BMP-2 Variants in Breast Epithelial to Mesenchymal Transition and Microcalcifications Origin
Abstract
1. Introduction
2. Materials and Methods
2.1. Histology
2.2. Immunohistochemistry
2.3. Transmission Electron Microscopy (TEM) of Breast Tissues
2.4. Energy Dispersive X-ray (EDX) Microanalysis
2.5. Calcium Oxalate Synthesis
2.6. Cell Culture
2.7. In Vitro Model for the Development of “Osteoblast-Like Cells”
2.8. Cell Culture Immunoflurescence
2.9. Statistical Analysis
3. Results
3.1. Morphological Classification of Breast Lesions
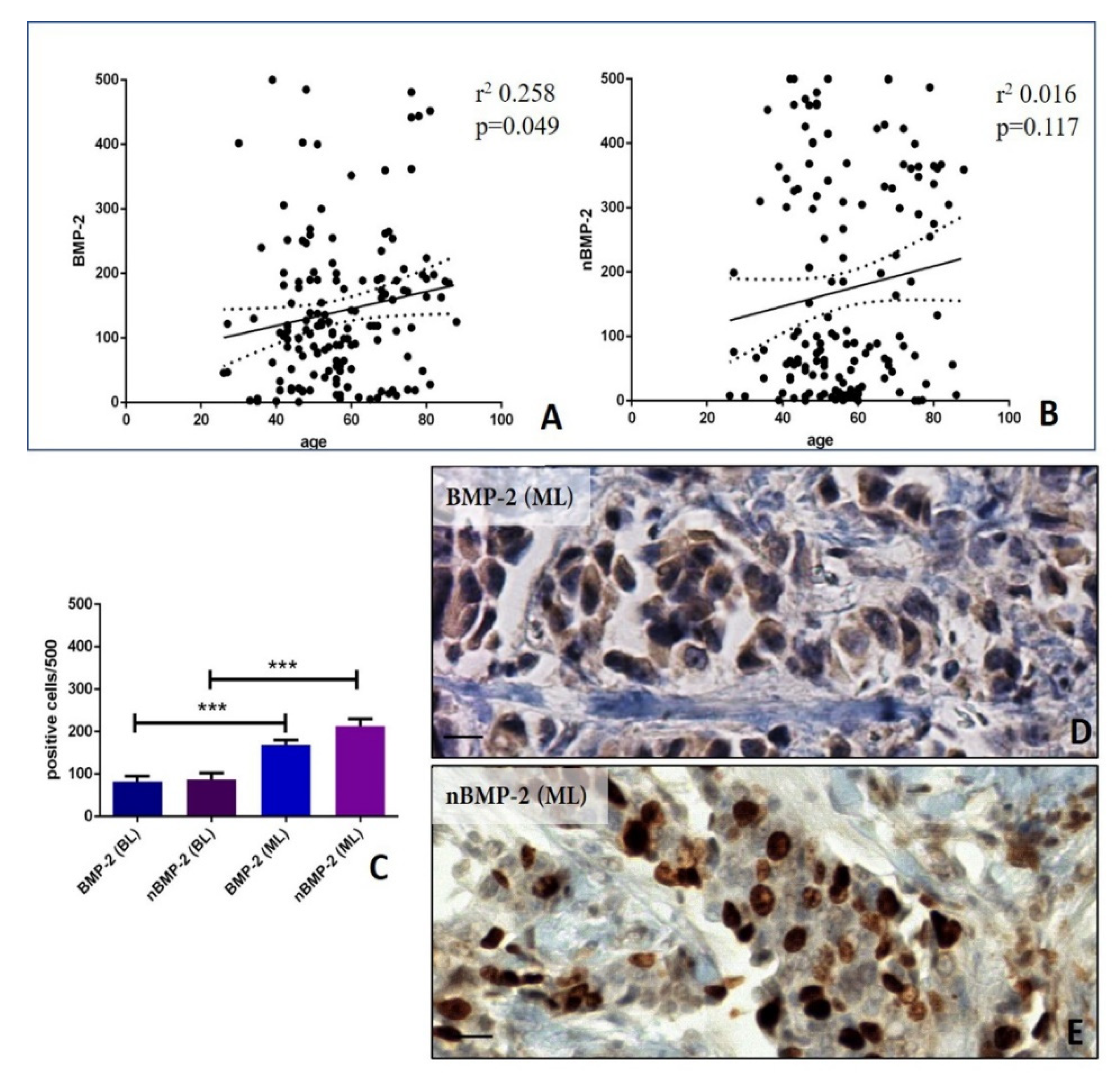
3.2. Analysis of nBMP-2/BMP-2 Expression in Breast Cancers
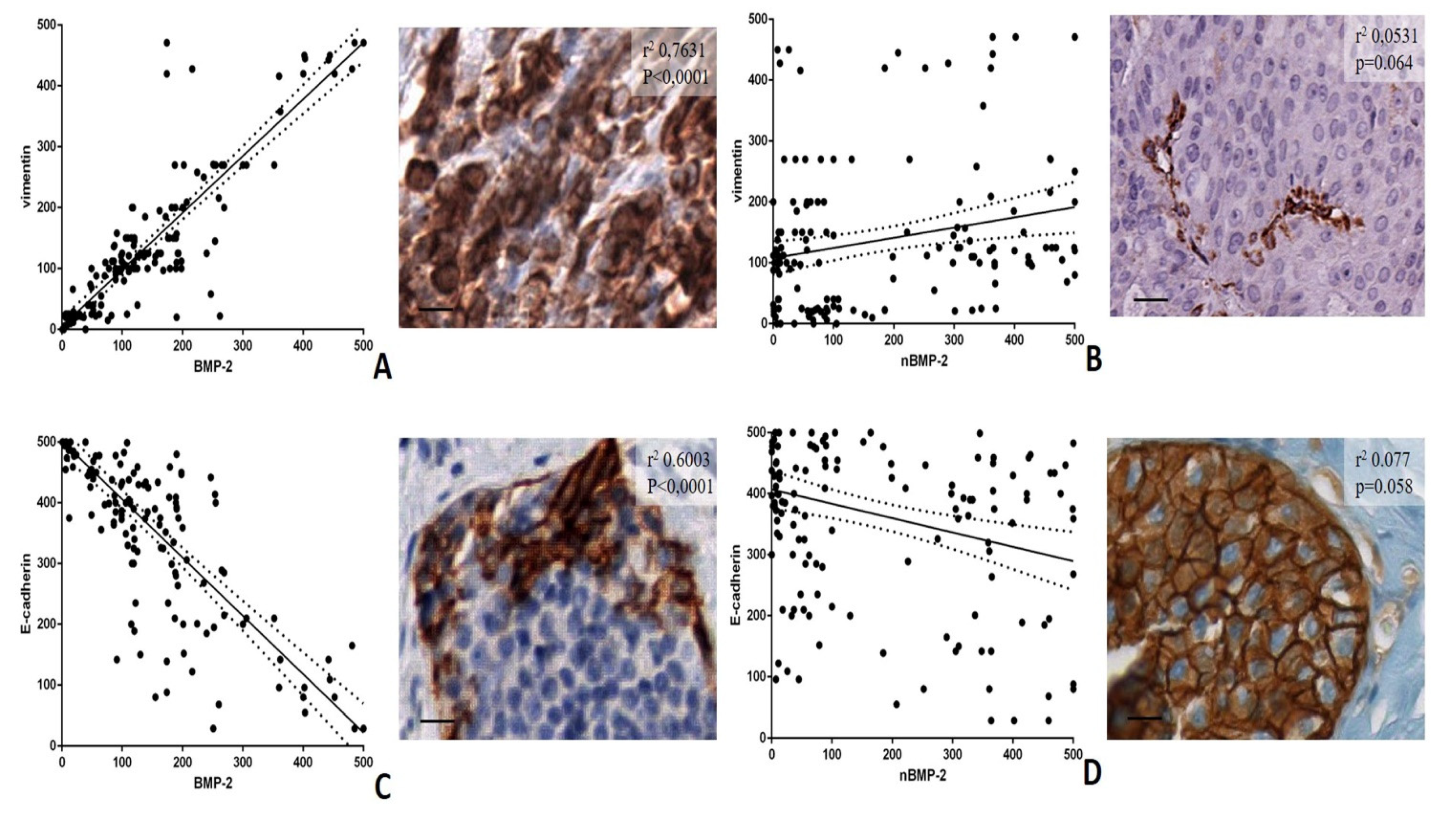
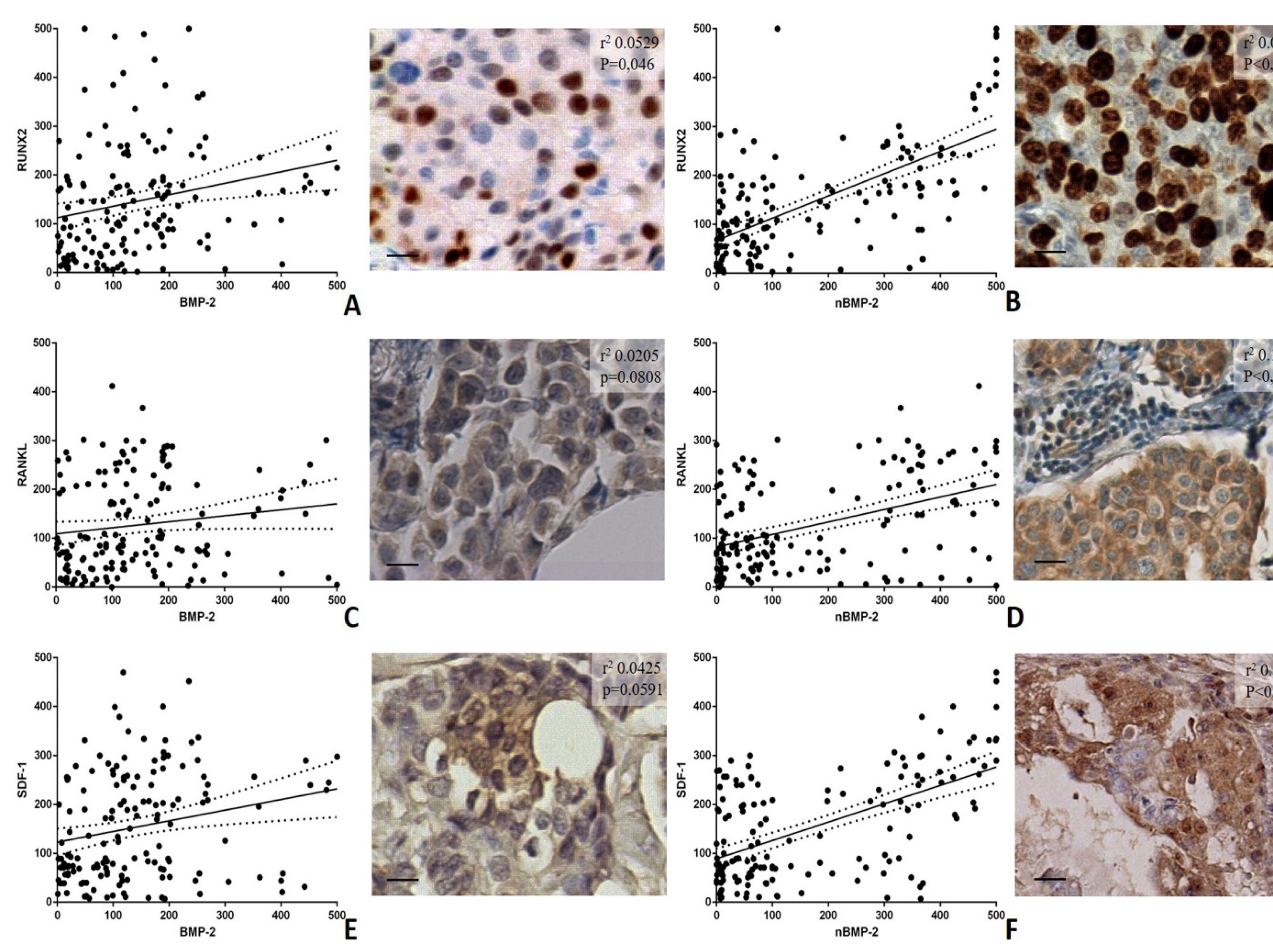
3.3. BMP-2/nBMP-2 and the Epithelial to Mesenchymal Transition
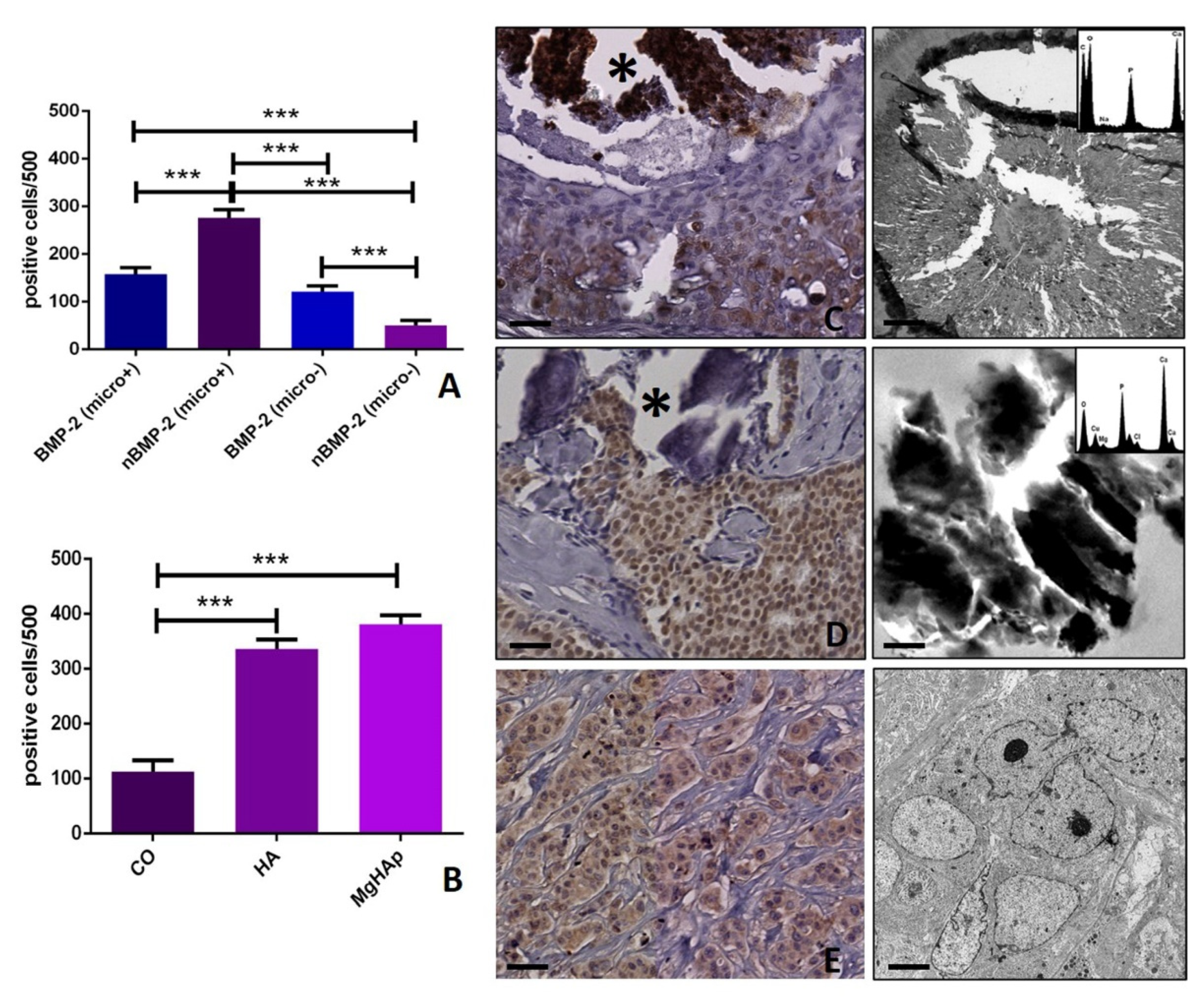
3.4. BMP-2/nBMP-2 and the Formation of Breast Microcalcifications
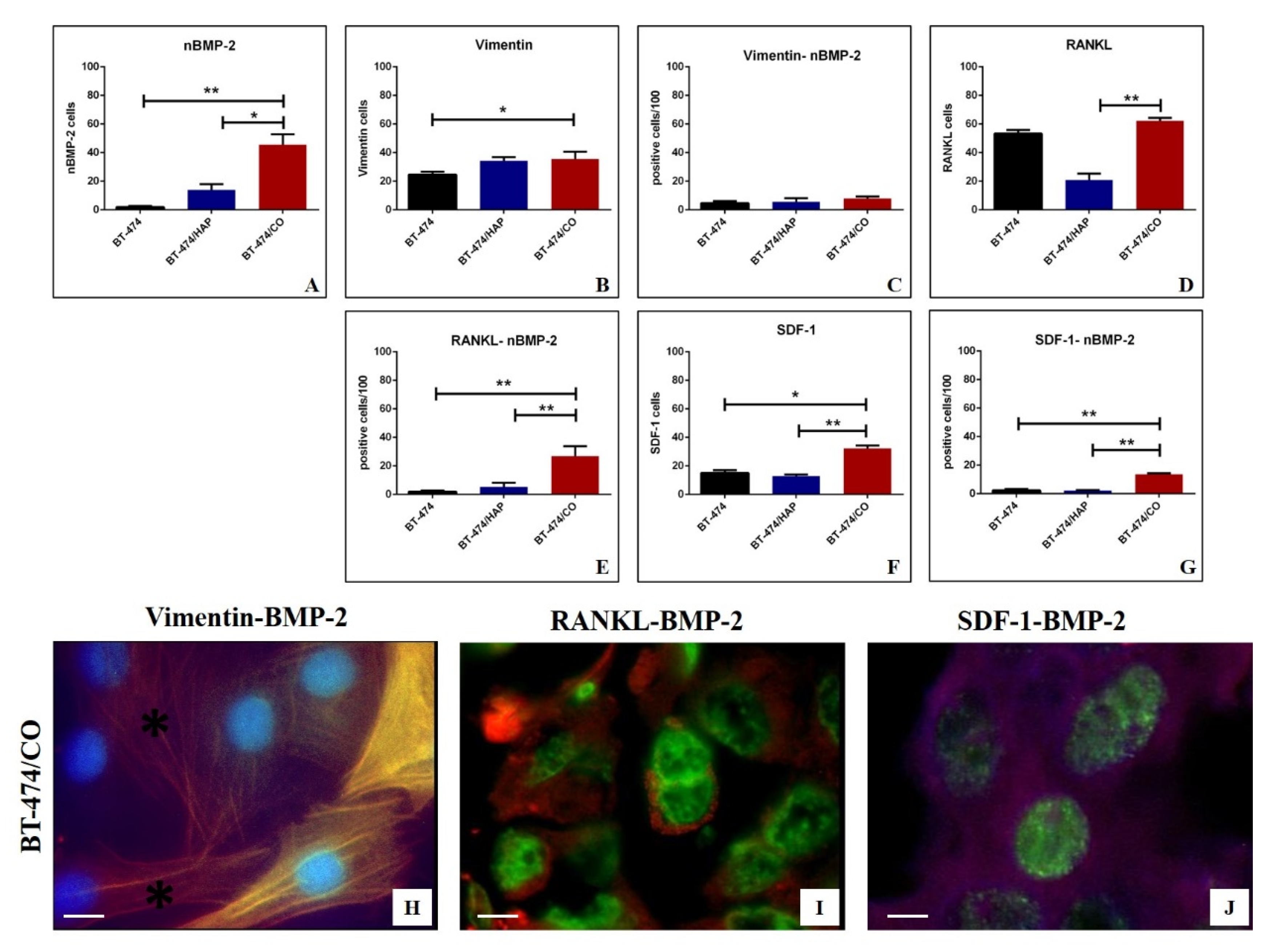
3.5. In Vitro Model
Immunofluorescence Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMPs | Bone morphogenetics proteins |
| BMP-2 | Bone morphogenetic protein-2 |
| BMP-4 | Bone morphogenetic protein-4 |
| BOLCs | Breast osteoblast-like cells |
| CO | Calcium oxalate |
| EDX | Energy dispersive X-ray |
| EMT | Epithelial to mesenchymal transition |
| ER | Estrogen receptor |
| H&E | Hematoxylin and eosin |
| HA | Hydroxyapatite |
| Mg–Hap | Magnesium-substituted hydroxyapatite |
| nBMP-2 | Nuclear variant of bone morphogenetic protein-2 |
| OPN | Osteopontin |
| PI3K/Akt | Phosphatidylinositol 3-kinases/protein kinase B |
| PTX3 | Pentraxin-related protein 3 |
| RANKL | Receptor activator of nuclear factor kappa-Β ligand |
| RUNX2 | Runt-related transcription factor 2 |
| SDF-1 | Stromal cell-derived factor |
| TEM | Transmission electron microscopy |
References
- Dafni, U.; Tsourti, Z.; Alatsathianos, I. Breast Cancer Statistics in the European Union: Incidence and Survival across European Countries. Breast Care 2019, 14, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, B.P. Epithelial-mesenchymal Transition—A Hallmark of Breast Cancer Metastasis. Cancer Hallm. 2013, 1, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.F.; Nofech-Mozes, S.; Bayani, J.; Bartlett, J. EMT in Breast Carcinoma—A Review. J. Clin. Med. 2016, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Antonacci, C.; Toschi, N.; Giannini, E.; Bonfiglio, R.; Buonomo, C.O.; Pistolese, C.A.; Tarantino, U.; Bonanno, E. Breast Osteoblast-like Cells: A Reliable Early Marker for Bone Metastases From Breast Cancer. Clin. Breast Cancer 2018, 18, e659–e669. [Google Scholar] [CrossRef]
- Huang, P.; Chen, A.; He, W.; Li, Z.; Zhang, G.; Liu, Z.; Liu, G.; Liu, X.; He, S.; Xiao, G.; et al. BMP-2 induces EMT and breast cancer stemness through Rb and CD44. Cell Death Discov. 2017, 3, 17039. [Google Scholar] [CrossRef]
- Freitas, C.M.T.; Burrell, H.R.; Valdoz, J.C.; Hamblin, G.J.; Raymond, C.M.; Cox, T.D.; Johnson, D.K.; Andersen, J.L.; Weber, K.S.; Bridgewater, L.C. The nuclear variant of bone morphogenetic protein 2 (nBMP2) is expressed in macrophages and alters calcium response. Sci. Rep. 2019, 9, 934. [Google Scholar] [CrossRef]
- Scimeca, M.; Bonfiglio, R.; Urbano, N.; Cerroni, C.; Anemona, L.; Montanaro, M.; Fazi, S.; Schillaci, O.; Mauriello, A.; Bonanno, E. Programmed death ligand 1 expression in prostate cancer cells is associated with deep changes of the tumor inflammatory infiltrate composition. Urol. Oncol. Semin. Orig. Investig. 2019, 37, e19–e31. [Google Scholar] [CrossRef]
- Scimeca, M.; Centofanti, F.; Celi, M.; Gasbarra, E.; Novelli, G.; Botta, A.; Tarantino, U. Vitamin D Receptor in Muscle Atrophy of Elderly Patients: A Key Element of Osteoporosis-Sarcopenia Connection. Aging Dis. 2018, 9, 952–964. [Google Scholar] [CrossRef]
- Scimeca, M.; Bischetti, S.; Lamsira, H.K.; Bonfiglio, R.; Bonanno, E. Energy Dispersive X-ray (EDX) microanalysis: A powerful tool in biomedical research and diagnosis. Eur. J. Histochem. 2018, 62, 2841. [Google Scholar] [CrossRef]
- Scimeca, M.; Pietroiusti, A.; Milano, F.; Anemona, L.; Orlandi, A.; Marsella, L.T.; Bonanno, E. Elemental Analysis of Histological Specimens: A Method to Unmask Nano Asbestos Fibers. Eur. J. Histochem. 2016, 60, 2573. [Google Scholar] [CrossRef]
- World Health Organization. Publication of the WHO Classification of Tumours, 5th Edition, Volume 2: Breast Tumours. Available online: https://www.iarc.fr/news-events/who-classification-of-tumours-5th-edition-volume-2-breast-tumours/ (accessed on 21 November 2019).
- Scimeca, M.; Bonfiglio, R.; Menichini, E.; Albonici, L.; Urbano, N.; De Caro, M.; Mauriello, A.; Schillaci, O.; Gambacurta, A.; Bonanno, E. Microcalcifications Drive Breast Cancer Occurrence and Development by Macrophage-Mediated Epithelial to Mesenchymal Transition. Int. J. Mol. Sci. 2019, 20, 5633. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Trivigno, D.; Bonfiglio, R.; Ciuffa, S.; Urbano, N.; Schillaci, O.; Bonanno, E. Breast cancer metastasis to bone: From epithelial to mesenchymal transition to breast osteoblast-like cells. Semin. Cancer Boil. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, R.; Scimeca, M.; Toschi, N.; Pistolese, C.A.; Giannini, E.; Antonacci, C.; Ciuffa, S.; Tancredi, V.; Tarantino, U.; Albonici, L.; et al. Radiological, Histological and Chemical Analysis of Breast Microcalcifications: Diagnostic Value and Biological Significance. J. Mammary Gland. Boil. Neoplasia 2018, 23, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Bonfiglio, R.; Montanaro, M.; Bonanno, E. Osteoblast-like cells in human cancers: New cell type and reliable markers for bone metastasis. Futur. Oncol. 2018, 14, 9–11. [Google Scholar] [CrossRef]
- Scimeca, M.; Giannini, E.; Antonacci, C.; Pistolese, C.A.; Spagnoli, L.G.; Bonanno, E. Microcalcifications in breast cancer: An active phenomenon mediated by epithelial cells with mesenchymal characteristics. BMC Cancer 2014, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Fritz, A.J.; Zaidi, S.K.; Van Wijnen, A.J.; Nickerson, J.A.; Imbalzano, A.N.; Lian, J.; Stein, J.L.; Stein, G.S. Epithelial-to-mesenchymal transition and cancer stem cells contribute to breast cancer heterogeneity. J. Cell. Physiol. 2018, 233, 9136–9144. [Google Scholar] [CrossRef]
- Bill, R.; Christofori, G. The relevance of EMT in breast cancer metastasis: Correlation or causality? FEBS Lett. 2015, 589, 1577–1587. [Google Scholar] [CrossRef]
- Liu, F.; Gu, L.-N.; Shan, B.-E.; Geng, C.-Z.; Sang, M. Biomarkers for EMT and MET in breast cancer: An update. Oncol. Lett. 2016, 12, 4869–4876. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, Y.; Long, X.; Xiao, P.; Ren, X.; Yu, J. BMP signaling and its paradoxical effects in tumorigenesis and dissemination. Oncotarget 2016, 7, 78206–78218. [Google Scholar] [CrossRef]
- Kanjilal, D.; Cottrell, J. Bone Morphogenetic Proteins (BMPs) and Bone Regeneration. Adv. Struct. Saf. Stud. 2018, 235–245. [Google Scholar] [CrossRef]
- Bonfiglio, R.; Scimeca, M.; Urbano, N.; Bonanno, E.; Schillaci, O. Breast microcalcifications: Biological and diagnostic perspectives. Futur. Oncol. 2018, 14, 3097–3099. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Y.; Lin, H.-H.; Tang, M.; Wang, Y.-K. Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget 2015, 6, 15966–15983. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, A.; Suresh, T.N.; Kumar, M.L. Expression of vimentin in breast carcinoma, its correlation with Ki67 and other histopathological parameters. Indian J. Cancer 2013, 50, 189. [Google Scholar] [CrossRef]
- Canas-Marques, R.; Schnitt, S.J. E-cadherin immunohistochemistry in breast pathology: Uses and pitfalls. Histopathology 2015, 68, 57–69. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Z.; Lu, N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adhes. Migr. 2015, 9, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Urbano, N.; Bonfiglio, R.; Schillaci, O.; Bonanno, E. Breast osteoblast-like cells: A new biomarker for the management of breast cancer. Br. J. Cancer 2018, 119, 1129–1132. [Google Scholar] [CrossRef]
- El-Gendi, S.; Mostafa, M.F. Runx2 Expression as a Potential Prognostic Marker in Invasive Ductal Breast Carcinoma. Pathol. Oncol. Res. 2015, 22, 461–470. [Google Scholar] [CrossRef]
- Atkins, G.J.; Kostakis, P.; Pan, B.; Farrugia, A.; Gronthos, S.; Evdokiou, A.; Harrison, K.; Findlay, D.M.; Zannettino, A.C. RANKL Expression Is Related to the Differentiation State of Human Osteoblasts. J. Bone Miner. Res. 2003, 18, 1088–1098. [Google Scholar] [CrossRef]
- Hosogane, N.; Huang, Z.; Rawlins, B.A.; Liu, X.; Boachie-Adjei, O.; Boskey, A.; Zhu, W. Stromal derived factor-1 regulates bone morphogenetic protein 2-induced osteogenic differentiation of primary mesenchymal stem cells. Int. J. Biochem. Cell Boil. 2010, 42, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.-H.; Wen, C.-S.; Wang, J.-Y.; Hsu, C.-Y.; Tsai, Y.-F.; Hung, S.; Tseng, L.-M.; Shyr, Y.-M. Role of estrogen receptors and Src signaling in mechanisms of bone metastasis by estrogen receptor positive breast cancers. J. Transl. Med. 2017, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- Jiramongkolchai, P.; Owens, P.; Hong, C.C. Emerging roles of the bone morphogenetic protein pathway in cancer: Potential therapeutic target for kinase inhibition. Biochem. Soc. Trans. 2016, 44, 1117–1134. [Google Scholar] [CrossRef] [PubMed]
- Urbano, N.; Scimeca, M.; Di Russo, C.; Bonanno, E.; Schillaci, O. Breast-Specific Gamma Imaging with [99mTc]Tc-Sestamibi: An In Vivo Analysis for Early Identification of Breast Cancer Lesions Expressing Bone Biomarkers. J. Clin. Med. 2020, 9, 747. [Google Scholar] [CrossRef]
- Urbano, N.; Scimeca, M.; Tancredi, V.; Bonanno, E.; Schillaci, O. 99mTC-Sestamibi Breast Imaging: Current Status, New ideas and Future Perspectives. Semin. Cancer Boil. 2020. [Google Scholar] [CrossRef]
| Antibody | Characteristics | Dilution | Retrieval |
|---|---|---|---|
| Anti-BMP2 | Mouse monoclonal clone 1A11; Novus Biologicals, Littleton, CO, USA | 1:500 | Citrate pH 6.0 |
| Anti-Vimentin | Mouse monoclonal clone V9; Ventana, Tucson, AZ, USA | Pre-diluted | EDTA citrate pH 7.8 |
| Anti-E-cadherin | Mouse monoclonal clone (36); Ventana, Tucson, AZ, USA | Pre-diluted | EDTA citrate pH 7.8 |
| Anti-RUNX2 | Mouse monoclonal clone 3F5; Novus Biologicals, Littleton, CO, USA | 1:100 | Citrate pH 6.0 |
| Anti-RANKL | Rabbit monoclonal clone 12A668; AbCam, Cambridge, UK | 1:100 | EDTA citrate pH 7.8 |
| anti-SDF-1 | Mouse monoclonal clone 79018; Novus Biologicals, Littleton, CO, USA | 1:100 | EDTA citrate pH 7.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scimeca, M.; Giocondo, R.; Montanaro, M.; Granaglia, A.; Bonfiglio, R.; Tancredi, V.; Mauriello, A.; Urbano, N.; Schillaci, O.; Bonanno, E. BMP-2 Variants in Breast Epithelial to Mesenchymal Transition and Microcalcifications Origin. Cells 2020, 9, 1381. https://doi.org/10.3390/cells9061381
Scimeca M, Giocondo R, Montanaro M, Granaglia A, Bonfiglio R, Tancredi V, Mauriello A, Urbano N, Schillaci O, Bonanno E. BMP-2 Variants in Breast Epithelial to Mesenchymal Transition and Microcalcifications Origin. Cells. 2020; 9(6):1381. https://doi.org/10.3390/cells9061381
Chicago/Turabian StyleScimeca, Manuel, Raffaella Giocondo, Manuela Montanaro, Annarita Granaglia, Rita Bonfiglio, Virginia Tancredi, Alessandro Mauriello, Nicoletta Urbano, Orazio Schillaci, and Elena Bonanno. 2020. "BMP-2 Variants in Breast Epithelial to Mesenchymal Transition and Microcalcifications Origin" Cells 9, no. 6: 1381. https://doi.org/10.3390/cells9061381
APA StyleScimeca, M., Giocondo, R., Montanaro, M., Granaglia, A., Bonfiglio, R., Tancredi, V., Mauriello, A., Urbano, N., Schillaci, O., & Bonanno, E. (2020). BMP-2 Variants in Breast Epithelial to Mesenchymal Transition and Microcalcifications Origin. Cells, 9(6), 1381. https://doi.org/10.3390/cells9061381








