylogenomic and functional analyses of salmon lice aquaporins uncover the molecular diversity of the superfamily in Arthropoda. BMC Genom. 2015, 16, 618. [Google Scholar] [CrossRef]
- Sonah, H.; Deshmukh, R.; Labbé, C.; Bélanger, R.R. Analysis of aquaporins in Brassicaceae species reveals high-level of conservation and dynamic role against biotic and abiotic stress in canola. Sci. Rep. 2017, 7, 2771. [Google Scholar] [CrossRef]
- Bezerra-Neto, J.P.; De Araújo, F.C.; Ferreira-Neto, J.R.; Da Silva, M.D.; Pandolfi, V.; Aburjaile, F.F.; Sakamoto, T.; de Silva, R.L.O.; Kido, É.A.; Amorim, L.L.B.; et al. Plant Aquaporins: Diversity, Evolution and Biotechnological Applications. Curr. Protein Pept. Sci. 2019, 20, 368–395. [Google Scholar] [CrossRef]
- Finn, R.N.; Cerda, J. Aquaporin Evolution in Fishes. Front. Physiol. 2011, 2, 44. [Google Scholar] [CrossRef]
- Van De Peer, Y.; Maere, S.; Meyer, A. The evolutionary significance of ancient genome duplications. Nat. Rev. Genet. 2009, 10, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Amores, A.; Catchen, J.; Ferrara, A.; Fontenot, Q.; Postlethwait, J.H. Genome evolution and meiotic maps by massively parallel DNA sequencing: Spotted gar, an outgroup for the teleost genome duplication. Genetics 2011, 188, 799–808. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Blum, S.; Feldman, M.W.; Lavi, U.; Hillel, J. Recent Duplication of the Common Carp (Cyprinus carpio L.) Genome as Revealed by Analyses of Microsatellite Loci. Mol. Biol. Evol. 2003, 20, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhang, X.; Wang, X.; Li, J.; Liu, G.; Kuang, Y.-Y.; Xu, J.; Zheng, X.; Ren, L.; Wang, G.; et al. Genome sequence and genetic diversity of the common carp, Cyprinus carpio. Nat. Genet. 2014, 46, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xu, J.; Liu, G.-J.; Chen, L.; Zhou, Z.; Peng, W.; Jiang, Y.; Zhao, Z.; Jia, Z.; Sun, Y.; et al. The allotetraploid origin and asymmetrical genome evolution of the common carp Cyprinus carpio. Nat. Commun. 2019, 10, 4625. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, C.; Brunet, F.; Chalopin, M.; Juanchich, A.; Bernard, M.; Noel, B.; Bento, P.; Da Silva, C.; Labadie, K.; Alberti, A.; et al. The rainbow trout genome provides novel insights into evolution after whole-genome duplication in vertebrates. Nat. Commun. 2014, 5, 3657. [Google Scholar] [CrossRef]
- MacQueen, D.J.; Johnston, I. A well-constrained estimate for the timing of the salmonid whole genome duplication reveals major decoupling from species diversification. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132881. [Google Scholar] [CrossRef]
- Lynch, M.; Conery, J.S. The Evolutionary Fate and Consequences of Duplicate Genes. Science 2000, 290, 1151–1155. [Google Scholar] [CrossRef]
- Wolf, Y.I.; Koonin, E.V. Genome reduction as the dominant mode of evolution. BioEssays 2013, 35, 829–837. [Google Scholar] [CrossRef]
- Zimmer, A.; Lang, D.; Richardt, S.; Frank, W.; Reski, R.; Rensing, S.A. Dating the early evolution of plants: Detection and molecular clock analyses of orthologs. Mol. Genet. Genom. 2007, 278, 393–402. [Google Scholar] [CrossRef]
- Betancur-R, R.; Wiley, E.O.; Arratia, G.; Acero, A.; Bailly, N.; Miya, M.; Lecointre, G.; Orti, G. Phylogenetic classification of bony fishes. BMC Evol. Biol. 2017, 17, 162. [Google Scholar] [CrossRef]
- Consortium, S.U.G.S. The genome of the sea urchin Strongylocentrotus purpuratus. Science 2006, 314, 951–952. [Google Scholar]
- Katoh, K.; Toh, H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 2008, 9, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Suyama, M.; Torrents, D.; Bork, P. PAL2NAL: Robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 2006, 34, W609–W612. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Swafford, D.L. PAUP*. Phylogenetic Analysis Using Parsimony (* and Other Models). Version 4.0b10 for Macintosh. (Sinauer Associates Inc, Sunderland, MASS. 2002). Available online: www.sinauer.com/detail.php?id=8060 (accessed on 1 July 2020).
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, N.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Han, M.V.; Zmasek, C.M. phyloXML: XML for evolutionary biology and comparative genomics. BMC Bioinform. 2009, 10, 356. [Google Scholar] [CrossRef]
- Nguyen, N.T.T.; Vincens, P.; Crollius, H.R.; Louis, A. Genomicus 2018: Karyotype evolutionary trees and on-the-fly synteny computing. Nucleic Acids Res. 2017, 46, D816–D822. [Google Scholar] [CrossRef]
- Hughes, L.C.; Orti, G.; Huang, Y.; Sun, Y.; Baldwin, C.C.; Thompson, A.W.; Arcila, D.; Betancur-R, R.; Li, C.; Becker, L.A.; et al. Comprehensive phylogeny of ray-finned fishes (Actinopterygii) based on transcriptomic and genomic data. Proc. Natl. Acad. Sci. USA 2018, 115, 6249–6254. [Google Scholar] [CrossRef]
- Chauvigné, F.; Boj, M.; Vilella, S.; Finn, R.N.; Cerdà, J. Subcellular localization of selectively permeable aquaporins in the male germline of a marine teleost reveals spatial redistribution in activated spermatozoa. Biol. Reprod. 2013, 89, 1–17. [Google Scholar] [CrossRef]
- Chauvigné, F.; Zapater, C.; Stavang, J.A.; Taranger, G.L.; Cerda, J.; Finn, R.N. The pH sensitivity of Aqp0 channels in tetraploid and diploid teleosts. FASEB J. 2015, 29, 2172–2184. [Google Scholar] [CrossRef]
- Chauvigné, F.; Boj, M.; Finn, R.N.; Cerda, J. Mitochondrial aquaporin-8-mediated hydrogen peroxide transport is essential for teleost spermatozoon motility. Sci. Rep. 2015, 5, 7789. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Ives, H.E. Water permeability and fluidity of renal basolateral membranes. Am. J. Physiol. 1986, 250, F633–F643. [Google Scholar] [CrossRef] [PubMed]
- Cerda, J.; Finn, R.N. Piscine aquaporins: An overview of recent advances. J. Exp. Zool. 2010, 313, 623–650. [Google Scholar] [CrossRef] [PubMed]
- Tine, M.; Kuhl, H.; Gagnaire, P.-A.; Louro, B.; Desmarais, E.; Martins, R.S.; Hecht, J.; Knaust, F.; Belkhir, K.; Klages, S.; et al. European sea bass genome and its variation provide insights into adaptation to euryhalinity and speciation. Nat. Commun. 2014, 5, 5770. [Google Scholar] [CrossRef]
- Cerda, J.; Chauvigné, F.; Finn, R.N. The Physiological Role and Regulation of Aquaporins in Teleost Germ Cells. Adv. Exp. Med. Biol. 2017, 969, 149–171. [Google Scholar]
- Morinaga, T.; Nakakoshi, M.; Hirao, A.; Imai, M.; Ishibashi, K. Mouse aquaporin 10 gene (AQP10) is a pseudogene. Biochem. Biophys. Res. Commun. 2002, 294, 630–634. [Google Scholar] [CrossRef]
- Tanaka, Y.; Morishita, Y.; Ishibashi, K. Aquaporin10 is a pseudogene in cattle and their relatives. Biochem. Biophys. Rep. 2015, 1, 16–21. [Google Scholar] [CrossRef]
- Irisarri, I.; Singh, P.; Koblmüller, S.; Torres-Dowdall, J.; Henning, F.; Franchini, P.; Fischer, C.; Lemmon, A.R.; Lemmon, E.M.; Thallinger, G.G.; et al. Phylogenomics uncovers early hybridization and adaptive loci shaping the radiation of Lake Tanganyika cichlid fishes. Nat. Commun. 2018, 9, 3159. [Google Scholar] [CrossRef]
- Sawyer, S.A. A Computer Package for the Statistical Detection of Gene Conversion. Distributed by the Author Department of Mathematics, Washington University in St Louis Sawyer wEbsite. 1999. Available online: http://www.math.wustl.edu/sawyer (accessed on 1 July 2020).
- Setiamarga, D.H.; Miya, M.; Yamanoue, Y.; Mabuchi, K.; Satoh, T.P.; Inoue, J.; Nishida, M. Interrelationships of Atherinomorpha (medakas, flyingfishes, killifishes, silversides, and their relatives): The first evidence based on whole mitogenome sequences. Mol. Phylogenet. Evol. 2008, 49, 598–605. [Google Scholar] [CrossRef]
- Dong, C.; Chen, L.; Feng, J.; Xu, J.; Mahboob, S.; Al-Ghanim, K.; Li, X.; Xu, P. Genome Wide Identification, Phylogeny, and Expression of Aquaporin Genes in Common Carp (Cyprinus carpio). PLoS ONE 2016, 11, e0166160. [Google Scholar] [CrossRef]
- De Boer, J.G.; Yazawa, R.; Davidson, W.S.; Koop, B.F. Bursts and horizontal evolution of DNA transposons in the speciation of pseudotetraploid salmonids. BMC Genom. 2007, 8, 422. [Google Scholar] [CrossRef] [PubMed]
- Kuraku, S.; Kuratani, S. Time Scale for Cyclostome Evolution Inferred with a Phylogenetic Diagnosis of Hagfish and Lamprey cDNA Sequences. Zool. Sci. 2006, 23, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Suleski, M.; Hedges, S.B. TimeTree: A Resource for Timelines, Timetrees, and Divergence Times. Mol. Biol. Evol. 2017, 34, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- Zardoya, R.; Villalba, S. A Phylogenetic Framework for the Aquaporin Family in Eukaryotes. J. Mol. Evol. 2001, 52, 391–404. [Google Scholar] [CrossRef]
- Meyer, A.; Schartl, M. Gene and genome duplications in vertebrates: The one-to-four (-to-eight in fish) rule and the evolution of novel gene functions. Curr. Opin. Cell Biol. 1999, 11, 699–704. [Google Scholar] [CrossRef]
- Hedges, S.B. Vertebrates (Vertebrata). In The Timetree of Life; Blair, S., Hedges, S.B., Eds.; Oxford University Press: New York, NY, USA, 2009; pp. 309–314. [Google Scholar]
- Kuraku, S.; Ota, K.G.; Kuratani, S. Jawless fishes (Cyclostomata). In The Timetree of Life; Blair, S., Hedges, S.B., Eds.; Oxford University Press: New York, NY, USA, 2009; pp. 317–319. [Google Scholar]
- Kuraku, S. Insights into Cyclostome Phylogenomics: Pre-2R or Post-2R. Zool. Sci. 2008, 25, 960–968. [Google Scholar] [CrossRef]
- Sacerdot, C.; Louis, A.; Bon, C.; Berthelot, C.; Crollius, H.R. Chromosome evolution at the origin of the ancestral vertebrate genome. Genome Biol. 2018, 19, 166. [Google Scholar] [CrossRef]
- Holland, L.Z.; Daza, D.O. A new look at an old question: When did the second whole genome duplication occur in vertebrate evolution? Genome Biol. 2018, 19, 209. [Google Scholar] [CrossRef]
- Simakov, O.; Marlétaz, F.; Yue, J.-X.; O’Connell, B.; Jenkins, J.; Brandt, A.; Calef, R.; Tung, C.-H.; Huang, T.-K.; Schmutz, J.; et al. Deeply conserved synteny resolves early events in vertebrate evolution. Nat. Ecol. Evol. 2020, 4, 820–830. [Google Scholar] [CrossRef]
- Nakatani, Y.; Takeda, H.; Kohara, Y.; Morishita, S. Reconstruction of the vertebrate ancestral genome reveals dynamic genome reorganization in early vertebrates. Genome Res. 2007, 17, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.; Lam, K.; Tay, B.-H.; Tay, A.; Brenner, S.; Venkatesh, B. Elephant shark (Callorhinchus milii) provides insights into the evolution of Hox gene clusters in gnathostomes. Proc. Natl. Acad. Sci. USA 2009, 106, 16327–16332. [Google Scholar] [CrossRef]
- Storz, J.F.; Opazo, J.C.; Hoffmann, F.G. Gene duplication, genome duplication, and the functional diversification of vertebrate globins. Mol. Phylogenet. Evol. 2012, 66, 469–478. [Google Scholar] [CrossRef]
- Pasquier, J.; Cabau, C.; Nguyen, T.; Jouanno, E.; Severac, D.; Braasch, I.; Journot, L.; Pontarotti, P.; Klopp, C.; Postlethwait, J.H.; et al. Gene evolution and gene expression after whole genome duplication in fish: The PhyloFish database. BMC Genom. 2016, 17, 368. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, M.; Gunnell, G.F.; Barba-Montoya, J.; Wilkins, A.; Yang, Z.; Yoder, A.D. Using Phylogenomic Data to Explore the Effects of Relaxed Clocks and Calibration Strategies on Divergence Time Estimation: Primates as a Test Case. Syst. Biol. 2018, 67, 594–615. [Google Scholar] [CrossRef]
- Makohon-Moore, S. The Evolution of Adipocytes during Human Origins: An Integrative Genomic, Cellular, and Molecular Approach. Ph.D. Thesis, Duke University, Durham, NC, USA, 2018; p. 216. [Google Scholar]
- Lien, S.; Koop, B.F.; Sandve, S.R.; Miller, J.; Kent, M.P.; Nome, T.; Hvidsten, T.R.; Leong, J.S.; Minkley, D.R.; Zimin, A.; et al. The Atlantic salmon genome provides insights into rediploidization. Nature 2016, 533, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Tipsmark, C.K.; Madsen, S.S.; Sørensen, K.J. Aquaporin expression dynamics in osmoregulatory tissues of Atlantic salmon during smoltification and seawater acclimation. J. Exp. Biol. 2010, 213, 368–379. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, O.; Chauvigné, F.; Ferré, A.; Nilsen, F.; Fjelldal, P.G.; Cerdà, J.; Finn, R.N. Unravelling the Complex Duplication History of Deuterostome Glycerol Transporters. Cells 2020, 9, 1663. https://doi.org/10.3390/cells9071663
Yilmaz O, Chauvigné F, Ferré A, Nilsen F, Fjelldal PG, Cerdà J, Finn RN. Unravelling the Complex Duplication History of Deuterostome Glycerol Transporters. Cells. 2020; 9(7):1663. https://doi.org/10.3390/cells9071663
Chicago/Turabian StyleYilmaz, Ozlem, François Chauvigné, Alba Ferré, Frank Nilsen, Per Gunnar Fjelldal, Joan Cerdà, and Roderick Nigel Finn. 2020. "Unravelling the Complex Duplication History of Deuterostome Glycerol Transporters" Cells 9, no. 7: 1663. https://doi.org/10.3390/cells9071663
APA StyleYilmaz, O., Chauvigné, F., Ferré, A., Nilsen, F., Fjelldal, P. G., Cerdà, J., & Finn, R. N. (2020). Unravelling the Complex Duplication History of Deuterostome Glycerol Transporters. Cells, 9(7), 1663. https://doi.org/10.3390/cells9071663




