Abstract
Esophageal cancer (EC) is an aggressive form of cancer, including squamous cell carcinoma (ESCC) and adenocarcinoma (EAC) as two predominant histological subtypes. Accumulating evidence supports the existence of cancer stem cells (CSCs) able to initiate and maintain EAC or ESCC. In this review, we aim to collect the current evidence on CSCs in esophageal cancer, including the biomarkers/characterization strategies of CSCs, heterogeneity of CSCs, and the key signaling pathways (Wnt/β-catenin, Notch, Hedgehog, YAP, JAK/STAT3) in modulating CSCs during esophageal cancer progression. Exploring the molecular mechanisms of therapy resistance in EC highlights DNA damage response (DDR), metabolic reprogramming, epithelial mesenchymal transition (EMT), and the role of the crosstalk of CSCs and their niche in the tumor progression. According to these molecular findings, potential therapeutic implications of targeting esophageal CSCs may provide novel strategies for the clinical management of esophageal cancer.
1. Introduction
Esophageal cancer (EC) is the 7th most commonly diagnosed cancer and the 6th leading cause of cancer-related death worldwide, with an estimated 572,000 new cases and 509,000 deaths in 2018 [1]. Esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC) are the two main histopathological subtypes of EC. EAC and ESCC vary in etiology and pathogenesis, genomic characteristics, geographical distribution, ethnic characteristics, and therapeutic sensitivity [2]. In addition to the common risk factors such as older age, male gender, tobacco smoking, and lower socioeconomic status, EAC is reported to be more related to obesity, gastroesophageal reflux disease (GERD), and Barrett’s esophagus, whereas ESCC is more associated to alcohol or hot beverages consumption and family history of cancer [3]. EAC exhibits frequent genomic amplifications of VEGFA, ERBB2, GATA4, GATA6, and CCNE1 as well as deletions of SMAD4, while ESCC generally presents amplifications of CCND1, SOX2, TERT, FGFR1, MDM2, NKX2-1, and/or TP63 as well as deletions of RB1 [4]. At the level of point mutations shows EAC frequent mutations in TP53, CDKN2A, ARID1A, and SMAD4 while ESCC is frequently mutated in TP53, CSMD3, NOTCH1, and PIK3CA [5,6]. EAC is more frequent in many western countries including Germany, while ESCC is the major histological type in eastern countries especially in China and Japan [7,8]. Years of efforts have improved the 5-year survival of EC from less than 5% in the 1960s to about 20% in recent decades [2]. Gradual improvement of multi-disciplinary management strategies of EC contributed to the improved therapeutic effect over time [9]. However, due to the lack of obvious symptoms at the early stage of the disease, EC patients usually have developed regional or distant metastasis at the time of diagnosis, which makes EC still a major global health care challenge. In addition, not all patients benefit from the multimodal therapies including neoadjuvant chemotherapy or perioperative chemoradiation and show no tumor response at all [10,11]. So far, the exact mechanisms underlying therapeutic resistance are often unclear.
Cancer stem cells (CSCs) are a small group of cancer cells with specific properties such as self-renewal, differentiation potential, proliferation, heterogeneity, and therapeutic resistance [12]. Since the first identification of CSC in acute myeloid leukemia (AML) by Bonnet et al. in 1990s [13], this particular subset of cells was reported in many solid tumors including gastrointestinal carcinoma [14,15]. The classic hierarchic CSC theory is that only CSCs have self-renewal ability and are able to differentiate into progenitor cells that lead to differentiated tumor cells. However, recent studies have shown the plasticity of CSCs while non-CSCs are capable of gaining stemness due to the changes in tumor microenvironment (TME) or the stimulations by cytotoxic treatments [16,17]. It is suggested that CSCs may be responsible for therapeutic resistance and are the major cellular source for tumor recurrence [12,17,18]. According to the CSCs theory, traditional cytotoxic treatments like chemotherapy and radiotherapy could eliminate rapidly proliferating non-CSC cells rather than the relatively quiescent CSCs and may stimulate non-CSCs to undergo stem-phenotypic transitions [16,17,18]. For EC patients, no significant survival benefit of an adjuvant chemotherapy or radiotherapy has been shown [19,20,21]. It has been reported that nearly 70% of patients showed limited or no response to current neoadjuvant chemotherapy and still 30–40% of patients did not achieve a satisfactory response after neoadjuvant chemoradiotherapy [10,22,23]. Moreover, long-term follow-up revealed that about 40–50% of patients developed local or distant recurrence even after radical multidisciplinary treatment [24,25,26]. In light of this relatively poor susceptibility of EC to chemo- or radiotherapy, it appears highly promising to understand the role of CSCs in EC and to explore therapeutic strategies aiming to eradicate CSCs.
In this review, we focus on the latest research findings on CSCs in EC from PubMed based on the medical subject headings of ‘esophageal cancer’, ‘esophageal adenocarcinoma’ or ‘esophageal squamous cell carcinoma’, ‘cancer stem cell’, ‘heterogeneity’ or ‘single cell’, ‘signaling pathways’, ‘chemotherapy’, ‘radiotherapy’ or ‘therapeutic resistance’, ‘prognosis’ or ‘survival’. Only peer reviewed articles written in English were included. We thoroughly discuss isolation of CSCs, their biological characteristics, TME crosstalk, therapeutic resistance, and potential novel perspectives of CSCs eradication in EC.
2. Isolation of Esophageal Cancer Stem Cells
The introduction of the “tumor stem cell” concept bears interesting opportunities to explore the pathogenesis of malignant tumors. Reliable and robust protocols for esophageal cancer stem cells (ECSCs) isolation and enrichment is a pivotal task to harmonize ECSC studies. In recent years, many experts have explored several separation methods of ECSCs, mainly belonging to the following two types of strategies: ECSC biomarker based and ECSC biomarker-free based.
2.1. ECSC Isolation—Biomarker Based
Using the specific surface or intracellular markers detectable by fluorescence-activated cell sorting (FACS) or antibodies conjugated to magnetic beads for screening of CSC is considered as one of the most authoritative methods (Figure 1).
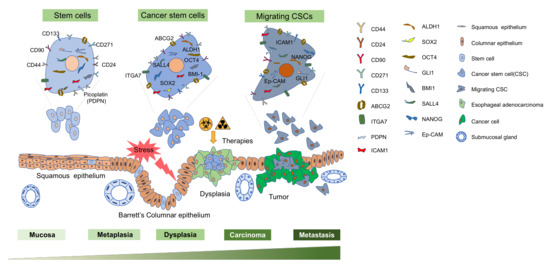
Figure 1.
A schematic of various esophageal cancer stem cell markers and the overview of the progression from normal squamous epithelium to dysplastic cell and finally developing into adenocarcinoma. Cell surface markers CD44, CD24, CD90, CD271, CD133, ABCG2, ITGA7, ICAM-1 and PDPN are used as single markers while CD44 and CD133 can be used in combination with other markers, including CD24, CD133 and CXCR4 to identify cancer stem cells (CSCs). Others are the transcription factors BMI1, NANOG, SOX2, OCT-4, GLI-1, SALL4, and Ep-CAM are implicated to enrich the CSCs. Various cell types have been proposed to give rise to metaplasia (the replacement of esophageal squamous epithelium by Barrett’s columnar epithelium in response to esophageal injury) which can progress to esophageal adenocarcinoma. Esophageal cancer cells are heterogeneous and include cancer stem cell populations. Chemotherapy and/or radiotherapy kill differentiated cancer cells but may fail to kill CSCs, which arise from stem cells, progenitor cells, or differentiated cells. Migrating cancer stem cells are considered to have a crucial role in initiating cancer metastasis.
CD44 and CD133 are multifunctional cell surface antigens that have a role in tumor proliferation, migration, invasion, and angiogenesis in several aspects of cancer cell phenotypes and have been extensively studied as single and combined CSC markers [27,28]. Single marker CD44 is suggested to be a prognostic marker for EAC and ESCC [29,30]. In particular, some studies have proposed CD44 as a CSC marker in ESCC [29,31]. Functional characteristics were also found for CD133 [32,33,34], CD271(p75NTR) [35,36,37], LgR5 [38,39,40], CD90 [41,42], ALDH1 [43,44,45], ABCG2 [33,46,47], ICAM-1[48] and ITGA7 [49]. Besides, CD44 and CD133 can be used in combination with CD24 (CD44+/CD24−) [50], CD133 (CD44+/CD133+)[51] and CXCR4 (CD133+/CXCR4+) [52] to identify esophageal CSCs (Table 1).

Table 1.
Cancer stem cell markers for prognosis of esophageal cancer.
Other read-outs for cancer stemness associated genes such as BMI-1 [61], Nanog [58,59,60], Sox2 [53,54,55,56,57], Oct-4 [54,62,65,66], SALL4 [55,73], GLI-1 [70,71,72], Ep-CAM [67,68,69], and Podoplanin (PDPN) [74,75,76,77] are involved in regulating CSC populations, leading to enhanced proliferation, invasiveness, therapy resistance, and metastatic capacity. They could potentially act as prognostic CSC markers in esophageal cancer.
2.2. ECSC Isolation—Biomarker-Free
In addition to the strategies mentioned above, there are several common methods for CSC isolation independent of specific markers. Firstly, side population (SP) cells are a subpopulation of cells that can exclude dyes such as Hoechst 33342 and therefore can be identified through FACS analysis. SP cells appear to be enriched with stem cells and share many biological characteristics with both normal and cancer stem cells [78], thus, they were regarded as stem cell-like cells in numerous types of cancers including leukemia [79], multiple myeloma [80], breast cancer [81]. Several studies have isolated stem cell-like subpopulations from esophageal cancer cells using side population strategy. For example, Huang et al. [62] isolated and identified SP cells in human esophageal cancer cell lines, the cells with the strongest dye efflux activity (SP cells) in EC9706 had higher clone formation efficiency than non-SP cells. Zhang et al. [82] demonstrated that radioresistant cell lines contained higher fractions of SP cells than parent cell lines of EC. In addition, Zhang et al. [83] also reported increased SP cells in 3D tumor spheres as compared to the 2D adherent cultured cells. Our previous study [84] detected SP cells using Hoechst 33342 staining in five different esophageal cancer cell lines and provided evidence that (1) the proportion of SP cells was variable in esophageal cancer cell lines, (2) SP cells exhibited stem cell properties and were associated with chemotherapy resistance, and (3) long-term exposure to chemotherapy drugs could enrich SP cells with EMT characteristics, which might be a source for recurrence and distant metastases. Secondly, serum-free suspension culture is widely accepted as an effective method for enrichment of CSCs. Many cancer types develop microsphere cells after serum-free suspension culture and exhibit stem cell-like characteristics [83,85,86,87]. The EAC cell line OE19 built tumor spheres, when cultured in serum-free medium, with increased expression levels of CD44 and they were more resistant to radiotherapy as the parent OE19 clone [88]. Consistently, in ESCC cell lines, sphere cells isolated through the same method showed higher radio-resistance than their parental cells [89]. Spheres from the ESCC line ECA109, cultured in serum-free medium, exhibited higher proliferation rates and tumorigenicity in vivo [90]. However, the well-accepted sphere formation assay may not be always the most effective method for CSC enrichment, and the same is true for analyses of acquired therapy resistance [91]. Thirdly, radiation resistance has been identified as a major characteristic of CSCs in vitro [92,93] and accumulating evidence indicates that CSCs are mediating resistance to radiation therapy in cancer patients [94]. Thus, radio-resistance can be used to isolate CSCs. Using an in vitro isogenic model of radioresistant EAC, Lynam-Lennon et al. [95] demonstrated that radioresistant EAC cells have enhanced tumorigenicity in vivo and increased expression of CSC-associated markers as well as enhanced holo-clone forming ability. In two other studies, fractionated irradiation was applied to acquire radio-resistant esophageal cancer cells [82,96]. Both studies demonstrated that radioresistant EAC and ESCC cells showed stem cell properties both in vitro and in vivo. Lastly, attached-cell Aldefluor method (ACAM) is used to identify stem-like cells in ESCC cell lines (KY-5, KY-10, TE-1, TE-8, YES-1, YES-2), where ACAM positive cells showed significantly higher ALDH activity and higher CD44 expression than the parental cells, which may represent a strategy to identify ECSCs [97].
Some studies defined tumor transplantation assays through serial tumor transplantation in the animal models as standard to characterize CSC subpopulations [27,98]. Limiting dilution analysis of tumor transplantation assays demonstrated that ESCC cells with higher CD44 expression showed a shorter latency for visible tumor initiation after subcutaneous tumor injections into NOD/SCID mice with low doses [29], which was especially observed in EC cells with higher expression of ALDH1 and ITGA7 [44,49]. Similarly, subcutaneous injection of OE19 SP cells as well as tumor spheres generated from Eca109 cells to nude mice showed higher tumorigenicity than their parental cells [84,90]. In addition to transplantation assays, lineage tracing is a powerful technique that allows researchers to follow the fate of individual cells and their progeny and was applied as an effective method to study stem cells [99]. Using genetic in vivo lineage tracing, Mariko et al. showed that LGR5+ tumor cells have self-renewal and differentiation capacity and functionally behave as CSCs in colon cancer [100]. As to esophagus, Jiang M et al. found p63+KRT5+KRT7+ basal cells in the upper gastrointestinal tract of mice serve as a source of progenitors for the transitional epithelium that can reproduce Barrett’s metaplasia [101]. Giroux et al. found that a long-lived progenitor cell population with expression of Krt15 is able to self-renew, proliferate, and generate differentiated cells murine esophageal epithelium [102]. Although there is still limited consensus on the identification of ECSCs, increasing amounts of studies are trying to focus on the ECSCs for both pre-clinical and clinical applications. Certainly, further investigations are still necessary to find more valid, reliable, and robust methods to identify CSCs in esophageal cancer.
2.3. Heterogeneity and Single-Cell Analysis of ECSCs
Tumors consist of genetically and epigenetically various cell subpopulations, which is referred to as intratumor heterogeneity. The tumor clones are not equally sensitive to current treatments and are considered a major reason for cancer treatment failure [103,104]. The CSC model is one of the most popular theories to explain intratumor heterogeneity [104]. Recent development of single cell analysis and next generation sequencing technologies allows dissection of intratumor genetic and epigenetic heterogeneity at single-cell resolution, providing new insights into the roles of CSCs in tumor initiation and intratumor heterogeneity [105].
Single-cell RNA sequencing (scRNA-seq) of primary ESCC and EAC tissues successfully distinguished tumor cells from non-tumor cells and showed intrinsic molecular heterogeneity of EAC and ESCC tumors [106]. Bulk RNA-seq and scRNA-seq of paclitaxel-resistant cells and parental cells revealed that molecular mechanisms of intrinsic paclitaxel resistance were distinct from those of acquired resistance at single-cell level. This may open new options to target paclitaxel resistance in ESCC [107]. The same methodology was also applied to analyze transcriptomic dynamics of ESCC cells with acquired radio-resistance throughout exposition of ESCC cells to different doses of irradiations in vitro. The results showed that a cellular heterogeneity with distinct subpopulations existed in irradiated ESCC cells, and dynamic gene regulations were found during the acquisition of radiation resistance [108,109]. Besides, a comparison of the transcriptomic profiles of EAC and ESCC cells with high and low stemness at single-cell level revealed a stemness-associated gene expression signature in ESCC and EAC cells. EAC CSCs highly expressed cell cycle-associated genes, while genes with regard to DNA replication and DNA damage repair were mainly increased in ESCC CSCs [110]. It was reported that beside intratumor heterogeneity, an intra-CSC heterogeneity was found in hepatocellular carcinoma, where different CSC subpopulations presented phenotypes, functions, and transcriptomic heterogeneity at a single-cell level [111]. In addition to single-cell transcriptomic analysis, single-molecule epigenomic technologies now provide an opportunity to study epigenetic regulations and dynamics such as DNA methylation, chromatin accessibility, and histone modifications at unprecedented resolution [112]. Moreover, although high-throughput single-cell methods have not yet arrived in proteomics, proteomics researchers hold an optimistic view that new technologies and strategies will soon be established to successfully tally proteins at single-cell level [113]. Therefore, given the encouraging perspective of single-cell analyses in cancer research, further studies focusing on intra-CSC heterogeneity in EC cells using single-cell analyses are needed and may provide new insights in targeting ECSCs.
3. ECSC Signaling Pathways
A regulatory network consisting of Wnt/β-catenin, transforming growth factor-β (TGF-β)/Smad, Notch, Hedgehog, Hippo, JAK/STAT3, and PI3K/AKT/c-MYC signaling pathways controls CSC properties [114,115,116,117,118,119,120]. These important signaling pathways regulate self-renewal, proliferation, and differentiation capacity of cancer stem cells. Dysregulation of these pathways may also contribute to the undesirable progression of esophageal cancer.
Overexpression of WNT10A plays an important role in ESCC through activation of the Wnt/β-catenin signaling pathway, inducing an increase of the CD44+/CD24− population, which can promote ESCC migration and invasion [121]. In addition, hypoxia-inducible-factor 1α (HIF-1α) has been revealed to be essential for regulating the stemness of ESCC by activating the Wnt/β-catenin pathway. Stable knockdown of HIF-1α in ESCC cells inhibited proliferation, migration, and tumor growth in vivo [122]. MicroRNA-455-3p was reported to play key roles in promoting chemoresistance in vitro and tumorigenesis of ESCC cells in vivo. Treatment with a miR-455-3p antagomir could sensitize ESCC cells to cisplatin and reduce the subpopulations of CD90+ and CD271+ (tumor-initiating cells) T-ICs via inactivation of Wnt/β-catenin and TGF-β signaling pathways [123]. Interestingly, SB525334, a TGF-β1 inhibitor, can significantly inhibit the migration and invasion of sphere-forming stem-like cells of KYSE70 and TE1, which display an increased self-renewal capacity, chemoresistance in vitro, and tumorigenesis in vivo [124].
The Notch signaling pathway plays an important role in regulating cell differentiation and proliferation during embryogenesis and normal tissue homeostasis, which have also been implicated in tumorigenesis including development of esophageal cancer [125]. Mastermind like1 (MAML1) is a key transcription coactivator of this pathway, which could promote the aggressiveness of ESCC through an upregulation of the EMT marker TWIST1 and increase the therapy resistance of ESCC stem cells, respectively [126,127]. In addition, Notch signaling is frequently activated in poorly differentiated tumors and drives a CSC phenotype. By using patient derived xenograft models and primary cell lines, several studies have demonstrated that Notch signaling is critical for CSC capacity and able to drive stemness and tumorigenicity of EAC [128].
Glioma-associated oncogene homolog 1 (Gli-1) is a key mediator of the Hedgehog (Hh) pathway. As a transcription factor of the Hh pathway, Gli-1 mediates therapy resistance in a study with 5-FU or radiation resistant EAC cell lines (SKGT4 (SK4) and Flo-1) [70]. And Gli-1 nuclear expression was identified as a strong and independent predictor of poor response to chemoradiation, early relapse and poor prognosis in ESCC after chemoradiotherapy (CRT) [70,129]. Gli-1 expression was observed in 28.3% of ESCC and showed strong correlation with the stemness genes SOX9 and CD44, which were associated to poor prognosis in ESCC patients [72]. Furthermore, Isohata et al. reported an existing crosstalk between Hh pathways and EMT pathways in ESCC since EMT regulator SIP1 is a downstream target of Gli-1 [130], indicating potential Hh pathway regulation on EMT state of ESCC. Furthermore, Patched1 (PTCH1), another key mediator of the Hedgehog (Hh) pathway, together with Sonic Hedgehog (SHH), one of mammalian HH ligands, were significantly enriched in EC resection tissue from the patients with minimal-residual disease (MRD) after receiving neoadjuvant chemoradiation (nCRT), and PTCH1 is upregulated in CD44+/CD24− CSC population in both EAC (OE33) and ESCC (OE21) cell lines [131]. This study demonstrated that the HH pathway might regulate CD44+/CD24− CSC populations and increase the cancer stemness and therapy resistance.
The Hippo pathway and its downstream effector Yes-associated protein (YAP) have been proposed to be regulators of organ size, cell proliferation, and stem cell properties in a variety of cancers [132,133,134,135]. Recent studies have demonstrated that genetic or pharmacological inhibition of YAP could repress CSC-like properties in vitro and attenuate tumor growth and CSC marker expression in ESCC xenograft models by directly activating its downstream target SOX9 [136]. Consistently, another study has shown that YAP1 driven SOX9 expression was a major determinant of CSC properties in both ESCC and EAC [137]. Moreover, YAP1 could confer therapy resistance and increase cell proliferation in EC cells by upregulating epidermal growth factor receptor (EGFR) [138].
The JAK/STAT3 signaling pathway plays a prominent role in mediating tumor cell proliferation, survival, invasion, and metastasis in different types of cancer [117]. Genistein, an angiogenesis inhibitor belonging to the category of isoflavones, suppressed the JAK1/2-STAT3 pathway by decreasing EGFR expression, significantly inhibiting esophageal cell proliferation in vitro, and tumorigenesis in vivo [139]. In addition, further research demonstrated that the suppression of the JAK/STAT3 pathway could inhibit ESCC cell proliferation in vitro [140,141].
Additionally, the PI3K/AKT/c-MYC signaling axis promotes cancer stem-like feature acquisition in ESCC cell lines [120]. The study found that the cell subset responsive to a Sox2 regulatory region (SRR2) reporter (RR cells) isolated from these ESCC cell lines contained significantly higher proportions of CD44-high and ALDH1A1-high cells. The authors demonstrated that the PI3K-AKT pathway regulates the RR phenotype and promotes its CSC-like features by upregulating c-MYC [120]. It should be noted that PI3K activation was observed to be related to human papillomavirus (HPV) oncogene repression in HPV-positive cervical cancer cells, contributing to therapy resistance, immune evasion, and tumor recurrence [142]. Given the clinical and experimental evidence showing a cross-talk between HPV infection status and CSC functions in oropharyngeal cancer as well as head and neck carcinomas, virus infection and related inflammation response may as well participate in the regulation of CSCs [143,144]. However, there is still limited evidence linking HPV or other viral infections to CSC in esophageal cancer. This aspect may deserve further investigation.
4. Therapeutic Resistance and CSC in EC
Primary and secondary resistance are major obstacles of conventional therapeutic strategies for esophageal cancer. CSCs are frequently resistant to established therapies and may be the primary cellular source underlying resistance. Several potential mechanisms mediating CSCs-induced therapeutic resistance have been reported, including relative quiescent status with enhanced DNA repair capacity, elevated drug export efficiency, improved protection against reactive oxygen species (ROS), and the protective CSC niche in the TME [145,146,147,148].
It is well-established that normal stem cells (SCs) present a reversible quiescent state that is insensitive to cytotoxic treatments, which mainly interfere with the mitotic system of proliferating cells [149,150,151]. Adult stem cells have a very robust DNA damage response (DDR) system to maintain genomic integrity and protect cellular homeostasis in response to stress [152,153]. These features also exist in CSCs [154,155]. In EC, CSCs isolated from the ESCC cell line EC9706 were resistant to DNA damage through impaired induction of p53 and declined G1 checkpoint arrest, and presented a slow-cycling status with a lower level of phosphorylated Stat3, c-Myc, and a higher level of p27 when compared with the non-CSCs [156]. Single cell analysis revealed that overexpression of cell cycle-associated genes, DNA replication modulating genes, as well as DNA damage repair regulating genes were significantly correlated with stem cell-like properties in both EAC and ESCC [110]. Besides, CD133+ ESCC cells are strongly resistant to conventional cytotoxic drugs [34]. It was reported that CD133+ glioma stem cells are resistant to radiotherapy through the activation of the DNA damage checkpoint with enhanced DNA damage repair response [157]. A similar mechanism may mediate therapy resistance of CD133+ cells in ESCC as well.
Multidrug resistance (MDR) describes the phenomenon that cancer cells can be cross-resistant to several structurally and functionally different drugs [158]. A major mechanism of MDR is an altered cell membrane transport system that can pump cytotoxic drugs out of the cancer cells before irreversible DNA damage occurs. For instance, overexpression of the ATP-binding cassette (ABC) transporter family is well established in drug resistant cells [159,160]. ABCB1 and ABCG2 are widely-studied members of the ABC family and are related to CSC induced chemoresistance in many solid tumors such as breast, colon, and lung cancer [161,162]. ABCB1 and ABCG2 were also found to be remarkably upregulated in ESCC CSCs with an enhanced resistance to cisplatin as compared to non-CSCs [83]. High expression of ABCG2 correlates with poor survival in ESCC patients [33,47]. ESCC cells with ABCG2 overexpression showed cross-resistance to both irinotecan and 5-FU through the activation of the AhR pathways, which could be reversed by targeting AhR to further inhibit ABCG2 expression [163,164]. To reverse MDR in cancer cells, modulating ROS may be a viable strategy. It has been suggested that MDR cancer cells are more susceptible to alterations in ROS levels and can be sensitized to cytotoxic drugs after improving the ROS level by targeting relative modulators [165,166]. However, ROS levels were found to be decreased in many types of CSCs [167]. Among the potential mechanisms, the aldehyde dehydrogenases (ALDHs) play an important role in reducing ROS level within CSCs. ALDHs are a group of enzymes that catalyze the oxidation of aldehydes into less toxic carboxylic acids, which are commonly regarded as detoxifying enzymes [168]. As the best-studied ALDH isoform, ALDH1 was reported to decrease ROS levels through the activation of antioxidant systems [169]. Detecting of ALDH1 activity was also widely used as a classic assay to identify CSCs in a variety of cancers including EC [170]. ALDH1+ cells in ESCC present typical stem cell-like properties as well as higher invasive and metastatic capabilities as compared to ALDH1− cells [44,45]. Clinical data suggested that EC patients with high expression of ALDH1 were more resistant to clinical interventions and had a poor long-term prognosis [44]. Taken together, the MDR of ECSCs may be attributed to the enhanced membrane pump-out ability and the decreased ROC level within tumor cells.
Normal stem cells reside in a “stem cell niche”, which refers to a dynamic microenvironment that balances the stem cell activity to govern tissue homeostasis under diverse conditions [171]. A similar concept “cancer stem cell niche” states that CSCs might localize in a protective niche within the TME, which is critical for maintaining the biological function of CSC [145,172]. As a major component of the TME, cancer-associated fibroblasts (CAFs) play a pivotal role in forming the CSC niche, promoting tumorigenesis and inducing therapeutic resistance [173]. ESCC cells co-cultured with CAFs showed significantly altered gene expression in the TME including matricellular proteins, growth factors, cytokines, chemokines, EMT-related genes, and components of inflammatory signaling pathways [174]. The cross-talk between EC cells and CAFs might be mediated by IL-6 through STAT3 and ERK1/2 signaling pathways and showed suppressed tumorigenesis both, in ESCC and EAC [174]. CAFs in ESCC were also reported to cause radio-resistance by regulating DNA damage response though promoting long noncoding RNA (lncRNA) DNM3OS expression via PDGFβ/PDGFRβ/FOXO1 signaling pathway [175]. EMT is another key process that may interact with CSC plasticity and TME. Studies have proved that stromal constituents of the TME can activate EMT through secreting various chemokines, cytokines, and activating several signaling pathways such as TGFβ, WNTs, NOTCH, and Hedgehog to maintain cancer stemness and promote tumor progression and metastasis in several types of cancers [176], including EC [177]. EC cells that underwent EMT presented an enhanced radiation resistance with improved DNA repair ability [178,179]. CSCs may also in turn modify their niche through activating EMT to reform adjacent stromal cells into a relatively undifferentiated status, which then reinforce the CSC plasticity as well as maintain the protective niche [147]. Some studies have reported that CSCs could usually be located in a hypoxic region in the TME [180], hypoxic condition of the CSC niche can also induce EMT as well as decrease inner ROS levels, which can further maintain cancer stemness and contribute to therapeutic resistance [181,182].
Up to date, many efforts are dedicated to exploring the underlying mechanisms of CSCs-induced therapeutic resistance and provide insights in cancer treatment. Among them, targeting CSCs to reverse treatment failure is one of the most promising strategies that may bring long-term benefits to cancer patients.
5. Therapeutic Strategies Targeting CSC in EC
The unsatisfying results of conventional chemo- and radio-therapeutic strategies highlight the clinical need of more effective therapeutic compounds, especially those which could prevent tumor recurrence. Among all the potential targets, CSCs together with the niche/TME have attracted attention as targets for pre-clinical and clinical approaches.
As discussed above, CSCs carrying variable surface markers allow the differentiation of highly malignant CSCs from normal cancer cells, many strategies based on surficial molecules and downstream compounds have also been developed to optimize treatment response. For instance, in 2016 a phase I study targeting CD44, a cancer cell progenitor marker of EAC, achieved a limited clinical benefit among AML patients. However, it suggested a promising combination therapy with other cytotoxic agents [183]. BMS-833923 is a potent and specific inhibitor of SMO in the Hh pathway [184], which is currently tested in a phase I trial (NCT00909402: completed, but results are not published yet) evaluating inhibition of SMO as a first-line therapy for unresectable metastatic EC patients in combination with Cisplatin and Capecitabine. Additionally, Taladegib (LY-2940680) an alternative small molecule interfering with the Hh cascade through binding to the SMO receptor is currently evaluated (NCT02530437: active, not recruiting) [185]. The amplification or drug induced overexpression of EGFR has long been considered as a marker for resistance and tumor progression [186]. Targeted therapy based on anti-EGFR monoclonal antibodies (mAb) e.g., nimotuzumab, plus irinotecan, a common medication for gastric cancer treatment, showed potential improvement in EGFR positive patients of advanced gastric cancer [187].
It is now widely accepted that the oncogenesis and tumor heterogeneity are not exclusively dependent on aberrations or mutations of tumor cells, but also accompanied by the dynamic changes of microenvironmental compositions as well as the state and properties of surrounding stromal cells [188,189]. Apart from malignant tumor cells, the highly diverse cell types in the TME, mainly including CAFs, immune cells, vascular endothelial cells, and mesenchymal stem cells [190,191,192], are genetically stable, thus could be utilized as multiple targets in cancer therapy. A series of efforts has been undertaken to investigate the prominent role of the CXCL12/CXCR4 axis in cancer progression of different cancer entities including both histologic subtypes of EC [193,194,195]. Combination therapies with the CXCR4 antagonist AMD3100 counteracted the resistance of pancreatic cancer cells to gemcitabine via Fak, ERK, and Akt pathways [196]. Furthermore, co-administration of AMD3100 and anti-PD-L1 antibody diminished stroma–cancer cell interaction together with an improved immune response [197]. Gockel et al. validated CXCR4 expression in 94.1% of resectable ESCC patients (54.9% weak expression vs. 45.1% strong expression) and 89.1% resectable EAC patients (71.7% weak expression vs. 29.3% strong expression), strong CXCR4 expression was supposed to be relevant to poor prognosis in both subtypes [198]. A reduced cell proliferation rate was observed in the EAC cell line OE19 treated with AMD3100, smaller primary tumor size, and fewer metastatic spread to lung, liver, and lymph node were further confirmed in OE19 injected mice under AMD3100 treatment [199]. Another approach targeting the TME arose from the observation of overexpressed VEGF/VEGF receptor system which initiates pathological angiogenesis in tumor niches. By blocking VEGFR-2 with ramucirumab, Fuchs et al. validated a significantly prolonged overall survival in a phase 3 trial among advanced gastric or gastro-esophageal junction adenocarcinoma (REGARD) patients [200]. Since rising evidence over past years supports the hypothesis that stem cells in gastroesophageal junction (GEJ) invoke very likely the occurrence of Barrett’s esophagus and EAC [201], antagonising VEGFR-2 could also be feasible in EAC.
Over the past decades, immunotherapy has been confirmed as an additional approach able to control different types of cancer and sometimes effective in patients resistant to conventional therapies [185]. Immune checkpoint receptors such as PD-1 and CTLA-4 are favorable drug targets as they release suppressive signals upon the activation by tumor cells. Up to 40% of GEJ adenocarcinoma cases express PD-L1 [202,203]. Blockade of PD-1 with the mAb Nivolumab revealed encouraging results with a survival benefit in advanced gastric or gastro-esophageal junction adenocarcinoma patients’ refractory to at least two chemotherapy regimens (ONO-4538-12, ATTRACTION-2) in a recent phase III trial in Asian patients [204]. Other novel immunological strategies involving genetically engineered immune cells (e.g., CAR T) could also elevate anti-CSC efficiency. Genetic knockdown of PD-1 alone, or in combination with chimeric antigen receptor (CAR) T cells, which carries a predefined affinity to a given tumor antigen, could significantly enhance immune reaction against a desired tumor type. Shannon et al. reported the persistent ability of CAR T cell CTL019 in monitoring ALL relapse [205]. However, in EAC patients one related clinical trial is still ongoing (NCT03706326: recruiting) and the result of another phase I study remains to be published (NCT03081715: completed). Similar to T cells, cytotoxic NK cells of the intrinsic immune system can induce apoptosis of viral infected or transformed autologous cells e.g., cancer cells via pore-forming and release of granzyme B [206]. CSCs are confirmed to be a small subset of cells with sparse MHC class I molecules (MHC-I), thus insinuates their blunt reaction to CD8+ T cells [207], which in term fails to silence NK cells as NK cell tolerance to self-tissue is maintained in the presence of MHC-I [208]. Additional studies have reported susceptibility of CSCs to allogeneic NK cells in diverse solid tumor types in pre-clinical models, such as colorectal cancer [209] and glioblastoma [210]. In another pre-clinical model, increased expression of the NKG2D ligands ULBP1, ULBP2, and MICA sensitized CD44+/CD24− breast CSCs to killing by previously activated NK cells by IL-2 and IL-15 [211]. Such investigations have ignited the passion of EC researchers to transform it into the clinical field (NCT02843581: completed) and the result is awaited by the community. The susceptibility of CSCs to cancer immunotherapy is poorly investigated and may represent an important mechanism underlying long-term benefit from these novel therapies.
According to the tumor cell subclone functional diversity model proposed by Kreso et al., conventional radio- and chemotherapy targeting dividing cancer cells collapsed to diminish dormant (stem cell-like) cells within tumor entities could account for the post-treatment repopulation of tumor cells [212]. The same group also successfully observed that the previously dormant cell lineage—a minor bunch among tumor initiating cells (T-ICs), survived from chemotherapy and contributed to tumor regrowth in colorectal cancer [213]. In glioblastoma, tumor regrowth was significantly halted after the ablation of a subset of stem cell like endogenous tumor cells [214]. Both studies favorably provided direct evidence of the tumor reproducing capacity of T-ICs. Thus, it is feasible to hitch CSCs to EC recurrence albeit similar investigations are still scarce, such mechanism reversely strengthens the weightiness of CSC targeting in EC administration with individualized therapy.
Eventually, emphasizing the importance of estimating prospective therapy response in precision medicine is warranted. For instance, a better OS in recurrent glioblastoma patients than previous data was achieved by using a systemic assay which firstly stratifies the cell kill rate to find an efficient one among different drug combinations targeting both CSCs and non-CSC cells [215]. This frontier attempt provided an auspicious way for the future treatment of recurrent EC patients or those with poor estimated prognosis.
6. Conclusions and Future Perspectives
The focus on ECSCs opens a new vision for translational research of EC and may result in further understanding of key mechanisms of EC etiology, progression, recurrence, and therapy resistance. As we discussed, CSCs could be isolated from esophageal tumors using either ECSC biomarker based or ECSC biomarker-free methods. A combined application of multiple markers or multiple methods to screen for CSCs in future studies may help to overcome the limitations derived from the heterogeneity of individual tumors. Furthermore, we emphasize the importance and advantages of integration of single cell analysis in esophageal cancer stem cell studies. This new method will help to understand both intertumor and intratumor heterogeneity of CSCs in EC and the clonal architecture of esophageal cancer for both adeno- and squamous cell carcinomas. The crosstalk between ECSCs and their niche (TME) not only plays a pivotal role during oncogenesis but also has profound effects on modulating therapeutic efficacy. Therefore, future strategies of combined treatments, that target CSCs and the TME may result in successful implementation of individualized therapy of EC patients.
Funding
This work was supported by Köln Fortune Program/Faculty of Medicine to YZ, University of Cologne (ID: 2680154501).
Acknowledgments
We appreciated the support of CSC scholarship (The China Scholarship Council) for Chenghui Zhou and Guangzhou Elite Scholarship Council (GESC) for Ningbo Fan.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ABC | ATP-binding cassette |
| ACAM | Attached-cell Aldefluor method |
| ALDHs | Aldehyde dehydrogenases |
| AML | Acute myeloid leukemia |
| BE | Barrett’s esophagus |
| CAFs | Cancer-associated fibroblasts |
| CAR | Chimeric antigen receptor |
| CSCs | Cancer stem cells |
| CRT | Chemoradiotherapy |
| DDR | DNA damage response |
| EAC | Esophageal adenocarcinoma |
| EC | Esophageal cancer |
| ECSCs | Esophageal cancer stem cells |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial mesenchymal transition |
| ESCC | Esophageal squamous cell carcinoma |
| FACS | Fluorescence-activated cell sorting |
| GEJ | Gastroesophageal junction |
| GERD | Gastroesophageal reflux disease |
| Hh | Hedgehog |
| HIF-1α | Hypoxia-inducible-factor 1α |
| HPV | Human Papillomavirus |
| ICAM1 | Intercellular adhesion molecule1 |
| lncRNA | Long noncoding RNA |
| Gli-1 | Glioma-associated oncogene homolog 1 |
| mAb | Monoclonal antibodies |
| MRD | Minimal residual disease |
| nCRT | Neoadjuvant chemoradiation |
| MAML1 | Mastermind like1 |
| MDR | Multidrug resistance |
| MHC-I | MHC class I molecules |
| REGARD | Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma |
| PDPN | Podoplanin |
| PTCH1 | Patched 1 |
| ROS | Reactive oxygen species |
| RR | Reporter-responsive |
| scRNA-seq | Single-cell RNA sequencing |
| SCs | Stem cells |
| SHH | Sonic Hedgehog |
| SP | Side population |
| SRR2 | Sox2 regulatory region |
| TGF-β | Transforming growths factor-β |
| TME | Tumor microenvironment |
| T-ICs | Tumor-initiating cells |
| YAP | Yes-associated protein |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, J.; Smyth, E.; Cunningham, D.; Lagergren, P. Oesophageal cancer. Lancet 2017, 390, 2383–2396. [Google Scholar] [CrossRef]
- Chung, C.S.; Lee, Y.C.; Wu, M.S. Prevention strategies for esophageal cancer: Perspectives of the East vs. West. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 869–883. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature 2017, 541, 169–175. [Google Scholar] [CrossRef]
- Frankell, A.M.; Jammula, S.; Li, X.; Contino, G.; Killcoyne, S.; Abbas, S.; Perner, J.; Bower, L.; Devonshire, G.; Ococks, E.; et al. The landscape of selection in 551 esophageal adenocarcinomas defines genomic biomarkers for the clinic. Nat. Genet. 2019, 51, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Chen, H.; Zhou, D.; Zhang, J.; Chen, Y.; Liu, Q.; Ai, D.; Zhu, H.; Chu, L.; Ren, W.; et al. Comparative genomic analysis of esophageal squamous cell carcinoma between Asian and Caucasian patient populations. Nat. Commun. 2017, 8, 1533. [Google Scholar] [CrossRef]
- Lin, Y.; Totsuka, Y.; He, Y.; Kikuchi, S.; Qiao, Y.; Ueda, J.; Wei, W.; Inoue, M.; Tanaka, H. Epidemiology of esophageal cancer in Japan and China. J. Epidemiol. 2013, 23, 233–242. [Google Scholar] [CrossRef]
- Abnet, C.C.; Arnold, M.; Wei, W.Q. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2018, 154, 360–373. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Corvera, C.; Das, P.; Denlinger, C.S.; Enzinger, P.C.; Fanta, P.; Farjah, F.; et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 855–883. [Google Scholar] [CrossRef]
- Van Hagen, P.; Hulshof, M.C.; van Lanschot, J.J.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.; Richel, D.J.; Nieuwenhuijzen, G.A.; Hospers, G.A.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.F. Clinical and Therapeutic Implications of Cancer Stem Cells. N. Engl. J. Med. 2019, 380, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of human brain tumour initiating cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef]
- O’Brien, C.A.; Pollett, A.; Gallinger, S.; Dick, J.E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007, 445, 106–110. [Google Scholar] [CrossRef]
- Da Silva-Diz, V.; Lorenzo-Sanz, L.; Bernat-Peguera, A.; Lopez-Cerda, M.; Munoz, P. Cancer cell plasticity: Impact on tumor progression and therapy response. Semin. Cancer Biol. 2018, 53, 48–58. [Google Scholar] [CrossRef]
- Vermeulen, L.; de Sousa e Melo, F.; Richel, D.J.; Medema, J.P. The developing cancer stem-cell model: Clinical challenges and opportunities. Lancet Oncol. 2012, 13, e83–e89. [Google Scholar] [CrossRef]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef]
- Ando, N.; Iizuka, T.; Ide, H.; Ishida, K.; Shinoda, M.; Nishimaki, T.; Takiyama, W.; Watanabe, H.; Isono, K.; Aoyama, N.; et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: A Japan Clinical Oncology Group Study—JCOG9204. J. Clin. Oncol. 2003, 21, 4592–4596. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.E.; Im, Y.H.; Kang, W.K.; Park, K.; Kim, K.; Shim, Y.M. Adjuvant chemotherapy with 5-fluorouracil and cisplatin in lymph node-positive thoracic esophageal squamous cell carcinoma. Ann. Thorac. Surg. 2005, 80, 1170–1175. [Google Scholar] [CrossRef]
- Iizuka, T. A comparison of chemotherapy and radiotherapy as adjuvant treatment to surgery for esophageal carcinoma. Japanese Esophageal Oncology Group. Chest 1993, 104, 203–207. [Google Scholar] [CrossRef]
- Ando, N.; Kato, H.; Igaki, H.; Shinoda, M.; Ozawa, S.; Shimizu, H.; Nakamura, T.; Yabusaki, H.; Aoyama, N.; Kurita, A.; et al. A Randomized Trial Comparing Postoperative Adjuvant Chemotherapy with Cisplatin and 5-Fluorouracil Versus Preoperative Chemotherapy for Localized Advanced Squamous Cell Carcinoma of the Thoracic Esophagus (JCOG9907). Ann. Surg. Oncol. 2012, 19, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Klevebro, F.; Alexandersson von Dobeln, G.; Wang, N.; Johnsen, G.; Jacobsen, A.B.; Friesland, S.; Hatlevoll, I.; Glenjen, N.I.; Lind, P.; Tsai, J.A.; et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann. Oncol. 2016, 27, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.; van Lanschot, J.J.B.; Hulshof, M.C.C.M.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef]
- Stahl, M.; Walz, M.K.; Riera-Knorrenschild, J.; Stuschke, M.; Sandermann, A.; Bitzer, M.; Wilke, H.; Budach, W. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): Long-term results of a controlled randomised trial. Eur. J. Cancer 2017, 81, 183–190. [Google Scholar] [CrossRef]
- Alderson, D.; Cunningham, D.; Nankivell, M.; Blazeby, J.M.; Griffin, S.M.; Crellin, A.; Grabsch, H.I.; Langer, R.; Pritchard, S.; Okines, A.; et al. Neoadjuvant cisplatin and fluorouracil versus epirubicin, cisplatin, and capecitabine followed by resection in patients with oesophageal adenocarcinoma (UK MRC OE05): An open-label, randomised phase 3 trial. Lancet Oncol. 2017, 18, 1249–1260. [Google Scholar] [CrossRef]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef]
- Zöller, M. CD44: Can a cancer-initiating cell profit from an abundantly expressed molecule? Nat. Rev. Cancer 2011, 11, 254–267. [Google Scholar] [CrossRef]
- Zhao, J.-S.; Li, W.-J.; Ge, D.; Zhang, P.-J.; Li, J.-J.; Lu, C.-L.; Ji, X.-D.; Guan, D.-X.; Gao, H.; Xu, L.-Y.; et al. Tumor initiating cells in esophageal squamous cell carcinomas express high levels of CD44. PLoS ONE 2011, 6, e21419. [Google Scholar] [CrossRef]
- Schizas, D.; Moris, D.; Kanavidis, P.; Michalinos, A.; Sioulas, A.; Pavlakis, K.; Machairas, A.; Liakakos, T. The Prognostic Value of CD44 Expression in Epithelial-Mesenchymal Transition: Preliminary Data from Patients with Gastric and Esophageal Cancer. In Vivo 2016, 30, 939–944. [Google Scholar] [CrossRef][Green Version]
- Le Bras, G.F.; Allison, G.L.; Richards, N.F.; Ansari, S.S.; Washington, M.K.; Andl, C.D. CD44 upregulation in E-cadherin-negative esophageal cancers results in cell invasion. PLoS ONE 2011, 6, e27063. [Google Scholar] [CrossRef]
- Sui, Y.-P.; Jian, X.-P.; Ma, L.I.; Xu, G.-Z.; Liao, H.-W.; Liu, Y.-P.; Wen, H.-C. Prognostic value of cancer stem cell marker CD133 expression in esophageal carcinoma: A meta-analysis. Mol. Clin. Oncol. 2016, 4, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Hang, D.; Dong, H.C.; Ning, T.; Dong, B.; Hou, D.L.; Xu, W.G. Prognostic value of the stem cell markers CD133 and ABCG2 expression in esophageal squamous cell carcinoma. Dis. Esophagus 2012, 25, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, W.W.; Han, L.; Chan, K.T.; Tsao, S.W.; Lee, N.P.Y.; Law, S.; Xu, L.Y.; Li, E.M.; Chan, K.W.; et al. MicroRNA-377 suppresses initiation and progression of esophageal cancer by inhibiting CD133 and VEGF. Oncogene 2017, 36, 3986–4000. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-D.; Yuan, Y.; Liu, X.-H.; Gong, D.-J.; Bai, C.-G.; Wang, F.; Luo, J.-H.; Xu, Z.-Y. Self-renewal and chemotherapy resistance of p75NTR positive cells in esophageal squamous cell carcinomas. BMC Cancer 2009, 9, 9. [Google Scholar] [CrossRef]
- Li, S.; Yue, D.; Chen, X.; Wang, L.; Li, J.; Ping, Y.; Gao, Q.; Wang, D.; Zhang, T.; Li, F.; et al. Epigenetic regulation of CD271, a potential cancer stem cell marker associated with chemoresistance and metastatic capacity. Oncol. Rep. 2015, 33, 425–432. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Okumura, T.; Hirano, K.; Watanabe, T.; Nagata, T.; Shimada, Y.; Tsukada, K. Detection of circulating tumor cells by p75NTR expression in patients with esophageal cancer. World J. Surg. Oncol. 2016, 14, 40. [Google Scholar] [CrossRef]
- Von Rahden, B.H.; Kircher, S.; Lazariotou, M.; Reiber, C.; Stuermer, L.; Otto, C.; Germer, C.T.; Grimm, M. LgR5 expression and cancer stem cell hypothesis: Clue to define the true origin of esophageal adenocarcinomas with and without Barrett’s esophagus? J. Exp. Clin. Cancer Res. 2011, 30, 23. [Google Scholar] [CrossRef]
- Becker, L.; Huang, Q.; Mashimo, H. Lgr5, an intestinal stem cell marker, is abnormally expressed in Barrett’s esophagus and esophageal adenocarcinoma. Dis. Esophagus 2010, 23, 168–174. [Google Scholar] [CrossRef]
- Lv, Z.; Yu, J.J.; Zhang, W.J.; Xiong, L.; Wang, F.; Li, L.F.; Zhou, X.L.; Gao, X.Y.; Ding, X.F.; Han, L.; et al. Expression and functional regulation of stemness gene Lgr5 in esophageal squamous cell carcinoma. Oncotarget 2017, 8, 26492–26504. [Google Scholar] [CrossRef]
- Tang, K.H.; Dai, Y.D.; Tong, M.; Chan, Y.P.; Kwan, P.S.; Fu, L.; Qin, Y.R.; Tsao, S.W.; Lung, H.L.; Lung, M.L.; et al. A CD90(+) tumor-initiating cell population with an aggressive signature and metastatic capacity in esophageal cancer. Cancer Res. 2013, 73, 2322–2332. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, C.; Zhu, H.; Tang, J.; Zhang, S.; Luo, J.; Sun, X. CD90 positive cells exhibit aggressive radioresistance in esophageal squamous cell carcinoma. J. Thorac. Dis. 2017, 9, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Wang, X.; Song, S.; Suzuki, A.; Taketa, T.; Sudo, K.; Wadhwa, R.; Hofstetter, W.L.; Komaki, R.; Maru, D.M.; et al. ALDH-1 expression levels predict response or resistance to preoperative chemoradiation in resectable esophageal cancer patients. Mol. Oncol. 2014, 8, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ren, Y.; Yu, X.; Qian, F.; Bian, B.S.; Xiao, H.L.; Wang, W.G.; Xu, S.L.; Yang, J.; Cui, W.; et al. ALDH1A1 defines invasive cancer stem-like cells and predicts poor prognosis in patients with esophageal squamous cell carcinoma. Mod. Pathol. 2014, 27, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.F.; Chen, P.T.; Lu, M.S.; Chen, W.C. Role of ALDH1 in the prognosis of esophageal cancer and its relationship with tumor microenvironment. Mol. Carcinog. 2018, 57, 78–88. [Google Scholar] [CrossRef]
- Huang, L.; Lu, Q.; Han, Y.; Li, Z.; Zhang, Z.; Li, X. ABCG2/V-ATPase was associated with the drug resistance and tumor metastasis of esophageal squamous cancer cells. Diagn Pathol. 2012, 7, 180. [Google Scholar] [CrossRef]
- Tsunoda, S.; Okumura, T.; Ito, T.; Kondo, K.; Ortiz, C.; Tanaka, E.; Watanabe, G.; Itami, A.; Sakai, Y.; Shimada, Y. ABCG2 expression is an independent unfavorable prognostic factor in esophageal squamous cell carcinoma. Oncology 2006, 71, 251–258. [Google Scholar] [CrossRef]
- Tsai, S.-T.; Wang, P.-J.; Liou, N.-J.; Lin, P.-S.; Chen, C.-H.; Chang, W.-C. ICAM1 Is a Potential Cancer Stem Cell Marker of Esophageal Squamous Cell Carcinoma. PLoS ONE 2015, 10, e0142834. [Google Scholar] [CrossRef]
- Ming, X.-Y.; Fu, L.; Zhang, L.-Y.; Qin, Y.-R.; Cao, T.-T.; Chan, K.W.; Ma, S.; Xie, D.; Guan, X.-Y. Integrin α7 is a functional cancer stem cell surface marker in oesophageal squamous cell carcinoma. Nat. Commun. 2016, 7, 13568. [Google Scholar] [CrossRef]
- Smit, J.K.; Faber, H.; Niemantsverdriet, M.; Baanstra, M.; Bussink, J.; Hollema, H.; van Os, R.P.; Plukker, J.T.M.; Coppes, R.P. Prediction of response to radiotherapy in the treatment of esophageal cancer using stem cell markers. Radiother. Oncol. 2013, 107, 434–441. [Google Scholar] [CrossRef]
- Okamoto, K.; Ninomiya, I.; Ohbatake, Y.; Hirose, A.; Tsukada, T.; Nakanuma, S.; Sakai, S.; Kinoshita, J.; Makino, I.; Nakamura, K.; et al. Expression status of CD44 and CD133 as a prognostic marker in esophageal squamous cell carcinoma treated with neoadjuvant chemotherapy followed by radical esophagectomy. Oncol. Rep. 2016, 36, 3333–3342. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Xu, F.; Gu, J.; Yuan, Y.; Zhao, G.; Yu, X.; Ge, D. Clinical and biological significance of stem-like CD133(+)CXCR4(+) cells in esophageal squamous cell carcinoma. J. Thorac. Cardiovasc. Surg. 2015, 150, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Bass, A.J.; Watanabe, H.; Mermel, C.H.; Yu, S.; Perner, S.; Verhaak, R.G.; Kim, S.Y.; Wardwell, L.; Tamayo, P.; Gat-Viks, I.; et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat. Genet. 2009, 41, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; He, W.; Lu, C.; Wang, Z.; Wang, J.; Giercksky, K.E.; Nesland, J.M.; Suo, Z. Oct3/4 and Sox2 are significantly associated with an unfavorable clinical outcome in human esophageal squamous cell carcinoma. Anticancer Res. 2009, 29, 1233–1241. [Google Scholar]
- Forghanifard, M.M.; Ardalan Khales, S.; Javdani-Mallak, A.; Rad, A.; Farshchian, M.; Abbaszadegan, M.R. Stemness state regulators SALL4 and SOX2 are involved in progression and invasiveness of esophageal squamous cell carcinoma. Med. Oncol. 2014, 31, 922. [Google Scholar] [CrossRef]
- Ten Kate, F.J.C.; van Olphen, S.H.; Bruno, M.J.; Wijnhoven, B.P.L.; van Lanschot, J.J.B.; Looijenga, L.H.J.; Fitzgerald, R.C.; Biermann, K. Loss of SRY-box2 (SOX2) expression and its impact on survival of patients with oesophageal adenocarcinoma. Br. J. Surg. 2017, 104, 1327–1337. [Google Scholar] [CrossRef]
- Chai, Y.; Li, Q.; Zhao, H.; Zhang, Z.; Yu, X.; Pang, L.; Liu, Z.; Zhao, J.; Wang, L.; Li, F. SOX2 antagonizes WWC1 to drive YAP1 activation in esophageal squamous cell carcinoma. Cancer Med. 2019, 8, 7055–7064. [Google Scholar] [CrossRef]
- Deng, L.; Xiang, X.; Yang, F.; Xiao, D.; Liu, K.; Chen, Z.; Zhang, R.; Feng, G. Functional evidence that the self-renewal gene NANOG regulates esophageal squamous cancer development. Biochem. Biophys. Res. Commun. 2017, 490, 161–168. [Google Scholar] [CrossRef]
- Du, Y.; Shi, L.; Wang, T.; Liu, Z.; Wang, Z. Nanog siRNA plus Cisplatin may enhance the sensitivity of chemotherapy in esophageal cancer. J. Cancer Res. Clin. Oncol. 2012, 138, 1759–1767. [Google Scholar] [CrossRef]
- Shimada, Y.; Okumura, T.; Sekine, S.; Moriyama, M.; Sawada, S.; Matsui, K.; Yoshioka, I.; Hojo, S.; Yoshida, T.; Nagata, T.; et al. Expression analysis of iPS cell-inductive genes in esophageal squamous cell carcinoma by tissue microarray. Anticancer Res. 2012, 32, 5507–5514. [Google Scholar]
- He, X.-T.; Cao, X.-F.; Ji, L.; Zhu, B.; Lv, J.; Wang, D.-D.; Lu, P.-H.; Cui, H.-G. Association between Bmi1 and clinicopathological status of esophageal squamous cell carcinoma. World J. Gastroenterol. 2009, 15, 2389–2394. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Gao, Q.; Guo, L.; Zhang, C.; Jiang, W.; Li, H.; Wang, J.; Han, X.; Shi, Y.; Lu, S.H. Isolation and identification of cancer stem-like cells in esophageal carcinoma cell lines. Stem Cells Dev. 2009, 18, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, K.; Jiang, X.; Wang, X.; Chen, Y.; Cui, X.; Pang, L.; Li, S.; Liu, C.; Zou, H.; et al. Clinicopathological significance of Bmi-1 overexpression in esophageal cancer: A meta-analysis. Biomark Med. 2018, 12, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Sang, M.X.; Zhu, S.C.; Liu, Z.K.; Ma, M. Radiosensitization of esophageal carcinoma cells by the silencing of BMI-1. Oncol. Rep. 2016, 35, 3669–3678. [Google Scholar] [CrossRef][Green Version]
- Vaiphei, K.; Sinha, S.K.; Kochhar, R. Comparative analysis of Oct4 in different histological subtypes of esophageal squamous cell carcinomas in different clinical conditions. Asian Pac. J. Cancer Prev. 2014, 15, 3519–3524. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Li, C.; Su, Y.; Fang, W.; Zhong, C.; Ji, W.; Zhang, Q.; Su, C. Transcription factor OCT4 promotes cell cycle progression by regulating CCND1 expression in esophageal carcinoma. Cancer Lett. 2014, 354, 77–86. [Google Scholar] [CrossRef]
- Stoecklein, N.H.; Siegmund, A.; Scheunemann, P.; Luebke, A.M.; Erbersdobler, A.; Verde, P.E.; Eisenberger, C.F.; Peiper, M.; Rehders, A.; Esch, J.S.A.; et al. Ep-CAM expression in squamous cell carcinoma of the esophagus: A potential therapeutic target and prognostic marker. BMC Cancer 2006, 6, 165. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, Y.; Pu, Y.; Liu, J.; Liu, B.; Yu, B.; Chen, K.; Fu, T.; Yang, C.J.; Liu, H.; et al. Using aptamers to elucidate esophageal cancer clinical samples. Sci. Rep. 2015, 5, 18516. [Google Scholar] [CrossRef]
- Matsuda, T.; Takeuchi, H.; Matsuda, S.; Hiraiwa, K.; Miyasho, T.; Okamoto, M.; Kawasako, K.; Nakamura, R.; Takahashi, T.; Wada, N.; et al. EpCAM, a potential therapeutic target for esophageal squamous cell carcinoma. Ann. Surg. Oncol. 2014, 21 (Suppl. 3), S356–S364. [Google Scholar] [CrossRef]
- Wadhwa, R.; Wang, X.; Baladandayuthapani, V.; Liu, B.; Shiozaki, H.; Shimodaira, Y.; Lin, Q.; Elimova, E.; Hofstetter, W.L.; Swisher, S.G.; et al. Nuclear expression of Gli-1 is predictive of pathologic complete response to chemoradiation in trimodality treated oesophageal cancer patients. Br. J. Cancer 2017, 117, 648–655. [Google Scholar] [CrossRef]
- Sims-Mourtada, J.; Izzo, J.G.; Apisarnthanarax, S.; Wu, T.T.; Malhotra, U.; Luthra, R.; Liao, Z.; Komaki, R.; van der Kogel, A.; Ajani, J.; et al. Hedgehog: An attribute to tumor regrowth after chemoradiotherapy and a target to improve radiation response. Clin. Cancer Res. 2006, 12, 6565–6572. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Cui, Y.; Ni, W.; Kim, S.; Xuan, Y. Gli1, a potential regulator of esophageal cancer stem cell, is identified as an independent adverse prognostic factor in esophageal squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2017, 143, 243–254. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhou, M.; Chen, X.; Yue, D.; Yang, L.; Qin, G.; Zhang, Z.; Gao, Q.; Wang, D.; Zhang, C.; et al. Inhibition of SALL4 reduces tumorigenicity involving epithelial-mesenchymal transition via Wnt/beta-catenin pathway in esophageal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2016, 35, 98. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.-K.; Chuang, W.-Y.; Yeh, C.-J.; Wu, Y.-C.; Liu, Y.-H.; Hsieh, M.-J.; Cheng, A.-J.; Hsueh, C.; Liu, H.-P. Prognostic significance of high podoplanin expression after chemoradiotherapy in esophageal squamous cell carcinoma patients. J. Surg. Oncol. 2012, 105, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wang, K.; Yang, S.; Wang, J.; Tan, B.; Bai, B.; Wang, N.; Jia, Y.; Jia, M.; Cheng, Y. Clinicopathology significance of podoplanin immunoreactivity in esophageal squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 2361–2371. [Google Scholar] [PubMed]
- Li, J.C.; Li, Y.; Ai, J.Y.; Chen, K.; Zhu, Y.H.; Fu, L.; Qin, Y.R.; Wang, L.J.; Guan, X.Y. Podoplaninpositive cancer cells at the edge of esophageal squamous cell carcinomas are involved in invasion. Mol. Med. Rep. 2014, 10, 1513–1518. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Q.; Yan, X.; Kato, Y.; Tanaka, M.; Inokuchi, S.; Yoshizawa, T.; Morohashi, S.; Kijima, H. Podoplanin-mediated TGF-beta-induced epithelial-mesenchymal transition and its correlation with bHLH transcription factor DEC in TE-11 cells. Int. J. Oncol. 2016, 48, 2310–2320. [Google Scholar] [CrossRef]
- Hadnagy, A.; Gaboury, L.; Beaulieu, R.; Balicki, D. SP analysis may be used to identify cancer stem cell populations. Exp. Cell Res. 2006, 312, 3701–3710. [Google Scholar] [CrossRef]
- Gross, E.; L’Faqihi-Olive, F.E.; Ysebaert, L.; Brassac, M.; Struski, S.; Kheirallah, S.; Fournié, J.J.; Laurent, G.; Quillet-Mary, A. B-chronic lymphocytic leukemia chemoresistance involves innate and acquired leukemic side population cells. Leukemia 2010, 24, 1885–1892. [Google Scholar] [CrossRef]
- Du, J.; Liu, S.; He, J.; Liu, X.; Qu, Y.; Yan, W.; Fan, J.; Li, R.; Xi, H.; Fu, W.; et al. MicroRNA-451 regulates stemness of side population cells via PI3K/Akt/mTOR signaling pathway in multiple myeloma. Oncotarget 2015, 6, 14993–15007. [Google Scholar] [CrossRef]
- Britton, K.M.; Kirby, J.A.; Lennard, T.W.; Meeson, A.P. Cancer stem cells and side population cells in breast cancer and metastasis. Cancers 2011, 3, 2106–2130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Komaki, R.; Wang, L.; Fang, B.; Chang, J.Y. Treatment of radioresistant stem-like esophageal cancer cells by an apoptotic gene-armed, telomerase-specific oncolytic adenovirus. Clin. Cancer Res. 2008, 14, 2813–2823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ma, L.; Xie, Y.K.; Miao, X.B.; Jin, C. Esophageal cancer tumorspheres involve cancer stem-like populations with elevated aldehyde dehydrogenase enzymatic activity. Mol. Med. Rep. 2012, 6, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bao, Q.; Schwarz, B.; Zhao, L.; Mysliwietz, J.; Ellwart, J.; Renner, A.; Hirner, H.; Niess, H.; Camaj, P.; et al. Stem cell-like side populations in esophageal cancer: A source of chemotherapy resistance and metastases. Stem Cells Dev. 2014, 23, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Möbest, D.; Goan, S.R.; Junghahn, I.; Winkler, J.; Fichtner, I.; Hermann, M.; Becker, M.; de Lima-Hahn, E.; Henschler, R. Differential kinetics of primitive hematopoietic cells assayed in vitro and in vivo during serum-free suspension culture of CD34+ blood progenitor cells. Stem Cells 1999, 17, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Trisciuoglio, D.; Tupone, M.G.; Desideri, M.; Di Martile, M.; Gabellini, C.; Buglioni, S.; Pallocca, M.; Alessandrini, G.; D’Aguanno, S.; Del Bufalo, D. BCL-X(L) overexpression promotes tumor progression-associated properties. Cell Death Dis. 2017, 8, 3216. [Google Scholar] [CrossRef]
- Xiao, G.; Li, X.; Li, G.; Zhang, B.; Xu, C.; Qin, S.; Du, N.; Wang, J.; Tang, S.C.; Zhang, J.; et al. MiR-129 blocks estrogen induction of NOTCH signaling activity in breast cancer stem-like cells. Oncotarget 2017, 8, 103261–103273. [Google Scholar] [CrossRef]
- Li, J.C.; Liu, D.; Yang, Y.; Wang, X.Y.; Pan, D.L.; Qiu, Z.D.; Su, Y.; Pan, J.J. Growth, clonability, and radiation resistance of esophageal carcinoma-derived stem-like cells. Asian Pac. J. Cancer Prev. 2013, 14, 4891–4896. [Google Scholar] [CrossRef][Green Version]
- Wang, J.L.; Yu, J.P.; Sun, Z.Q.; Sun, S.P. Radiobiological characteristics of cancer stem cells from esophageal cancer cell lines. World J. Gastroenterol. 2014, 20, 18296–18305. [Google Scholar] [CrossRef]
- Xu, D.D.; Zhou, P.J.; Wang, Y.; Zhang, L.; Fu, W.Y.; Ruan, B.B.; Xu, H.P.; Hu, C.Z.; Tian, L.; Qin, J.H.; et al. Reciprocal activation between STAT3 and miR-181b regulates the proliferation of esophageal cancer stem-like cells via the CYLD pathway. Mol. Cancer 2016, 15, 40. [Google Scholar] [CrossRef]
- Pastrana, E.; Silva-Vargas, V.; Doetsch, F. Eyes wide open: A critical review of sphere-formation as an assay for stem cells. Cell Stem Cell 2011, 8, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Lanzardo, S.; Conti, L.; Rooke, R.; Ruiu, R.; Accart, N.; Bolli, E.; Arigoni, M.; Macagno, M.; Barrera, G.; Pizzimenti, S.; et al. Immunotargeting of Antigen xCT Attenuates Stem-like Cell Behavior and Metastatic Progression in Breast Cancer. Cancer Res. 2016, 76, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C.; Tsai, J.T.; Chao, T.Y.; Ma, H.I.; Liu, W.H. The STAT3/Slug Axis Enhances Radiation-Induced Tumor Invasion and Cancer Stem-like Properties in Radioresistant Glioblastoma. Cancers 2018, 10, 512. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.L.; Li, Y.J.; Liao, K.; Shi, L.; Zhang, N.; Liu, S.; Hu, Y.Y.; Li, S.L.; Wang, Y. 2-Methoxyestradiol inhibits the proliferation and migration and reduces the radioresistance of nasopharyngeal carcinoma CNE-2 stem cells via NF-κB/HIF-1 signaling pathway inactivation and EMT reversal. Oncol. Rep. 2017, 37, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Lynam-Lennon, N.; Heavey, S.; Sommerville, G.; Bibby, B.A.; Ffrench, B.; Quinn, J.; Gasch, C.; O’Leary, J.J.; Gallagher, M.F.; Reynolds, J.V.; et al. MicroRNA-17 is downregulated in esophageal adenocarcinoma cancer stem-like cells and promotes a radioresistant phenotype. Oncotarget 2017, 8, 11400–11413. [Google Scholar] [CrossRef]
- Che, S.M.; Zhang, X.Z.; Liu, X.L.; Chen, X.; Hou, L. The radiosensitization effect of NS398 on esophageal cancer stem cell-like radioresistant cells. Dis. Esophagus 2011, 24, 265–273. [Google Scholar] [CrossRef]
- Almanaa, T.N.; Geusz, M.E.; Jamasbi, R.J. A new method for identifying stem-like cells in esophageal cancer cell lines. J. Cancer 2013, 4, 536–548. [Google Scholar] [CrossRef]
- Nguyen, L.V.; Vanner, R.; Dirks, P.; Eaves, C.J. Cancer stem cells: An evolving concept. Nat. Rev. Cancer 2012, 12, 133–143. [Google Scholar] [CrossRef]
- Wu, S.S.; Lee, J.H.; Koo, B.K. Lineage Tracing: Computational Reconstruction Goes Beyond the Limit of Imaging. Mol. Cells 2019, 42, 104–112. [Google Scholar] [CrossRef]
- Shimokawa, M.; Ohta, Y.; Nishikori, S.; Matano, M.; Takano, A.; Fujii, M.; Date, S.; Sugimoto, S.; Kanai, T.; Sato, T. Visualization and targeting of LGR5(+) human colon cancer stem cells. Nature 2017, 545, 187–192. [Google Scholar] [CrossRef]
- Jiang, M.; Li, H.; Zhang, Y.; Yang, Y.; Lu, R.; Liu, K.; Lin, S.; Lan, X.; Wang, H.; Wu, H.; et al. Transitional basal cells at the squamous-columnar junction generate Barrett’s oesophagus. Nature 2017, 550, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Giroux, V.; Lento, A.A.; Islam, M.; Pitarresi, J.R.; Kharbanda, A.; Hamilton, K.E.; Whelan, K.A.; Long, A.; Rhoades, B.; Tang, Q.; et al. Long-lived keratin 15+ esophageal progenitor cells contribute to homeostasis and regeneration. J. Clin. Investig. 2017, 127, 2378–2391. [Google Scholar] [CrossRef] [PubMed]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Math, M.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef]
- Prasetyanti, P.R.; Medema, J.P. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol. Cancer 2017, 16, 41. [Google Scholar] [CrossRef]
- Gawad, C.; Koh, W.; Quake, S.R. Single-cell genome sequencing: Current state of the science. Nat. Rev. Genet. 2016, 17, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yu, J.; Li, Y.; Hou, Q.; Zhou, R.; Zhang, N.; Jing, Z.; Jiang, M.; Li, Z.; Hua, Y.; et al. Single-cell RNA sequencing reveals diverse intratumoral heterogeneities and gene signatures of two types of esophageal cancers. Cancer Lett. 2018, 438, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, S.; Yu, J.; Li, Y.; Zhang, X.Y.; Yang, L.; Zhang, H.; Hou, Q.; Jiang, M.; Brunicardi, F.C.; et al. Single-cell Transcriptome Analyses Reveal Molecular Signals to Intrinsic and Acquired Paclitaxel Resistance in Esophageal Squamous Cancer Cells. Cancer Lett. 2018, 420, 156–167. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Hou, Q.; Huang, M.; Zhang, H.; Jiang, Z.; Yue, J.; Wu, S. Single-cell RNA-seq of esophageal squamous cell carcinoma cell line with fractionated irradiation reveals radioresistant gene expression patterns. BMC Genom. 2019, 20, 611. [Google Scholar] [CrossRef]
- Wu, H.; Yu, J.; Kong, D.; Xu, Y.; Zhang, Z.; Shui, J.; Li, Z.; Luo, H.; Wang, K. Population and singlecell transcriptome analyses reveal diverse transcriptional changes associated with radioresistance in esophageal squamous cell carcinoma. Int. J. Oncol. 2019, 55, 1237–1248. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Hou, Q.; Zhou, R.; Li, Z.; Wu, S.; Yu, J.; Jiang, M. Single-cell intratumoral stemness analysis reveals the involvement of cell cycle and DNA damage repair in two different types of esophageal cancer. Oncol. Rep. 2019, 41, 3201–3208. [Google Scholar] [CrossRef]
- Zheng, H.; Pomyen, Y.; Hernandez, M.O.; Li, C.; Livak, F.; Tang, W.; Dang, H.; Greten, T.F.; Davis, J.L.; Zhao, Y.; et al. Single-cell analysis reveals cancer stem cell heterogeneity in hepatocellular carcinoma. Hepatology 2018, 68, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Shema, E.; Bernstein, B.E.; Buenrostro, J.D. Single-cell and single-molecule epigenomics to uncover genome regulation at unprecedented resolution. Nat. Genet. 2019, 51, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Marx, V. A dream of single-cell proteomics. Nat. Methods 2019, 16, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Bray, S.J. Notch signalling in context. Nat. Rev. Mol. Cell Biol. 2016, 17, 722–735. [Google Scholar] [CrossRef]
- Girardi, D.; Barrichello, A.; Fernandes, G.; Pereira, A. Targeting the Hedgehog Pathway in Cancer: Current Evidence and Future Perspectives. Cells 2019, 8, 153. [Google Scholar] [CrossRef]
- Hoffmeyer, K.; Raggioli, A.; Rudloff, S.; Anton, R.; Hierholzer, A.; Del Valle, I.; Hein, K.; Vogt, R.; Kemler, R. Wnt/β-catenin signaling regulates telomerase in stem cells and cancer cells. Science 2012, 336, 1549–1554. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Mo, J.S.; Park, H.W.; Guan, K.L. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014, 15, 642–656. [Google Scholar] [CrossRef]
- Kim, S.Y.; Baek, K.H. TGF-β signaling pathway mediated by deubiquitinating enzymes. Cell Mol. Life Sci. 2019, 76, 653–665. [Google Scholar] [CrossRef]
- Zhang, H.F.; Wu, C.; Alshareef, A.; Gupta, N.; Zhao, Q.; Xu, X.E.; Jiao, J.W.; Li, E.M.; Xu, L.Y.; Lai, R. The PI3K/AKT/c-MYC Axis Promotes the Acquisition of Cancer Stem-Like Features in Esophageal Squamous Cell Carcinoma. Stem Cells 2016, 34, 2040–2051. [Google Scholar] [CrossRef]
- Long, A.; Giroux, V.; Whelan, K.A.; Hamilton, K.E.; Tétreault, M.P.; Tanaka, K.; Lee, J.S.; Klein-Szanto, A.J.; Nakagawa, H.; Rustgi, A.K. WNT10A promotes an invasive and self-renewing phenotype in esophageal squamous cell carcinoma. Carcinogenesis 2015, 36, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Liu, R.D.; Chen, X.Q.; Wang, B.; Li, L.F.; Guo, Y.S.; Chen, X.J.; Ren, X.Q. HIF-1α promotes the stemness of oesophageal squamous cell carcinoma by activating the Wnt/β-catenin pathway. Oncol. Rep. 2019, 42, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhu, J.; Wu, G.; Cao, L.; Tan, Z.; Zhang, S.; Jiang, L.; Wu, J.; Li, M.; Song, L.; et al. Antagonizing miR-455-3p inhibits chemoresistance and aggressiveness in esophageal squamous cell carcinoma. Mol. Cancer 2017, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Zhang, Z.; Li, J.; Chen, X.; Ping, Y.; Liu, S.; Shi, X.; Li, L.; Wang, L.; Huang, L.; et al. Transforming growth factor-beta1 promotes the migration and invasion of sphere-forming stem-like cell subpopulations in esophageal cancer. Exp. Cell Res. 2015, 336, 141–149. [Google Scholar] [CrossRef]
- Moghbeli, M.; Abbaszadegan, M.R.; Golmakani, E.; Forghanifard, M.M. Correlation of Wnt and NOTCH pathways in esophageal squamous cell carcinoma. J. Cell Commun. Signal. 2016, 10, 129–135. [Google Scholar] [CrossRef]
- Forghanifard, M.M.; Azaraz, S.; Ardalan Khales, S.; Morshedi Rad, D.; Abbaszadegan, M.R. MAML1 promotes ESCC aggressiveness through upregulation of EMT marker TWIST1. Mol. Biol. Rep. 2020. [Google Scholar] [CrossRef]
- Moghbeli, M.; Mosannen Mozaffari, H.; Memar, B.; Forghanifard, M.M.; Gholamin, M.; Abbaszadegan, M.R. Role of MAML1 in targeted therapy against the esophageal cancer stem cells. J. Transl. Med. 2019, 17, 126. [Google Scholar] [CrossRef]
- Wang, Z.; Da Silva, T.G.; Jin, K.; Han, X.; Ranganathan, P.; Zhu, X.; Sanchez-Mejias, A.; Bai, F.; Li, B.; Fei, D.L.; et al. Notch signaling drives stemness and tumorigenicity of esophageal adenocarcinoma. Cancer Res. 2014, 74, 6364–6374. [Google Scholar] [CrossRef]
- Yoshikawa, R.; Nakano, Y.; Tao, L.; Koishi, K.; Matsumoto, T.; Sasako, M.; Tsujimura, T.; Hashimoto-Tamaoki, T.; Fujiwara, Y. Hedgehog signal activation in oesophageal cancer patients undergoing neoadjuvant chemoradiotherapy. Br. J. Cancer 2008, 98, 1670–1674. [Google Scholar] [CrossRef]
- Isohata, N.; Aoyagi, K.; Mabuchi, T.; Daiko, H.; Fukaya, M.; Ohta, H.; Ogawa, K.; Yoshida, T.; Sasaki, H. Hedgehog and epithelial-mesenchymal transition signaling in normal and malignant epithelial cells of the esophagus. Int. J. Cancer 2009, 125, 1212–1221. [Google Scholar] [CrossRef]
- Wang, D.; Nagle, P.W.; Wang, H.H.; Smit, J.K.; Faber, H.; Baanstra, M.; Karrenbeld, A.; Chiu, R.K.; Plukker, J.T.M.; Coppes, R.P. Hedgehog Pathway as a Potential Intervention Target in Esophageal Cancer. Cancers 2019, 11, 821. [Google Scholar] [CrossRef] [PubMed]
- Giraud, J.; Molina-Castro, S.; Seeneevassen, L.; Sifré, E.; Izotte, J.; Tiffon, C.; Staedel, C.; Boeuf, H.; Fernandez, S.; Barthelemy, P.; et al. Verteporfin targeting YAP1/TAZ-TEAD transcriptional activity inhibits the tumorigenic properties of gastric cancer stem cells. Int. J. Cancer 2020, 146, 2255–2267. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.J.; Lin, C.Y.; Liao, W.Y.; Hour, T.C.; Wang, H.D.; Chuu, C.P. CD44 Promotes Migration and Invasion of Docetaxel-Resistant Prostate Cancer Cells Likely via Induction of Hippo-Yap Signaling. Cells 2019, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Basu-Roy, U.; Bayin, N.S.; Rattanakorn, K.; Han, E.; Placantonakis, D.G.; Mansukhani, A.; Basilico, C. Sox2 antagonizes the Hippo pathway to maintain stemness in cancer cells. Nat. Commun. 2015, 6, 6411. [Google Scholar] [CrossRef] [PubMed]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Z.; Yu, X.; Huang, X.; Liu, Z.; Chai, Y.; Yang, L.; Wang, Q.; Li, M.; Zhao, J.; et al. Unbalanced YAP-SOX9 circuit drives stemness and malignant progression in esophageal squamous cell carcinoma. Oncogene 2019, 38, 2042–2055. [Google Scholar] [CrossRef]
- Song, S.; Ajani, J.A.; Honjo, S.; Maru, D.M.; Chen, Q.; Scott, A.W.; Heallen, T.R.; Xiao, L.; Hofstetter, W.L.; Weston, B.; et al. Hippo coactivator YAP1 upregulates SOX9 and endows esophageal cancer cells with stem-like properties. Cancer Res. 2014, 74, 4170–4182. [Google Scholar] [CrossRef]
- Song, S.; Honjo, S.; Jin, J.; Chang, S.S.; Scott, A.W.; Chen, Q.; Kalhor, N.; Correa, A.M.; Hofstetter, W.L.; Albarracin, C.T.; et al. The Hippo Coactivator YAP1 Mediates EGFR Overexpression and Confers Chemoresistance in Esophageal Cancer. Clin. Cancer Res. 2015, 21, 2580–2590. [Google Scholar] [CrossRef]
- Gao, J.; Xia, R.; Chen, J.; Gao, J.; Luo, X.; Ke, C.; Ren, C.; Li, J.; Mi, Y. Inhibition of esophageal-carcinoma cell proliferation by genistein via suppression of JAK1/2-STAT3 and AKT/MDM2/p53 signaling pathways. Aging 2020, 12. [Google Scholar] [CrossRef]
- Lu, Z.; Lu, C.; Li, C.; Jiao, Y.; Li, Y.; Zhang, G. Dracorhodin perchlorate induces apoptosis and G2/M cell cycle arrest in human esophageal squamous cell carcinoma through inhibition of the JAK2/STAT3 and AKT/FOXO3a pathways. Mol. Med. Rep. 2019, 20, 2091–2100. [Google Scholar] [CrossRef]
- Ha, Y.N.E.; Dai, Y.; Wufuer, D.; Pidayi, M.; Anasihan, G.; Chen, L. Second-generation Src/Abl inhibitor bosutinib effectively induces apoptosis in human esophageal squamous cell carcinoma (ESCC) cells via inhibiting Src/Abl signaling. Neoplasma 2020, 67, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Bossler, F.; Kuhn, B.J.; Gunther, T.; Kraemer, S.J.; Khalkar, P.; Adrian, S.; Lohrey, C.; Holzer, A.; Shimobayashi, M.; Durst, M.; et al. Repression of Human Papillomavirus Oncogene Expression under Hypoxia Is Mediated by PI3K/mTORC2/AKT Signaling. MBio 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Hufbauer, M.; Maltseva, M.; Meinrath, J.; Lechner, A.; Beutner, D.; Huebbers, C.U.; Akgul, B. HPV16 increases the number of migratory cancer stem cells and modulates their miRNA expression profile in oropharyngeal cancer. Int. J. Cancer 2018, 143, 1426–1439. [Google Scholar] [CrossRef] [PubMed]
- Reid, P.; Marcu, L.G.; Olver, I.; Moghaddasi, L.; Staudacher, A.H.; Bezak, E. Diversity of cancer stem cells in head and neck carcinomas: The role of HPV in cancer stem cell heterogeneity, plasticity and treatment response. Radiother. Oncol. 2019, 135, 1–12. [Google Scholar] [CrossRef]
- Prieto-Vila, M.; Takahashi, R.U.; Usuba, W.; Kohama, I.; Ochiya, T. Drug Resistance Driven by Cancer Stem Cells and Their Niche. Int. J. Mol. Sci. 2017, 18, 2574. [Google Scholar] [CrossRef]
- Lytle, N.K.; Barber, A.G.; Reya, T. Stem cell fate in cancer growth, progression and therapy resistance. Nat. Rev. Cancer 2018, 18, 669–680. [Google Scholar] [CrossRef]
- Carnero, A.; Garcia-Mayea, Y.; Mir, C.; Lorente, J.; Rubio, I.T.; ME, L.L. The cancer stem-cell signaling network and resistance to therapy. Cancer Treat. Rev. 2016, 49, 25–36. [Google Scholar] [CrossRef]
- Gasch, C.; Ffrench, B.; O’Leary, J.J.; Gallagher, M.F. Catching moving targets: Cancer stem cell hierarchies, therapy-resistance & considerations for clinical intervention. Mol. Cancer 2017, 16, 43. [Google Scholar] [CrossRef]
- Cheung, T.H.; Rando, T.A. Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 2013, 14, 329–340. [Google Scholar] [CrossRef]
- Li, L.; Bhatia, R. Stem cell quiescence. Clin. Cancer Res. 2011, 17, 4936–4941. [Google Scholar] [CrossRef]
- Cho, I.J.; Lui, P.P.; Obajdin, J.; Riccio, F.; Stroukov, W.; Willis, T.L.; Spagnoli, F.; Watt, F.M. Mechanisms, Hallmarks, and Implications of Stem Cell Quiescence. Stem Cell Rep. 2019, 12, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Vitale, I.; Manic, G.; De Maria, R.; Kroemer, G.; Galluzzi, L. DNA Damage in Stem Cells. Mol. Cell 2017, 66, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.K.; Blanpain, C.; Rossi, D.J. DNA damage response in adult stem cells: Pathways and consequences. Nat. Rev. Mol. Cell Biol. 2011, 12, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Moore, N.; Lyle, S. Quiescent, slow-cycling stem cell populations in cancer: A review of the evidence and discussion of significance. J. Oncol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Danes, A.; Larsimont, J.C.; Liagre, M.; Munoz-Couselo, E.; Lapouge, G.; Brisebarre, A.; Dubois, C.; Suppa, M.; Sukumaran, V.; Del Marmol, V.; et al. A slow-cycling LGR5 tumour population mediates basal cell carcinoma relapse after therapy. Nature 2018, 562, 434–438. [Google Scholar] [CrossRef]
- Chen, Y.; Li, D.; Wang, D.; Liu, X.; Yin, N.; Song, Y.; Lu, S.H.; Ju, Z.; Zhan, Q. Quiescence and attenuated DNA damage response promote survival of esophageal cancer stem cells. J. Cell Biochem. 2012, 113, 3643–3652. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Bugde, P.; Biswas, R.; Merien, F.; Lu, J.; Liu, D.-X.; Chen, M.; Zhou, S.; Li, Y. The therapeutic potential of targeting ABC transporters to combat multi-drug resistance. Expert Opin. Ther. Targets 2017, 21, 511–530. [Google Scholar] [CrossRef]
- Kathawala, R.J.; Gupta, P.; Ashby, C.R., Jr.; Chen, Z.S. The modulation of ABC transporter-mediated multidrug resistance in cancer: A review of the past decade. Drug Resist. Updat. 2015, 18, 1–17. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Westover, D.; Ling, X.; Lam, H.; Welch, J.; Jin, C.; Gongora, C.; Del Rio, M.; Wani, M.; Li, F. FL118, a novel camptothecin derivative, is insensitive to ABCG2 expression and shows improved efficacy in comparison with irinotecan in colon and lung cancer models with ABCG2-induced resistance. Mol. Cancer 2015, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Katayama, K.; Mitsuhashi, J.; Sugimoto, Y. Functions of the breast cancer resistance protein (BCRP/ABCG2) in chemotherapy. Adv. Drug Deliv. Rev. 2009, 61, 26–33. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.; Yu, L.; Liu, S.; Fu, J.; Cho, C.H. Constitutive AhR activation leads to concomitant ABCG2-mediated multidrug resistance in cisplatin-resistant esophageal carcinoma cells. Mol. Carcinog. 2012, 51, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mathur, A.; Zhang, Y.; Xi, S.; Atay, S.; Hong, J.A.; Datrice, N.; Upham, T.; Kemp, C.D.; Ripley, R.T.; et al. Mithramycin represses basal and cigarette smoke-induced expression of ABCG2 and inhibits stem cell signaling in lung and esophageal cancer cells. Cancer Res. 2012, 72, 4178–4192. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, J.Q.; Assaraf, Y.G.; Ren, L.; Gupta, P.; Wei, L.; Ashby, C.R., Jr.; Yang, D.H.; Chen, Z.S. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist. Updat. 2018, 41, 1–25. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Z.; Liu, X.; Agarwal, P.; Zhao, S.; Conroy, D.W.; Ji, G.; Yu, J.; Jaroniec, C.P.; Liu, Z.; et al. Targeted production of reactive oxygen species in mitochondria to overcome cancer drug resistance. Nat. Commun. 2018, 9, 562. [Google Scholar] [CrossRef]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef]
- Dinavahi, S.S.; Bazewicz, C.G.; Gowda, R.; Robertson, G.P. Aldehyde Dehydrogenase Inhibitors for Cancer Therapeutics. Trends Pharmacol. Sci. 2019, 40, 774–789. [Google Scholar] [CrossRef]
- Mizuno, T.; Suzuki, N.; Makino, H.; Furui, T.; Morii, E.; Aoki, H.; Kunisada, T.; Yano, M.; Kuji, S.; Hirashima, Y.; et al. Cancer stem-like cells of ovarian clear cell carcinoma are enriched in the ALDH-high population associated with an accelerated scavenging system in reactive oxygen species. Gynecol. Oncol. 2015, 137, 299–305. [Google Scholar] [CrossRef]
- Xu, X.; Chai, S.; Wang, P.; Zhang, C.; Yang, Y.; Yang, Y.; Wang, K. Aldehyde dehydrogenases and cancer stem cells. Cancer Lett. 2015, 369, 50–57. [Google Scholar] [CrossRef]
- Voog, J.; Jones, D.L. Stem cells and the niche: A dynamic duo. Cell Stem Cell 2010, 6, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Kise, K.; Kinugasa-Katayama, Y.; Takakura, N. Tumor microenvironment for cancer stem cells. Adv. Drug Deliv. Rev. 2016, 99, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Karakasheva, T.A.; Lin, E.W.; Tang, Q.; Qiao, E.; Waldron, T.J.; Soni, M.; Klein-Szanto, A.J.; Sahu, V.; Basu, D.; Ohashi, S.; et al. IL-6 Mediates Cross-Talk between Tumor Cells and Activated Fibroblasts in the Tumor Microenvironment. Cancer Res. 2018, 78, 4957–4970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hua, Y.; Jiang, Z.; Yue, J.; Shi, M.; Zhen, X.; Zhang, X.; Yang, L.; Zhou, R.; Wu, S. Cancer-associated Fibroblast-promoted LncRNA DNM3OS Confers Radioresistance by Regulating DNA Damage Response in Esophageal Squamous Cell Carcinoma. Clin. Cancer Res. 2019, 25, 1989–2000. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Lin, E.W.; Karakasheva, T.A.; Hicks, P.D.; Bass, A.J.; Rustgi, A.K. The tumor microenvironment in esophageal cancer. Oncogene 2016, 35, 5337–5349. [Google Scholar] [CrossRef]
- Zhao, Y.; Yi, J.; Tao, L.; Huang, G.; Chu, X.; Song, H.; Chen, L. Wnt signaling induces radioresistance through upregulating HMGB1 in esophageal squamous cell carcinoma. Cell Death Dis. 2018, 9, 433. [Google Scholar] [CrossRef]
- Su, H.; Jin, X.; Zhang, X.; Zhao, L.; Lin, B.; Li, L.; Fei, Z.; Shen, L.; Fang, Y.; Pan, H.; et al. FH535 increases the radiosensitivity and reverses epithelial-to-mesenchymal transition of radioresistant esophageal cancer cell line KYSE-150R. J. Transl. Med. 2015, 13, 104. [Google Scholar] [CrossRef]
- Das, B.; Tsuchida, R.; Malkin, D.; Koren, G.; Baruchel, S.; Yeger, H. Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells 2008, 26, 1818–1830. [Google Scholar] [CrossRef]
- Melzer, C.; von der Ohe, J.; Lehnert, H.; Ungefroren, H.; Hass, R. Cancer stem cell niche models and contribution by mesenchymal stroma/stem cells. Mol. Cancer 2017, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Colwell, N.; Larion, M.; Giles, A.J.; Seldomridge, A.N.; Sizdahkhani, S.; Gilbert, M.R.; Park, D.M. Hypoxia in the glioblastoma microenvironment: Shaping the phenotype of cancer stem-like cells. Neuro Oncol. 2017, 19, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Vey, N.; Delaunay, J.; Martinelli, G.; Fiedler, W.; Raffoux, E.; Prebet, T.; Gomez-Roca, C.; Papayannidis, C.; Kebenko, M.; Paschka, P.; et al. Phase I clinical study of RG7356, an anti-CD44 humanized antibody, in patients with acute myeloid leukemia. Oncotarget 2016, 7, 32532–32542. [Google Scholar] [CrossRef]
- Justilien, V.; Fields, A.P. Molecular pathways: Novel approaches for improved therapeutic targeting of Hedgehog signaling in cancer stem cells. Clin. Cancer Res. 2015, 21, 505–513. [Google Scholar] [CrossRef]
- Pan, Q.; Li, Q.; Liu, S.; Ning, N.; Zhang, X.; Xu, Y.; Chang, A.E.; Wicha, M.S. Concise Review: Targeting Cancer Stem Cells Using Immunologic Approaches. Stem Cells 2015, 33, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- Satoh, T.; Lee, K.H.; Rha, S.Y.; Sasaki, Y.; Park, S.H.; Komatsu, Y.; Yasui, H.; Kim, T.-Y.; Yamaguchi, K.; Fuse, N.; et al. Randomized phase II trial of nimotuzumab plus irinotecan versus irinotecan alone as second-line therapy for patients with advanced gastric cancer. Gastric. Cancer 2015, 18, 824–832. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef]
- Barbato, L.; Bocchetti, M.; Di Biase, A.; Regad, T. Cancer Stem Cells and Targeting Strategies. Cells 2019, 8, 926. [Google Scholar] [CrossRef] [PubMed]
- Plaks, V.; Kong, N.; Werb, Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Daniel, S.K.; Seo, Y.D.; Pillarisetty, V.G. The CXCL12-CXCR4/CXCR7 axis as a mechanism of immune resistance in gastrointestinal malignancies. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sleightholm, R.L.; Neilsen, B.K.; Li, J.; Steele, M.M.; Singh, R.K.; Hollingsworth, M.A.; Oupicky, D. Emerging roles of the CXCL12/CXCR4 axis in pancreatic cancer progression and therapy. Pharmacol. Ther. 2017, 179, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Łukaszewicz-Zając, M.; Mroczko, B.; Szmitkowski, M. Chemokines and their receptors in esophageal cancer—The systematic review and future perspectives. Tumour. Biol. 2015, 36, 5707–5714. [Google Scholar] [CrossRef]
- Singh, S.; Srivastava, S.K.; Bhardwaj, A.; Owen, L.B.; Singh, A.P. CXCL12-CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: A novel target for therapy. Br. J. Cancer 2010, 103, 1671–1679. [Google Scholar] [CrossRef]
- Feig, C.; Jones, J.O.; Kraman, M.; Wells, R.J.; Deonarine, A.; Chan, D.S.; Connell, C.M.; Roberts, E.W.; Zhao, Q.; Caballero, O.L.; et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 20212–20217. [Google Scholar] [CrossRef]
- Gockel, I.; Schimanski, C.C.; Heinrich, C.; Wehler, T.; Frerichs, K.; Drescher, D.; von Langsdorff, C.; Domeyer, M.; Biesterfeld, S.; Galle, P.R.; et al. Expression of chemokine receptor CXCR4 in esophageal squamous cell and adenocarcinoma. BMC Cancer 2006, 6, 290. [Google Scholar] [CrossRef][Green Version]
- Gros, S.J.; Kurschat, N.; Drenckhan, A.; Dohrmann, T.; Forberich, E.; Effenberger, K.; Reichelt, U.; Hoffman, R.M.; Pantel, K.; Kaifi, J.T.; et al. Involvement of CXCR4 chemokine receptor in metastastic HER2-positive esophageal cancer. PLoS ONE 2012, 7, e47287. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014, 383, 31–39. [Google Scholar] [CrossRef]
- Sayin, S.I.; Baumeister, T.; Wang, T.C.; Quante, M. Origins of Metaplasia in the Esophagus: Is This a GE Junction Stem Cell Disease? Dig. Dis. Sci. 2018, 63, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Raufi, A.G.; Klempner, S.J. Immunotherapy for advanced gastric and esophageal cancer: Preclinical rationale and ongoing clinical investigations. J. Gastrointest Oncol. 2015, 6, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.K.; Boku, N.; Satoh, T.; Ryu, M.H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.S.; Muro, K.; Kang, W.K.; et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 2461–2471. [Google Scholar] [CrossRef]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef]
- Tallerico, R.; Garofalo, C.; Carbone, E. A New Biological Feature of Natural Killer Cells: The Recognition of Solid Tumor-Derived Cancer Stem Cells. Front. Immunol. 2016, 7, 179. [Google Scholar] [CrossRef]
- Raulet, D.H.; Vance, R.E. Self-tolerance of natural killer cells. Nat. Rev. Immunol. 2006, 6, 520–531. [Google Scholar] [CrossRef]
- Tallerico, R.; Todaro, M.; Di Franco, S.; Maccalli, C.; Garofalo, C.; Sottile, R.; Palmieri, C.; Tirinato, L.; Pangigadde, P.N.; La Rocca, R.; et al. Human NK cells selective targeting of colon cancer-initiating cells: A role for natural cytotoxicity receptors and MHC class I molecules. J. Immunol. 2013, 190, 2381–2390. [Google Scholar] [CrossRef]
- Castriconi, R.; Daga, A.; Dondero, A.; Zona, G.; Poliani, P.L.; Melotti, A.; Griffero, F.; Marubbi, D.; Spaziante, R.; Bellora, F.; et al. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J. Immunol. 2009, 182, 3530–3539. [Google Scholar] [CrossRef]
- Yin, T.; Wang, G.; He, S.; Liu, Q.; Sun, J.; Wang, Y. Human cancer cells with stem cell-like phenotype exhibit enhanced sensitivity to the cytotoxicity of IL-2 and IL-15 activated natural killer cells. Cell. Immunol. 2016, 300, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Kreso, A.; Dick, J.E. Evolution of the cancer stem cell model. Cell Stem Cell 2014, 14, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Kreso, A.; O’Brien, C.A.; van Galen, P.; Gan, O.I.; Notta, F.; Brown, A.M.; Ng, K.; Ma, J.; Wienholds, E.; Dunant, C.; et al. Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science 2013, 339, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Yu, T.S.; McKay, R.M.; Burns, D.K.; Kernie, S.G.; Parada, L.F. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 2012, 488, 522–526. [Google Scholar] [CrossRef]
- Ranjan, T.; Howard, C.M.; Yu, A.; Xu, L.; Aziz, K.; Jho, D.; Leonardo, J.; Hameed, M.A.; Karlovits, S.M.; Wegner, R.E.; et al. Cancer Stem Cell Chemotherapeutics Assay for Prospective Treatment of Recurrent Glioblastoma and Progressive Anaplastic Glioma: A Single-Institution Case Series. Transl. Oncol. 2020, 13, 100755. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).