Rab27a Contributes to the Processing of Inflammatory Pain in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Behavior
2.3. Immunohistochemistry
2.4. In Situ Hybridization
2.5. Western Blot
2.6. Real-Time RT-PCR
2.7. Statistics
3. Results
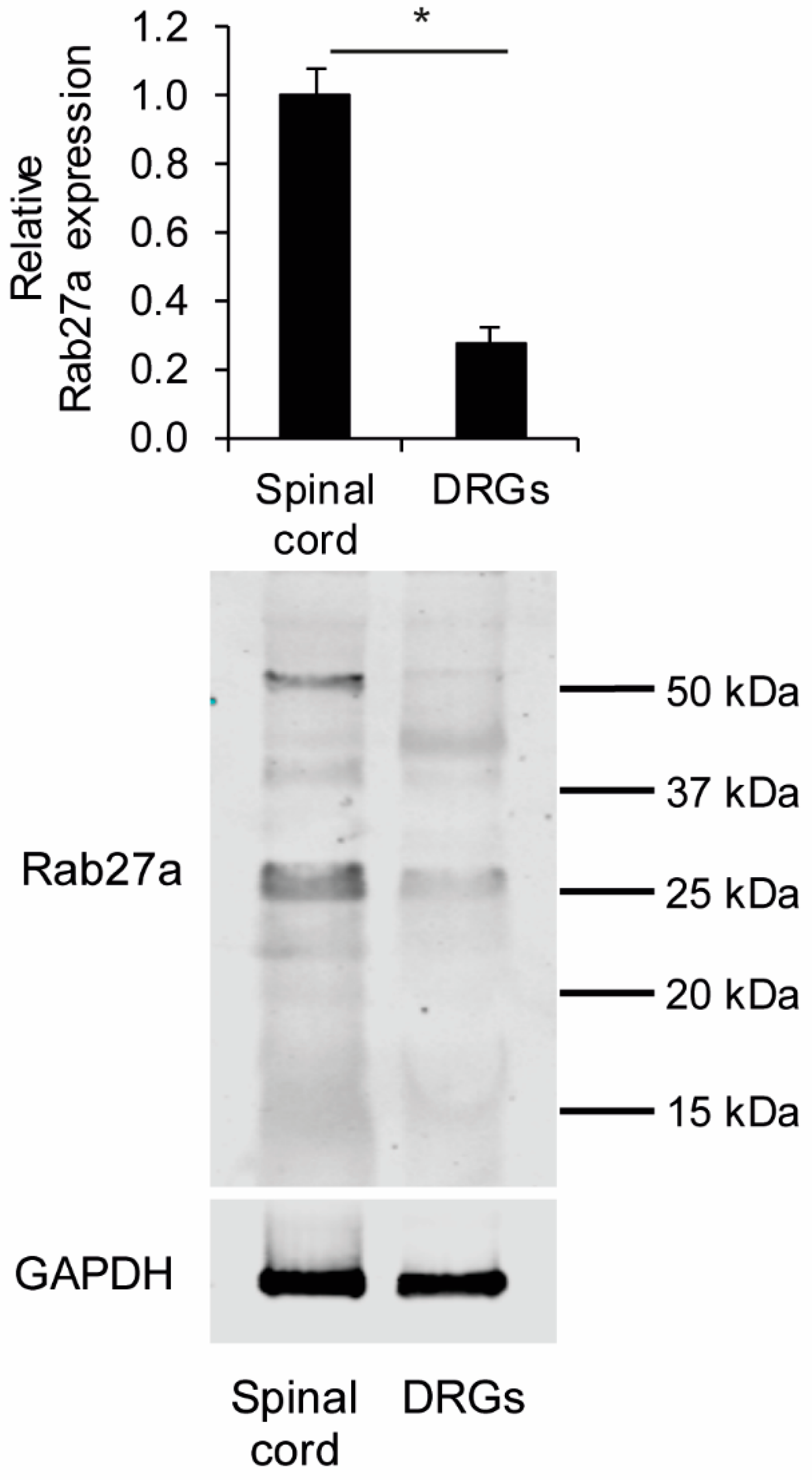
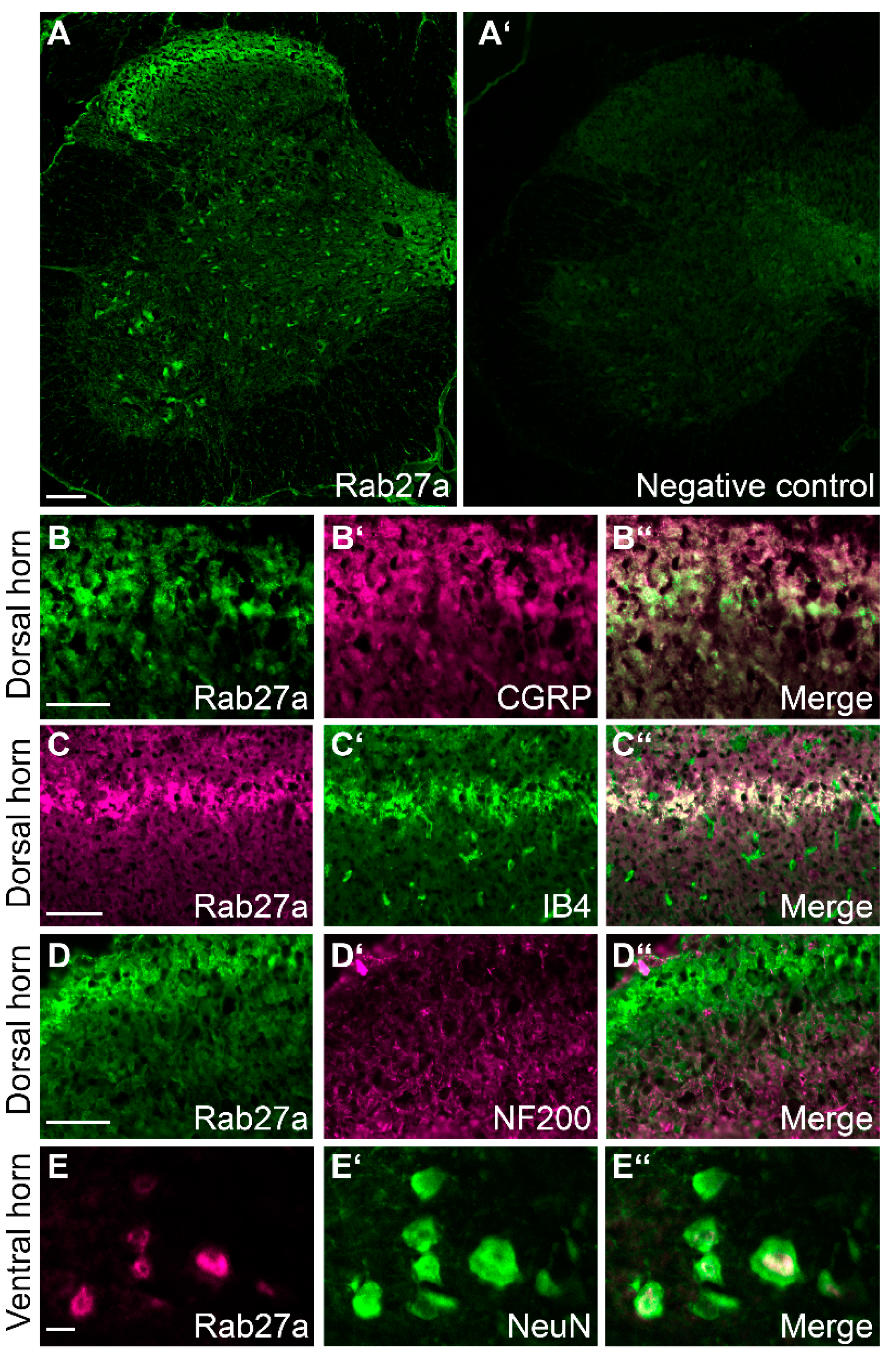
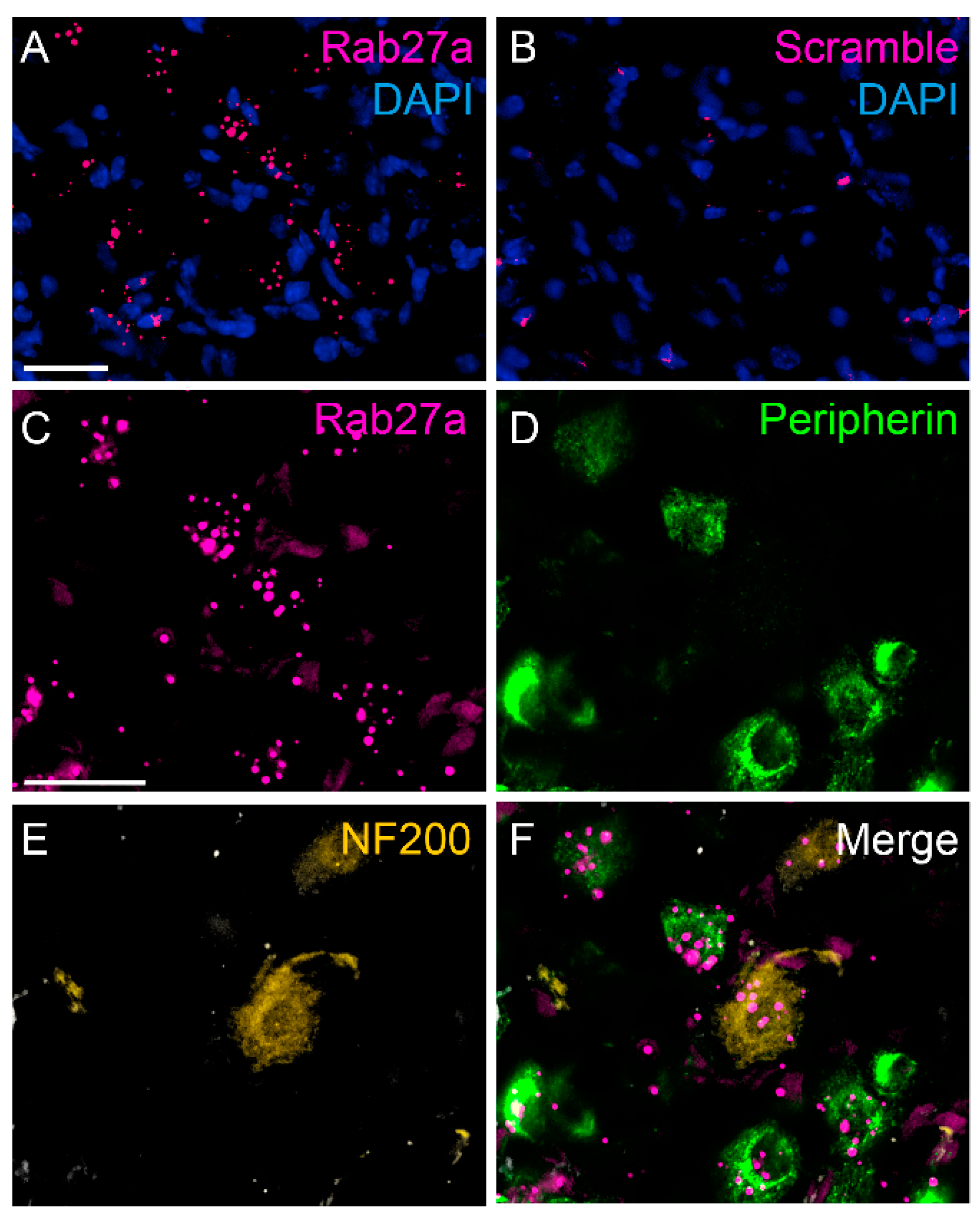
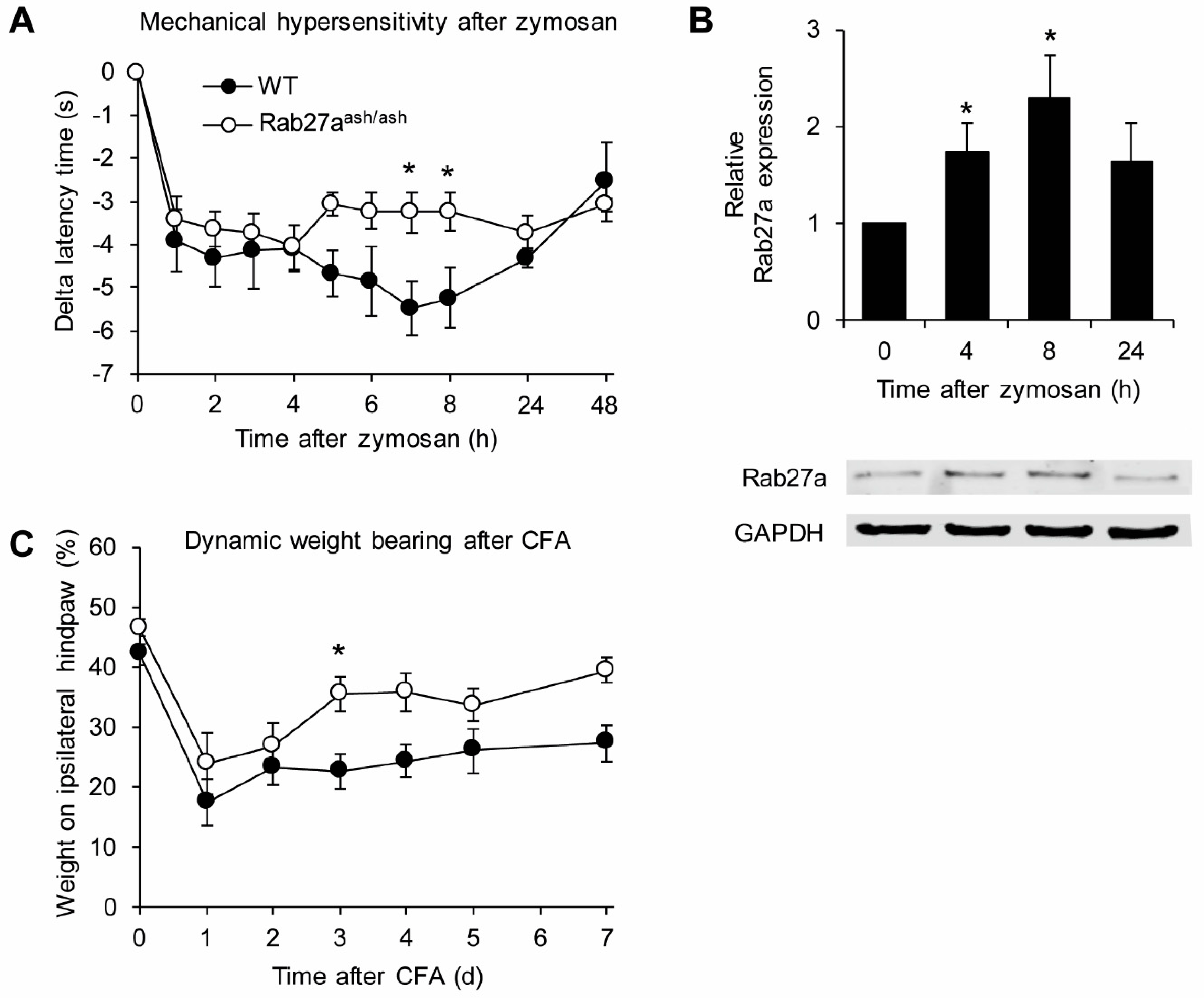
3.1. Rab27a Expression in the Spinal Cord and in Dorsal Root Ganglia
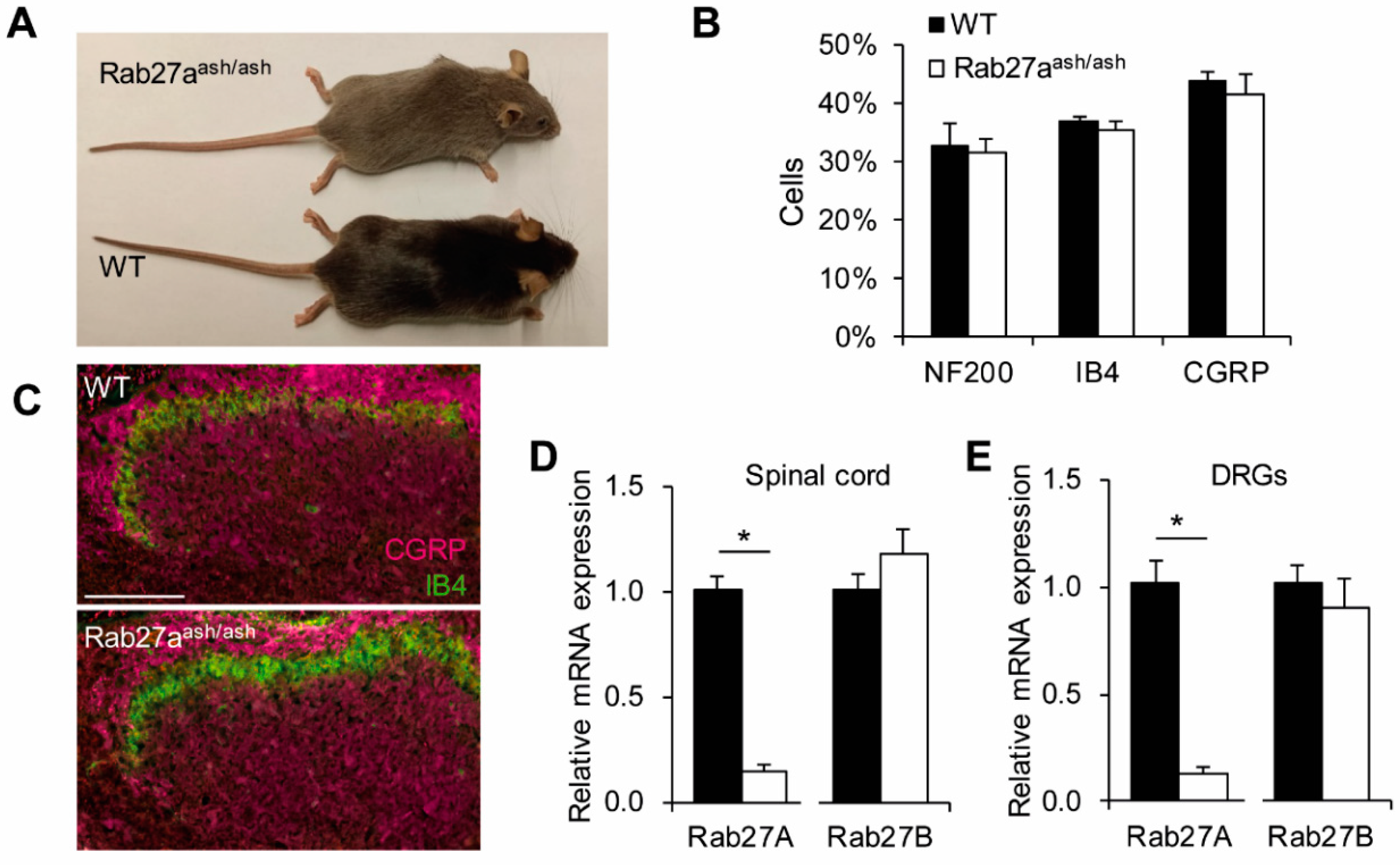
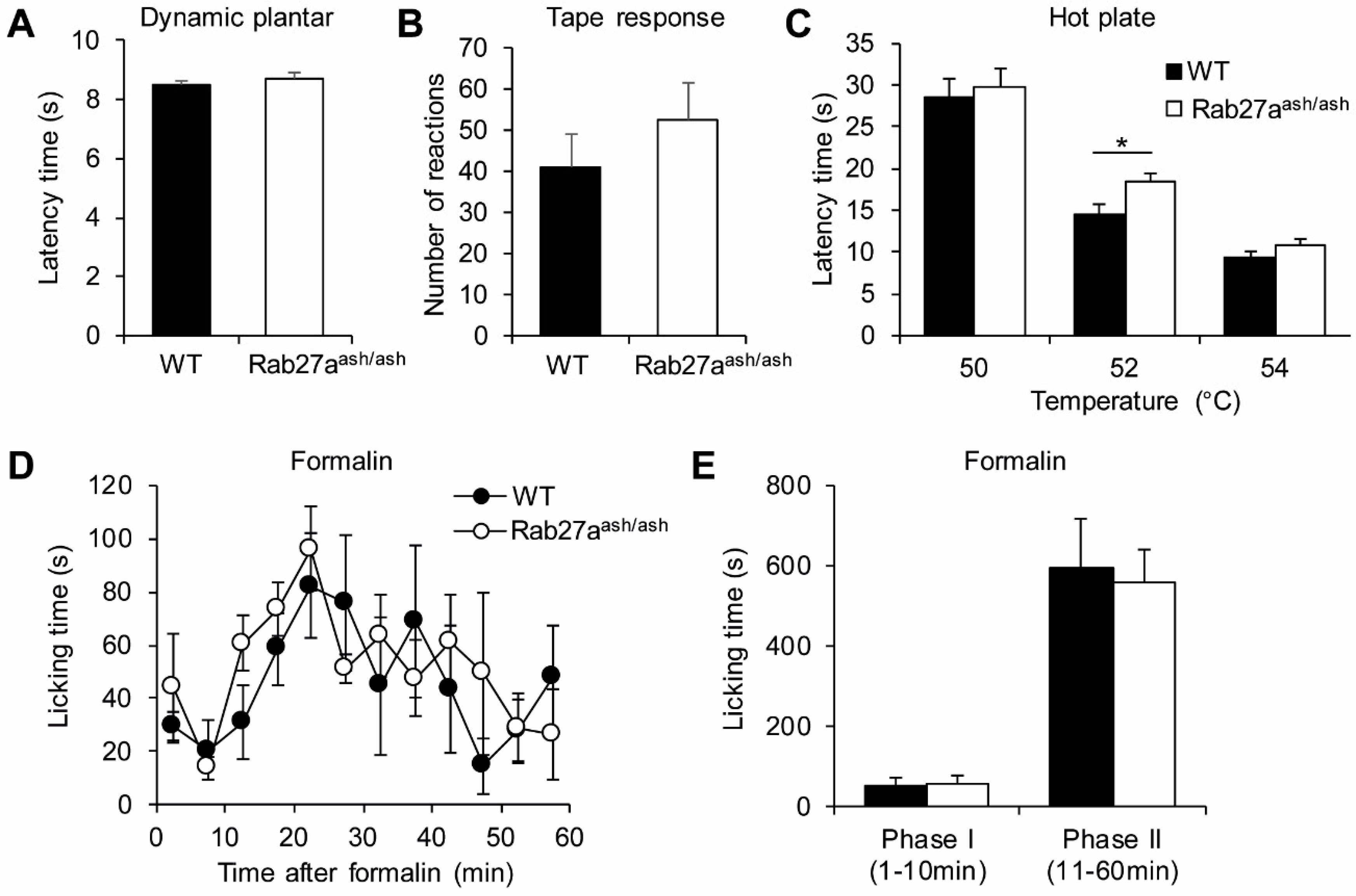
3.2. Rab27aash/ash Mice Display Normal Basal Sensitivity
3.3. Rab27aash/ash Mice Display Reduced Inflammatory Pain Behavior
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease. Pain 2019, 160, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-R.; Xu, Z.-Z.; Gao, Y.-J. Emerging targets in Neuroinflammation-Driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef]
- Johannes, C.B.; Le, T.K.; Zhou, X.; Johnston, J.A.; Dworkin, R.H. The prevalence of chronic pain in United States adults: Results of an Internet-Based survey. J. Pain 2010, 11, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Pan, C.; He, S.-M.; Lang, B.; Gao, G.-D.; Wang, X.-L.; Wang, Y. The Ras superfamily of small GTPases in Non-Neoplastic cerebral diseases. Front. Mol. Neurosci. 2019, 12, 121. [Google Scholar] [CrossRef]
- Kallenborn-Gerhardt, W.; Möser, C.V.; Lorenz, J.E.; Steger, M.; Heidler, J.; Scheving, R.; Petersen, J.; Kennel, L.; Flauaus, C.; Lu, R.; et al. Rab7—A novel redox target that modulates inflammatory pain processing. Pain 2017, 158, 1354–1365. [Google Scholar] [CrossRef]
- Chen, H.; Hu, Y.; Xie, K.; Chen, Y.; Wang, H.; Bian, Y.; Wang, Y.; Dong, A.; Yu, Y. Effect of autophagy on allodynia, hyperalgesia and astrocyte activation in a rat model of neuropathic pain. Int. J. Mol. Med. 2018, 42, 2009–2019. [Google Scholar] [CrossRef]
- Mousa, S.A.; Shaqura, B.I.M.; Khalefa, C.; Zollner, L.; Schaad, J.; Schneider, T.S.; Shippenberg, J.F.; Richter, R.; Hellweg, M.; Shakibaei, M. Schafer. Rab7 silencing prevents Mu-Opioid receptor lysosomal targeting and rescues opioid responsiveness to strengthen diabetic neuropathic pain therapy. Diabetes 2013, 62, 1308–1319. [Google Scholar] [CrossRef]
- Roosterman, D.; Cottrell, G.S.; Schmidlin, F.; Steinhoff, M.; Bunnett, N.W. Recycling and resensitization of the neurokinin 1 receptor. J. Biol. Chem. 2004, 279, 30670–30679. [Google Scholar] [CrossRef]
- Chen, W.; Guo, S.; Wang, S. MicroRNA-16 alleviates inflammatory pain by targeting Ras-Related protein 23 (RAB23) and inhibiting p38 MAPK activation. Med Sci. Monit. 2016, 22, 3894–3901. [Google Scholar] [CrossRef]
- Bolasco, G.; Tracey-White, D.C.; Tolmachova, T.; Thorley, A.J.; Tetley, T.D.; Seabra, M.; Hume, A.N. Loss of Rab27 function results in abnormal lung epithelium structure in mice. Am. J. Physiol. Physiol. 2010, 300, C466–C476. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meeths, M.; Bryceson, Y.T.; Rudd, E.; Zheng, C.; Wood, S.M.; Ramme, K.; Beutel, K.; Hasle, H.; Heilmann, C.; Hultenby, K.; et al. Clinical presentation of Griscelli syndrome type 2 and spectrum ofRAB27Amutations. Pediatr. Blood Cancer 2009, 54, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Ménasché, G.; Pastural, E.; Feldmann, J.; Certain, S.; Ersoy, F.; Dupuis, S.; Wulffraat, N.; Bianchi, D.; Fischer, A.; Le Deist, F.; et al. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat. Genet. 2000, 25, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Ramadass, M.; Catz, S.D. Molecular mechanisms regulating secretory organelles and endosomes in neutrophils and their implications for inflammation. Immunol. Rev. 2016, 273, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Cabrera, A.N.; Matos-Martínez, M.; Sánchez-Villegas, M.C.; Lázaro-Castillo, L.M.; Méndez-León, J.; Martínez-Amigon, J.; Aguilar, M.; García-Escobar, B. The Griscelli-Prunieras syndrome: A case report. Bol. Méd. Hosp. Infant. México 1993, 50, 503–507. [Google Scholar]
- Haddad, E.K.; Wu, X.; Hammer, J.A.; Henkart, P.A. Defective granule exocytosis in Rab27a-Deficient lymphocytes from ashen mice. J. Cell Biol. 2001, 152, 835–842. [Google Scholar] [CrossRef]
- Hume, A.N.; Collinson, L.; Rapak, A.; Gomes, A.; Hopkins, C.R.; Seabra, M. Rab27a regulates the peripheral distribution of melanosomes in Melanocytes. J. Cell Biol. 2001, 152, 795–808. [Google Scholar] [CrossRef]
- Stinchcombe, J.C.; Barral, D.C.; Mules, E.H.; Booth, S.; Hume, A.N.; Machesky, L.M.; Seabra, M.; Griffiths, G.M. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J. Cell Biol. 2001, 152, 825–834. [Google Scholar] [CrossRef]
- Wilson, S.; Yip, R.; Swing, D.A.; O’Sullivan, T.N.; Zhang, Y.; Novak, E.K.; Swank, R.T.; Russell, L.B.; Copeland, N.G.; Jenkins, N.A. A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc. Natl. Acad. Sci. USA 2000, 97, 7933–7938. [Google Scholar] [CrossRef]
- Ginsberg, S.D.; Mufson, E.J.; Alldred, M.J.; Counts, S.E.; Wuu, J.; Nixon, R.A.; Che, S. Upregulation of select rab GTPases in cholinergic basal forebrain neurons in mild cognitive impairment and Alzheimer’s disease. J. Chem. Neuroanat. 2011, 42, 102–110. [Google Scholar] [CrossRef]
- Hui, L.; Geiger, N.; Bloor-Young, D.; Churchill, G.C.; Geiger, J.D.; Chen, X. Release of calcium from endolysosomes increases calcium influx through N-type calcium channels: Evidence for acidic Store-Operated calcium entry in neurons. Cell Calcium 2015, 58, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Tang, S.; Han, X.; Jiang, Z.; Dong, L.; Liu, C.; Liang, X.; Dong, J.; Qiu, C.; Wang, Y.; et al. KIBRA controls exosome secretion via inhibiting the proteasomal degradation of Rab27a. Nat. Commun. 2019, 10, 1639. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, Z.; Wei, Z.; Cheng, Q.; Li, X.; Li, W.; Duan, S.; Gu, X. Lysosomal exocytosis in Schwann cells contributes to axon remyelination. Glia 2011, 60, 295–305. [Google Scholar] [CrossRef]
- Chen, G.; Su, W.-F.; Gu, Y.; Wei, Z.-Y.; Shen, Y.-T.; Jin, Z.-H.; Yuan, Y.; Gu, X.-S. Rab27a/Slp2-a complex is involved in Schwann cell myelination. Neural Regen. Res. 2016, 11, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Seabra, M.; Mules, E.H.; Hume, A.N. Rab GTPases, intracellular traffic and disease. Trends Mol. Med. 2002, 8, 23–30. [Google Scholar] [CrossRef]
- Barral, D.C.; Ramalho, J.S.; Anders, R.; Hume, A.N.; Knapton, H.J.; Tolmachova, T.; Collinson, L.M.; Goulding, D.; Authi, K.S.; Seabra, M.C. Functional redundancy of Rab27 proteins and the pathogenesis of Griscelli syndrome. J. Clin. Investig. 2002, 110, 247–257. [Google Scholar] [CrossRef]
- Ranade, S.S.; Woo, S.-H.; Dubin, A.E.; Moshourab, R.; Wetzel, C.; Petrus, M.; Mathur, J.; Bégay, V.; Coste, B.; Mainquist, J.; et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 2014, 516, 121–125. [Google Scholar] [CrossRef]
- Eddy, N.B.; Leimbach, D. Synthetic analgesics. II. Dithienylbutenyl-and dithienylbutylamines. J. Pharm. Exp. 1953, 107, 385–393. [Google Scholar]
- Cortright, D.N.; Krause, J.E.; Broom, D.C. TRP channels and pain. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2007, 1772, 978–988. [Google Scholar] [CrossRef]
- Mogil, J.S.; Wilson, S.G.; Bon, K.; Lee, S.E.; Chung, K.; Raber, P.; Pieper, J.O.; Hain, H.S.; Belknap, J.K.; Hubert, L.; et al. Heritability of nociception I: Responses of 11 inbred mouse strains on 12 measures of nociception. Pain 1999, 80, 67–82. [Google Scholar] [CrossRef]
- Kallenborn-Gerhardt, W.; Schröder, K.; Del Turco, D.; Lu, R.; Kynast, K.; Kosowski, J.; Niederberger, E.; Shah, A.M.; Brandes, R.P.; Geisslinger, G.; et al. NADPH Oxidase-4 maintains neuropathic pain after peripheral nerve injury. J. Neurosci. 2012, 32, 10136–10145. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Bausch, A.E.; Kallenborn-Gerhardt, W.; Stoetzer, C.; De Bruin, N.; Ruth, P.; Geisslinger, G.; Leffler, A.; Lukowski, R.; Schmidtko, A. Slack channels expressed in sensory neurons control neuropathic pain in mice. J. Neurosci. 2015, 35, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Schmidtko, A.; Luo, C.; Gao, W.; Geisslinger, G.; Kuner, R.; Tegeder, I. Genetic deletion of synapsin II reduces neuropathic pain due to reduced glutamate but increased GABA in the spinal cord dorsal horn. Pain 2008, 139, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Hunskaar, S.; Fasmer, O.B.; Hole, K. Formalin test in mice, a useful technique for evaluating mild analgesics. J. Neurosci. Methods 1985, 14, 69–76. [Google Scholar] [CrossRef]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M. TRPA1 mediates Formalin-Induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Flauaus, C.; Kennel, L.; Petersen, J.; Drees, O.; Kallenborn-Gerhardt, W.; Ruth, P.; Lukowski, R.; Schmidtko, A. KCa 3.1 channels modulate the processing of noxious chemical stimuli in mice. Neuropharmacology 2017, 125, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Meller, S.; Gebhart, G. Intraplantar zymosan as a reliable, quantifiable model of thermal and mechanical hyperalgesia in the rat. Eur. J. Pain 1997, 1, 43–52. [Google Scholar] [CrossRef]
- Pitzer, C.; Kuner, R.; Tappe-Theodor, A. Voluntary and evoked behavioral correlates in inflammatory pain conditions under different social housing conditions. Pain Rep. 2016, 1, e564. [Google Scholar] [CrossRef]
- Schmidtko, A.; Gao, W.; König, P.; Heine, S.; Motterlini, R.; Ruth, P.; Schlossmann, J.; Koesling, R.; Niederberger, E.; Tegeder, I.; et al. cGMP Produced by No-Sensitive guanylyl cyclase essentially contributes to inflammatory and neuropathic pain by using targets different from cGMP-Dependent protein kinase I. J. Neurosci. 2008, 28, 8568–8576. [Google Scholar] [CrossRef]
- Schnell, S.A.; Staines, W.; Wessendorf, M.W. Reduction of Lipofuscin-Like autofluorescence in fluorescently labeled tissue. J. Histochem. Cytochem. 1999, 47, 719–730. [Google Scholar] [CrossRef]
- Takahashi, A.; Okada, R.; Nagao, K.; Kawamata, Y.; Hanyu, A.; Yoshimoto, S.; Takasugi, M.; Watanabe, S.; Kanemaki, M.T.; Obuse, C.; et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat. Commun. 2017, 8, 15287. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, A.; Hochgerner, H.; Lönnerberg, P.; Johnsson, A.; Memic, F.; Van Der Zwan, J.; Häring, M.; Braun, E.; Borm, L.E.; La Manno, G.; et al. Molecular architecture of the mouse nervous system. Cell 2018, 174, 999–1014.e22. [Google Scholar] [CrossRef] [PubMed]
- Catz, S.D. The role of Rab27a in the regulation of neutrophil function. Cell. Microbiol. 2014, 16, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M. Rab27 effectors, pleiotropic regulators in secretory pathways. Traffic 2013, 14, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Krzewski, K.; Cullinane, A.R. Evidence for defective Rab GTPase-dependent cargo traffic in immune disorders. Exp. Cell Res. 2013, 319, 2360–2367. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Z.-H.; Fang, R.; Fang, J.; He, S.; Liu, T. Functional implications of Rab27 GTPases in Cancer. Cell Commun. Signal. 2018, 16, 44. [Google Scholar] [CrossRef]
- Tolmachova, T.; Anders, R.; Stinchcombe, J.; Bossi, G.; Griffiths, G.M.; Huxley, C.; Seabra, M. A general role for Rab27a in secretory cells. Mol. Biol. Cell 2004, 15, 332–344. [Google Scholar] [CrossRef]
- Yamaoka, M.; Ishizaki, T.; Kimura, T. GTP- and GDP-Dependent Rab27a effectors in pancreatic Beta-Cells. Biol. Pharm. Bull. 2015, 38, 663–668. [Google Scholar] [CrossRef]
- LaCroix-Fralish, M.L.; Austin, J.-S.; Zheng, F.Y.; Levitin, D.J.; Mogil, J.S. Patterns of pain: Meta-Analysis of microarray studies of pain. Pain 2011, 152, 1888–1898. [Google Scholar] [CrossRef]
- Caterina, M.J. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A Heat-Activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Vandewauw, I.; De Clercq, K.; Mulier, M.; Held, K.; Pinto, S.; Van Ranst, N.; Segal, A.; Voet, T.; Vennekens, R.; Zimmermann, K.; et al. A TRP channel trio mediates acute noxious heat sensing. Nature 2018, 555, 662–666. [Google Scholar] [CrossRef]
- Caterina, M.J.; Rosen, T.A.; Tominaga, M.; Brake, A.J.; Julius, D. A Capsaicin-Receptor homologue with a high threshold for noxious heat. Nature 1999, 398, 436–441. [Google Scholar] [CrossRef]
- Cho, H.; Yang, Y.D.; Lee, J.; Lee, B.; Kim, T.; Jang, Y.; Back, S.K.; Na, H.S.; Harfe, B.D.; Wang, F.; et al. The Calcium-Activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat. Neurosci. 2012, 15, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Viana, F.; Voets, T. Heat pain and cold pain. In The Oxford Handbook of the Neurobiology of Pain; Oxford University Press (OUP): Oxford, UK, 2019. [Google Scholar]
- Lee, H.; Iida, T.; Mizuno, A.; Suzuki, M.; Caterina, M.J. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J. Neurosci. 2005, 25, 1304–1310. [Google Scholar] [CrossRef]
- Moqrich, A.; Hwang, S.W.; Earley, T.J.; Petrus, M.; Murray, A.N.; Spencer, K.S.R.; Andahazy, M.; Story, G.M.; Patapoutian, A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005, 307, 1468–1472. [Google Scholar] [CrossRef]
- Vriens, J.; Owsianik, G.; Hofmann, T.; Philipp, S.E.; Stab, J.; Chen, X.; Benoit, M.; Xue, F.; Janssens, A.; Kerselaers, S.; et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 2011, 70, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.K.; Horiuchi, H.; Fukuda, M. Rab27a regulates epithelial sodium channel (ENaC) activity through Synaptotagmin-Like protein (SLP-5) and Munc13-4 effector mechanism. Biochem. Biophys. Res. Commun. 2006, 344, 651–657. [Google Scholar] [CrossRef]
- Saxena, S.K.; Kaur, S. Rab27a negatively regulates CFTR chloride channel function in colonic epithelia: Involvement of the effector proteins in the regulatory mechanism. Biochem. Biophys. Res. Commun. 2006, 346, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Reichhart, N.; Markowski, M.; Ishiyama, S.; Wagner, A.; Crespo-Garcia, S.; Schorb, T.; Ramalho, J.; Milenkovic, V.M.; Föckler, R.; Seabra, M.; et al. Rab27a GTPase modulates L-Type Ca2+ channel function via interaction with the II–III linker of CaV1.3 subunit. Cell. Signal. 2015, 27, 2231–2240. [Google Scholar] [CrossRef]
- Chen, D.; Guo, J.; Miki, T.; Tachibana, M.; Gahl, W.A. Molecular cloning and characterization of Rab27a and Rab27b, novel human rab proteins shared by melanocytes and platelets. Biochem. Mol. Med. 1997, 60, 27–37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gomi, H.; Mori, K.; Itohara, S.; Izumi, T. Rab27b is expressed in a wide range of exocytic cells and involved in the delivery of secretory granules near the plasma membrane. Mol. Biol. Cell 2007, 18, 4377–4386. [Google Scholar] [CrossRef] [PubMed]
- Gallo, L.I.; Dalghi, M.G.; Clayton, D.R.; Ruiz, W.G.; Khandelwal, P.; Apodaca, G. RAB27B requirement for Stretch-Induced exocytosis in bladder umbrella cells. Am. J. Physiol. Physiol. 2018, 314, C349–C365. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Brzezinska, A.A.; Tolmachova, T.; Munafo, D.B.; Ellis, B.A.; Seabra, M.; Hong, H.; Catz, S.D. Rab27a and Rab27b regulate neutrophil azurophilic granule exocytosis and NADPH oxidase activity by independent mechanisms. Traffic 2010, 11, 533–547. [Google Scholar] [CrossRef]
- Ostrowski, M.; Carmo, N.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nature 2009, 12, 19–30. [Google Scholar] [CrossRef]
- Singh, R.K.; Mizuno, K.; Wasmeier, C.; Wavre-Shapton, S.T.; Recchi, C.; Catz, S.D.; Futter, C.; Tolmachova, T.; Hume, A.N.; Seabra, M. Distinct and opposing roles for Rab27a/Mlph/MyoVa and Rab27b/Munc13-4 in mast cell secretion. FEBS J. 2012, 280, 892–903. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gross, T.; Wack, G.; Syhr, K.M.J.; Tolmachova, T.; Seabra, M.C.; Geisslinger, G.; Niederberger, E.; Schmidtko, A.; Kallenborn-Gerhardt, W. Rab27a Contributes to the Processing of Inflammatory Pain in Mice. Cells 2020, 9, 1488. https://doi.org/10.3390/cells9061488
Gross T, Wack G, Syhr KMJ, Tolmachova T, Seabra MC, Geisslinger G, Niederberger E, Schmidtko A, Kallenborn-Gerhardt W. Rab27a Contributes to the Processing of Inflammatory Pain in Mice. Cells. 2020; 9(6):1488. https://doi.org/10.3390/cells9061488
Chicago/Turabian StyleGross, Tilman, Gesine Wack, Katharina M. J. Syhr, Tanya Tolmachova, Miguel C. Seabra, Gerd Geisslinger, Ellen Niederberger, Achim Schmidtko, and Wiebke Kallenborn-Gerhardt. 2020. "Rab27a Contributes to the Processing of Inflammatory Pain in Mice" Cells 9, no. 6: 1488. https://doi.org/10.3390/cells9061488
APA StyleGross, T., Wack, G., Syhr, K. M. J., Tolmachova, T., Seabra, M. C., Geisslinger, G., Niederberger, E., Schmidtko, A., & Kallenborn-Gerhardt, W. (2020). Rab27a Contributes to the Processing of Inflammatory Pain in Mice. Cells, 9(6), 1488. https://doi.org/10.3390/cells9061488




