PSPC1 Potentiates IGF1R Expression to Augment Cell Adhesion and Motility
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Constructs
2.2. Reagents
2.3. Adhesion Assay
2.4. 3D Matrigel Assay
2.5. Cell Lysate and Immunoblot Analysis
2.6. Immunofluorescence Analysis
2.7. Migration, Invasion and Haptotaxis Assays
2.8. Transcriptome Analysis and Proteomic Analysis
2.9. RRM Domain Mutation and Deletion Construction of PSPC1
3. Results
3.1. Expression of PSPC1 Promotes Cell Migration and Cytoskeleton Re-Assembly
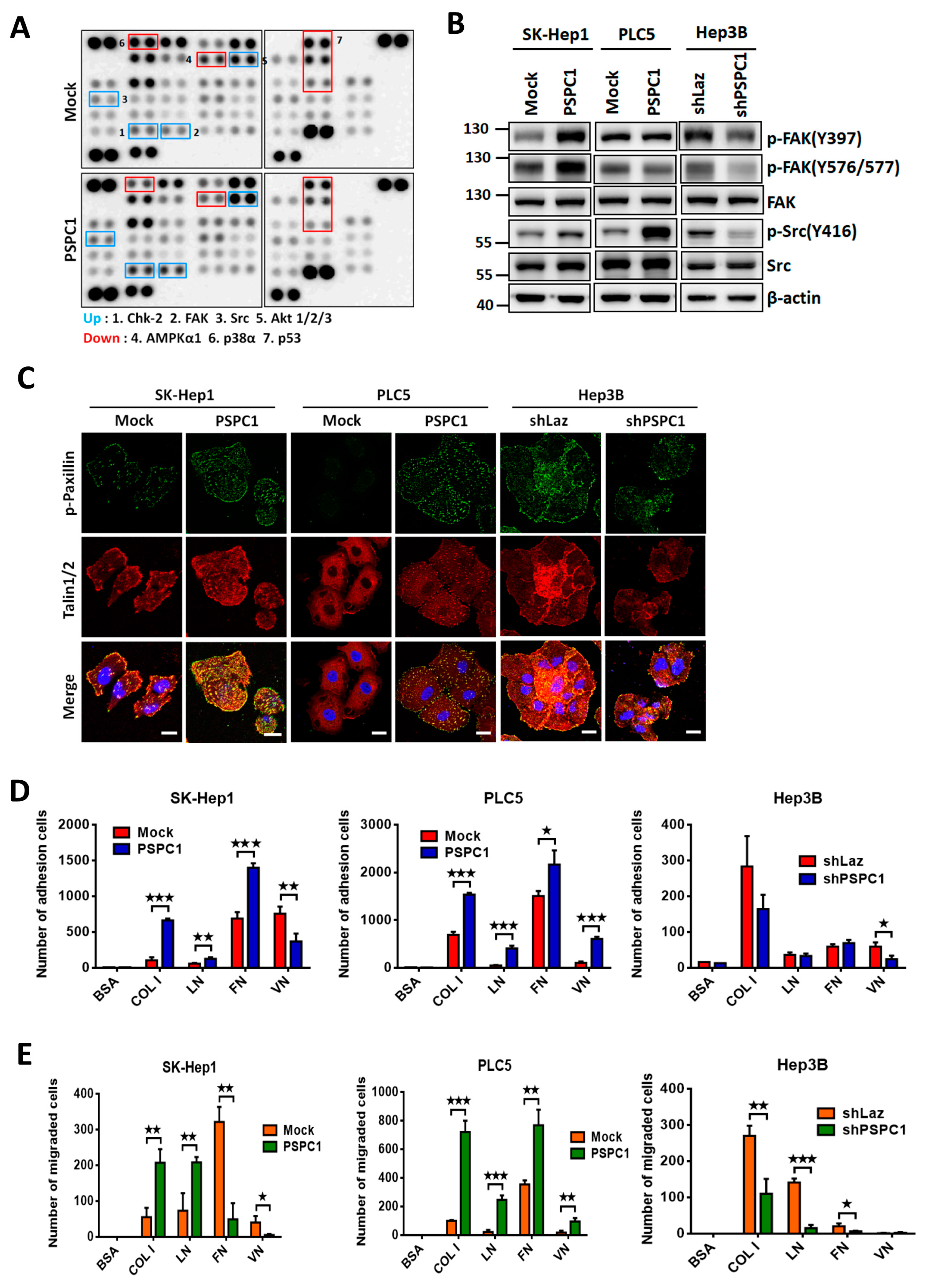
3.2. PSPC1 Induced Focal Adhesion Formation and Chemotaxis to ECM
3.3. PSPC1 Modulates IGF1R Expression Involved in Focal Adhesion Pathway
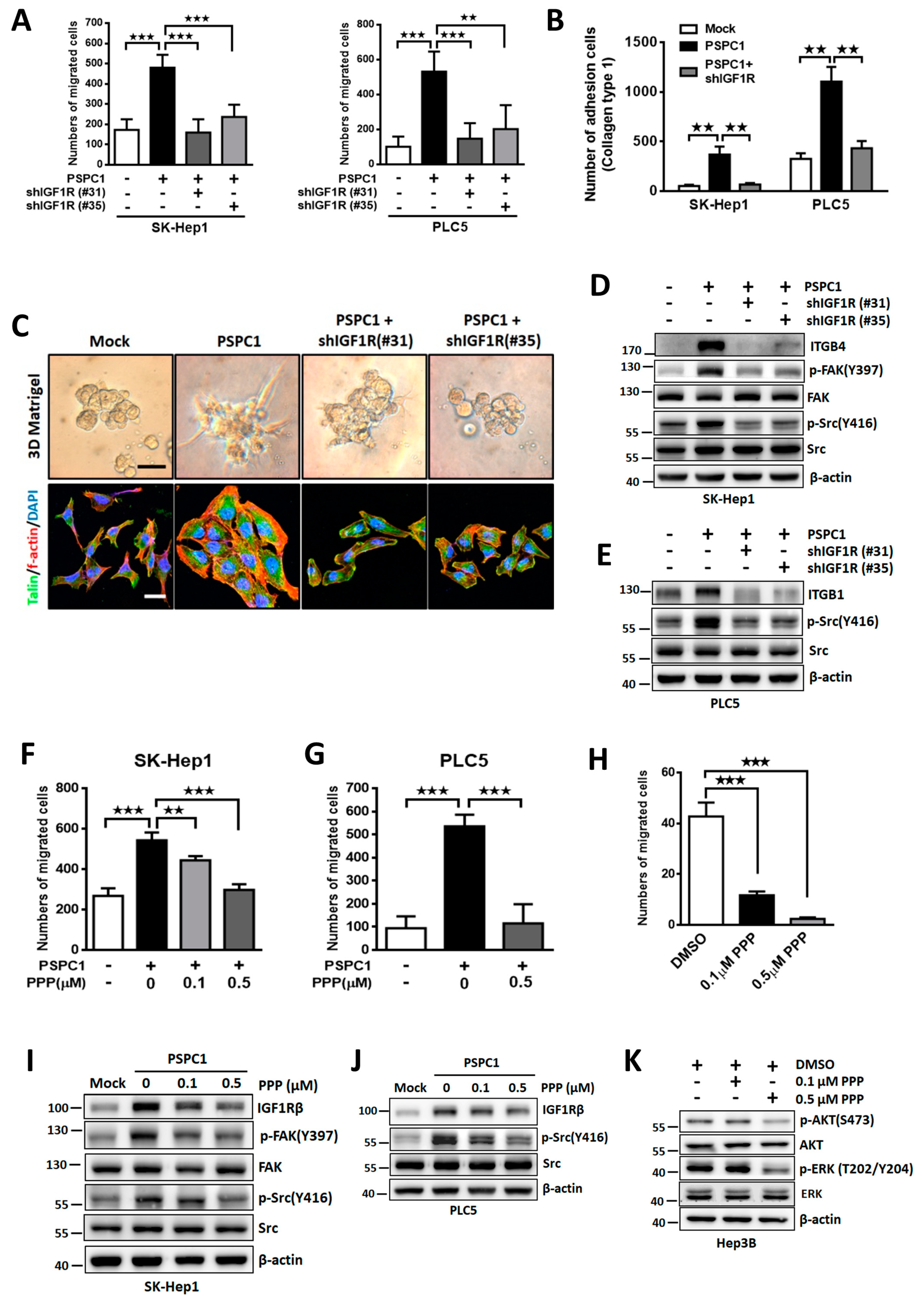
3.4. Blocking IGF1R Signaling Suppresses PSPC1-Induced Cell Motility and Adhesion
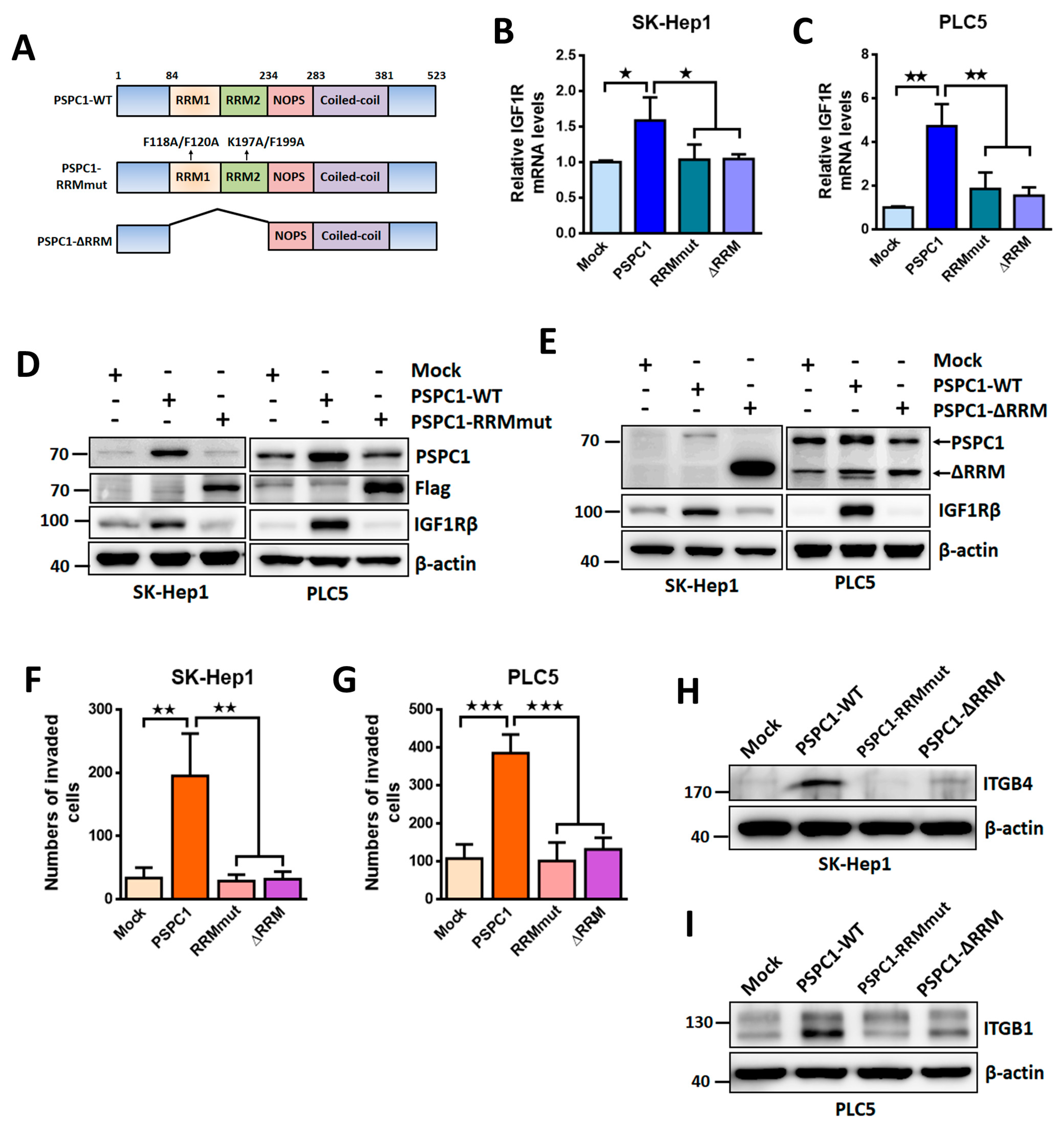
3.5. PSPC1 RNA Recognition Motif (RRM) Required for PSPC1/IGF1R Axis Activated Cell Motility
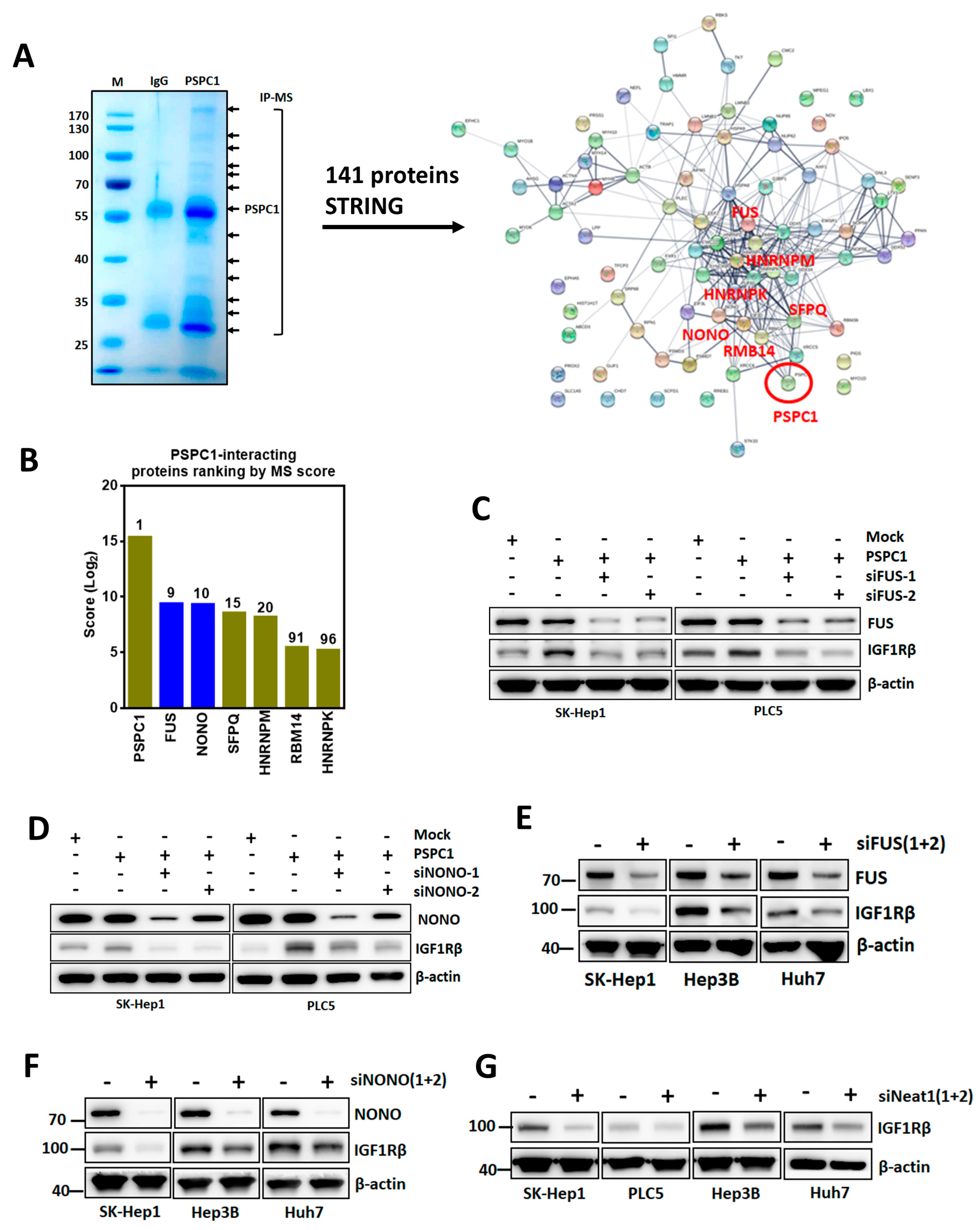
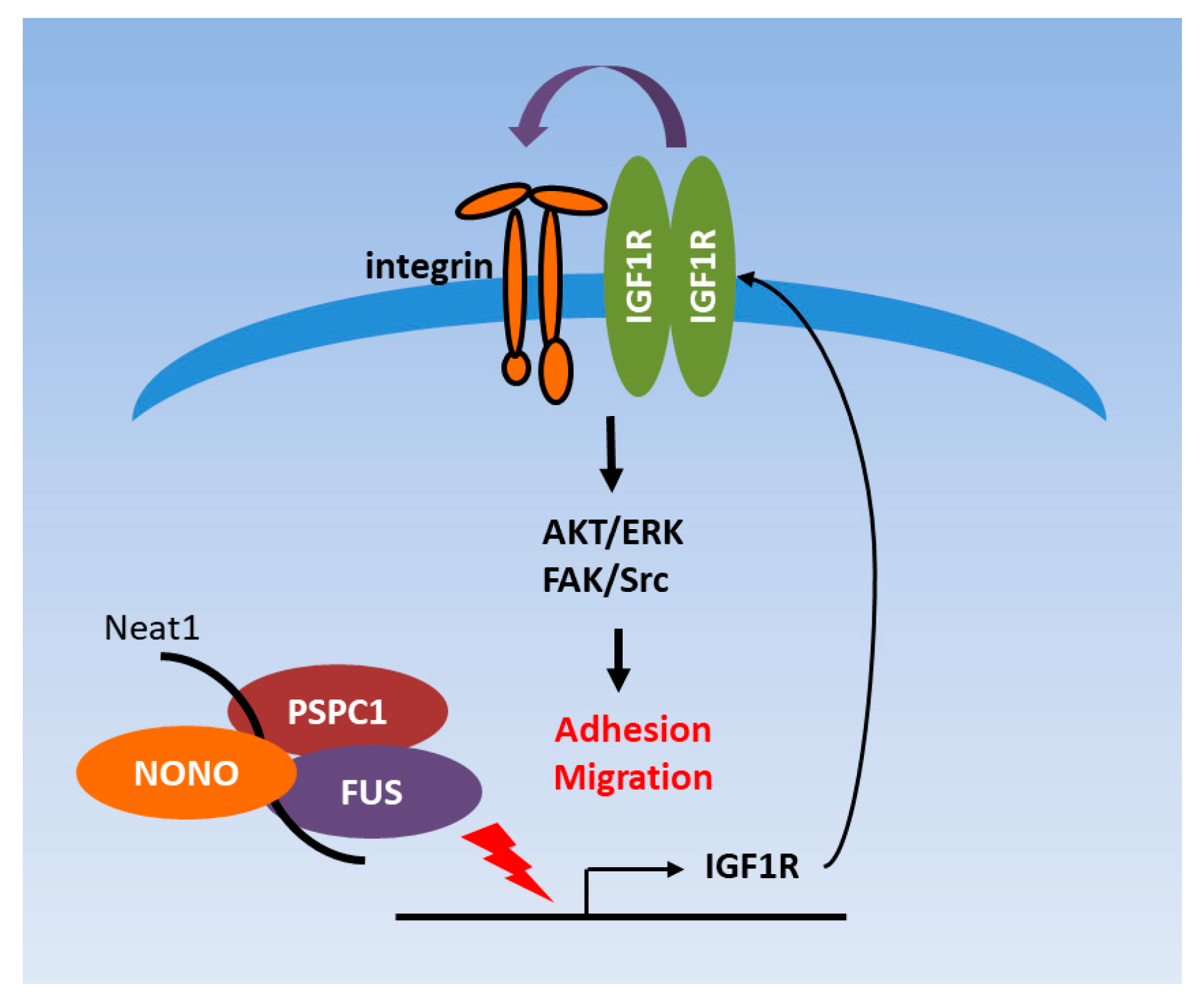
3.6. PSPC1 Collaborated with Paraspeckle Components to Modulate IGF1R Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Knott, G.J.; Bond, C.S.; Fox, A.H. The DBHS proteins SFPQ, NONO and PSPC1: A multipurpose molecular scaffold. Nucleic Acids Res. 2016, 44, 3989–4004. [Google Scholar] [CrossRef] [PubMed]
- Passon, D.M.; Lee, M.; Rackham, O.; Stanley, W.A.; Sadowska, A.; Filipovska, A.; Fox, A.H.; Bond, C.S. Structure of the heterodimer of human NONO and paraspeckle protein component 1 and analysis of its role in subnuclear body formation. Proc. Natl. Acad. Sci. USA 2012, 109, 4846–4850. [Google Scholar] [CrossRef] [PubMed]
- Guallar, D.; Bi, X.; Pardavila, J.A.; Huang, X.; Saenz, C.; Shi, X.; Zhou, H.; Faiola, F.; Ding, J.; Haruehanroengra, P.; et al. RNA-dependent chromatin targeting of TET2 for endogenous retrovirus control in pluripotent stem cells. Nat. Genet. 2018, 50, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Kong, L.; Lu, X.; Zhang, G.; Chi, L.; Jiang, Y.; Wu, Y.; Yan, C.; Duerksen-Hughes, P.; Zhu, X.; et al. Paraspeckle protein 1 (PSPC1) is involved in the cisplatin induced DNA damage response—Role in G1/S checkpoint. PLoS ONE 2014, 9, e97174. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, G.; Shan, S.; Shang, Y.; Chi, L.; Li, H.; Cao, Y.; Zhu, X.; Zhang, M.; Yang, J. Depletion of Paraspeckle Protein 1 Enhances Methyl Methanesulfonate-Induced Apoptosis through Mitotic Catastrophe. PLoS ONE 2016, 11, e0146952. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, J.; Rajbhandari, P.; Damianov, A.; Han, A.; Sallam, T.; Waki, H.; Villanueva, C.J.; Lee, S.D.; Nielsen, R.; Mandrup, S.; et al. RNA-binding protein PSPC1 promotes the differentiation-dependent nuclear export of adipocyte RNAs. J. Clin. Investig. 2017, 127, 987–1004. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fan, P.; Zhao, Y.; Zhang, S.; Lu, J.; Xie, W.; Jiang, Y.; Lei, F.; Xu, N.; Zhang, Y. NEAT1 modulates herpes simplex virus-1 replication by regulating viral gene transcription. Cell. Mol. Life Sci. 2017, 74, 1117–1131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, S.B.; Wang, M.R.; Yao, R.W.; Wu, D.; Yang, L.; Chen, L.L. Genome-wide screening of NEAT1 regulators reveals cross-regulation between paraspeckles and mitochondria. Nat. Cell Biol. 2018, 20, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Skelt, L.; Notaro, A.; Highley, J.R.; Fox, A.H.; La Bella, V.; Buchman, V.L.; Shelkovnikova, T.A. ALS-linked FUS mutations confer loss and gain of function in the nucleus by promoting excessive formation of dysfunctional paraspeckles. Acta Neuropathol. Commun. 2019, 7, 7. [Google Scholar] [CrossRef]
- West, J.A.; Mito, M.; Kurosaka, S.; Takumi, T.; Tanegashima, C.; Chujo, T.; Yanaka, K.; Kingston, R.E.; Hirose, T.; Bond, C.; et al. Structural, super-resolution microscopy analysis of paraspeckle nuclear body organization. J. Cell Biol. 2016, 214, 817–830. [Google Scholar] [CrossRef]
- Yeh, H.W.; Hsu, E.C.; Lee, S.S.; Lang, Y.D.; Lin, Y.C.; Chang, C.Y.; Lee, S.Y.; Gu, D.L.; Shih, J.H.; Ho, C.M.; et al. PSPC1 mediates TGF-beta1 autocrine signalling and Smad2/3 target switching to promote EMT, stemness and metastasis. Nat. Cell Biol. 2018, 20, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.D.; Chen, H.Y.; Ho, C.M.; Shih, J.H.; Hsu, E.C.; Shen, R.; Lee, Y.C.; Chen, J.W.; Wu, C.Y.; Yeh, H.W.; et al. PSPC1-interchanged interactions with PTK6 and beta-catenin synergize oncogenic subcellular translocations and tumor progression. Nat. Commun. 2019, 10, 5716. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.B.; Zhou, L.; Liang, Z.Y.; Zhou, W.X.; Jin, Y. Expression of GRK2 and IGF1R in hepatocellular carcinoma: Clinicopathological and prognostic significance. J. Clin. Pathol. 2017, 70, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Han, X.D.; Qiu, Y.; Xiong, J.; Yu, Y.; Wang, B.; Zhu, Z.Z.; Qian, B.P.; Chen, Y.X.; Wang, S.F.; et al. Increased expression of insulin-like growth factor-1 receptor is correlated with tumor metastasis and prognosis in patients with osteosarcoma. J. Surg. Oncol. 2012, 105, 235–243. [Google Scholar] [CrossRef]
- Dale, O.T.; Aleksic, T.; Shah, K.A.; Han, C.; Mehanna, H.; Rapozo, D.C.; Sheard, J.D.; Goodyear, P.; Upile, N.S.; Robinson, M.; et al. IGF-1R expression is associated with HPV-negative status and adverse survival in head and neck squamous cell cancer. Carcinogenesis 2015, 36, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Heskamp, S.; Boerman, O.C.; Molkenboer-Kuenen, J.D.; Wauters, C.A.; Strobbe, L.J.; Mandigers, C.M.; Bult, P.; Oyen, W.J.; van der Graaf, W.T.; van Laarhoven, H.W. Upregulation of IGF-1R expression during neoadjuvant therapy predicts poor outcome in breast cancer patients. PLoS ONE 2015, 10, e0117745. [Google Scholar] [CrossRef]
- Yeo, C.D.; Park, K.H.; Park, C.K.; Lee, S.H.; Kim, S.J.; Yoon, H.K.; Lee, Y.S.; Lee, E.J.; Lee, K.Y.; Kim, T.J. Expression of insulin-like growth factor 1 receptor (IGF-1R) predicts poor responses to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in non-small cell lung cancer patients harboring activating EGFR mutations. Lung Cancer Amst. Neth. 2015, 87, 311–317. [Google Scholar] [CrossRef]
- Pollak, M.N.; Schernhammer, E.S.; Hankinson, S.E. Insulin-like growth factors and neoplasia. Nat. Rev. Cancer 2004, 4, 505–518. [Google Scholar] [CrossRef]
- Mayer, I.A.; Arteaga, C.L. The PI3K/AKT Pathway as a Target for Cancer Treatment. Annu. Rev. Med. 2016, 67, 11–28. [Google Scholar] [CrossRef]
- She, Q.B.; Halilovic, E.; Ye, Q.; Zhen, W.; Shirasawa, S.; Sasazuki, T.; Solit, D.B.; Rosen, N. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell 2010, 18, 39–51. [Google Scholar] [CrossRef]
- Guo, W.; Giancotti, F.G. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 2004, 5, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yu, D. Targeting Src family kinases in anti-cancer therapies: Turning promise into triumph. Trends Pharmacol. Sci. 2012, 33, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Stanicka, J.; Rieger, L.; O’Shea, S.; Cox, O.; Coleman, M.; O’Flanagan, C.; Addario, B.; McCabe, N.; Kennedy, R.; O’Connor, R. FES-related tyrosine kinase activates the insulin-like growth factor-1 receptor at sites of cell adhesion. Oncogene 2018, 37, 3131–3150. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.K.; Hanson, D.A.; Schlaepfer, D.D. Focal adhesion kinase: In command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005, 6, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Iams, W.T.; Lovly, C.M. Molecular Pathways: Clinical Applications and Future Direction of Insulin-like Growth Factor-1 Receptor Pathway Blockade. Clin. Cancer Res. 2015, 21, 4270–4277. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Murphy-Ullrich, J.E. The de-adhesive activity of matricellular proteins: Is intermediate cell adhesion an adaptive state? J. Clin. Investig. 2001, 107, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Tojkander, S.; Gateva, G.; Lappalainen, P. Actin stress fibers—Assembly, dynamics and biological roles. J. Cell Sci. 2012, 125, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, A.; Fedele, C.; Trerotola, M.; Ganguly, K.K.; Languino, L.R. IGF-IR promotes prostate cancer growth by stabilizing alpha5beta1 integrin protein levels. PLoS ONE 2013, 8, e76513. [Google Scholar] [CrossRef]
- Goel, H.L.; Sayeed, A.; Breen, M.; Zarif, M.J.; Garlick, D.S.; Leav, I.; Davis, R.J.; Fitzgerald, T.J.; Morrione, A.; Hsieh, C.C.; et al. Beta1 integrins mediate resistance to ionizing radiation in vivo by inhibiting c-Jun amino terminal kinase 1. J. Cell Physiol. 2013, 228, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Kiely, P.A.; Leahy, M.; O’Gorman, D.; O’Connor, R. RACK1-mediated integration of adhesion and insulin-like growth factor I (IGF-I) signaling and cell migration are defective in cells expressing an IGF-I receptor mutated at tyrosines 1250 and 1251. J. Biol. Chem. 2005, 280, 7624–7633. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, J.S.; Hwang, S.J.; Lee, H.Y. Insulin-like growth factor binding protein-3 inhibits cell adhesion via suppression of integrin beta4 expression. Oncotarget 2015, 6, 15150–15163. [Google Scholar] [CrossRef] [PubMed]
- Vasilcanu, R.; Vasilcanu, D.; Rosengren, L.; Natalishvili, N.; Sehat, B.; Yin, S.; Girnita, A.; Axelson, M.; Girnita, L.; Larsson, O. Picropodophyllin induces downregulation of the insulin-like growth factor 1 receptor: Potential mechanistic involvement of Mdm2 and beta-arrestin1. Oncogene 2008, 27, 1629–1638. [Google Scholar] [CrossRef]
- Girnita, A.; Girnita, L.; del Prete, F.; Bartolazzi, A.; Larsson, O.; Axelson, M. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res. 2004, 64, 236–242. [Google Scholar] [CrossRef]
- Fox, A.H.; Bond, C.S.; Lamond, A.I. P54nrb forms a heterodimer with PSP1 that localizes to paraspeckles in an RNA-dependent manner. Mol. Biol. Cell 2005, 16, 5304–5315. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, S.; Ikei, A.; Taguchi, Y.; Tabuchi, Y.; Fujimoto, N.; Obinata, M.; Uesugi, S.; Kurihara, Y. PSPC1, NONO, and SFPQ are expressed in mouse Sertoli cells and may function as coregulators of androgen receptor-mediated transcription. Biol. Reprod. 2006, 75, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, T.; Nakagawa, S.; Tanigawa, A.; Sasaki, Y.F.; Goshima, N.; Hirose, T. Alternative 3’-end processing of long noncoding RNA initiates construction of nuclear paraspeckles. EMBO J. 2012, 31, 4020–4034. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Yamazaki, T.; Nakagawa, S. Molecular anatomy of the architectural NEAT1 noncoding RNA: The domains, interactors, and biogenesis pathway required to build phase-separated nuclear paraspeckles. Wiley Interdiscip. Rev. RNA 2019, 10, e1545. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.W.; Lee, S.S.; Chang, C.Y.; Lang, Y.D.; Jou, Y.S. A New Switch for TGFbeta in Cancer. Cancer Res. 2019, 79, 3797–3805. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.W.; Jou, Y.S. PSPC1 potentiates TGF-beta-dependent metastatic dissemination. Mol. Cell. Oncol. 2018, 5, e1472058. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.D.; Jou, Y.S. PSPC1: A contextual determinant of tumor progression. Mol. Cellular Oncol. 2020, 7, 1721253. [Google Scholar] [CrossRef] [PubMed]
- Denduluri, S.K.; Idowu, O.; Wang, Z.; Liao, Z.; Yan, Z.; Mohammed, M.K.; Ye, J.; Wei, Q.; Wang, J.; Zhao, L.; et al. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis. 2015, 2, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.R.; Zhau, H.E.; Odero-Marah, V.A.; Osunkoya, A.O.; Kimbro, K.S.; Tighiouart, M.; Liu, T.; Simons, J.W.; O’Regan, R.M. Insulin-like growth factor-I-dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2008, 68, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Desai, V.; Wang, J.; Epstein, D.M.; Miglarese, M.; Buck, E. Epithelial-mesenchymal transition predicts sensitivity to the dual IGF-1R/IR inhibitor OSI-906 in hepatocellular carcinoma cell lines. Mol. Cancer Ther. 2012, 11, 503–513. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goel, H.L.; Breen, M.; Zhang, J.; Das, I.; Aznavoorian-Cheshire, S.; Greenberg, N.M.; Elgavish, A.; Languino, L.R. Beta1A integrin expression is required for type 1 insulin-like growth factor receptor mitogenic and transforming activities and localization to focal contacts. Cancer Res. 2005, 65, 6692–6700. [Google Scholar] [CrossRef]
- Yu, X.; Li, Z.; Zheng, H.; Chan, M.T.; Wu, W.K. NEAT1: A novel cancer-related long non-coding RNA. Cell Prolif. 2017, 50. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, J.; Chen, C.; Zhang, R.; Wang, K. Pan-cancer analysis of long non-coding RNA NEAT1 in various cancers. Genes Dis. 2018, 5, 27–35. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jen, H.-W.; Gu, D.-L.; Lang, Y.-D.; Jou, Y.-S. PSPC1 Potentiates IGF1R Expression to Augment Cell Adhesion and Motility. Cells 2020, 9, 1490. https://doi.org/10.3390/cells9061490
Jen H-W, Gu D-L, Lang Y-D, Jou Y-S. PSPC1 Potentiates IGF1R Expression to Augment Cell Adhesion and Motility. Cells. 2020; 9(6):1490. https://doi.org/10.3390/cells9061490
Chicago/Turabian StyleJen, Hsin-Wei, De-Leung Gu, Yaw-Dong Lang, and Yuh-Shan Jou. 2020. "PSPC1 Potentiates IGF1R Expression to Augment Cell Adhesion and Motility" Cells 9, no. 6: 1490. https://doi.org/10.3390/cells9061490
APA StyleJen, H.-W., Gu, D.-L., Lang, Y.-D., & Jou, Y.-S. (2020). PSPC1 Potentiates IGF1R Expression to Augment Cell Adhesion and Motility. Cells, 9(6), 1490. https://doi.org/10.3390/cells9061490





