Elusive Roles of the Different Ceramidases in Human Health, Pathophysiology, and Tissue Regeneration
Abstract
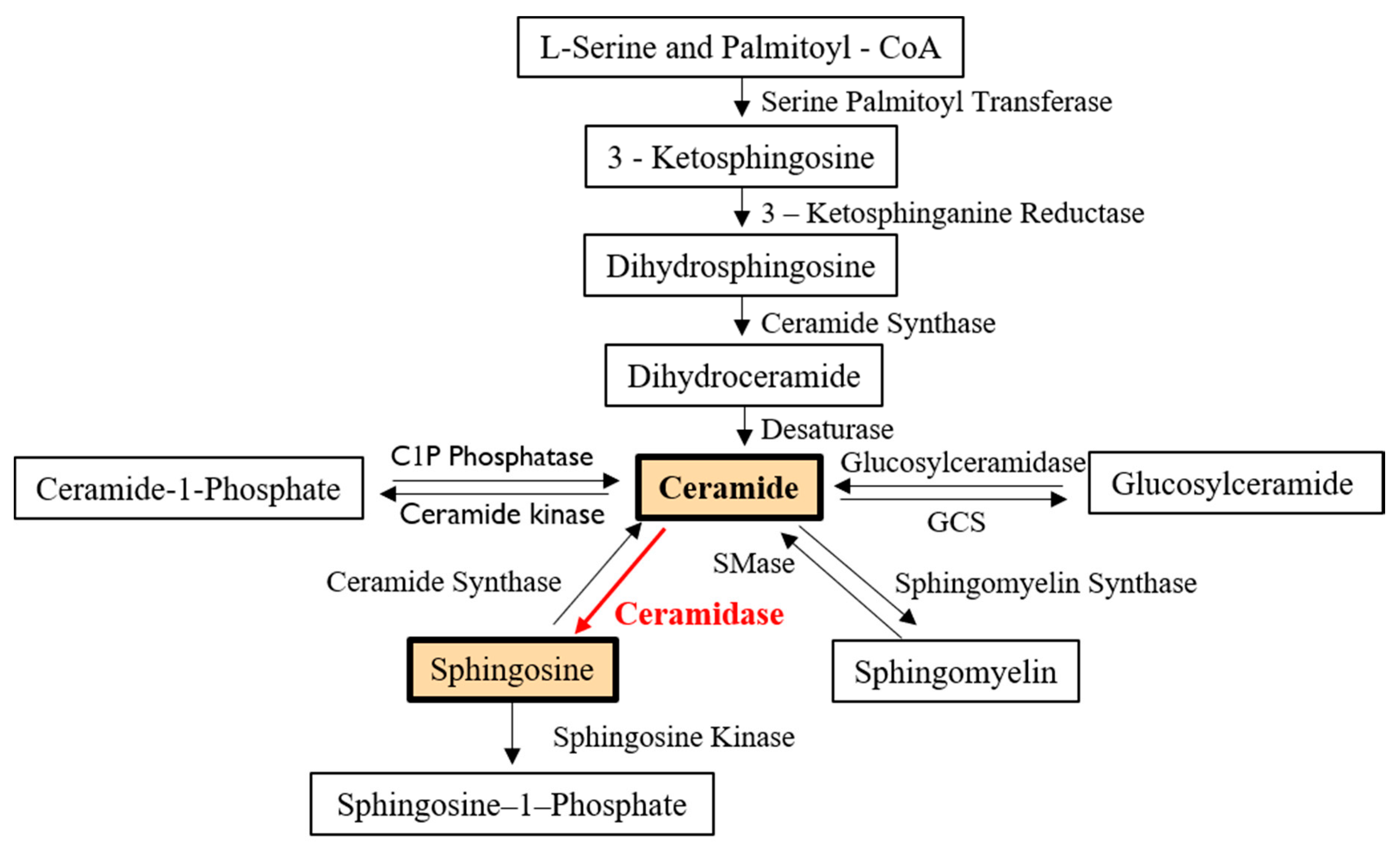
1. Introduction
2. Characteristics and Regulatory Pathways of Ceramidases
2.1. General Characteristics
2.1.1. Acid Ceramidase
2.1.2. Neutral Ceramidase
2.1.3. Alkaline Ceramidase
2.2. Regulatory Pathways
2.2.1. Acid Ceramidase
2.2.2. Neutral Ceramidase
2.2.3. Alkaline Ceramidase
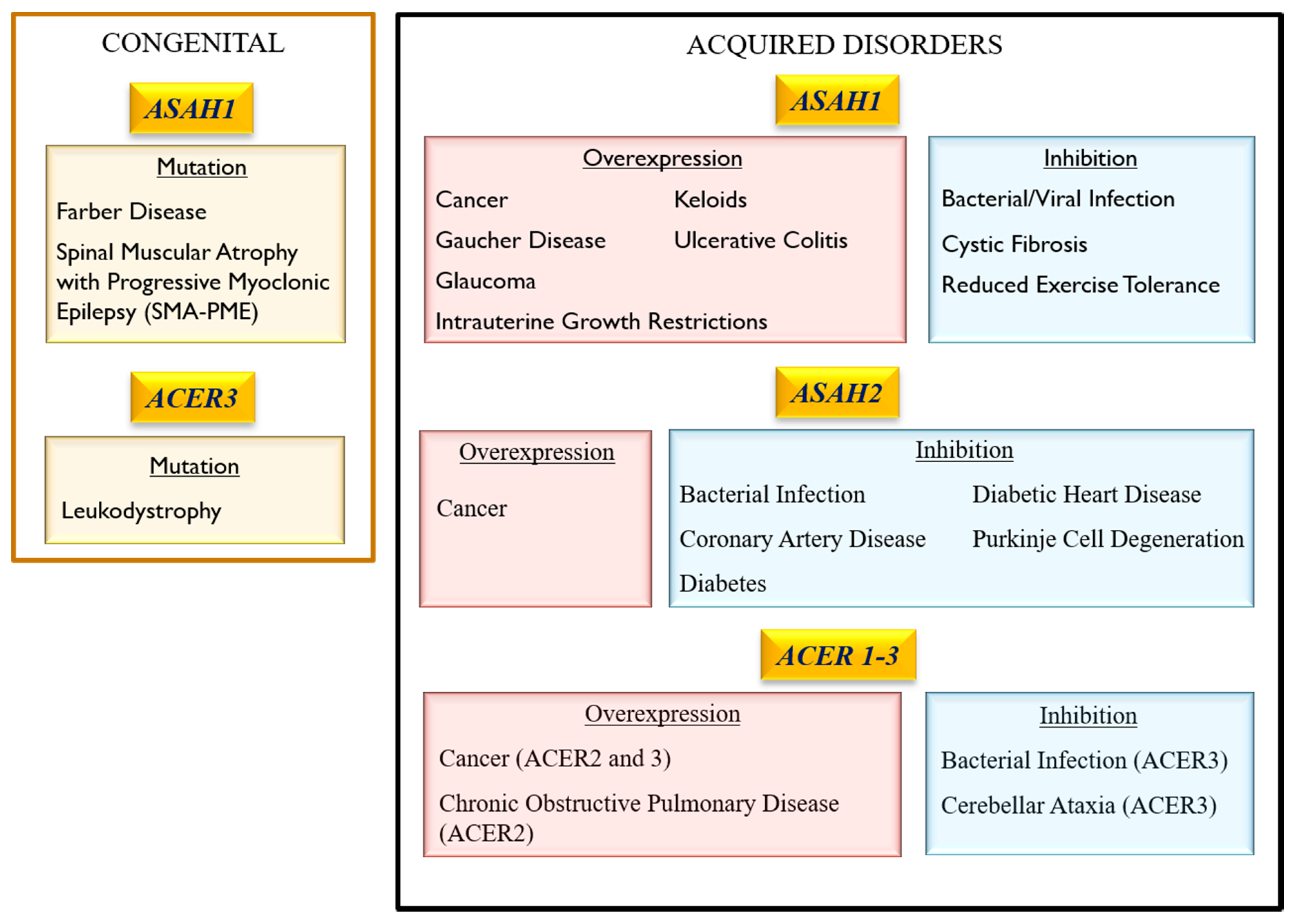
3. Association of Ceramidase Gene Mutations with Human Inheritable Diseases
3.1. Acid Ceramidase
3.1.1. Farber Lipogranulomatosis (FRBRL)
3.1.2. Spinal Muscular Atrophy with Progressive Myoclonic Epilepsy (SMA-PME)
3.1.3. Intrauterine Growth Restrictions (IUGR)
3.1.4. Krabbe Disease
3.2. Neutral ceramidase
3.3. Alkaline Ceramidase
Progressive Leukodystrophy
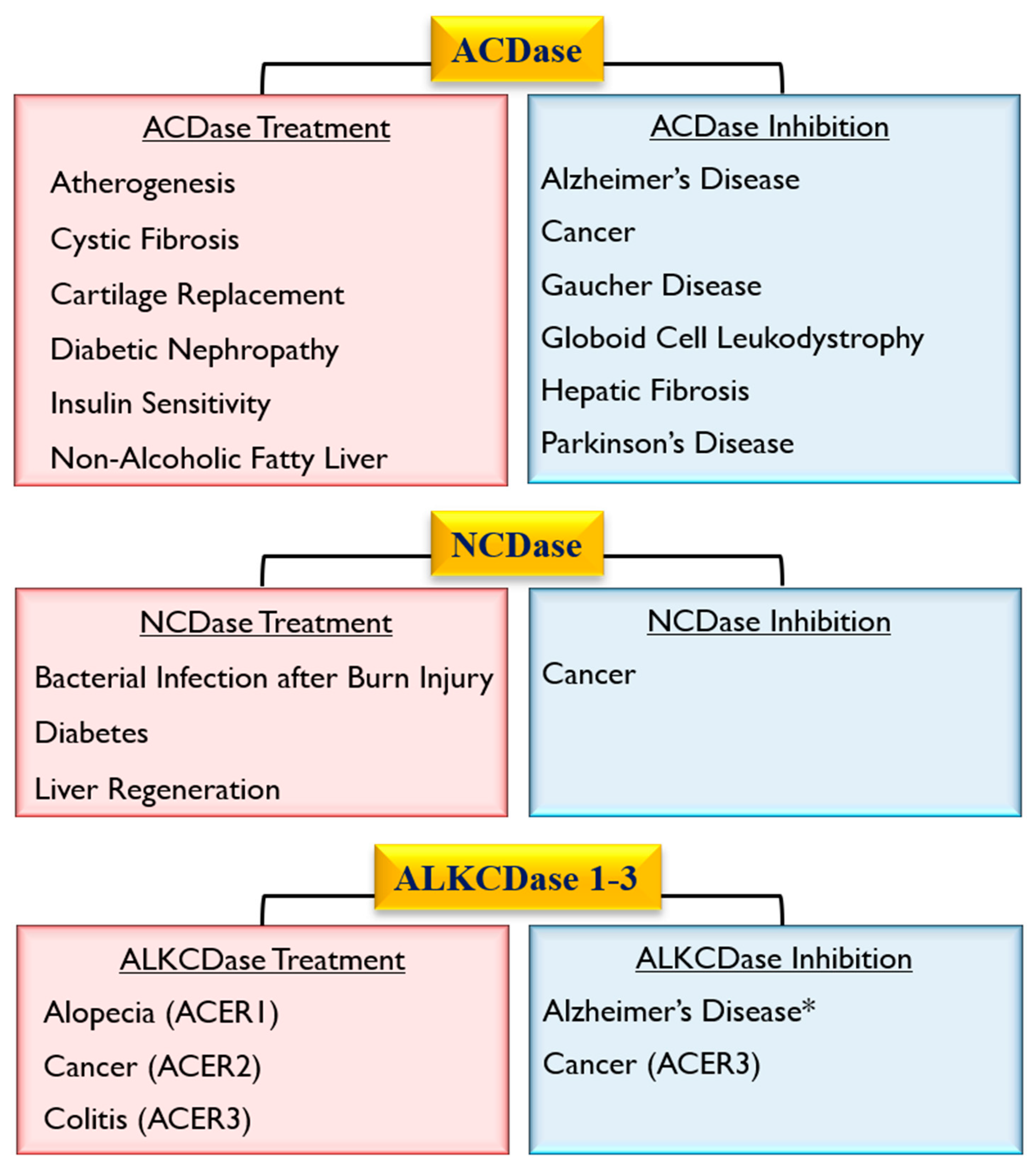
4. Role of Ceramidase Activity in Human Non-heritable Diseases
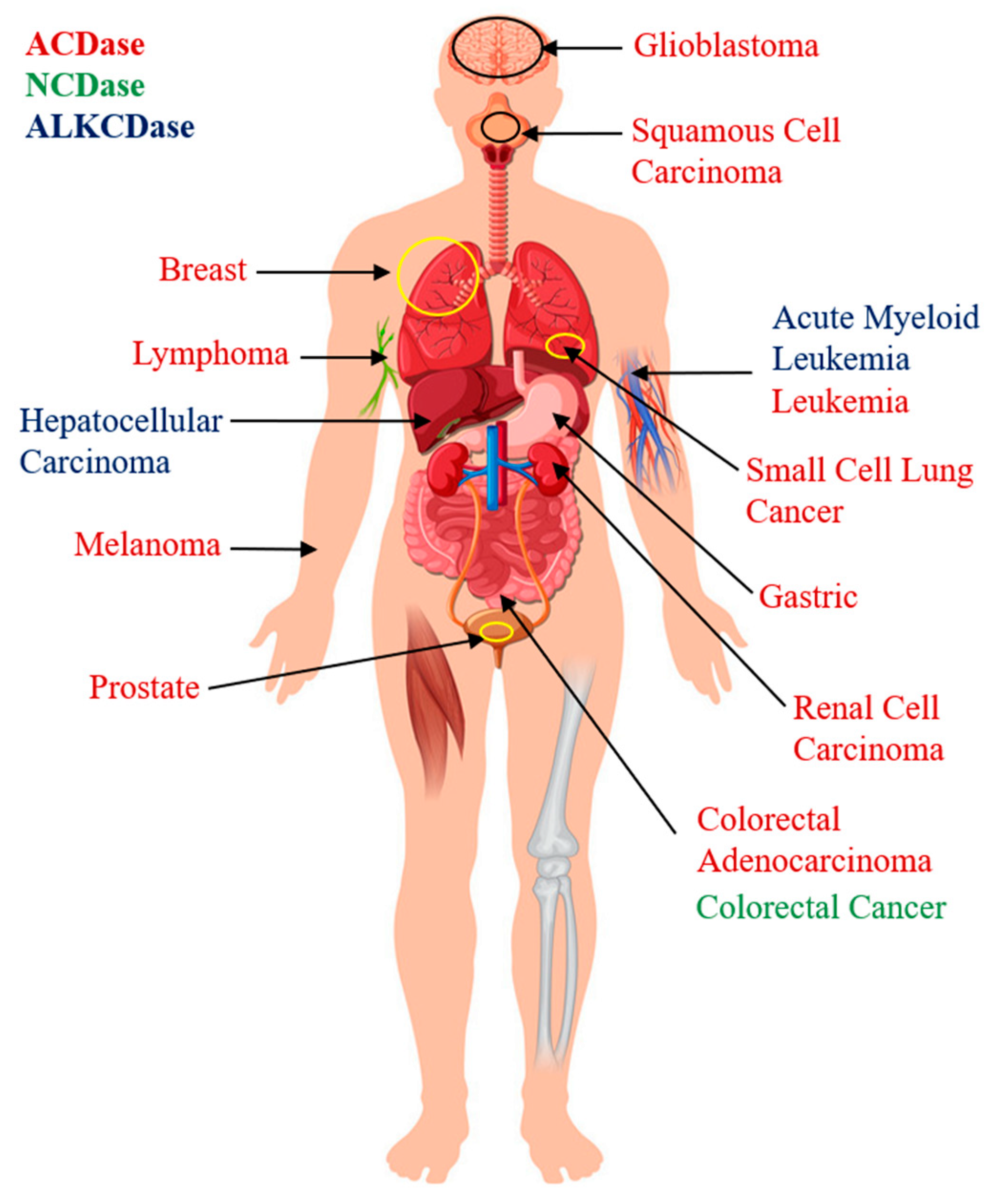
4.1. Role of Ceramidases in Cancer Pathology
4.1.1. Acid Ceramidase
4.1.2. Neutral Ceramidase
4.1.3. Alkaline Ceramidase
4.2. Role of Ceramidases in the Onset of Age-Related Neurodegenerative Diseases
Acid Ceramidase
4.3. Role of Ceramidases in Cardio-Pulmonary Disease
4.3.1. Acid Ceramidase
4.3.2. Neutral Ceramidase
4.3.3. Alkaline Ceramidase
4.4. Role of Ceramidases in Metabolic Disorders
4.4.1. Acid Ceramidase
4.4.2. Neutral Ceramidase
4.4.3. Alkaline Ceramidase
5. Role of Ceramidase Activity in Infectious Diseases
5.1. Role of Ceramidase Activity in Bacterial Infection
5.1.1. Acid Ceramidase
5.1.2. Neutral Ceramidase
5.1.3. Alkaline Ceramidase
5.2. Role of Ceramidase Activity in Viral Infection
6. Role of Ceramidases in Tissue Regeneration and Healing
6.1. Acid Ceramidase
6.2. Neutral Ceramidase
6.3. Alkaline Ceramidase
7. Conclusions
Funding
Conflicts of Interest
References
- Bartke, N.; Hannun, Y.A. Bioactive sphingolipids: Metabolism and function. J. Lipid Res. 2008, 50, S91–S96. [Google Scholar] [CrossRef] [PubMed]
- Książek, M.; Chacińska, M.; Chabowski, A.; Baranowski, M. Sources, metabolism, and regulation of circulating sphingosine-1-phosphate. J. Lipid Res. 2015, 56, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Coant, N.; Sakamoto, W.; Mao, C.; Hannun, Y.A. Ceramidases, roles in sphingolipid metabolism and in health and disease. Adv. Boil. Regul. 2016, 63, 122–131. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Dworski, S.; Zhu, C.; DeAngelis, V.; Solyom, A.; Medin, J.A.; Simonaro, C.M.; Schuchman, E.H. Enzyme replacement therapy for Farber disease: Proof-of-concept studies in cells and mice. BBA Clin. 2017, 7, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Gebai, A.; Gorelik, A.; Li, Z.; Illes, K.; Nagar, B. Structural basis for the activation of acid ceramidase. Nat. Commun. 2018, 9, 1621. [Google Scholar] [CrossRef]
- Mao, C.; Obeid, L.M. Ceramidases: Regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochim. et Biophys. Acta (BBA) - Bioenerg. 2008, 1781, 424–434. [Google Scholar] [CrossRef]
- Houben, E.; Holleran, W.M.; Yaginuma, T.; Mao, C.; Obeid, L.M.; Rogiers, V.; Takagi, Y.; Elias, P.M.; Uchida, Y. Differentiation-associated expression of ceramidase isoforms in cultured keratinocytes and epidermis. J. Lipid Res. 2006, 47, 1063–1070. [Google Scholar] [CrossRef]
- Sakamoto, W.; Coant, N.; Canals, D.; Obeid, L.M.; Hannun, Y.A. Functions of neutral ceramidase in the Golgi apparatus. J. Lipid Res. 2018, 59, 2116–2125. [Google Scholar] [CrossRef]
- Casasampere, M.; Camacho, L.; Cingolani, F.; Casas, J.; Egido-Gabás, M.; Abad, J.L.; Bedia, C.; Xu, R.; Wang, K.; Canals, D.; et al. Activity of neutral and alkaline ceramidases on fluorogenicN-acylated coumarin-containing aminodiols. J. Lipid Res. 2015, 56, 2019–2028. [Google Scholar] [CrossRef]
- Tan, S.-F.; Liu, X.; Fox, T.E.; Barth, B.M.; Sharma, A.; Turner, S.D.; Awwad, A.; Dewey, A.; Doi, K.; Spitzer, B.; et al. Acid ceramidase is upregulated in AML and represents a novel therapeutic target. Oncotarget 2016, 7, 83208–83222. [Google Scholar] [CrossRef]
- Hu, W.; Xu, R.; Sun, W.; Szulc, Z.M.; Bielawski, J.; Obeid, L.M.; Mao, C. Alkaline Ceramidase 3 (ACER3) Hydrolyzes Unsaturated Long-chain Ceramides, and Its Down-regulation Inhibits Both Cell Proliferation and Apoptosis*. J. Boil. Chem. 2010, 285, 7964–7976. [Google Scholar] [CrossRef] [PubMed]
- Liakath-Ali, K.; Vancollie, V.; Lelliott, C.J.; Speak, A.O.; Lafont, D.; Protheroe, H.J.; Ingvorsen, C.; Galli, A.; Green, A.; Gleeson, D.; et al. Alkaline ceramidase 1 is essential for mammalian skin homeostasis and regulating whole-body energy expenditure. J. Pathol. 2016, 239, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, L.E. Use of Liposome Preparation to Treat Mycobacterial Infections. Immunobiol. 1994, 191, 578–583. [Google Scholar] [CrossRef]
- Lin, C.-L.; Xu, R.; Yi, J.K.; Li, F.; Chen, J.; Jones, E.C.; Slutsky, J.B.; Huang, L.; Rigas, B.; Cao, J.; et al. Alkaline Ceramidase 1 Protects Mice from Premature Hair Loss by Maintaining the Homeostasis of Hair Follicle Stem Cells. Stem Cell Rep. 2017, 9, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, K.; Mileva, I.; Hannun, Y.A.; Obeid, L.M.; Mao, C. Alkaline ceramidase 2 and its bioactive product sphingosine are novel regulators of the DNA damage response. Oncotarget 2016, 7, 18440–18457. [Google Scholar] [CrossRef]
- Vasiliauskaite-Brooks, I.; Healey, R.D.; Rochaix, P.; Saint-Paul, J.; Sounier, R.; Grison, C.; Waltrich-Augusto, T.; Fortier, M.; Hoh, F.; Saied, E.M.; et al. Structure of a human intramembrane ceramidase explains enzymatic dysfunction found in leukodystrophy. Nat. Commun. 2018, 9, 5437. [Google Scholar] [CrossRef]
- Boojar, M.M.A.; Boojar, M.M.A.; Golmohammad, S.; Bahrehbar, I. Data on cell survival, apoptosis, ceramide metabolism and oxidative stress in A-494 renal cell carcinoma cell line treated with hesperetin and hesperetin-7-O-acetate. Data Brief. 2018, 20, 596–601. [Google Scholar] [CrossRef]
- Kurokawa, H.; Ito, H.; Matsui, H. Monascus purpureus induced apoptosis on gastric cancer cell by scavenging mitochondrial reactive oxygen species. J. Clin. Biochem. Nutr. 2017, 61, 189–195. [Google Scholar] [CrossRef]
- Liu, F.; Li, X.; Lu, C.; Bai, A.; Bielawski, J.; Bielawska, A.; Marshall, B.; Schoenlein, P.V.; Lebedyeva, I.O.; Liu, K. Ceramide activates lysosomal cathepsin B and cathepsin D to attenuate autophagy and induces ER stress to suppress myeloid-derived suppressor cells. Oncotarget 2016, 7, 83907–83925. [Google Scholar] [CrossRef]
- Boojar, M.M.A.; Hassanipour, M.; Mehr, S.E.; Boojar, M.M.A.; Dehpour, A.R. New Aspects of Silibinin Stereoisomers and their 3-O-galloyl Derivatives on Cytotoxicity and Ceramide Metabolism in Hep G2 hepatocarcinoma Cell Line. Iran. J. Pharm. Res. IJPR 2016, 15, 421–433. [Google Scholar]
- Rahman, A.; Pallichankandy, S.; Thayyullathil, F.; Galadari, S. Critical role of H2O2 in mediating sanguinarine-induced apoptosis in prostate cancer cells via facilitating ceramide generation, ERK1/2 phosphorylation, and Par-4 cleavage. Free. Radic. Boil. Med. 2019, 134, 527–544. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.-L.; Park, J.Y.; Kim, E.H.; Jang, H.J.; Information, P.E.K.F.C. Targeting acid ceramidase sensitises head and neck cancer to cisplatin. Eur. J. Cancer 2016, 52, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, S.; Yoshitomi, T.; Sakharkar, M.K.; Nagasaki, Y. Redox nanoparticles inhibit curcumin oxidative degradation and enhance its therapeutic effect on prostate cancer. J. Control. Release 2015, 209, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Ozer, M.Y.; Oztopcu-Vatan, P.; Kus, G. The investigation of ceranib-2 on apoptosis and drug interaction with carboplatin in human non small cell lung cancer cells in vitro. Cytotechnology 2017, 70, 387–396. [Google Scholar] [CrossRef]
- Yu, F.P.S.; Molino, S.; Sikora, J.; Rasmussen, S.; Rybova, J.; Tate, E.; Geurts, A.M.; Turner, P.V.; McKillop, W.M.; Medin, J.A. Hepatic pathology and altered gene transcription in a murine model of acid ceramidase deficiency. Lab. Investig. 2019, 99, 1572–1592. [Google Scholar] [CrossRef]
- Tirodkar, T.S.; Lu, P.; Bai, A.; Scheffel, M.J.; Gencer, S.; Garrett-Mayer, E.; Bielawska, A.; Ogretmen, B.; Voelkel-Johnson, C. Expression of Ceramide Synthase 6 Transcriptionally Activates Acid Ceramidase in a c-Jun N-terminal Kinase (JNK)-dependent Manner. J. Boil. Chem. 2015, 290, 13157–13167. [Google Scholar] [CrossRef]
- Signoretto, E.; Zierle, J.; Bhuyan, A.A.M.; Castagna, M.; Lang, F. Ceranib-2-induced suicidal erythrocyte death. Cell Biochem. Funct. 2016, 34, 359–366. [Google Scholar] [CrossRef]
- Sugano, E.; Edwards, G.; Saha, S.; Wilmott, L.A.; Grambergs, R.C.; Mondal, K.; Qi, H.; Stiles, M.; Tomita, H.; Mandal, N. Overexpression of acid ceramidase (ASAH1) protects retinal cells (ARPE19) from oxidative stress. J. Lipid Res. 2018, 60, 30–43. [Google Scholar] [CrossRef]
- Braun, F.; Rinschen, M.M.; Bartels, V.; Frommolt, P.; Habermann, B.; Hoeijmakers, J.H.; Schumacher, B.; Dollé, M.E.; Müller, R.-U.; Benzing, T.; et al. Altered lipid metabolism in the aging kidney identified by three layered omic analysis. Aging 2016, 8, 441–454. [Google Scholar] [CrossRef]
- Airola, M.V.; Allen, W.J.; Pulkoski-Gross, M.J.; Obeid, L.M.; Rizzo, R.C.; Hannun, Y.A. Structural basis for ceramide recognition and hydrolysis by human Neutral Ceramidase. Structure 2015, 23, 1482–1491. [Google Scholar] [CrossRef]
- Coant, N.; García-Barros, M.; Zhang, Q.; Obeid, L.M.; Hannun, Y.A. AKT as a key target for growth promoting functions of neutral ceramidase in colon cancer cells. Oncogene 2018, 37, 3852–3863. [Google Scholar] [CrossRef] [PubMed]
- García-Barros, M.; Coant, N.; Kawamori, T.; Wada, M.; Snider, A.J.; Truman, J.-P.; Wu, B.X.; Furuya, H.; Clarke, C.J.; Bialkowska, A.B.; et al. Role of neutral ceramidase in colon cancer. FASEB J. 2016, 30, 4159–4171. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.E.; Katunaric, B.; Hockenberry, J.C.; Gutterman, D.D.; Freed, J.K. Manipulation of the Sphingolipid Rheostat Influences the Mediator of Flow-Induced Dilation in the Human Microvasculature. J. Am. Hear. Assoc. 2019, 8, e013153. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, K.; Mather, A.R.; Marimuthu, S.; Shah, P.P.; Snider, A.J.; Obeid, L.M.; Hannun, Y.A.; Beverly, L.J.; Siskind, L.J. Loss of neutral ceramidase protects cells from nutrient- and energy -deprivation-induced cell death. Biochem. J. 2016, 473, 743–755. [Google Scholar] [CrossRef]
- Coant, N.; Hannun, Y.A. Neutral ceramidase: Advances in mechanisms, cell regulation, and roles in cancer. Adv. Boil. Regul. 2019, 71, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Okino, N.; Tani, M. New insight into the structure, reaction mechanism, and biological functions of neutral ceramidase. Biochim. et Biophys. Acta (BBA) - Mol. Cell Boil. Lipids 2014, 1841, 682–691. [Google Scholar] [CrossRef]
- Sun, W.; Xu, R.; Hu, W.; Jin, J.; Crellin, H.A.; Bielawski, J.; Szulc, Z.M.; Thiers, B.H.; Obeid, L.M.; Mao, C. Upregulation of the Human Alkaline Ceramidase 1 and Acid Ceramidase Mediates Calcium-Induced Differentiation of Epidermal Keratinocytes. J. Investig. Dermatol. 2008, 128, 389–397. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Jin, Y.; Wang; He, Q.; Liu, Z.; Ai, Q.; Lei, Y.; Li, Y.; Song, F.; et al. Alkaline ceramidase 2 is a novel direct target of p53 and induces autophagy and apoptosis through ROS generation. Sci. Rep. 2017, 7, 44573. [Google Scholar] [CrossRef]
- Xu, R.; Garcia-Barros, M.; Wen, S.; Li, F.; Lin, C.-L.; Hannun, Y.A.; Obeid, L.M.; Mao, C. Tumor suppressor p53 links ceramide metabolism to DNA damage response through alkaline ceramidase 2. Cell Death Differ. 2017, 25, 841–856. [Google Scholar] [CrossRef]
- Chen, C.; Yin, Y.; Li, C.; Chen, J.; Xie, J.; Lu, Z.; Li, M.; Wang, Y.; Zhang, C.C. ACER3 supports development of acute myeloid leukemia. Biochem. Biophys. Res. Commun. 2016, 478, 33–38. [Google Scholar] [CrossRef]
- Yin, Y.; Xu, M.; Gao, J.; Li, M. Alkaline ceramidase 3 promotes growth of hepatocellular carcinoma cells via regulating S1P/S1PR2/PI3K/AKT signaling. Pathol. Res. Pract. 2018, 214, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Sikora, J.; Dworski, S.; Jones, E.E.; Kamani, M.A.; Micsenyi, M.C.; Sawada, T.; Le Faouder, P.; Bertrand-Michel, J.; Dupuy, A.; Dunn, C.K.; et al. Acid Ceramidase Deficiency in Mice Results in a Broad Range of Central Nervous System Abnormalities. Am. J. Pathol. 2017, 187, 864–883. [Google Scholar] [CrossRef] [PubMed]
- Dworski, S.; Berger, A.; Furlonger, C.; Moreau, J.M.; Yoshimitsu, M.; Trentadue, J.; Au, B.C.; Paige, C.J.; A Medin, J. Markedly perturbed hematopoiesis in acid ceramidase deficient mice. Haematologica 2015, 100, e162–e165. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.P.S.; Islam, D.; Sikora, J.; Dworski, S.; Gurka’, J.; Lopez-Vasquez, L.; Liu, M.; Kuebler, W.M.; Levade, T.; Zhang, H.; et al. Chronic lung injury and impaired pulmonary function in a mouse model of acid ceramidase deficiency. Am. J. Physiol. Cell. Mol. Physiol. 2018, 314, L406–L420. [Google Scholar] [CrossRef]
- Dworski, S.; Lu, P.; Khan, A.; Maranda, B.; Mitchell, J.; Parini, R.; Di Rocco, M.; Hugle, B.; Yoshimitsu, M.; Magnusson, B.; et al. Acid Ceramidase Deficiency is characterized by a unique plasma cytokine and ceramide profile that is altered by therapy. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2016, 1863, 386–394. [Google Scholar] [CrossRef]
- Yu, F.P.S.; Dworski, S.; Medin, J.A. Deletion of MCP-1 Impedes Pathogenesis of Acid Ceramidase Deficiency. Sci. Rep. 2018, 8, 1808. [Google Scholar] [CrossRef]
- Gan, J.J.; Garcia, V.; Tian, J.; Tagliati, M.; Parisi, J.E.; Chung, J.M.; Lewis, R.; Baloh, R.; Levade, T.; Pierson, T.M. Acid ceramidase deficiency associated with spinal muscular atrophy with progressive myoclonic epilepsy. Neuromuscul. Disord. 2015, 25, 959–963. [Google Scholar] [CrossRef]
- Rubboli, G.; Veggiotti, P.; Pini, A.; Berardinelli, A.; Cantalupo, G.; Bertini, E.; Tiziano, D.F.; D’Amico, A.; Piazza, E.; Abiusi, E.; et al. Spinal muscular atrophy associated with progressive myoclonic epilepsy: A rare condition caused by mutations in ASAH1. Epilepsia 2015, 56, 692–698. [Google Scholar] [CrossRef]
- Chauvin, S.; Yinon, Y.; Xu, J.; Ermini, L.; Sallais, J.; Tagliaferro, A.; Todros, T.; Post, M.; Caniggia, I. Aberrant TGFbeta Signalling Contributes to Dysregulation of Sphingolipid Metabolism in Intrauterine Growth Restriction. J. Clin. Endocrinol. Metab. 2015, 100, E986–E996. [Google Scholar] [CrossRef]
- Tappino, B.; Biancheri, R.; Mort, M.; Regis, S.; Corsolini, F.; Rossi, A.; Stroppiano, M.; Lualdi, S.; Fiumara, A.; Bembi, B.; et al. Identification and characterization of 15 novel GALC gene mutations causing Krabbe disease. Hum. Mutat. 2010, 31, E1894–E1915. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Benitez, B.A.; Nagree, M.S.; Dearborn, J.T.; Jiang, X.; Guzman, M.A.; Woloszynek, J.C.; Giaramita, A.; Yip, B.K.; et al. Genetic ablation of acid ceramidase in Krabbe disease confirms the psychosine hypothesis and identifies a new therapeutic target. Proc. Natl. Acad. Sci. USA 2019, 116, 20097–20103. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Dreier, J.L.; Ellis, J.M.; Allende, M.L.; Kalkofen, D.N.; Sanders, K.M.; Bielawski, J.; Bielawska, A.; Hannun, Y.A.; Proia, R.L. Neutral Ceramidase Encoded by theAsah2Gene Is Essential for the Intestinal Degradation of Sphingolipids. J. Boil. Chem. 2005, 281, 7324–7331. [Google Scholar] [CrossRef] [PubMed]
- Edvardson, S.; Yi, J.K.; Jalas, C.; Xu, R.; Webb, B.D.; Snider, J.; Fedick, A.; Kleinman, E.; Treff, N.R.; Mao, C.; et al. Deficiency of the alkaline ceramidase ACER3 manifests in early childhood by progressive leukodystrophy. J. Med. Genet. 2016, 53, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Parveen, F.; Bender, D.; Law, S.-H.; Mishra, V.K.; Chen, C.-C.; Ke, L. Role of Ceramidases in Sphingolipid Metabolism and Human Diseases. Cells 2019, 8, 1573. [Google Scholar] [CrossRef]
- Doan, N.; Nguyen, H.S.; Montoure, A.; Al-Gizawiy, M.M.; Mueller, W.M.; Kurpad, S.; Rand, S.D.; Connelly, J.M.; Chitambar, C.R.; Schmainda, K.M.; et al. Acid ceramidase is a novel drug target for pediatric brain tumors. Oncotarget 2017, 8, 24753–24761. [Google Scholar] [CrossRef] [PubMed]
- Doan, N.; Nguyen, H.S.; Al-Gizawiy, M.M.; Mueller, W.M.; Sabbadini, R.A.; Rand, S.D.; Connelly, J.M.; Chitambar, C.R.; Schmainda, K.; Mirza, S.P. Acid ceramidase confers radioresistance to glioblastoma cells. Oncol. Rep. 2017, 38, 1932–1940. [Google Scholar] [CrossRef]
- Doan, N.B.; Alhajala, H.; Al-Gizawiy, M.M.; Mueller, W.M.; Rand, S.D.; Connelly, J.M.; Cochran, E.J.; Chitambar, C.R.; Clark, P.; Kuo, J.; et al. Acid ceramidase and its inhibitors: A de novo drug target and a new class of drugs for killing glioblastoma cancer stem cells with high efficiency. Oncotarget 2017, 8, 112662–112674. [Google Scholar] [CrossRef]
- Klobucar, M.; Grbcic, P.; Pavelic, S.K.; Jonjic, N.; Visentin, S.; Sedic, M. Acid ceramidase inhibition sensitizes human colon cancer cells to oxaliplatin through downregulation of transglutaminase 2 and beta1 integrin/FAK-mediated signalling. Biochem. Biophys. Res. Commun. 2018, 503, 843–848. [Google Scholar] [CrossRef]
- Sänger, N.; Ruckhäberle, E.; Győrffy, B.; Engels, K.; Heinrich, T.; Fehm, T.; Graf, A.; Holtrich, U.; Becker, S.; Karn, T. Acid ceramidase is associated with an improved prognosis in both DCIS and invasive breast cancer. Mol. Oncol. 2014, 9, 58–67. [Google Scholar] [CrossRef]
- Mizutani, N.; Inoue, M.; Omori, Y.; Ito, H.; Tamiya-Koizumi, K.; Takagi, A.; Kojima, T.; Nakamura, M.; Iwaki, S.; Nakatochi, M.; et al. Increased acid ceramidase expression depends on upregulation of androgen-dependent deubiquitinases, USP2, in a human prostate cancer cell line, LNCaP. J. Biochem. 2015, 158, 309–319. [Google Scholar] [CrossRef]
- Bowden, D.; Sutton, P.A.; Wall, M.; Jithesh, P.V.; Jenkins, R.; Palmer, D.; Goldring, C.; Parsons, J.L.; Park, B.K.; Kitteringham, N.; et al. Proteomic profiling of rectal cancer reveals acid ceramidase is implicated in radiation response. J. Proteom. 2018, 179, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Realini, N.; Palese, F.; Pizzirani, D.; Pontis, S.; Basit, A.; Bach, A.; Ganesan, A.; Piomelli, D. Acid Ceramidase in Melanoma: Expression, Localization, and Effects of Pharmacological Inhibition. J. Biol. Chem. 2016, 291, 2422–2434. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, J.; Garandeau, D.; Pandiani, C.; Gaudel, C.; Bille, K.; Nottet, N.; Garcia, V.; Colosetti, P.; Pagnotta, S.; Bahadoran, P.; et al. Lysosomal acid ceramidase ASAH1 controls the transition between invasive and proliferative phenotype in melanoma cells. Oncogene 2018, 38, 1282–1295. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.-F.; Dunton, W.; Liu, X.; Fox, T.E.; Morad, S.A.F.; Desai, D.; Doi, K.; Conaway, M.R.; Amin, S.; Claxton, D.F.; et al. Acid ceramidase promotes drug resistance in acute myeloid leukemia through NF-κB-dependent P-glycoprotein upregulation. J. Lipid Res. 2019, 60, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- White-Gilbertson, S.; Lu, P.; Norris, J.S.; Voelkel-Johnson, C. Genetic and pharmacological inhibition of acid ceramidase prevents asymmetric cell division by neosis. J. Lipid Res. 2019, 60, 1225–1235. [Google Scholar] [CrossRef]
- Cho, S.M.; Lee, H.K.; Liu, Q.; Wang, M.-W.; Kwon, H.J. A Guanidine-Based Synthetic Compound Suppresses Angiogenesis via Inhibition of Acid Ceramidase. ACS Chem. Boil. 2018, 14, 11–19. [Google Scholar] [CrossRef]
- Espaillat, M.P.; Snider, A.J.; Qiu, Z.; Channer, B.; Coant, N.; Schuchman, E.H.; Kew, R.R.; Sheridan, B.S.; A Hannun, Y.; Obeid, L.M. Loss of acid ceramidase in myeloid cells suppresses intestinal neutrophil recruitment. FASEB J. 2017, 32, 2339–2353. [Google Scholar] [CrossRef]
- Lai, M.; Realini, N.; La Ferla, M.; Passalacqua, I.; Matteoli, G.; Ganesan, A.; Pistello, M.; Mazzanti, C.M.; Piomelli, D. Complete Acid Ceramidase ablation prevents cancer-initiating cell formation in melanoma cells. Sci. Rep. 2017, 7, 7411. [Google Scholar] [CrossRef]
- Bai, A.; Bielawska, A.; Rahmaniyan, M.; Kraveka, J.M.; Bielawski, J.; Hannun, Y.A. Dose dependent actions of LCL521 on acid ceramidase and key sphingolipid metabolites. Bioorganic Med. Chem. 2018, 26, 6067–6075. [Google Scholar] [CrossRef]
- Bai, A.; Mao, C.; Jenkins, R.W.; Szulc, Z.M.; Bielawska, A.; Hannun, Y.A. Anticancer actions of lysosomally targeted inhibitor, LCL521, of acid ceramidase. PLoS ONE 2017, 12, e0177805. [Google Scholar] [CrossRef]
- Baspinar, M.; Ozyurt, R.; Kus, G.; Kutlay, O.; Ozkurt, M.; Erkasap, N.; Kabadere, S.; Yasar, N.F.; Erkasap, S. Effects of ceranib-2 on cell survival and TNF-alpha in colon cancer cell line. Bratisl. Med. J. 2017, 118, 391–393. [Google Scholar] [CrossRef]
- Vethakanraj, H.S.; Sesurajan, B.P.; Padmanaban, V.P.; Jayaprakasam, M.; Murali, S.; Sekar, A.K. Anticancer effect of acid ceramidase inhibitor ceranib-2 in human breast cancer cell lines MCF-7, MDA MB-231 by the activation of SAPK/JNK, p38 MAPK apoptotic pathways, inhibition of the Akt pathway, downregulation of ERα. Anti-Cancer Drugs 2018, 29, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Vethakanraj, H.S.; Babu, T.A.; Sudarsanan, G.B.; Duraisamy, P.K.; Kumar, S.A. Targeting ceramide metabolic pathway induces apoptosis in human breast cancer cell lines. Biochem. Biophys. Res. Commun. 2015, 464, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Vejselova, D.; Kutlu, H.M.; Kuş, G. Examining impacts of ceranib-2 on the proliferation, morphology and ultrastructure of human breast cancer cells. Cytotechnology 2016, 68, 2721–2728. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Thayyullathil, F.; Pallichankandy, S.; Galadari, S. Hydrogen peroxide/ceramide/Akt signaling axis play a critical role in the antileukemic potential of sanguinarine. Free. Radic. Boil. Med. 2016, 96, 273–289. [Google Scholar] [CrossRef]
- Adada, M.; Canals, D.; Jeong, N.; Kelkar, A.D.; Hernandez-Corbacho, M.; Pulkoski-Gross, M.J.; Donaldson, J.C.; Hannun, Y.A.; Obeid, L.M. Intracellular sphingosine kinase 2-derived sphingosine-1-phosphate mediates epidermal growth factor-induced ezrin-radixin-moesin phosphorylation and cancer cell invasion. FASEB J. 2015, 29, 4654–4669. [Google Scholar] [CrossRef]
- Klosinski, L.P.; Yao, J.; Yin, F.; Fonteh, A.N.; Harrington, M.G.; Christensen, T.A.; Trushina, E.; Brinton, R.D. White Matter Lipids as a Ketogenic Fuel Supply in Aging Female Brain: Implications for Alzheimer’s Disease. EBioMedicine 2015, 2, 1888–1904. [Google Scholar] [CrossRef]
- Wang, K.; Xu, R.; Schrandt, J.; Shah, P.; Gong, Y.Z.; Preston, C.; Wang, L.; Yi, J.K.; Lin, C.-L.; Sun, W.; et al. Alkaline Ceramidase 3 Deficiency Results in Purkinje Cell Degeneration and Cerebellar Ataxia Due to Dyshomeostasis of Sphingolipids in the Brain. PLoS Genet. 2015, 11, e1005591. [Google Scholar] [CrossRef]
- Wimo, A.; Winblad, B.; Jönsson, L. The worldwide societal costs of dementia: Estimates for 2009. Alzheimer’s Dement. 2010, 6, 98–103. [Google Scholar] [CrossRef]
- Davis, W. The ATP-Binding Cassette Transporter-2 (ABCA2) Overexpression Modulates Sphingosine Levels and Transcription of the Amyloid Precursor Protein (APP) Gene. Curr. Alzheimer Res. 2015, 12, 847–859. [Google Scholar] [CrossRef]
- Kim, M.J.; Jeon, S.; Burbulla, L.F.; Krainc, D. Acid ceramidase inhibition ameliorates α-synuclein accumulation upon loss of GBA1 function. Hum. Mol. Genet. 2018, 27, 1972–1988. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.J.; Marques, A.R.A.; Appelman, M.D.; Verhoek, M.; Strijland, A.; Mirzaian, M.; Scheij, S.; Ouairy, C.M.; Lahav, D.; Wisse, P.; et al. Lysosomal glycosphingolipid catabolism by acid ceramidase: Formation of glycosphingoid bases during deficiency of glycosidases. FEBS Lett. 2016, 590, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.Z.; Valencia, A.-K.; Piqueras, M.C.; Enriquez-Algeciras, M.; Bhattacharya, S.K. Optic Nerve Lipidomics Reveal Impaired Glucosylsphingosine Lipids Pathway in Glaucoma. Investig. Opthalmology Vis. Sci. 2019, 60, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Huang, D.; Hong, J.; Bhat, O.M.; Yuan, X.; Li, P.-L. Control of lysosomal TRPML1 channel activity and exosome release by acid ceramidase in mouse podocytes. Am. J. Physiol. Physiol. 2019, 317, C481–C491. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xu, R.; Low, B.E.; Lin, C.-L.; García-Barros, M.; Schrandt, J.; Mileva, I.; Snider, A.; Luo, C.K.; Jiang, X.-C.; et al. Alkaline ceramidase 2 is essential for the homeostasis of plasma sphingoid bases and their phosphates. FASEB J. 2018, 32, 3058–3069. [Google Scholar] [CrossRef]
- Grassme, H.; Henry, B.; Ziobro, R.; Becker, K.A.; Riethmuller, J.; Gardner, A.; Seitz, A.P.; Steinmann, J.; Lang, S.; Ward, C.; et al. beta1-Integrin Accumulates in Cystic Fibrosis Luminal Airway Epithelial Membranes and Decreases Sphingosine, Promoting Bacterial Infections. Cell Host Microbe. 2017, 21, 707–718.e8. [Google Scholar] [CrossRef]
- Novgorodov, S.A.; Riley, C.L.; Yu, J.; Keffler, J.A.; Clarke, C.J.; Van Laer, A.O.; Baicu, C.F.; Zile, M.R.; Gudz, T.I. Lactosylceramide contributes to mitochondrial dysfunction in diabetes. J. Lipid Res. 2016, 57, 546–562. [Google Scholar] [CrossRef]
- Nedvedova, I.; Kolar, D.; Neckar, J.; Kalous, M.; Pravenec, M.; Šilhavý, J.; Korenkova, V.; Kolar, F.; Zurmanova, J. Cardioprotective Regimen of Adaptation to Chronic Hypoxia Diversely Alters Myocardial Gene Expression in SHR and SHR-mtBN Conplastic Rat Strains. Front. Endocrinol. 2019, 9. [Google Scholar] [CrossRef]
- Jeong, I.; Lim, J.-H.; Oh, D.K.; Kim, W.J.; Oh, Y.-M. Gene expression profile of human lung in a relatively early stage of COPD with emphysema. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2643–2655. [Google Scholar] [CrossRef]
- Lewis, L.S.; Huffman, K.M.; Smith, I.J.; Donahue, M.P.; Slentz, C.A.; Houmard, J.A.; Hubal, M.J.; Hoffman, E.P.; Hauser, E.R.; Siegler, I.C.; et al. Genetic Variation in Acid Ceramidase Predicts Non-completion of an Exercise Intervention. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- Weikel, K.A.; Cacicedo, J.M.; Ruderman, N.B.; Ido, Y. Glucose and palmitate uncouple AMPK from autophagy in human aortic endothelial cells. Am. J. Physiol. Cell Physiol. 2015, 308, C249–C263. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Bhat, O.M.; Li, G.; Dempsey, S.K.; Zhang, Q.; Ritter, J.K.; Li, W.; Li, P.-L. Lysosomal regulation of extracellular vesicle excretion during d-ribose-induced NLRP3 inflammasome activation in podocytes. Biochim. et Biophys. Acta (BBA) - Bioenerg. 2019, 1866, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, Q.; Hong, J.; Ritter, J.K.; Li, P.-L. Inhibition of pannexin-1 channel activity by adiponectin in podocytes: Role of acid ceramidase activation. Biochim. et Biophys. Acta (BBA) - Mol. Cell Boil. Lipids 2018, 1863, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.Y.; Holland, W.L.; Kusminski, C.M.; Sun, K.; Sharma, A.; Pearson, M.J.; Sifuentes, A.J.; McDonald, J.G.; Gordillo, R.; Scherer, P.E.; et al. Targeted Induction of Ceramide Degradation Leads to Improved Systemic Metabolism and Reduced Hepatic Steatosis. Cell Metab. 2015, 22, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Presa, N.; Clugston, R.D.; Lingrell, S.; Kelly, S.E.; Merrill, A.H.; Jana, S.; Kassiri, Z.; Gomez-Muñoz, A.; Vance, D.E.; Jacobs, R.; et al. Vitamin E alleviates non-alcoholic fatty liver disease in phosphatidylethanolamine N-methyltransferase deficient mice. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2019, 1865, 14–25. [Google Scholar] [CrossRef]
- Choi, S.R.; Lim, J.H.; Kim, M.Y.; Kim, E.N.; Kim, Y.; Choi, B.S.; Kim, Y.-S.; Kim, H.W.; Lim, K.-M.; Kim, M.J.; et al. Adiponectin receptor agonist AdipoRon decreased ceramide, and lipotoxicity, and ameliorated diabetic nephropathy. Metabolism 2018, 85, 348–360. [Google Scholar] [CrossRef]
- Luo, F.; Feng, Y.; Ma, H.; Liu, C.; Chen, G.; Wei, X.; Mao, X.; Li, X.; Xu, Y.; Tang, S.; et al. Neutral ceramidase activity inhibition is involved in palmitate-induced apoptosis in INS-1 cells. Endocr. J. 2017, 64, 767–776. [Google Scholar] [CrossRef]
- Aguirre, C.; Castillo, V.; Llanos, M. Oral Administration of the Endocannabinoid Anandamide during Lactation: Effects on Hypothalamic Cannabinoid Type 1 Receptor and Food Intake in Adult Mice. J. Nutr. Metab. 2017, 2017, 1–5. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhu, R.; Jin, J. Neutral ceramidase-enriched exosomes prevent palmitic acid-induced insulin resistance in H4IIEC3 hepatocytes. FEBS Open Bio 2016, 6, 1078–1084. [Google Scholar] [CrossRef]
- Lipinski, K.V.W.; Weske, S.; Keul, P.; Peters, S.; Baba, H.A.; Heusch, G.; Gräler, M.H.; Levkau, B. Hepatocyte nuclear factor 1A deficiency causes hemolytic anemia in mice by altering erythrocyte sphingolipid homeostasis. Blood 2017, 130, 2786–2798. [Google Scholar] [CrossRef]
- Wang, K.; Li, C.; Lin, X.; Sun, H.; Xu, R.; Li, Q.; Wei, Y.; Li, Y.; Qian, J.; Liu, C.; et al. Targeting alkaline ceramidase 3 alleviates the severity of nonalcoholic steatohepatitis by reducing oxidative stress. Cell Death Dis. 2020, 11, 28. [Google Scholar] [CrossRef]
- Keitsch, S.; Riethmüller, J.; Soddemann, M.; Sehl, C.; Wilker, B.; Edwards, M.J.; Caldwell, C.C.; Fraunholz, M.; Gulbins, E.; Becker, K.A.; et al. Pulmonary infection of cystic fibrosis mice with Staphylococcus aureus requires expression of α-toxin. Boil. Chem. 2018, 399, 1203–1213. [Google Scholar] [CrossRef]
- Azuma, M.M.; Balani, P.; Boisvert, H.; Gil, M.; Egashira, K.; Yamaguchi, T.; Hasturk, H.; Duncan, M.; Kawai, T.; Movila, A. Endogenous acid ceramidase protects epithelial cells from Porphyromonas gingivalis-induced inflammation in vitro. Biochem. Biophys. Res. Commun. 2017, 495, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.C.; Seitz, A.P.; Edwards, M.J.; Gulbins, E.; Caldwell, C.C. Frontline Science: Sphingosine rescues burn-injured mice from pulmonary Pseudomonas aeruginosa infection. J. Leukoc. Biol. 2016, 100, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xu, R.; Snider, A.J.; Schrandt, J.; Li, Y.; Bialkowska, A.B.; Li, M.; Zhou, J.; A Hannun, Y.; Obeid, L.M.; et al. Alkaline ceramidase 3 deficiency aggravates colitis and colitis-associated tumorigenesis in mice by hyperactivating the innate immune system. Cell Death Dis. 2016, 7, e2124. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, M. The ghost of pandemics past: Revisiting two centuries of influenza in Sweden. Med. Humanit. 2016, 43, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.C.; Dudas, G.; Rambaut, A.; Andersen, K.G. The evolution of Ebola virus: Insights from the 2013–2016 epidemic. Nature 2016, 538, 193–200. [Google Scholar] [CrossRef]
- Weaver, S.C.; Costa, F.; Blanco, M.A.G.; Ko, A.; Ribeiro, G.S.; Saade, G.; Shi, P.-Y.; Vasilakis, N. Zika virus: History, emergence, biology, and prospects for control. Antivir. Res. 2016, 130, 69–80. [Google Scholar] [CrossRef]
- Prompetchara, E.; Ketloy, C.; Palaga, T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020, 38, 1–9. [Google Scholar] [CrossRef]
- Nguyen, A.; Guedán, A.; Mousnier, A.; Swieboda, D.; Zhang, Q.; Horkai, D.; Le Novère, N.; Solari, R.; Wakelam, M. Host lipidome analysis during rhinovirus replication in HBECs identifies potential therapeutic targets. J. Lipid Res. 2018, 59, 1671–1684. [Google Scholar] [CrossRef]
- Grafen, A.; Schumacher, F.; Chithelen, J.; Kleuser, B.; Beyersdorf, N.; Schneider-Schaulies, J. Use of Acid Ceramidase and Sphingosine Kinase Inhibitors as Antiviral Compounds Against Measles Virus Infection of Lymphocytes in vitro. Front. Cell Dev. Boil. 2019, 7, 218. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Bohn, P.; Bhat, H.; Jastrow, H.; Walkenfort, B.; Cansiz, F.; Fink, J.; Bauer, M.; Olszewski, D.; Ramos-Nascimento, A.; et al. Acid ceramidase of macrophages traps herpes simplex virus in multivesicular bodies and protects from severe disease. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Plöhn, S.; Edelmann, B.; Japtok, L.; He, X.; Hose, M.; Hansen, W.; Schuchman, E.H.; Eckstein, A.; Berchner-Pfannschmidt, U. CD40 Enhances Sphingolipids in Orbital Fibroblasts: Potential Role of Sphingosine-1-Phosphate in Inflammatory T-Cell Migration in Graves’ Orbitopathy. Investig. Opthalmology Vis. Sci. 2018, 59, 5391–5397. [Google Scholar] [CrossRef] [PubMed]
- Santos-Cortez, R.L.P.; Hu, Y.; Sun, F.; Benahmed-Miniuk, F.; Tao, J.; Kanaujiya, J.; Ademola, S.; Fadiora, S.; Odesina, V.; A Nickerson, D.; et al. Identification of ASAH1 as a susceptibility gene for familial keloids. Eur. J. Hum. Genet. 2017, 25, 1155–1161. [Google Scholar] [CrossRef]
- Chen, J.Y.; Newcomb, B.; Zhou, C.; Pondick, J.V.; Ghoshal, S.; York, S.R.; Motola, D.L.; Coant, N.; Yi, J.K.; Mao, C.; et al. Tricyclic Antidepressants Promote Ceramide Accumulation to Regulate Collagen Production in Human Hepatic Stellate Cells. Sci. Rep. 2017, 7, 44867. [Google Scholar] [CrossRef]
- Frohbergh, M.E.; Guevara, J.; Grelsamer, R.P.; Barbe, M.F.; He, X.; Simonaro, C.M.; Schuchman, E.H. Acid ceramidase treatment enhances the outcome of autologous chondrocyte implantation in a rat osteochondral defect model. Osteoarthr. Cartil. 2015, 24, 752–762. [Google Scholar] [CrossRef]
- Bonafe, L.; Kariminejad, A.; Li, J.; Royer-Bertrand, B.; Garcia, V.; Mahdavi, S.; Bozorgmehr, B.; Lachman, R.L.; Mittaz‐Crettol, L.; Campos‐Xavier, B.; et al. Brief Report: Peripheral Osteolysis in Adults Linked to ASAH1 (Acid Ceramidase) Mutations: A New Presentation of Farber’s Disease. Arthritis Rheumatol. 2016, 68, 2323–2327. [Google Scholar] [CrossRef]
- Nojima, H.; Freeman, C.M.; Schuster, R.M.; Japtok, L.; Kleuser, B.; Edwards, M.J.; Gulbins, E.; Lentsch, A.B. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J. Hepatol. 2015, 64, 60–68. [Google Scholar] [CrossRef]
- Pethő, A.; Chen, Y.; George, A. Exosomes in Extracellular Matrix Bone Biology. Curr. Osteoporos. Rep. 2018, 16, 58–64. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, C.; Akkaoui, J.; Yamada, C.; Ho, A.; Mao, C.; Movila, A. Elusive Roles of the Different Ceramidases in Human Health, Pathophysiology, and Tissue Regeneration. Cells 2020, 9, 1379. https://doi.org/10.3390/cells9061379
Duarte C, Akkaoui J, Yamada C, Ho A, Mao C, Movila A. Elusive Roles of the Different Ceramidases in Human Health, Pathophysiology, and Tissue Regeneration. Cells. 2020; 9(6):1379. https://doi.org/10.3390/cells9061379
Chicago/Turabian StyleDuarte, Carolina, Juliet Akkaoui, Chiaki Yamada, Anny Ho, Cungui Mao, and Alexandru Movila. 2020. "Elusive Roles of the Different Ceramidases in Human Health, Pathophysiology, and Tissue Regeneration" Cells 9, no. 6: 1379. https://doi.org/10.3390/cells9061379
APA StyleDuarte, C., Akkaoui, J., Yamada, C., Ho, A., Mao, C., & Movila, A. (2020). Elusive Roles of the Different Ceramidases in Human Health, Pathophysiology, and Tissue Regeneration. Cells, 9(6), 1379. https://doi.org/10.3390/cells9061379




