Cancer Biology and Prevention in Diabetes
Abstract
1. Introduction
2. Prevalence of Cancer in Diabetes
3. Antidiabetic Drugs in Cancer
3.1. Insulin and Insulin Analogs
3.2. Sulfonylureas
3.3. Metformin and Cancer
3.4. Thiazolidinediones, Peroxisome Proliferator-Activated Receptor-γ and Cancer
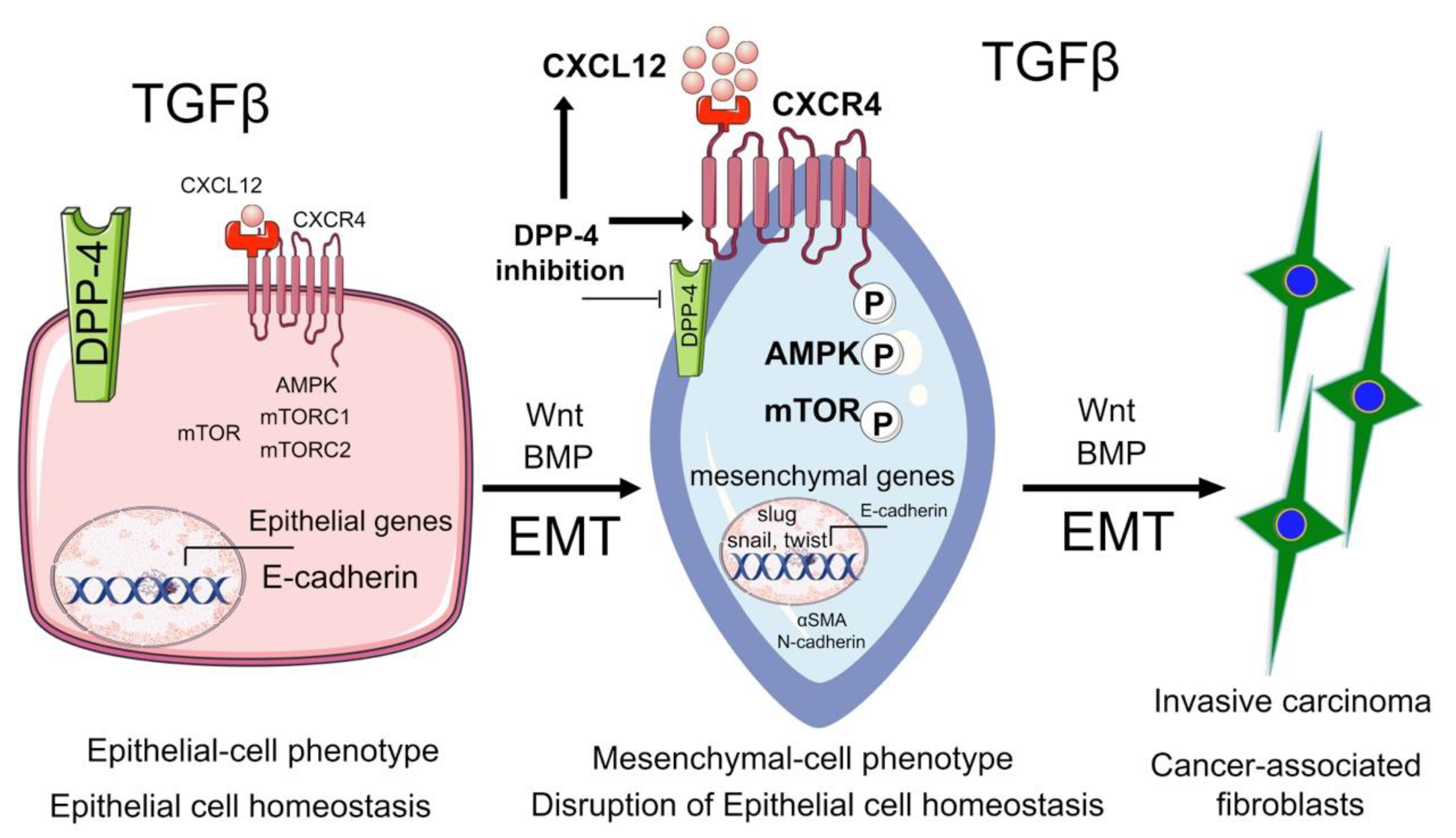
3.5. Incretin Drugs and DPP4 Inhibitors in Cancer
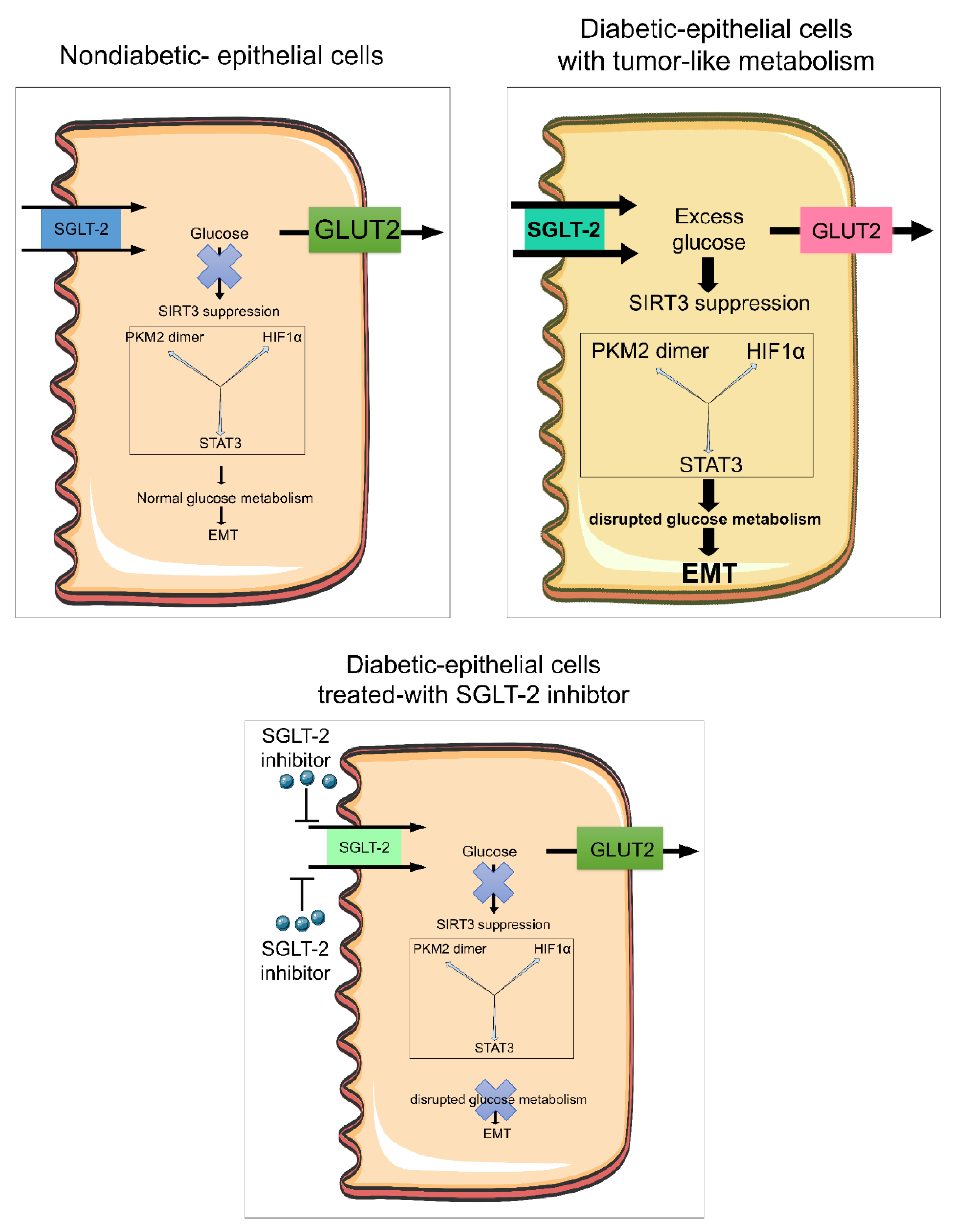
3.6. Sodium-Glucose Cotransporter 2 Inhibitors in Cancer
4. Cancer Therapies and Diabetes
5. Biology of EMT in Cancer
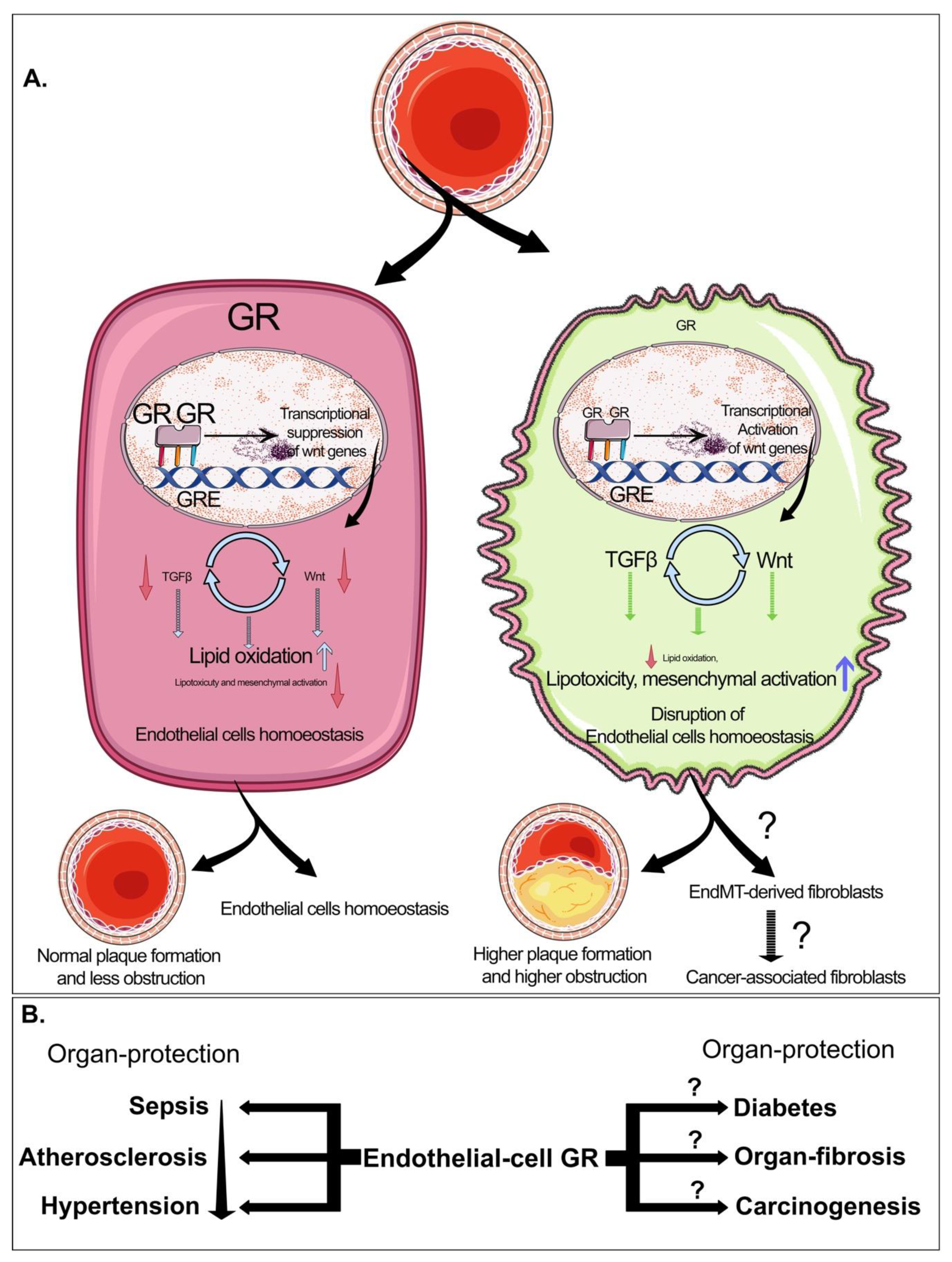
6. Biology of EndMT in Cancer
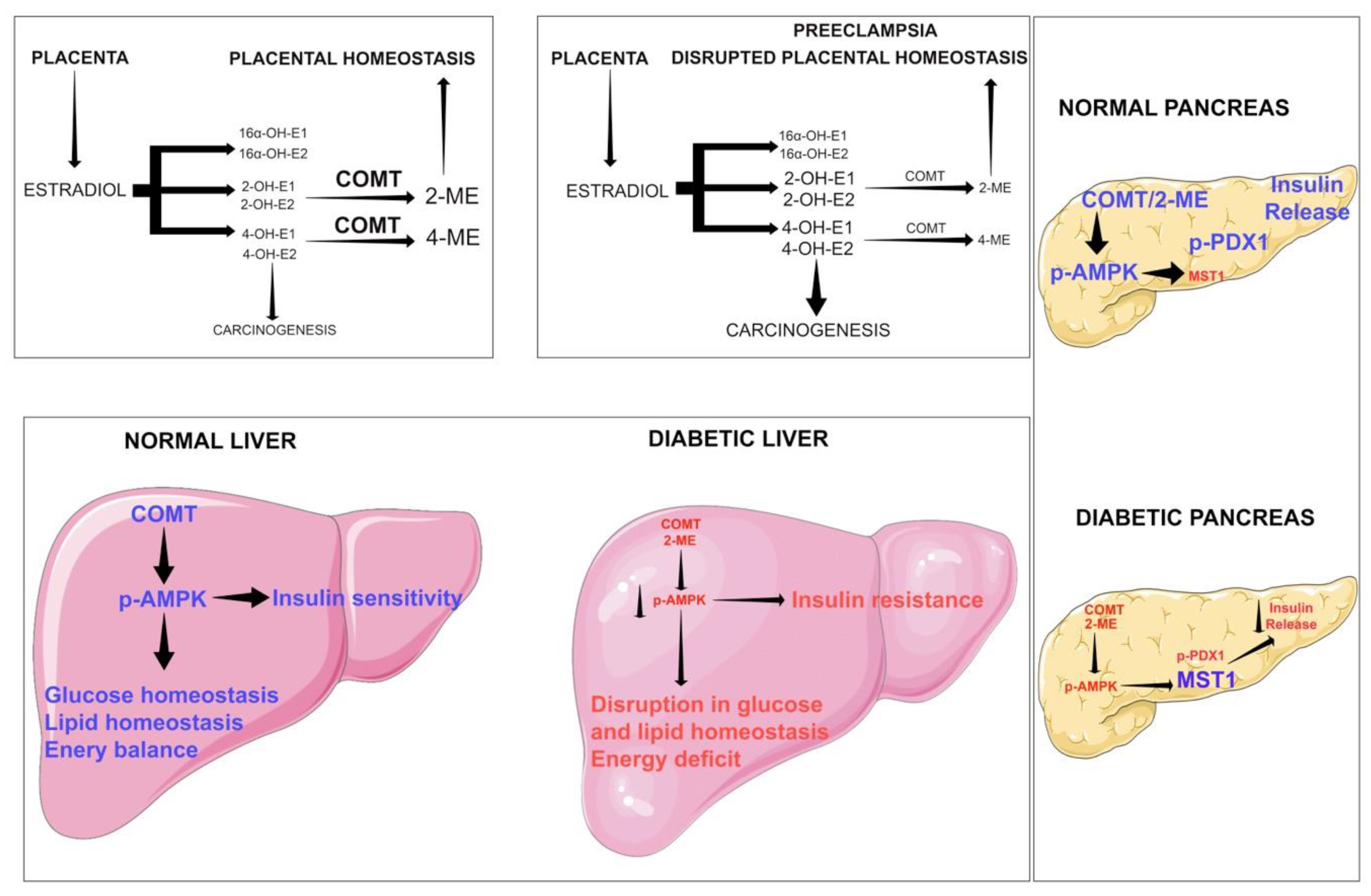
7. Biology of Catechol-o-Methyltransferase in Cancer
8. Biology of AMPK in Cancer
9. Biology of Glucocorticoid Receptors in Diabetes and Cancer
10. Perspective and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Emerging Risk Factors, C.; Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- King, H.; Aubert, R.E.; Herman, W.H. Global burden of diabetes, 1995–2025: Prevalence, numerical estimates, and projections. Diabetes Care 1998, 21, 1414–1431. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. Idf diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef]
- Duarte, A.A.; Mohsin, S.; Golubnitschaja, O. Diabetes care in figures: Current pitfalls and future scenario. EPMA J. 2018, 9, 125–131. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Viscoli, C.M.; Young, L.H.; Kernan, W.N. Diabetes prevention and cardiovascular complications. Diabetologia 2019, 62, 2161–2162. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Abudawood, M. Diabetes and cancer: A comprehensive review. J. Res. Med. Sci. 2019, 24, 94. [Google Scholar] [CrossRef]
- Harding, J.L.; Andes, L.J.; Gregg, E.W.; Cheng, Y.J.; Weir, H.K.; Bullard, K.M.; Burrows, N.R.; Imperatore, G. Trends in cancer mortality among people with vs. without diabetes in the USA, 1988–2015. Diabetologia 2020, 63, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Golubnitschaja, O. Diabetes care: Risk factors, prediction, prevention, and individualized treatment. Infect. Disord. Drug Targets 2008, 8, 68–69. [Google Scholar] [CrossRef]
- Sen, S.; He, Y.; Koya, D.; Kanasaki, K. Cancer biology in diabetes. J. Diabetes Investig. 2014, 5, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005, 330, 1304–1305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J. Metformin and reduced risk of cancer in the hong kong diabetes registry: Real effect or immortal time bias? J. Gen. Intern. Med. 2019, 34, 1154–1157. [Google Scholar] [CrossRef]
- Wynn, A.; Vacheron, A.; Zuber, J.; Solomon, S.S. Metformin associated with increased survival in type 2 diabetes patients with pancreatic cancer and lymphoma. Am. J. Med. Sci. 2019, 358, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Coller, H.A. Is cancer a metabolic disease? Am. J. Pathol. 2014, 184, 4–17. [Google Scholar] [CrossRef]
- Argiles, J.M.; Busquets, S.; Stemmler, B.; Lopez-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Jimenez, C.; Gutierrez-Salmeron, M.; Chocarro-Calvo, A.; Garcia-Martinez, J.M.; Castano, A.; De la Vieja, A. From obesity to diabetes and cancer: Epidemiological links and role of therapies. Br. J. Cancer 2016, 114, 716–722. [Google Scholar] [CrossRef]
- Cebioglu, M.; Schild, H.H.; Golubnitschaja, O. Diabetes mellitus as a risk factor for cancer: Stress or viral etiology? Infect. Disord. Drug Targets 2008, 8, 76–87. [Google Scholar] [CrossRef]
- Zendehdel, K.; Nyren, O.; Ostenson, C.G.; Adami, H.O.; Ekbom, A.; Ye, W. Cancer incidence in patients with type 1 diabetes mellitus: A population-based cohort study in sweden. J. Natl. Cancer Inst. 2003, 95, 1797–1800. [Google Scholar] [CrossRef]
- Swerdlow, A.J.; Laing, S.P.; Qiao, Z.; Slater, S.D.; Burden, A.C.; Botha, J.L.; Waugh, N.R.; Morris, A.D.; Gatling, W.; Gale, E.A.; et al. Cancer incidence and mortality in patients with insulin-treated diabetes: A uk cohort study. Br. J. Cancer 2005, 92, 2070–2075. [Google Scholar] [CrossRef]
- Stevens, R.J.; Roddam, A.W.; Beral, V. Pancreatic cancer in type 1 and young-onset diabetes: Systematic review and meta-analysis. Br. J. Cancer 2007, 96, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Dseagu, V.L.; Shelton, N.; Mindell, J.S. Epidemiological evidence of a relationship between type-1 diabetes mellitus and cancer: A review of the existing literature. Int. J. Cancer 2013, 132, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Novosyadlyy, R.; Lann, D.E.; Vijayakumar, A.; Rowzee, A.; Lazzarino, D.A.; Fierz, Y.; Carboni, J.M.; Gottardis, M.M.; Pennisi, P.A.; Molinolo, A.A.; et al. Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res. 2010, 70, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Vigneri, P.; Frasca, F.; Sciacca, L.; Pandini, G.; Vigneri, R. Diabetes and cancer. Endocr. Relat. Cancer 2009, 16, 1103–1123. [Google Scholar] [CrossRef] [PubMed]
- Shebl, F.M.; Andreotti, G.; Rashid, A.; Gao, Y.T.; Yu, K.; Shen, M.C.; Wang, B.S.; Li, Q.; Han, T.Q.; Zhang, B.H.; et al. Diabetes in relation to biliary tract cancer and stones: A population-based study in shanghai, china. Br. J. Cancer 2010, 103, 115–119. [Google Scholar] [CrossRef]
- Waters, K.M.; Henderson, B.E.; Stram, D.O.; Wan, P.; Kolonel, L.N.; Haiman, C.A. Association of diabetes with prostate cancer risk in the multiethnic cohort. Am. J. Epidemiol. 2009, 169, 937–945. [Google Scholar] [CrossRef]
- Wu, Y.; Brodt, P.; Sun, H.; Mejia, W.; Novosyadlyy, R.; Nunez, N.; Chen, X.; Mendoza, A.; Hong, S.H.; Khanna, C.; et al. Insulin-like growth factor-i regulates the liver microenvironment in obese mice and promotes liver metastasis. Cancer Res. 2010, 70, 57–67. [Google Scholar] [CrossRef]
- Erion, D.M.; Shulman, G.I. Diacylglycerol-mediated insulin resistance. Nat. Med. 2010, 16, 400–402. [Google Scholar] [CrossRef]
- Samuel, V.T.; Petersen, K.F.; Shulman, G.I. Lipid-induced insulin resistance: Unravelling the mechanism. Lancet 2010, 375, 2267–2277. [Google Scholar] [CrossRef]
- Rosse, C.; Linch, M.; Kermorgant, S.; Cameron, A.J.; Boeckeler, K.; Parker, P.J. Pkc and the control of localized signal dynamics. Nat. Rev. Mol. Cell Biol. 2010, 11, 103–112. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of u.S. Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Salmeron, M.; Chocarro-Calvo, A.; Garcia-Martinez, J.M.; de la Vieja, A.; Garcia-Jimenez, C. Epidemiological bases and molecular mechanisms linking obesity, diabetes, and cancer. Endocrinol. Diabetes Nutr. 2017, 64, 109–117. [Google Scholar] [CrossRef]
- Orgel, E.; Mittelman, S.D. The links between insulin resistance, diabetes, and cancer. Curr. Diab. Rep. 2013, 13, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, A.; Malaguarnera, R. Insulin receptor and cancer. Endocr. Relat. Cancer 2011, 18, R125–R147. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.H.; Kikkawa, U.; Matsuzaki, H.; Chen, J.H.; Chang, W.C. Insulin receptor substrate-1 prevents autophagy-dependent cell death caused by oxidative stress in mouse nih/3t3 cells. J. Biomed. Sci. 2012, 19, 64. [Google Scholar] [CrossRef]
- Hemkens, L.G.; Grouven, U.; Bender, R.; Gunster, C.; Gutschmidt, S.; Selke, G.W.; Sawicki, P.T. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: A cohort study. Diabetologia 2009, 52, 1732–1744. [Google Scholar] [CrossRef]
- Sciacca, L.; Le Moli, R.; Vigneri, R. Insulin analogs and cancer. Front. Endocrinol. 2012, 3, 21. [Google Scholar] [CrossRef]
- Tseng, C.H. Human insulin therapy is associated with an increased risk of lung cancer: A population-based retrospective cohort study. Front. Endocrinol. 2019, 10, 443. [Google Scholar] [CrossRef]
- Mannucci, E.; Monami, M.; Balzi, D.; Cresci, B.; Pala, L.; Melani, C.; Lamanna, C.; Bracali, I.; Bigiarini, M.; Barchielli, A.; et al. Doses of insulin and its analogues and cancer occurrence in insulin-treated type 2 diabetic patients. Diabetes Care 2010, 33, 1997–2003. [Google Scholar] [CrossRef]
- Pocock, S.J.; Smeeth, L. Insulin glargine and malignancy: An unwarranted alarm. Lancet 2009, 374, 511–513. [Google Scholar] [CrossRef]
- Jonasson, J.M.; Ljung, R.; Talback, M.; Haglund, B.; Gudbjornsdottir, S.; Steineck, G. Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in sweden. Diabetologia 2009, 52, 1745–1754. [Google Scholar] [CrossRef]
- Luo, J.; Chlebowski, R.; Wactawski-Wende, J.; Schlecht, N.F.; Tinker, L.; Margolis, K.L. Diabetes and lung cancer among postmenopausal women. Diabetes Care 2012, 35, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, H.B.; Shi, X.F.; Song, Y. Conventional hypoglycaemic agents and the risk of lung cancer in patients with diabetes: A meta-analysis. PLoS ONE 2014, 9, e99577. [Google Scholar] [CrossRef] [PubMed]
- But, A.; De Bruin, M.L.; Bazelier, M.T.; Hjellvik, V.; Andersen, M.; Auvinen, A.; Starup-Linde, J.; Schmidt, M.K.; Furu, K.; de Vries, F.; et al. Cancer risk among insulin users: Comparing analogues with human insulin in the caring five-country cohort study. Diabetologia 2017, 60, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Mayer, D.; Chantelau, E. Treatment with insulin glargine (lantus) increases the proliferative potency of the serum of patients with type-1 diabetes: A pilot study on mcf-7 breast cancer cells. Arch. Physiol. Biochem. 2010, 116, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B. Newer insulin analogs: Advances in basal insulin replacement. Diabetes Obes. Metab. 2013, 15 (Suppl. 1), 6–10. [Google Scholar] [CrossRef] [PubMed]
- Currie, C.J.; Poole, C.D.; Gale, E.A. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009, 52, 1766–1777. [Google Scholar] [CrossRef]
- Bowker, S.L.; Majumdar, S.R.; Veugelers, P.; Johnson, J.A. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006, 29, 254–258. [Google Scholar] [CrossRef]
- Monami, M.; Balzi, D.; Lamanna, C.; Barchielli, A.; Masotti, G.; Buiatti, E.; Marchionni, N.; Mannucci, E. Are sulphonylureas all the same? A cohort study on cardiovascular and cancer-related mortality. Diabetes Metab. Res. Rev. 2007, 23, 479–484. [Google Scholar] [CrossRef]
- Monami, M.; Lamanna, C.; Balzi, D.; Marchionni, N.; Mannucci, E. Sulphonylureas and cancer: A case-control study. Acta Diabetol. 2009, 46, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Castiglione, A.; Ghigo, E.; Gentile, L.; Durazzo, M.; Cavallo-Perin, P.; Ciccone, G. Mortality outcomes of different sulphonylurea drugs: The results of a 14-year cohort study of type 2 diabetic patients. Eur. J. Endocrinol. 2013, 169, 117–126. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, C.H.; Lin, J.W.; Wu, L.C.; Lai, M.S.; Chuang, L.M. Oral insulin secretagogues, insulin, and cancer risk in type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2012, 97, E1170–E1175. [Google Scholar] [CrossRef] [PubMed]
- Tuccori, M.; Wu, J.W.; Yin, H.; Majdan, A.; Azoulay, L. The use of glyburide compared with other sulfonylureas and the risk of cancer in patients with type 2 diabetes. Diabetes Care 2015, 38, 2083–2089. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; So, W.Y.; Ma, R.C.; Yu, L.W.; Ko, G.T.; Kong, A.P.; Ng, V.W.; Luk, A.O.; Ozaki, R.; Tong, P.C.; et al. Use of sulphonylurea and cancer in type 2 diabetes-the hong kong diabetes registry. Diabetes Res. Clin. Pract. 2010, 90, 343–351. [Google Scholar] [CrossRef]
- Hendriks, A.M.; Schrijnders, D.; Kleefstra, N.; de Vries, E.G.E.; Bilo, H.J.G.; Jalving, M.; Landman, G.W.D. Sulfonylurea derivatives and cancer, friend or foe? Eur. J. Pharmacol. 2019, 861, 172598. [Google Scholar] [CrossRef]
- Alsaggaf, R.; Pfeiffer, R.M.; Wang, Y.; St George, D.M.M.; Zhan, M.; Wagner, K.R.; Amr, S.; Greene, M.H.; Gadalla, S.M. Diabetes, metformin and cancer risk in myotonic dystrophy type i. Int. J. Cancer 2019. [Google Scholar] [CrossRef]
- Merry, B.J. Molecular mechanisms linking calorie restriction and longevity. Int. J. Biochem. Cell. Biol. 2002, 34, 1340–1354. [Google Scholar] [CrossRef]
- Stevens, R.J.; Ali, R.; Bankhead, C.R.; Bethel, M.A.; Cairns, B.J.; Camisasca, R.P.; Crowe, F.L.; Farmer, A.J.; Harrison, S.; Hirst, J.A.; et al. Cancer outcomes and all-cause mortality in adults allocated to metformin: Systematic review and collaborative meta-analysis of randomised clinical trials. Diabetologia 2012, 55, 2593–2603. [Google Scholar] [CrossRef]
- Shaw, R.J.; Lamia, K.A.; Vasquez, D.; Koo, S.H.; Bardeesy, N.; Depinho, R.A.; Montminy, M.; Cantley, L.C. The kinase lkb1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005, 310, 1642–1646. [Google Scholar] [CrossRef]
- Dowling, R.J.; Zakikhani, M.; Fantus, I.G.; Pollak, M.; Sonenberg, N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007, 67, 10804–10812. [Google Scholar] [CrossRef]
- Shackelford, D.B.; Shaw, R.J. The lkb1-ampk pathway: Metabolism and growth control in tumour suppression. Nat. Rev. Cancer 2009, 9, 563–575. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.; et al. Ampk phosphorylates and inhibits srebp activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Hebrard, S.; Leclerc, J.; Zarrinpashneh, E.; Soty, M.; Mithieux, G.; Sakamoto, K.; Andreelli, F.; Viollet, B. Metformin inhibits hepatic gluconeogenesis in mice independently of the lkb1/ampk pathway via a decrease in hepatic energy state. J. Clin. Investig. 2010, 120, 2355–2369. [Google Scholar] [CrossRef] [PubMed]
- Kalender, A.; Selvaraj, A.; Kim, S.Y.; Gulati, P.; Brule, S.; Viollet, B.; Kemp, B.E.; Bardeesy, N.; Dennis, P.; Schlager, J.J.; et al. Metformin, independent of ampk, inhibits mtorc1 in a rag gtpase-dependent manner. Cell Metab. 2010, 11, 390–401. [Google Scholar] [CrossRef]
- Buzzai, M.; Jones, R.G.; Amaravadi, R.K.; Lum, J.J.; DeBerardinis, R.J.; Zhao, F.; Viollet, B.; Thompson, C.B. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007, 67, 6745–6752. [Google Scholar] [CrossRef]
- Kisfalvi, K.; Eibl, G.; Sinnett-Smith, J.; Rozengurt, E. Metformin disrupts crosstalk between g protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Res. 2009, 69, 6539–6545. [Google Scholar] [CrossRef] [PubMed]
- Phoenix, K.N.; Vumbaca, F.; Fox, M.M.; Evans, R.; Claffey, K.P. Dietary energy availability affects primary and metastatic breast cancer and metformin efficacy. Breast Cancer Res. Treat. 2009, 123, 333–344. [Google Scholar] [CrossRef]
- Bonanni, B.; Puntoni, M.; Cazzaniga, M.; Pruneri, G.; Serrano, D.; Guerrieri-Gonzaga, A.; Gennari, A.; Trabacca, M.S.; Galimberti, V.; Veronesi, P.; et al. Dual effect of metformin on breast cancer proliferation in a randomized presurgical trial. J. Clin. Oncol. 2012, 30, 2593–2600. [Google Scholar] [CrossRef]
- Higurashi, T.; Takahashi, H.; Endo, H.; Hosono, K.; Yamada, E.; Ohkubo, H.; Sakai, E.; Uchiyama, T.; Hata, Y.; Fujisawa, N.; et al. Metformin efficacy and safety for colorectal polyps: A double-blind randomized controlled trial. BMC Cancer 2012, 12, 118. [Google Scholar] [CrossRef]
- Thakkar, B.; Aronis, K.N.; Vamvini, M.T.; Shields, K.; Mantzoros, C.S. Metformin and sulfonylureas in relation to cancer risk in type ii diabetes patients: A meta-analysis using primary data of published studies. Metabolism 2013, 62, 922–934. [Google Scholar] [CrossRef]
- Cazzaniga, M.; Bonanni, B.; Guerrieri-Gonzaga, A.; Decensi, A. Is it time to test metformin in breast cancer clinical trials? Cancer Epidemiol. Biomark. Prev. 2009, 18, 701–705. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goodwin, P.J.; Ligibel, J.A.; Stambolic, V. Metformin in breast cancer: Time for action. J. Clin. Oncol. 2009, 27, 3271–3273. [Google Scholar] [CrossRef]
- Suissa, S.; Azoulay, L. Metformin and the risk of cancer: Time-related biases in observational studies. Diabetes Care 2012, 35, 2665–2673. [Google Scholar] [CrossRef]
- Suissa, S.; Azoulay, L. Metformin and cancer: Mounting evidence against an association. Diabetes Care 2014, 37, 1786–1788. [Google Scholar] [CrossRef] [PubMed]
- Mamtani, R.; Pfanzelter, N.; Haynes, K.; Finkelman, B.S.; Wang, X.; Keefe, S.M.; Haas, N.B.; Vaughn, D.J.; Lewis, J.D. Incidence of bladder cancer in patients with type 2 diabetes treated with metformin or sulfonylureas. Diabetes Care 2014, 37, 1910–1917. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lega, I.C.; Austin, P.C.; Gruneir, A.; Goodwin, P.J.; Rochon, P.A.; Lipscombe, L.L. Association between metformin therapy and mortality after breast cancer: A population-based study. Diabetes Care 2013, 36, 3018–3026. [Google Scholar] [CrossRef] [PubMed]
- Margel, D.; Urbach, D.R.; Lipscombe, L.L.; Bell, C.M.; Kulkarni, G.; Austin, P.C.; Fleshner, N. Metformin use and all-cause and prostate cancer-specific mortality among men with diabetes. J. Clin. Oncol. 2013, 31, 3069–3075. [Google Scholar] [CrossRef]
- Smiechowski, B.B.; Azoulay, L.; Yin, H.; Pollak, M.N.; Suissa, S. The use of metformin and the incidence of lung cancer in patients with type 2 diabetes. Diabetes Care 2013, 36, 124–129. [Google Scholar] [CrossRef]
- Smiechowski, B.; Azoulay, L.; Yin, H.; Pollak, M.N.; Suissa, S. The use of metformin and colorectal cancer incidence in patients with type ii diabetes mellitus. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1877–1883. [Google Scholar] [CrossRef]
- Oh, T.K.; Song, I.A. Metformin use and the risk of cancer in patients with diabetes: A nationwide sample cohort study. Cancer Prev. Res. 2020, 13, 195–202. [Google Scholar] [CrossRef]
- Feng, Z.; Zhou, X.; Liu, N.; Wang, J.; Chen, X.; Xu, X. Metformin use and prostate cancer risk: A meta-analysis of cohort studies. Medicine 2019, 98, e14955. [Google Scholar] [CrossRef]
- Fasshauer, M.; Paschke, R. Regulation of adipocytokines and insulin resistance. Diabetologia 2003, 46, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Motomura, W.; Okumura, T.; Takahashi, N.; Obara, T.; Kohgo, Y. Activation of peroxisome proliferator-activated receptor gamma by troglitazone inhibits cell growth through the increase of p27kip1 in human. Pancreatic carcinoma cells. Cancer Res. 2000, 60, 5558–5564. [Google Scholar] [PubMed]
- Luconi, M.; Mangoni, M.; Gelmini, S.; Poli, G.; Nesi, G.; Francalanci, M.; Pratesi, N.; Cantini, G.; Lombardi, A.; Pepi, M.; et al. Rosiglitazone impairs proliferation of human adrenocortical cancer: Preclinical study in a xenograft mouse model. Endocr. Relat. Cancer 2010, 17, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Cellai, I.; Petrangolini, G.; Tortoreto, M.; Pratesi, G.; Luciani, P.; Deledda, C.; Benvenuti, S.; Ricordati, C.; Gelmini, S.; Ceni, E.; et al. In vivo effects of rosiglitazone in a human neuroblastoma xenograft. Br. J. Cancer 2010, 102, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, M.; Okumura, T.; Tanno, S.; Sawamukai, M.; Motomura, W.; Takahashi, N.; Kohgo, Y. Ppar gamma ligand-induced apoptosis through a p53-dependent mechanism in human gastric cancer cells. Cancer Sci. 2003, 94, 338–343. [Google Scholar] [CrossRef]
- Bosetti, C.; Rosato, V.; Buniato, D.; Zambon, A.; La Vecchia, C.; Corrao, G. Cancer risk for patients using thiazolidinediones for type 2 diabetes: A meta-analysis. Oncologist 2013, 18, 148–156. [Google Scholar] [CrossRef]
- Govindarajan, R.; Ratnasinghe, L.; Simmons, D.L.; Siegel, E.R.; Midathada, M.V.; Kim, L.; Kim, P.J.; Owens, R.J.; Lang, N.P. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J. Clin. Oncol. 2007, 25, 1476–1481. [Google Scholar] [CrossRef]
- Kurishima, K.; Watanabe, H.; Ishikawa, H.; Satoh, H.; Hizawa, N. Survival of patients with lung cancer and diabetes mellitus. Mol. Clin. Oncol. 2017, 6, 907–910. [Google Scholar] [CrossRef]
- Hall, G.C.; Roberts, C.M.; Boulis, M.; Mo, J.; MacRae, K.D. Diabetes and the risk of lung cancer. Diabetes Care 2005, 28, 590–594. [Google Scholar] [CrossRef]
- Chang, C.H.; Lin, J.W.; Wu, L.C.; Lai, M.S.; Chuang, L.M.; Chan, K.A. Association of thiazolidinediones with liver cancer and colorectal cancer in type 2 diabetes mellitus. Hepatology 2012, 55, 1462–1472. [Google Scholar] [CrossRef] [PubMed]
- Monami, M.; Lamanna, C.; Marchionni, N.; Mannucci, E. Rosiglitazone and risk of cancer: A meta-analysis of randomized clinical trials. Diabetes Care 2008, 31, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Elashoff, M.; Matveyenko, A.V.; Gier, B.; Elashoff, R.; Butler, P.C. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011, 141, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Amori, R.E.; Lau, J.; Pittas, A.G. Efficacy and safety of incretin therapy in type 2 diabetes: Systematic review and meta-analysis. JAMA 2007, 298, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Campbell-Thompson, M.; Gurlo, T.; Dawson, D.W.; Atkinson, M.; Butler, P.C. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes 2013, 62, 2595–2604. [Google Scholar] [CrossRef] [PubMed]
- Mayo, K.E.; Miller, L.J.; Bataille, D.; Dalle, S.; Goke, B.; Thorens, B.; Drucker, D.J. International union of pharmacology. Xxxv. The glucagon receptor family. Pharmacol. Rev. 2003, 55, 167–194. [Google Scholar] [CrossRef] [PubMed]
- Volz, A.; Goke, R.; Lankat-Buttgereit, B.; Fehmann, H.C.; Bode, H.P.; Goke, B. Molecular cloning, functional expression, and signal transduction of the gip-receptor cloned from a human insulinoma. FEBS Lett. 1995, 373, 23–29. [Google Scholar] [CrossRef]
- Yamada, Y.; Hayami, T.; Nakamura, K.; Kaisaki, P.J.; Someya, Y.; Wang, C.Z.; Seino, S.; Seino, Y. Human gastric inhibitory polypeptide receptor: Cloning of the gene (gipr) and cdna. Genomics 1995, 29, 773–776. [Google Scholar] [CrossRef]
- Widenmaier, S.B.; Ao, Z.; Kim, S.J.; Warnock, G.; McIntosh, C.H. Suppression of p38 mapk and jnk via akt-mediated inhibition of apoptosis signal-regulating kinase 1 constitutes a core component of the beta-cell pro-survival effects of glucose-dependent insulinotropic polypeptide. J. Biol. Chem. 2009, 284, 30372–30382. [Google Scholar] [CrossRef]
- Ceperuelo-Mallafre, V.; Duran, X.; Pachon, G.; Roche, K.; Garrido-Sanchez, L.; Vilarrasa, N.; Tinahones, F.J.; Vicente, V.; Pujol, J.; Vendrell, J.; et al. Disruption of gip/gipr axis in human adipose tissue is linked to obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2014, 99, E908–E919. [Google Scholar] [CrossRef]
- Regazzo, D.; Barbot, M.; Scaroni, C.; Albiger, N.; Occhi, G. The pathogenic role of the gip/gipr axis in human endocrine tumors: Emerging clinical mechanisms beyond diabetes. Rev. Endocr. Metab. Disord. 2020, 21, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Waser, B.; Rehmann, R.; Sanchez, C.; Fourmy, D.; Reubi, J.C. Glucose-dependent insulinotropic polypeptide receptors in most gastroenteropancreatic and bronchial neuroendocrine tumors. J. Clin. Endocrinol. Metab. 2012, 97, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.K.; Maxwell, J.E.; Carr, J.C.; Wang, D.; O’Dorisio, M.S.; O’Dorisio, T.M.; Howe, J.R. Gipr expression in gastric and duodenal neuroendocrine tumors. J. Surg. Res. 2014, 190, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Gourni, E.; Waser, B.; Clerc, P.; Fourmy, D.; Reubi, J.C.; Maecke, H.R. The glucose-dependent insulinotropic polypeptide receptor: A novel target for neuroendocrine tumor imaging-first preclinical studies. J. Nucl. Med. 2014, 55, 976–982. [Google Scholar] [CrossRef]
- Willekens, S.M.A.; Joosten, L.; Boerman, O.C.; Brom, M.; Gotthardt, M. Characterization of (111)in-labeled glucose-dependent insulinotropic polypeptide as a radiotracer for neuroendocrine tumors. Sci. Rep. 2018, 8, 2948. [Google Scholar] [CrossRef]
- Kanasaki, K.; Shi, S.; Kanasaki, M.; He, J.; Nagai, T.; Nakamura, Y.; Ishigaki, Y.; Kitada, M.; Srivastava, S.P.; Koya, D. Linagliptin-mediated dpp-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes 2014, 63, 2120–2131. [Google Scholar] [CrossRef]
- Shi, S.; Srivastava, S.P.; Kanasaki, M.; He, J.; Kitada, M.; Nagai, T.; Nitta, K.; Takagi, S.; Kanasaki, K.; Koya, D. Interactions of dpp-4 and integrin beta1 influences endothelial-to-mesenchymal transition. Kidney Int. 2015, 88, 479–489. [Google Scholar] [CrossRef]
- Srivastava, S.P.; Goodwin, J.E.; Kanasaki, K.; Koya, D. Inhibition of angiotensin-converting enzyme ameliorates renal fibrosis by mitigating dpp-4 level and restoring antifibrotic micrornas. Genes 2020, 11, 211. [Google Scholar] [CrossRef]
- Cordero, O.J.; Salgado, F.J.; Nogueira, M. On the origin of serum cd26 and its altered concentration in cancer patients. Cancer Immunol. Immunother. 2009, 58, 1723–1747. [Google Scholar] [CrossRef]
- Stremenova, J.; Krepela, E.; Mares, V.; Trim, J.; Dbaly, V.; Marek, J.; Vanickova, Z.; Lisa, V.; Yea, C.; Sedo, A. Expression and enzymatic activity of dipeptidyl peptidase-iv in human astrocytic tumours are associated with tumour grade. Int. J. Oncol. 2007, 31, 785–792. [Google Scholar] [CrossRef]
- Yamaguchi, U.; Nakayama, R.; Honda, K.; Ichikawa, H.; Hasegawa, T.; Shitashige, M.; Ono, M.; Shoji, A.; Sakuma, T.; Kuwabara, H.; et al. Distinct gene expression-defined classes of gastrointestinal stromal tumor. J. Clin. Oncol. 2008, 26, 4100–4108. [Google Scholar] [CrossRef] [PubMed]
- Bauvois, B.; De Meester, I.; Dumont, J.; Rouillard, D.; Zhao, H.X.; Bosmans, E. Constitutive expression of cd26/dipeptidylpeptidase iv on peripheral blood b lymphocytes of patients with b chronic lymphocytic leukaemia. Br. J. Cancer 1999, 79, 1042–1048. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khin, E.E.; Kikkawa, F.; Ino, K.; Kajiyama, H.; Suzuki, T.; Shibata, K.; Tamakoshi, K.; Nagasaka, T.; Mizutani, S. Dipeptidyl peptidase iv expression in endometrial endometrioid adenocarcinoma and its inverse correlation with tumor grade. Am. J. Obstet. Gynecol. 2003, 188, 670–676. [Google Scholar] [CrossRef]
- Liang, P.I.; Yeh, B.W.; Li, W.M.; Chan, T.C.; Chang, I.W.; Huang, C.N.; Li, C.C.; Ke, H.L.; Yeh, H.C.; Wu, W.J.; et al. Dpp4/cd26 overexpression in urothelial carcinoma confers an independent prognostic impact and correlates with intrinsic biological aggressiveness. Oncotarget 2017, 8, 2995–3008. [Google Scholar] [CrossRef]
- Morimoto, C.; Schlossman, S.F. The structure and function of cd26 in the t-cell immune response. Immunol. Rev. 1998, 161, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, M.; Buse, J.B.; Gray, C.L.; Pate, V.; Marquis, M.A.; Sturmer, T. Dipeptidyl-peptidase-4 inhibitors and pancreatic cancer: A cohort study. Diabetes Obes. Metab. 2014, 16, 1247–1256. [Google Scholar] [CrossRef]
- Nagel, A.K.; Ahmed-Sarwar, N.; Werner, P.M.; Cipriano, G.C.; Van Manen, R.P.; Brown, J.E. Dipeptidyl peptidase-4 inhibitor-associated pancreatic carcinoma: A review of the faers database. Ann. Pharmacother. 2016, 50, 27–31. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, J.; Yuan, Y.; Zou, Z.; Lai, X.; Rahmani, D.M.; Wang, F.; Xi, Y.; Huang, Q.; Bu, S. Dipeptidyl peptidase-4 inhibitors and cancer risk in patients with type 2 diabetes: A meta-analysis of randomized clinical trials. Sci. Rep. 2017, 7, 8273. [Google Scholar] [CrossRef]
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; Buse, J.B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S.; et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2015, 373, 232–242. [Google Scholar] [CrossRef]
- Spinar, J.; Smahelova, A. [Savor timi 53—Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus]. Vnitr. Lek. 2013, 59, 1003–1007. [Google Scholar]
- Leiter, L.A.; Teoh, H.; Mosenzon, O.; Cahn, A.; Hirshberg, B.; Stahre, C.A.; Hoekstra, J.B.; Alvarsson, M.; Im, K.; Scirica, B.M.; et al. Frequency of cancer events with saxagliptin in the savor-timi 53 trial. Diabetes Obes. Metab. 2016, 18, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Monami, M.; Dicembrini, I.; Mannucci, E. Dipeptidyl peptidase-4 inhibitors and pancreatitis risk: A meta-analysis of randomized clinical trials. Diabetes Obes. Metab. 2014, 16, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Boffetta, P.; Lucas, A.L. The role of common pharmaceutical agents on the prevention and treatment of pancreatic cancer. Gut Liver 2016, 10, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Metzemaekers, M.; Van Damme, J.; Mortier, A.; Proost, P. Regulation of chemokine activity—A focus on the role of dipeptidyl peptidase iv/cd26. Front. Immunol. 2016, 7, 483. [Google Scholar] [CrossRef] [PubMed]
- Christopherson, K.W., 2nd; Frank, R.R.; Jagan, S.; Paganessi, L.A.; Gregory, S.A.; Fung, H.C. Cd26 protease inhibition improves functional response of unfractionated cord blood, bone marrow, and mobilized peripheral blood cells to cxcl12/sdf-1. Exp. Hematol. 2012, 40, 945–952. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mego, M.; Cholujova, D.; Minarik, G.; Sedlackova, T.; Gronesova, P.; Karaba, M.; Benca, J.; Cingelova, S.; Cierna, Z.; Manasova, D.; et al. Cxcr4-sdf-1 interaction potentially mediates trafficking of circulating tumor cells in primary breast cancer. BMC Cancer 2016, 16, 127. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.; Magalhaes, M.A.; Coniglio, S.J.; Condeelis, J.S.; Segall, J.E. Opposing roles of cxcr4 and cxcr7 in breast cancer metastasis. Breast Cancer Res. 2011, 13, R128. [Google Scholar] [CrossRef]
- Mortier, A.; Gouwy, M.; Van Damme, J.; Proost, P.; Struyf, S. Cd26/dipeptidylpeptidase iv-chemokine interactions: Double-edged regulation of inflammation and tumor biology. J. Leukoc. Biol. 2016, 99, 955–969. [Google Scholar] [CrossRef]
- Kajiyama, H.; Kikkawa, F.; Suzuki, T.; Shibata, K.; Ino, K.; Mizutani, S. Prolonged survival and decreased invasive activity attributable to dipeptidyl peptidase iv overexpression in ovarian carcinoma. Cancer Res. 2002, 62, 2753–2757. [Google Scholar]
- Inamoto, T.; Yamochi, T.; Ohnuma, K.; Iwata, S.; Kina, S.; Inamoto, S.; Tachibana, M.; Katsuoka, Y.; Dang, N.H.; Morimoto, C. Anti-cd26 monoclonal antibody-mediated g1-s arrest of human renal clear cell carcinoma caki-2 is associated with retinoblastoma substrate dephosphorylation, cyclin-dependent kinase 2 reduction, p27(kip1) enhancement, and disruption of binding to the extracellular matrix. Clin. Cancer Res. 2006, 12, 3470–3477. [Google Scholar]
- Kissow, H.; Hartmann, B.; Holst, J.J.; Viby, N.E.; Hansen, L.S.; Rosenkilde, M.M.; Hare, K.J.; Poulsen, S.S. Glucagon-like peptide-1 (glp-1) receptor agonism or dpp-4 inhibition does not accelerate neoplasia in carcinogen treated mice. Regul. Pept. 2012, 179, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Femia, A.P.; Raimondi, L.; Maglieri, G.; Lodovici, M.; Mannucci, E.; Caderni, G. Long-term treatment with sitagliptin, a dipeptidyl peptidase-4 inhibitor, reduces colon carcinogenesis and reactive oxygen species in 1,2-dimethylhydrazine-induced rats. Int. J. Cancer 2013, 133, 2498–2503. [Google Scholar] [CrossRef] [PubMed]
- Scirica, B.M.; Bhatt, D.L.; Braunwald, E.; Steg, P.G.; Davidson, J.; Hirshberg, B.; Ohman, P.; Frederich, R.; Wiviott, S.D.; Hoffman, E.B.; et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2013, 369, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Aoe, K.; Amatya, V.J.; Fujimoto, N.; Ohnuma, K.; Hosono, O.; Hiraki, A.; Fujii, M.; Yamada, T.; Dang, N.H.; Takeshima, Y.; et al. Cd26 overexpression is associated with prolonged survival and enhanced chemosensitivity in malignant pleural mesothelioma. Clin. Cancer Res. 2012, 18, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Takagaki, Y.; Yoshitomi, Y.; Ikeda, T.; Li, J.; Kitada, M.; Kumagai, A.; Kawakita, E.; Shi, S.; Kanasaki, K.; et al. Inhibition of dipeptidyl peptidase-4 accelerates epithelial-mesenchymal transition and breast cancer metastasis via the cxcl12/cxcr4/mtor axis. Cancer Res. 2019, 79, 735–746. [Google Scholar] [CrossRef]
- Srivastava, S.P.; Hedayat, A.F.; Kanasaki, K.; Goodwin, J.E. Microrna crosstalk influences epithelial-to-mesenchymal, endothelial-to-mesenchymal, and macrophage-to-mesenchymal transitions in the kidney. Front. Pharmacol. 2019, 10, 904. [Google Scholar] [CrossRef]
- Amar, S.K.; Srivastav, A.K.; Srivastava, S.P. Advances of the current therapeutic approach for the management of breast cancer. In Current Advances in Breast Cancer Research: A Molecular Approach; Bentham Science Publishers: Sharjah, UAE, 2020; pp. 328–345. [Google Scholar]
- Engelman, J.A. Targeting pi3k signalling in cancer: Opportunities, challenges and limitations. Nat. Rev. Cancer 2009, 9, 550–562. [Google Scholar] [CrossRef]
- Bracho-Valdes, I.; Moreno-Alvarez, P.; Valencia-Martinez, I.; Robles-Molina, E.; Chavez-Vargas, L.; Vazquez-Prado, J. Mtorc1- and mtorc2-interacting proteins keep their multifunctional partners focused. IUBMB Life 2011, 63, 896–914. [Google Scholar] [CrossRef]
- Chang, L.H.; Chen, C.H.; Huang, D.Y.; Pai, H.C.; Pan, S.L.; Teng, C.M. Thrombin induces expression of twist and cell motility via the hypoxia-inducible factor-1alpha translational pathway in colorectal cancer cells. J. Cell. Physiol. 2011, 226, 1060–1068. [Google Scholar] [CrossRef]
- Chen, G.; Chen, S.M.; Wang, X.; Ding, X.F.; Ding, J.; Meng, L.H. Inhibition of chemokine (cxc motif) ligand 12/chemokine (cxc motif) receptor 4 axis (cxcl12/cxcr4)-mediated cell migration by targeting mammalian target of rapamycin (mtor) pathway in human gastric carcinoma cells. J. Biol. Chem. 2012, 287, 12132–12141. [Google Scholar] [CrossRef]
- Fujita, Y.; Inagaki, N. Renal sodium glucose cotransporter 2 inhibitors as a novel therapeutic approach to treatment of type 2 diabetes: Clinical data and mechanism of action. J. Diabetes Investig. 2014, 5, 265–275. [Google Scholar] [CrossRef]
- Zou, H.; Zhou, B.; Xu, G. Sglt2 inhibitors: A novel choice for the combination therapy in diabetic kidney disease. Cardiovasc. Diabetol. 2017, 16, 65. [Google Scholar] [CrossRef]
- Ferrannini, E.; Solini, A. Sglt2 inhibition in diabetes mellitus: Rationale and clinical prospects. Nat. Rev. Endocrinol. 2012, 8, 495–502. [Google Scholar] [CrossRef]
- Marsenic, O. Glucose control by the kidney: An emerging target in diabetes. Am. J. Kidney Dis. 2009, 53, 875–883. [Google Scholar] [CrossRef]
- Monami, M.; Nardini, C.; Mannucci, E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: A meta-analysis of randomized clinical trials. Diabetes Obes. Metab. 2014, 16, 457–466. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the american diabetes association and the european association for the study of diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef]
- Tang, H.; Dai, Q.; Shi, W.; Zhai, S.; Song, Y.; Han, J. Sglt2 inhibitors and risk of cancer in type 2 diabetes: A systematic review and meta-analysis of randomised controlled trials. Diabetologia 2017, 60, 1862–1872. [Google Scholar] [CrossRef]
- Ptaszynska, A.; Cohen, S.M.; Messing, E.M.; Reilly, T.P.; Johnsson, E.; Johnsson, K. Assessing bladder cancer risk in type 2 diabetes clinical trials: The dapagliflozin drug development program as a ‘case study’. Diabetes Ther. 2015, 6, 357–375. [Google Scholar] [CrossRef]
- Lin, H.W.; Tseng, C.H. A review on the relationship between sglt2 inhibitors and cancer. Int. J. Endocrinol. 2014, 2014, 719578. [Google Scholar] [CrossRef]
- De Jonghe, S.; Proctor, J.; Vinken, P.; Feyen, B.; Wynant, I.; Marien, D.; Geys, H.; Mamidi, R.N.; Johnson, M.D. Carcinogenicity in rats of the sglt2 inhibitor canagliflozin. Chem. Biol. Interact. 2014, 224, 1–12. [Google Scholar] [CrossRef]
- Taub, M.E.; Ludwig-Schwellinger, E.; Ishiguro, N.; Kishimoto, W.; Yu, H.; Wagner, K.; Tweedie, D. Sex-, species-, and tissue-specific metabolism of empagliflozin in male mouse kidney forms an unstable hemiacetal metabolite (m466/2) that degrades to 4-hydroxycrotonaldehyde, a reactive and cytotoxic species. Chem. Res. Toxicol. 2015, 28, 103–115. [Google Scholar] [CrossRef]
- Kuang, H.; Liao, L.; Chen, H.; Kang, Q.; Shu, X.; Wang, Y. Therapeutic effect of sodium glucose co-transporter 2 inhibitor dapagliflozin on renal cell carcinoma. Med. Sci. Monit. 2017, 23, 3737–3745. [Google Scholar] [CrossRef]
- Wu, J.H.; Foote, C.; Blomster, J.; Toyama, T.; Perkovic, V.; Sundstrom, J.; Neal, B. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2016, 4, 411–419. [Google Scholar] [CrossRef]
- Reilly, T.P.; Graziano, M.J.; Janovitz, E.B.; Dorr, T.E.; Fairchild, C.; Lee, F.; Chen, J.; Wong, T.; Whaley, J.M.; Tirmenstein, M. Carcinogenicity risk assessment supports the chronic safety of dapagliflozin, an inhibitor of sodium-glucose co-transporter 2, in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2014, 5, 73–96. [Google Scholar] [CrossRef] [PubMed]
- Cangoz, S.; Chang, Y.Y.; Chempakaseril, S.J.; Guduru, R.C.; Huynh, L.M.; John, J.S.; John, S.T.; Joseph, M.E.; Judge, R.; Kimmey, R.; et al. The kidney as a new target for antidiabetic drugs: Sglt2 inhibitors. J. Clin. Pharm. Ther. 2013, 38, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Scafoglio, C.; Hirayama, B.A.; Kepe, V.; Liu, J.; Ghezzi, C.; Satyamurthy, N.; Moatamed, N.A.; Huang, J.; Koepsell, H.; Barrio, J.R.; et al. Functional expression of sodium-glucose transporters in cancer. Proc. Natl. Acad. Sci. USA 2015, 112, E4111–E4119. [Google Scholar] [CrossRef] [PubMed]
- Chao, E.C.; Henry, R.R. Sglt2 inhibition--a novel strategy for diabetes treatment. Nat. Rev. Drug Discov. 2010, 9, 551–559. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Takagi, S.; Nitta, K.; Kitada, M.; Srivastava, S.P.; Takagaki, Y.; Kanasaki, K.; Koya, D. Renal protective effects of empagliflozin via inhibition of emt and aberrant glycolysis in proximal tubules. JCI Insight 2020, 5, e129034. [Google Scholar] [CrossRef]
- Srivastava, S.P.; Li, J.; Kitada, M.; Fujita, H.; Yamada, Y.; Goodwin, J.E.; Kanasaki, K.; Koya, D. Sirt3 deficiency leads to induction of abnormal glycolysis in diabetic kidney with fibrosis. Cell. Death Dis. 2018, 9, 997. [Google Scholar] [CrossRef]
- Srivastava, S.P.; Goodwin, J.E.; Kanasaki, K.; Koya, D. Metabolic reprogramming by n-acetyl-seryl-aspartyl-lysyl-proline protects against diabetic kidney disease. Br. J. Pharmacol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hirschey, M.D.; Shimazu, T.; Goetzman, E.; Jing, E.; Schwer, B.; Lombard, D.B.; Grueter, C.A.; Harris, C.; Biddinger, S.; Ilkayeva, O.R.; et al. Sirt3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 2010, 464, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Bindu, S.; Pillai, V.B.; Kanwal, A.; Samant, S.; Mutlu, G.M.; Verdin, E.; Dulin, N.; Gupta, M.P. Sirt3 blocks myofibroblast differentiation and pulmonary fibrosis by preventing mitochondrial DNA damage. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L68–L78. [Google Scholar] [CrossRef] [PubMed]
- Lovisa, S.; Kalluri, R. Fatty acid oxidation regulates the activation of endothelial-to-mesenchymal transition. Trends Mol. Med. 2018, 24, 432–434. [Google Scholar] [CrossRef]
- Verges, B.; Cariou, B. Mtor inhibitors and diabetes. Diabetes Res. Clin. Pract. 2015, 110, 101–108. [Google Scholar] [CrossRef]
- Verges, B.; Walter, T.; Cariou, B. Endocrine side effects of anti-cancer drugs: Effects of anti-cancer targeted therapies on lipid and glucose metabolism. Eur. J. Endocrinol. 2014, 170, R43–R55. [Google Scholar] [CrossRef]
- Geuna, E.; Roda, D.; Rafii, S.; Jimenez, B.; Capelan, M.; Rihawi, K.; Montemurro, F.; Yap, T.A.; Kaye, S.B.; De Bono, J.S.; et al. Complications of hyperglycaemia with pi3k-akt-mtor inhibitors in patients with advanced solid tumours on phase i clinical trials. Br. J. Cancer 2015, 113, 1541–1547. [Google Scholar] [CrossRef][Green Version]
- Feng, J.P.; Yuan, X.L.; Li, M.; Fang, J.; Xie, T.; Zhou, Y.; Zhu, Y.M.; Luo, M.; Lin, M.; Ye, D.W. Secondary diabetes associated with 5-fluorouracil-based chemotherapy regimens in non-diabetic patients with colorectal cancer: Results from a single-centre cohort study. Colorectal. Dis. 2013, 15, 27–33. [Google Scholar] [CrossRef]
- Saglam, H.S.; Kose, O.; Kumsar, S.; Budak, S.; Adsan, O. Fasting blood glucose and lipid profile alterations following twelve-month androgen deprivation therapy in men with prostate cancer. Sci. World J. 2012, 2012, 696329. [Google Scholar] [CrossRef]
- Hara, N. Prostate carcinogenesis with diabetes and androgen-deprivation-therapy-related diabetes: An update. Exp. Diabetes Res. 2012, 2012, 801610. [Google Scholar] [CrossRef]
- Ma, Y.S.; Yang, I.P.; Tsai, H.L.; Huang, C.W.; Juo, S.H.; Wang, J.Y. High glucose modulates antiproliferative effect and cytotoxicity of 5-fluorouracil in human colon cancer cells. DNA Cell Biol. 2014, 33, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Nan, D.N.; Fernandez-Ayala, M.; Vega Villegas, M.E.; Garcia-Castano, A.; Rivera, F.; Lopez-Brea, M.; Gonzalez-Macias, J. Diabetes mellitus following cisplatin treatment. Acta Oncol. 2003, 42, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.P.; Vos, P.; Vinh-Hung, V.; Borok, T.L.; Dutta, S.; Karlsson, U.; Lee, H.; Martinez, T.; Jo, B.H.; Nguyen, L.M.; et al. Altered glucose metabolism during chemoradiation for head and neck cancer. Anticancer Res. 2009, 29, 4683–4687. [Google Scholar] [PubMed]
- Meacham, L.R.; Sklar, C.A.; Li, S.; Liu, Q.; Gimpel, N.; Yasui, Y.; Whitton, J.A.; Stovall, M.; Robison, L.L.; Oeffinger, K.C. Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: A report for the childhood cancer survivor study. Arch. Intern. Med. 2009, 169, 1381–1388. [Google Scholar] [CrossRef]
- Hills, C.E.; Squires, P.E. The role of tgf-beta and epithelial-to mesenchymal transition in diabetic nephropathy. Cytokine Growth Factor Rev. 2011, 22, 131–139. [Google Scholar]
- Grande, M.T.; Sanchez-Laorden, B.; Lopez-Blau, C.; De Frutos, C.A.; Boutet, A.; Arevalo, M.; Rowe, R.G.; Weiss, S.J.; Lopez-Novoa, J.M.; Nieto, M.A. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat. Med. 2015, 21, 989–997. [Google Scholar] [CrossRef]
- Lovisa, S.; LeBleu, V.S.; Tampe, B.; Sugimoto, H.; Vadnagara, K.; Carstens, J.L.; Wu, C.C.; Hagos, Y.; Burckhardt, B.C.; Pentcheva-Hoang, T.; et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat. Med. 2015, 21, 998–1009. [Google Scholar] [CrossRef]
- Marquez-Exposito, L.; Lavoz, C.; Rodrigues-Diez, R.R.; Rayego-Mateos, S.; Orejudo, M.; Cantero-Navarro, E.; Ortiz, A.; Egido, J.; Selgas, R.; Mezzano, S.; et al. Gremlin regulates tubular epithelial to mesenchymal transition via vegfr2: Potential role in renal fibrosis. Front. Pharmacol. 2018, 9, 1195. [Google Scholar] [CrossRef]
- Srivastava, S.P.; Koya, D.; Kanasaki, K. Micrornas in kidney fibrosis and diabetic nephropathy: Roles on emt and endmt. Biomed. Res. Int. 2013, 2013, 125469. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Alidadiani, N.; Ghaderi, S.; Dilaver, N.; Bakhshamin, S.; Bayat, M. Epithelial mesenchymal transition transcription factor (tf): The structure, function and microrna feedback loop. Gene 2018, 674, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, N.; Mo, N.; Lu, S.; Song, E.; Ren, C.; Li, Z. Quercetin inhibits kidney fibrosis and the epithelial to mesenchymal transition of the renal tubular system involving suppression of the sonic hedgehog signaling pathway. Food Funct. 2019, 10, 3782–3797. [Google Scholar] [CrossRef] [PubMed]
- Massague, J. Tgfbeta in cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Settleman, J. Emt, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef]
- Tian, X.J.; Zhang, H.; Xing, J. Coupled reversible and irreversible bistable switches underlying tgfbeta-induced epithelial to mesenchymal transition. Biophys. J. 2013, 105, 1079–1089. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, X.J.; Zhang, H.; Teng, Y.; Li, R.; Bai, F.; Elankumaran, S.; Xing, J. Tgf-beta-induced epithelial-to-mesenchymal transition proceeds through stepwise activation of multiple feedback loops. Sci. Signal 2014, 7, ra91. [Google Scholar] [CrossRef]
- Biswas, K.; Jolly, M.K.; Ghosh, A. Stability and mean residence times for hybrid epithelial/mesenchymal phenotype. Phys. Biol. 2019, 16, 25003. [Google Scholar] [CrossRef]
- Glover, E.K.; Jordan, N.; Sheerin, N.S.; Ali, S. Regulation of endothelial-to-mesenchymal transition by micrornas in chronic allograft dysfunction. Transplantation 2019, 103, e64–e73. [Google Scholar] [CrossRef]
- Li, J.; Shi, S.; Srivastava, S.P.; Kitada, M.; Nagai, T.; Nitta, K.; Kohno, M.; Kanasaki, K.; Koya, D. Fgfr1 is critical for the anti-endothelial mesenchymal transition effect of n-acetyl-seryl-aspartyl-lysyl-proline via induction of the map4k4 pathway. Cell Death Dis. 2017, 8, e2965. [Google Scholar] [CrossRef]
- Curci, C.; Castellano, G.; Stasi, A.; Divella, C.; Loverre, A.; Gigante, M.; Simone, S.; Cariello, M.; Montinaro, V.; Lucarelli, G.; et al. Endothelial-to-mesenchymal transition and renal fibrosis in ischaemia/reperfusion injury are mediated by complement anaphylatoxins and akt pathway. Nephrol. Dial. Transplant. 2014, 29, 799–808. [Google Scholar] [CrossRef]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, J.C.; Dimmeler, S.; Harvey, R.P.; Finkel, T.; Aikawa, E.; Krenning, G.; Baker, A.H. Endothelial to mesenchymal transition in cardiovascular disease: Jacc state-of-the-art review. J. Am. Coll. Cardiol. 2019, 73, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Good, R.B.; Gilbane, A.J.; Trinder, S.L.; Denton, C.P.; Coghlan, G.; Abraham, D.J.; Holmes, A.M. Endothelial to mesenchymal transition contributes to endothelial dysfunction in pulmonary arterial hypertension. Am. J. Pathol. 2015, 185, 1850–1858. [Google Scholar] [CrossRef]
- Cho, J.G.; Lee, A.; Chang, W.; Lee, M.S.; Kim, J. Endothelial to mesenchymal transition represents a key link in the interaction between inflammation and endothelial dysfunction. Front. Immunol. 2018, 9, 294. [Google Scholar] [CrossRef] [PubMed]
- Kitao, A.; Sato, Y.; Sawada-Kitamura, S.; Harada, K.; Sasaki, M.; Morikawa, H.; Shiomi, S.; Honda, M.; Matsui, O.; Nakanuma, Y. Endothelial to mesenchymal transition via transforming growth factor-beta1/smad activation is associated with portal venous stenosis in idiopathic portal hypertension. Am. J. Pathol. 2009, 175, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Ranchoux, B.; Antigny, F.; Rucker-Martin, C.; Hautefort, A.; Pechoux, C.; Bogaard, H.J.; Dorfmuller, P.; Remy, S.; Lecerf, F.; Plante, S.; et al. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation 2015, 131, 1006–1018. [Google Scholar] [CrossRef]
- Nakano, Y.; Oyamada, M.; Dai, P.; Nakagami, T.; Kinoshita, S.; Takamatsu, T. Connexin43 knockdown accelerates wound healing but inhibits mesenchymal transition after corneal endothelial injury in vivo. Investig. Ophthalmol. Vis. Sci. 2008, 49, 93–104. [Google Scholar] [CrossRef]
- Lee, J.G.; Jung, E.; Heur, M. Fibroblast growth factor 2 induces proliferation and fibrosis via snai1-mediated activation of cdk2 and zeb1 in corneal endothelium. J. Biol. Chem. 2018, 293, 3758–3769. [Google Scholar] [CrossRef]
- Medici, D. Endothelial-mesenchymal transition in regenerative medicine. Stem Cells. Int. 2016, 2016, 6962801. [Google Scholar] [CrossRef]
- Man, S.; Sanchez Duffhues, G.; Ten Dijke, P.; Baker, D. The therapeutic potential of targeting the endothelial-to-mesenchymal transition. Angiogenesis 2019, 22, 3–13. [Google Scholar] [CrossRef]
- Pardali, E.; Sanchez-Duffhues, G.; Gomez-Puerto, M.C.; Ten Dijke, P. Tgf-beta-induced endothelial-mesenchymal transition in fibrotic diseases. Int. J. Mol. Sci. 2017, 18, 2157. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Zhou, J. Cxcr7 attenuates the tgf-beta-induced endothelial-to-mesenchymal transition and pulmonary fibrosis. Mol. Biosyst. 2017, 13, 2116–2124. [Google Scholar] [CrossRef] [PubMed]
- Dufton, N.P.; Peghaire, C.R.; Osuna-Almagro, L.; Raimondi, C.; Kalna, V.; Chuahan, A.; Webb, G.; Yang, Y.; Birdsey, G.M.; Lalor, P.; et al. Dynamic regulation of canonical tgfbeta signalling by endothelial transcription factor erg protects from liver fibrogenesis. Nat. Commun. 2017, 8, 895. [Google Scholar] [CrossRef]
- Nagai, T.; Kanasaki, M.; Srivastava, S.P.; Nakamura, Y.; Ishigaki, Y.; Kitada, M.; Shi, S.; Kanasaki, K.; Koya, D. N-acetyl-seryl-aspartyl-lysyl-proline inhibits diabetes-associated kidney fibrosis and endothelial-mesenchymal transition. Biomed. Res. Int. 2014, 2014, 696475. [Google Scholar] [CrossRef] [PubMed]
- Nitta, K.; Shi, S.; Nagai, T.; Kanasaki, M.; Kitada, M.; Srivastava, S.P.; Haneda, M.; Kanasaki, K.; Koya, D. Oral administration of n-acetyl-seryl-aspartyl-lysyl-proline ameliorates kidney disease in both type 1 and type 2 diabetic mice via a therapeutic regimen. Biomed. Res. Int. 2016, 2016, 9172157. [Google Scholar] [CrossRef] [PubMed]
- Medici, D.; Kalluri, R. Endothelial-mesenchymal transition and its contribution to the emergence of stem cell phenotype. Semin. Cancer Biol. 2012, 22, 379–384. [Google Scholar] [CrossRef]
- Srivastava, S.P.; Shi, S.; Kanasaki, M.; Nagai, T.; Kitada, M.; He, J.; Nakamura, Y.; Ishigaki, Y.; Kanasaki, K.; Koya, D. Effect of antifibrotic micrornas crosstalk on the action of n-acetyl-seryl-aspartyl-lysyl-proline in diabetes-related kidney fibrosis. Sci. Rep. 2016, 6, 29884. [Google Scholar] [CrossRef]
- Sun, Q.; Miao, J.; Luo, J.; Yuan, Q.; Cao, H.; Su, W.; Zhou, Y.; Jiang, L.; Fang, L.; Dai, C.; et al. The feedback loop between mir-21, pdcd4 and ap-1 functions as a driving force for renal fibrogenesis. J. Cell Sci. 2018, 131, jcs202317. [Google Scholar] [CrossRef]
- Zhou, H.; Mehta, S.; Srivastava, S.P.; Grabinska, K.; Zhang, X.; Wong, C.; Hedayat, A.; Perrotta, P.; Fernandez-Hernando, C.; Sessa, W.C.; et al. Endothelial cell-glucocorticoid receptor interactions and regulation of wnt signaling. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Pandey, A.K.; Verma, G.; Vig, S.; Srivastava, S.; Srivastava, A.K.; Datta, M. Mir-29a levels are elevated in the db/db mice liver and its overexpression leads to attenuation of insulin action on pepck gene expression in hepg2 cells. Mol. Cell. Endocrinol. 2011, 332, 125–133. [Google Scholar] [CrossRef]
- Kaur, K.; Pandey, A.K.; Srivastava, S.; Srivastava, A.K.; Datta, M. Comprehensive mirnome and in silico analyses identify the wnt signaling pathway to be altered in the diabetic liver. Mol. Biosyst. 2011, 7, 3234–3244. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.P.; Shi, S.; Koya, D.; Kanasaki, K. Lipid mediators in diabetic nephropathy. Fibrogenesis Tissue Repair 2014, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, E.M.; Potenta, S.; Xie, L.; Zeisberg, M.; Kalluri, R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007, 67, 10123–10128. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.; Roswall, P.; Cortez, E.; Hanahan, D.; Pietras, K. Pericytes promote endothelial cell survival through induction of autocrine vegf-a signaling and bcl-w expression. Blood 2011, 118, 2906–2917. [Google Scholar] [CrossRef] [PubMed]
- Kanasaki, K.; Palmsten, K.; Sugimoto, H.; Ahmad, S.; Hamano, Y.; Xie, L.; Parry, S.; Augustin, H.G.; Gattone, V.H.; Folkman, J.; et al. Deficiency in catechol-o-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature 2008, 453, 1117–1121. [Google Scholar] [CrossRef]
- Shand, F.H.; Langenbach, S.Y.; Keenan, C.R.; Ma, S.P.; Wheaton, B.J.; Schuliga, M.J.; Ziogas, J.; Stewart, A.G. In vitro and in vivo evidence for anti-inflammatory properties of 2-methoxyestradiol. J. Pharmacol. Exp. Ther. 2011, 336, 962–972. [Google Scholar] [CrossRef]
- Tunbridge, E.M.; Harrison, P.J.; Weinberger, D.R. Catechol-o-methyltransferase, cognition, and psychosis: Val158met and beyond. Biol. Psychiatry 2006, 60, 141–151. [Google Scholar] [CrossRef]
- Annerbrink, K.; Westberg, L.; Nilsson, S.; Rosmond, R.; Holm, G.; Eriksson, E. Catechol o-methyltransferase val158-met polymorphism is associated with abdominal obesity and blood pressure in men. Metabolism 2008, 57, 708–711. [Google Scholar] [CrossRef]
- Tworoger, S.S.; Chubak, J.; Aiello, E.J.; Yasui, Y.; Ulrich, C.M.; Farin, F.M.; Stapleton, P.L.; Irwin, M.L.; Potter, J.D.; Schwartz, R.S.; et al. The effect of cyp19 and comt polymorphisms on exercise-induced fat loss in postmenopausal women. Obes. Res. 2004, 12, 972–981. [Google Scholar] [CrossRef]
- Zhu, B.T. Catechol-o-methyltransferase (comt)-mediated methylation metabolism of endogenous bioactive catechols and modulation by endobiotics and xenobiotics: Importance in pathophysiology and pathogenesis. Curr. Drug Metab. 2002, 3, 321–349. [Google Scholar] [CrossRef]
- Kanasaki, M.; Srivastava, S.P.; Yang, F.; Xu, L.; Kudoh, S.; Kitada, M.; Ueki, N.; Kim, H.; Li, J.; Takeda, S.; et al. Deficiency in catechol-o-methyltransferase is linked to a disruption of glucose homeostasis in mice. Sci. Rep. 2017, 7, 7927. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.T.; Jablonski, K.A.; Chen, L.; Harden, M.; Tolkin, B.R.; Kaptchuk, T.J.; Bray, G.A.; Ridker, P.M.; Florez, J.C.; Diabetes Prevention Program Research, G.; et al. Catechol-o-methyltransferase association with hemoglobin a1c. Metabolism 2016, 65, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Harskamp, R.E.; Zeeman, G.G. Preeclampsia: At risk for remote cardiovascular disease. Am. J. Med. Sci. 2007, 334, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Craici, I.; Wagner, S.; Garovic, V.D. Preeclampsia and future cardiovascular risk: Formal risk factor or failed stress test? Ther. Adv. Cardiovasc. Dis. 2008, 2, 249–259. [Google Scholar] [CrossRef]
- Nixon, A.J.; Neuberg, D.; Hayes, D.F.; Gelman, R.; Connolly, J.L.; Schnitt, S.; Abner, A.; Recht, A.; Vicini, F.; Harris, J.R. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage i or ii breast cancer. J. Clin. Oncol. 1994, 12, 888–894. [Google Scholar] [CrossRef]
- Bergman-Jungestrom, M.; Wingren, S. Catechol-o-methyltransferase (comt) gene polymorphism and breast cancer risk in young women. Br. J. Cancer 2001, 85, 859–862. [Google Scholar] [CrossRef]
- Bergman-Jungestrom, M.; Gentile, M.; Lundin, A.C.; Wingren, S. Association between cyp17 gene polymorphism and risk of breast cancer in young women. Int. J. Cancer 1999, 84, 350–353. [Google Scholar] [CrossRef]
- Spurdle, A.B.; Hopper, J.L.; Dite, G.S.; Chen, X.; Cui, J.; McCredie, M.R.; Giles, G.G.; Southey, M.C.; Venter, D.J.; Easton, D.F.; et al. Cyp17 promoter polymorphism and breast cancer in australian women under age forty years. J. Natl. Cancer Inst. 2000, 92, 1674–1681. [Google Scholar] [CrossRef]
- Lundin, A.C.; Soderkvist, P.; Eriksson, B.; Bergman-Jungestrom, M.; Wingren, S. Association of breast cancer progression with a vitamin d receptor gene polymorphism. South-east sweden breast cancer group. Cancer Res. 1999, 59, 2332–2334. [Google Scholar]
- Ekbom, A.; Trichopoulos, D.; Adami, H.O.; Hsieh, C.C.; Lan, S.J. Evidence of prenatal influences on breast cancer risk. Lancet 1992, 340, 1015–1018. [Google Scholar] [CrossRef]
- Zhu, B.T.; Conney, A.H. Functional role of estrogen metabolism in target cells: Review and perspectives. Carcinogenesis 1998, 19, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Weisz, J.; Clawson, G.A.; Creveling, C.R. Biogenesis and inactivation of catecholestrogens. Adv. Pharmacol. 1998, 42, 828–833. [Google Scholar] [PubMed]
- Liehr, J.G. Dual role of oestrogens as hormones and pro-carcinogens: Tumour initiation by metabolic activation of oestrogens. Eur. J. Cancer Prev. 1997, 6, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Liehr, J.G.; Ricci, M.J. 4-hydroxylation of estrogens as marker of human mammary tumors. Proc. Natl. Acad. Sci. USA 1996, 93, 3294–3296. [Google Scholar] [CrossRef]
- Yager, J.D.; Liehr, J.G. Molecular mechanisms of estrogen carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1996, 36, 203–232. [Google Scholar] [CrossRef]
- Cavalieri, E.L.; Stack, D.E.; Devanesan, P.D.; Todorovic, R.; Dwivedy, I.; Higginbotham, S.; Johansson, S.L.; Patil, K.D.; Gross, M.L.; Gooden, J.K.; et al. Molecular origin of cancer: Catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc. Natl. Acad. Sci. USA 1997, 94, 10937–10942. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J. Oxyradicals and DNA damage. Carcinogenesis 2000, 21, 361–370. [Google Scholar] [CrossRef]
- Lachman, H.M.; Papolos, D.F.; Saito, T.; Yu, Y.M.; Szumlanski, C.L.; Weinshilboum, R.M. Human catechol-o-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 1996, 6, 243–250. [Google Scholar] [CrossRef]
- Scanlon, P.D.; Raymond, F.A.; Weinshilboum, R.M. Catechol-o-methyltransferase: Thermolabile enzyme in erythrocytes of subjects homozygous for allele for low activity. Science 1979, 203, 63–65. [Google Scholar] [CrossRef]
- Millikan, R.C.; Pittman, G.S.; Tse, C.K.; Duell, E.; Newman, B.; Savitz, D.; Moorman, P.G.; Boissy, R.J.; Bell, D.A. Catechol-o-methyltransferase and breast cancer risk. Carcinogenesis 1998, 19, 1943–1947. [Google Scholar] [CrossRef]
- Kahn, B.B.; Alquier, T.; Carling, D.; Hardie, D.G. Amp-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005, 1, 15–25. [Google Scholar] [CrossRef]
- Fu, A.; Eberhard, C.E.; Screaton, R.A. Role of ampk in pancreatic beta cell function. Mol. Cell. Endocrinol. 2013, 366, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, S.; Higurashi, T.; Nakajima, A. Ampk: Therapeutic target for diabetes and cancer prevention. Curr. Pharm. Des. 2017, 23, 3629–3644. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Saud, S.M.; Young, M.R.; Chen, G.; Hua, B. Targeting ampk for cancer prevention and treatment. Oncotarget 2015, 6, 7365–7378. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Yang, C.S.; DiPaola, R.S.; Tan, X.L. Repurposing of metformin and aspirin by targeting ampk-mtor and inflammation for pancreatic cancer prevention and treatment. Cancer Prev. Res. 2014, 7, 388–397. [Google Scholar] [CrossRef]
- Hardie, D.G. Molecular pathways: Is ampk a friend or a foe in cancer? Clin. Cancer Res. 2015, 21, 3836–3840. [Google Scholar] [CrossRef]
- Hardie, D.G. The lkb1-ampk pathway-friend or foe in cancer? Cancer Cell 2013, 23, 131–132. [Google Scholar] [CrossRef]
- Hawley, S.A.; Ross, F.A.; Gowans, G.J.; Tibarewal, P.; Leslie, N.R.; Hardie, D.G. Phosphorylation by akt within the st loop of ampk-alpha1 down-regulates its activation in tumour cells. Biochem. J. 2014, 459, 275–287. [Google Scholar] [CrossRef]
- Zheng, B.; Jeong, J.H.; Asara, J.M.; Yuan, Y.Y.; Granter, S.R.; Chin, L.; Cantley, L.C. Oncogenic b-raf negatively regulates the tumor suppressor lkb1 to promote melanoma cell proliferation. Mol. Cell 2009, 33, 237–247. [Google Scholar] [CrossRef]
- Jones, R.G.; Plas, D.R.; Kubek, S.; Buzzai, M.; Mu, J.; Xu, Y.; Birnbaum, M.J.; Thompson, C.B. Amp-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell 2005, 18, 283–293. [Google Scholar] [CrossRef]
- Jeon, S.M.; Chandel, N.S.; Hay, N. Ampk regulates nadph homeostasis to promote tumour cell survival during energy stress. Nature 2012, 485, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Zadra, G.; Batista, J.L.; Loda, M. Dissecting the dual role of ampk in cancer: From experimental to human studies. Mol. Cancer Res. 2015, 13, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- LeBleu, V.S.; O’Connell, J.T.; Gonzalez Herrera, K.N.; Wikman, H.; Pantel, K.; Haigis, M.C.; Carvalho, F.M.; Damascena, A.; Domingos Chinen, L.T.; Rocha, R.M. Pgc-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nature Cell Biol. 2014, 16, 992–1003. [Google Scholar] [CrossRef]
- Chaube, B.; Malvi, P.; Singh, S.V.; Mohammad, N.; Viollet, B.; Bhat, M.K. Ampk maintains energy homeostasis and survival in cancer cells via regulating p38/pgc-1alpha-mediated mitochondrial biogenesis. Cell Death Discov. 2015, 1, 15063. [Google Scholar] [CrossRef] [PubMed]
- Winder, W.W.; Holmes, B.F.; Rubink, D.S.; Jensen, E.B.; Chen, M.; Holloszy, J.O. Activation of amp-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J. Appl. Physiol. (1985) 2000, 88, 2219–2226. [Google Scholar] [CrossRef]
- Bergeron, R.; Ren, J.M.; Cadman, K.S.; Moore, I.K.; Perret, P.; Pypaert, M.; Young, L.H.; Semenkovich, C.F.; Shulman, G.I. Chronic activation of amp kinase results in nrf-1 activation and mitochondrial biogenesis. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1340–E1346. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, D.; Yuan, X.; Liu, Y.; Guo, X.; Li, J.; Song, J. Glucocorticoid receptor-irs-1 axis controls emt and the metastasis of breast cancers. J. Mol. Cell Biol. 2019, 11, 1042–1055. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, T.N.; Knight, J.K.; Goodwin, J.E. The glucocorticoid receptor in cardiovascular health and disease. Cells 2019, 8, 1227. [Google Scholar] [CrossRef]
- Harris, D.; Barts, A.; Connors, J.; Dahl, M.; Elliott, T.; Kong, J.; Keane, T.; Thompson, D.; Stafford, S.; Ur, E.; et al. Glucocorticoid-induced hyperglycemia is prevalent and unpredictable for patients undergoing cancer therapy: An observational cohort study. Curr. Oncol. 2013, 20, e532–e538. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Morita, M.; Hirao, T.; Yamaguchi, M.; Shiratori, R.; Kikuya, M.; Chibana, H.; Ito, K. Shift in energy metabolism caused by glucocorticoids enhances the effect of cytotoxic anti-cancer drugs against acute lymphoblastic leukemia cells. Oncotarget 2017, 8, 94271–94285. [Google Scholar] [CrossRef][Green Version]
- Pan, D.; Kocherginsky, M.; Conzen, S.D. Activation of the glucocorticoid receptor is associated with poor prognosis in estrogen receptor-negative breast cancer. Cancer Res. 2011, 71, 6360–6370. [Google Scholar] [CrossRef]
- Hummel, K.P.; Dickie, M.M.; Coleman, D.L. Diabetes, a new mutation in the mouse. Science 1966, 153, 1127–1128. [Google Scholar] [CrossRef]
- Bauerle, K.T.; Harris, C. Glucocorticoids and diabetes. Mo. Med. 2016, 113, 378–383. [Google Scholar] [PubMed]
- Delaunay, F.; Khan, A.; Cintra, A.; Davani, B.; Ling, Z.C.; Andersson, A.; Ostenson, C.G.; Gustafsson, J.; Efendic, S.; Okret, S. Pancreatic beta cells are important targets for the diabetogenic effects of glucocorticoids. J. Clin. Investig. 1997, 100, 2094–2098. [Google Scholar] [CrossRef]
- Fine, N.H.F.; Doig, C.L.; Elhassan, Y.S.; Vierra, N.C.; Marchetti, P.; Bugliani, M.; Nano, R.; Piemonti, L.; Rutter, G.A.; Jacobson, D.A.; et al. Glucocorticoids reprogram beta-cell signaling to preserve insulin secretion. Diabetes 2018, 67, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Opherk, C.; Tronche, F.; Kellendonk, C.; Kohlmuller, D.; Schulze, A.; Schmid, W.; Schutz, G. Inactivation of the glucocorticoid receptor in hepatocytes leads to fasting hypoglycemia and ameliorates hyperglycemia in streptozotocin-induced diabetes mellitus. Mol. Endocrinol. 2004, 18, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Roh, H.C.; Kumari, M.; Rosen, E.D. Adipocyte glucocorticoid receptor is important in lipolysis and insulin resistance due to exogenous steroids, but not insulin resistance caused by high fat feeding. Mol. Metab. 2017, 6, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Burckart, G.J. Nuclear factor kappa b: Important transcription factor and therapeutic target. J. Clin. Pharmacol. 1998, 38, 981–993. [Google Scholar] [CrossRef]
- Longui, C.A. Glucocorticoid therapy: Minimizing side effects. J. Pediatr. (Rio J.) 2007, 83, S163–S177. [Google Scholar] [CrossRef]
- Goodwin, J.E.; Feng, Y.; Velazquez, H.; Sessa, W.C. Endothelial glucocorticoid receptor is required for protection against sepsis. Proc. Natl. Acad. Sci. USA 2013, 110, 306–311. [Google Scholar] [CrossRef]
- Goodwin, J.E.; Zhang, X.; Rotllan, N.; Feng, Y.; Zhou, H.; Fernandez-Hernando, C.; Yu, J.; Sessa, W.C. Endothelial glucocorticoid receptor suppresses atherogenesis-brief report. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 779–782. [Google Scholar] [CrossRef] [PubMed]
- King, E.M.; Holden, N.S.; Gong, W.; Rider, C.F.; Newton, R. Inhibition of nf-kappab-dependent transcription by mkp-1: Transcriptional repression by glucocorticoids occurring via p38 mapk. J. Biol. Chem. 2009, 284, 26803–26815. [Google Scholar] [CrossRef] [PubMed]
- Meduri, G.U.; Muthiah, M.P.; Carratu, P.; Eltorky, M.; Chrousos, G.P. Nuclear factor-kappab- and glucocorticoid receptor alpha- mediated mechanisms in the regulation of systemic and pulmonary inflammation during sepsis and acute respiratory distress syndrome. Evidence for inflammation-induced target tissue resistance to glucocorticoids. Neuroimmunomodulation 2005, 12, 321–338. [Google Scholar]
- Qian, S.; Golubnitschaja, O.; Zhan, X. Chronic inflammation: Key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. EPMA J. 2019, 10, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhan, X. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA J. 2018, 9, 77–102. [Google Scholar] [CrossRef]
- Janssens, J.P.; Schuster, K.; Voss, A. Preventive, predictive, and personalized medicine for effective and affordable cancer care. EPMA J. 2018, 9, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Avishai, E.; Yeghiazaryan, K.; Golubnitschaja, O. Impaired wound healing: Facts and hypotheses for multi-professional considerations in predictive, preventive and personalised medicine. EPMA J. 2017, 8, 23–33. [Google Scholar] [CrossRef]
- Colak, S.; Ten Dijke, P. Targeting tgf-beta signaling in cancer. Trends Cancer 2017, 3, 56–71. [Google Scholar] [CrossRef]
- Ma, X.; Cui, Z.; Du, Z.; Lin, H. Transforming growth factor-beta signaling, a potential mechanism associated with diabetes mellitus and pancreatic cancer? J. Cell. Physiol. 2020. [Google Scholar] [CrossRef]
- Kim, B.G.; Li, C.; Qiao, W.; Mamura, M.; Kasprzak, B.; Anver, M.; Wolfraim, L.; Hong, S.; Mushinski, E.; Potter, M.; et al. Smad4 signalling in t cells is required for suppression of gastrointestinal cancer. Nature 2006, 441, 1015–1019. [Google Scholar] [CrossRef]
- Yingling, J.M.; Blanchard, K.L.; Sawyer, J.S. Development of tgf-beta signalling inhibitors for cancer therapy. Nat. Rev. Drug Discov. 2004, 3, 1011–1022. [Google Scholar] [CrossRef]
- Zou, J.; Luo, H.; Zeng, Q.; Dong, Z.; Wu, D.; Liu, L. Protein kinase ck2alpha is overexpressed in colorectal cancer and modulates cell proliferation and invasion via regulating emt-related genes. J. Transl. Med. 2011, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Bhola, N.E.; Balko, J.M.; Dugger, T.C.; Kuba, M.G.; Sanchez, V.; Sanders, M.; Stanford, J.; Cook, R.S.; Arteaga, C.L. Tgf-beta inhibition enhances chemotherapy action against triple-negative breast cancer. J. Clin. Investig. 2013, 123, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Singh, H.; Satyanarayana, M.; Srivastava, S.P.; Tiwari, P.; Singh, A.B.; Dwivedi, A.K.; Singh, S.K.; Srivastava, M.; Nath, C.; et al. Flavone-based novel antidiabetic and antidyslipidemic agents. J. Med. Chem. 2012, 55, 4551–4567. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Srivastava, S.P.; Srivastava, D.S.; Srivastava, A.K.; Haq, W.; Katti, S.B. Thiazolidin-4-one and thiazinan-4-one derivatives analogous to rosiglitazone as potential antihyperglycemic and antidyslipidemic agents. Eur. J. Med. Chem. 2013, 63, 611–620. [Google Scholar] [CrossRef]
- Arha, D.; Pandeti, S.; Mishra, A.; Srivastava, S.P.; Srivastava, A.K.; Narender, T.; Tamrakar, A.K. Deoxyandrographolide promotes glucose uptake through glucose transporter-4 translocation to plasma membrane in l6 myotubes and exerts antihyperglycemic effect in vivo. Eur. J. Pharmacol. 2015, 768, 207–216. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.; Tripathi, V.D.; Maurya, R.A.; Srivastava, S.P.; Bhatia, G.; Tamrakar, A.K.; Srivastava, A.K. Design and synthesis of 2,4-disubstituted polyhydroquinolines as prospective antihyperglycemic and lipid modulating agents. Bioorg. Med. Chem. 2010, 18, 4138–4148. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.; Gupta, L.P.; Ahmad, P.; Srivastava, S.P.; Rahuja, N.; Tamrakar, A.K.; Srivastava, A.K. Synthesis of propiophenone derivatives as new class of antidiabetic agents reducing body weight in db/db mice. Bioorg. Med. Chem. 2012, 20, 2172–2179. [Google Scholar] [CrossRef]
- Balaramnavar, V.M.; Srivastava, R.; Rahuja, N.; Gupta, S.; Rawat, A.K.; Varshney, S.; Chandasana, H.; Chhonker, Y.S.; Doharey, P.K.; Kumar, S.; et al. Identification of novel ptp1b inhibitors by pharmacophore based virtual screening, scaffold hopping and docking. Eur. J. Med. Chem. 2014, 87, 578–594. [Google Scholar] [CrossRef]
- Jaiswal, N.; Bhatia, V.; Srivastava, S.P.; Srivastava, A.K.; Tamrakar, A.K. Antidiabetic effect of eclipta alba associated with the inhibition of alpha-glucosidase and aldose reductase. Nat. Prod. Res. 2012, 26, 2363–2367. [Google Scholar] [CrossRef]
- Mishra, A.; Srivastava, R.; Srivastava, S.P.; Gautam, S.; Tamrakar, A.K.; Maurya, R.; Srivastava, A.K. Antidiabetic activity of heart wood of pterocarpus marsupium roxb. And analysis of phytoconstituents. Indian J. Exp. Biol. 2013, 51, 363–374. [Google Scholar] [PubMed]
- Srivastava, S.P.; Mishra, A.; Bhatia, V.; Narender, T.; Srivastava, A.K. Acacia catechu hard wood: Potential anti-diabetic cum anti-dyslipidemic. Med. Chem. Res. 2010, 20, 1732–1739. [Google Scholar] [CrossRef]
- Srivsatava, R.; Srivastava, S.P.; Jaiswal, N.; Mishra, A.; Maurya, R.; Srivastava, A.K. Antidiabetic and antidyslipidemic activities of cuminum cyminum l. In validated animal models. Med. Chem. Res. 2010, 20, 1656–1666. [Google Scholar] [CrossRef]
- Bhatia, V.; Srivastava, S.P.; Srivastava, R.; Mishra, A.; Narender, T.; Maurya, R.; Srivastava, A.K. Antihyperglycaemic and aldose reductase inhibitory potential of acacia catechu hard wood and tectona grandis leaves. Med. Chem. Res. 2010, 20, 1724–1731. [Google Scholar] [CrossRef]
- Jaiswal, N. Inhibition of alpha-glucosidase by acacia nilotica prevents hyperglycemia along with improvement of diabetic complications via aldose reductase inhibition. J. Diabetes Metab. 2013. [Google Scholar] [CrossRef]
- Shukla, P.; Srivastava, S.P.; Srivastava, R.; Rawat, A.K.; Srivastava, A.K.; Pratap, R. Synthesis and antidyslipidemic activity of chalcone fibrates. Bioorg. Med. Chem. Lett. 2011, 21, 3475–3478. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, S.P.; Goodwin, J.E. Cancer Biology and Prevention in Diabetes. Cells 2020, 9, 1380. https://doi.org/10.3390/cells9061380
Srivastava SP, Goodwin JE. Cancer Biology and Prevention in Diabetes. Cells. 2020; 9(6):1380. https://doi.org/10.3390/cells9061380
Chicago/Turabian StyleSrivastava, Swayam Prakash, and Julie E. Goodwin. 2020. "Cancer Biology and Prevention in Diabetes" Cells 9, no. 6: 1380. https://doi.org/10.3390/cells9061380
APA StyleSrivastava, S. P., & Goodwin, J. E. (2020). Cancer Biology and Prevention in Diabetes. Cells, 9(6), 1380. https://doi.org/10.3390/cells9061380




