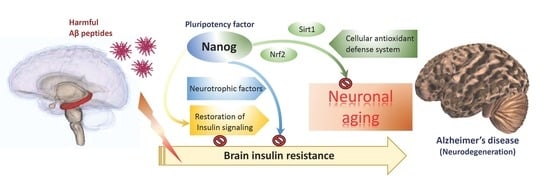
The Pluripotency Factor Nanog Protects against Neuronal Amyloid β-Induced Toxicity and Oxidative Stress through Insulin Sensitivity Restoration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture, Transfection and Viability Assay
2.3. mRNA Expression Analysis by Reverse-Transcription Quantitative PCR (qPCR)
2.4. Western Blot Analysis
2.5. DAPI Staining on Nuclei
2.6. Detection of Superoxide by Dihydroethidium (DHE) Staining
2.7. Analysis of Mitochondrial Membrane Potential by JC-1 Staining
2.8. SA-β-Galactosidase Staining
2.9. Experimental Animals
2.10. T-Maze Test and Object Recognition Behavior Tests
2.11. Statistical Analysis
3. Results
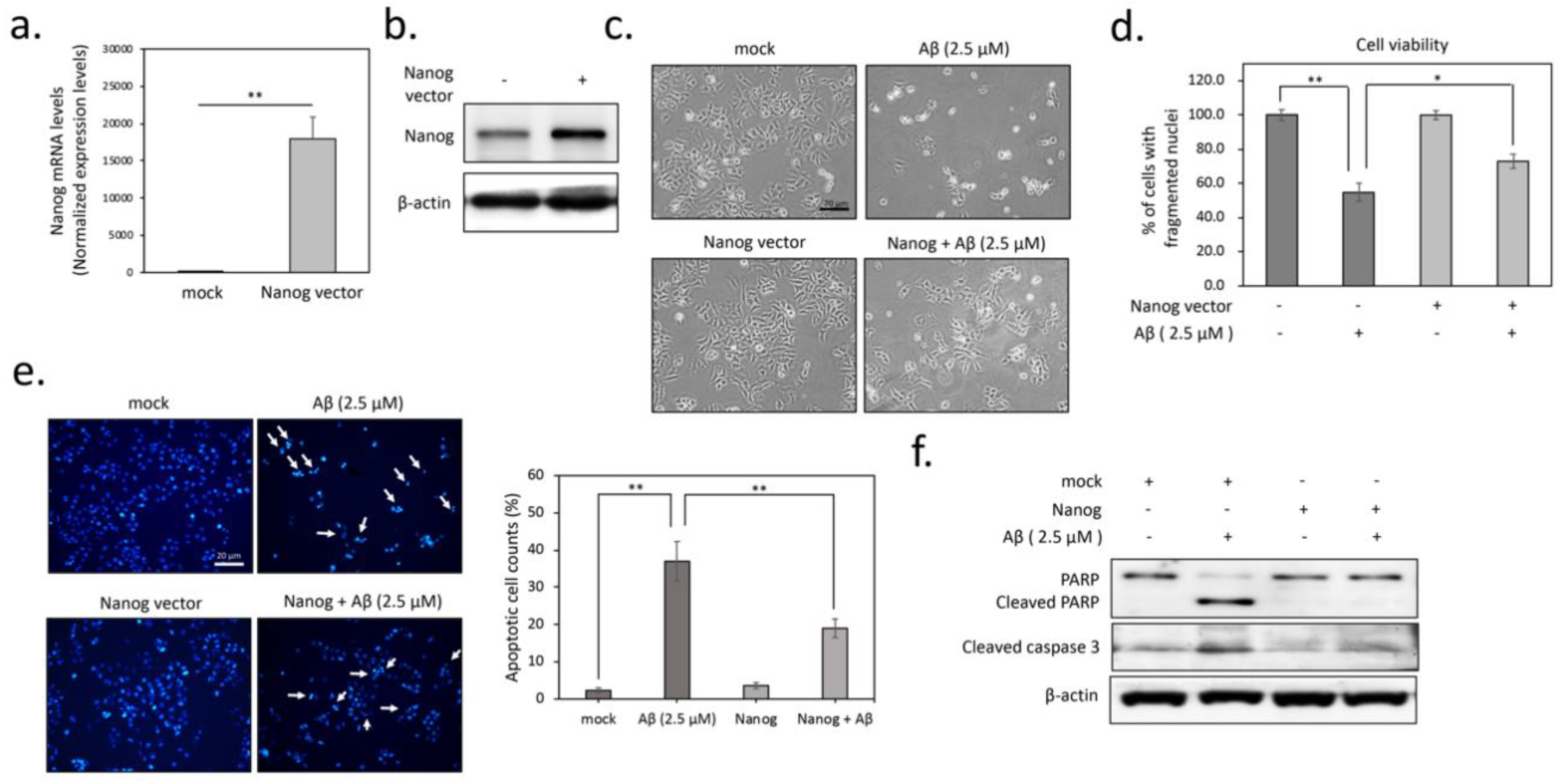
3.1. Overexpression of Nanog Significantly Reduced Aβ-Mediated Cytotoxicity
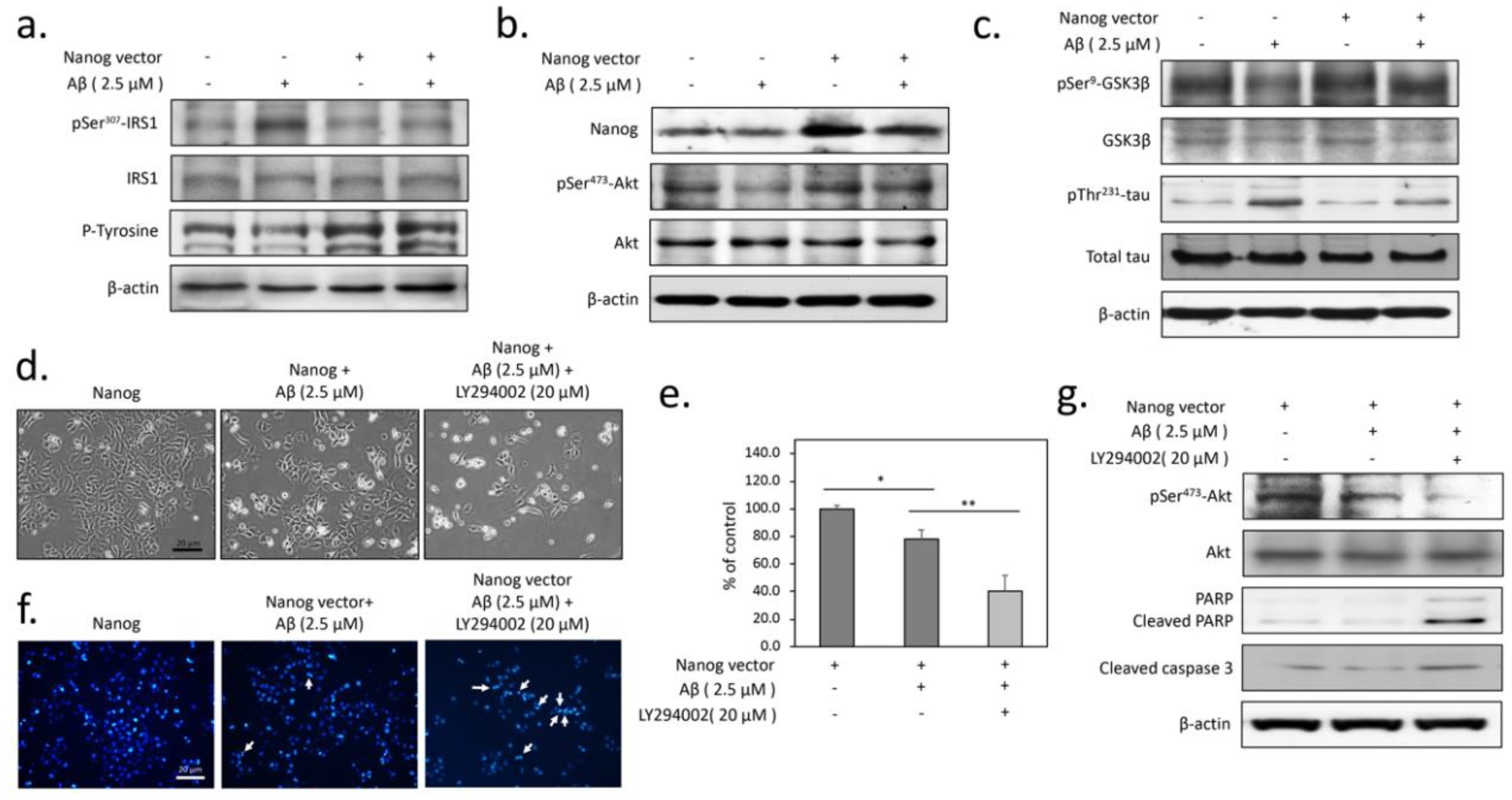
3.2. The Protection from Aβ-Induced Apoptosis by Nanog is Dependent on Insulin Signaling in SK-N-MC Neuronal Cells
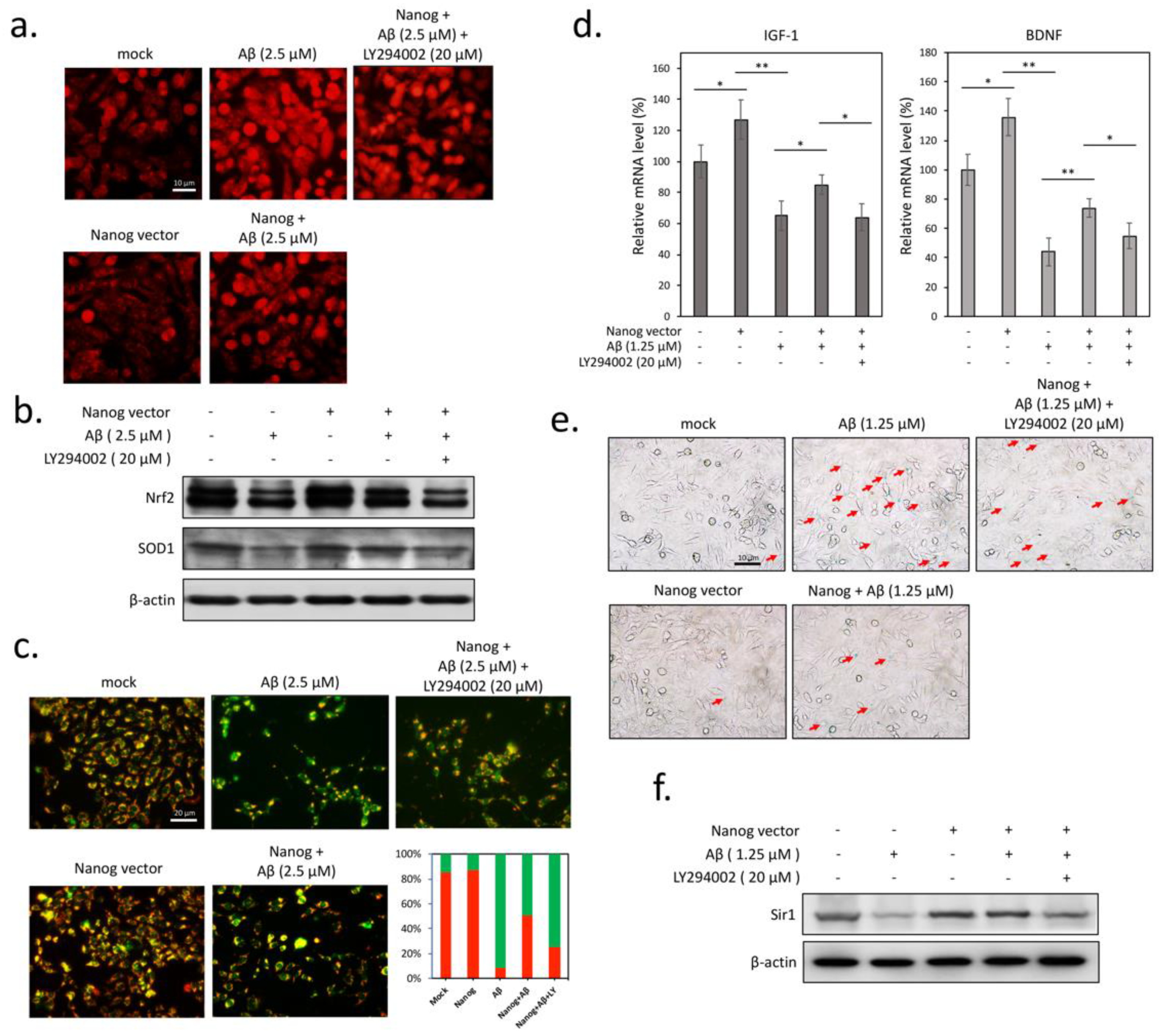
3.3. Aβ-Induced Superoxide Accumulation and Mitochondrial Dysfunction are Attenuated by Nanog Overexpression
3.4. Overexpression of Nanog Protects Rat Brain against Aβ-Induced Cognitive Impairments
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, X.Q.; Mobley, W.C. Alzheimer Disease Pathogenesis: Insights From Molecular and Cellular Biology Studies of Oligomeric Abeta and Tau Species. Front. Neurosci. 2019, 13, 659. [Google Scholar] [CrossRef]
- Lin, C.L.; Huang, W.N.; Li, H.H.; Huang, C.N.; Hsieh, S.; Lai, C.; Lu, F.J. Hydrogen-rich water attenuates amyloid beta-induced cytotoxicity through upregulation of Sirt1-FoxO3a by stimulation of AMP-activated protein kinase in SK-N-MC cells. Chem. -Biol. Interact. 2015, 240, 12–21. [Google Scholar] [CrossRef]
- Marchant, N.L.; Reed, B.R.; Sanossian, N.; Madison, C.M.; Kriger, S.; Dhada, R.; Mack, W.J.; DeCarli, C.; Weiner, M.W.; Mungas, D.M.; et al. The aging brain and cognition: Contribution of vascular injury and abeta to mild cognitive dysfunction. Jama Neurol. 2013, 70, 488–495. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, X.C.; Song, Y.; Pan, X.D.; Dai, X.M.; Zhang, J.; Cui, X.L.; Wu, X.L.; Zhu, Y.G. Amyloid beta Protein Aggravates Neuronal Senescence and Cognitive Deficits in 5XFAD Mouse Model of Alzheimer’s Disease. Chin. Med. J. 2016, 129, 1835–1844. [Google Scholar] [CrossRef]
- Mecocci, P.; Boccardi, V.; Cecchetti, R.; Bastiani, P.; Scamosci, M.; Ruggiero, C.; Baroni, M. A Long Journey into Aging, Brain Aging, and Alzheimer’s Disease Following the Oxidative Stress Tracks. J. Alzheimer’s Dis. 2018, 62, 1319–1335. [Google Scholar] [CrossRef]
- Sala Frigerio, C.; Wolfs, L.; Fattorelli, N.; Thrupp, N.; Voytyuk, I.; Schmidt, I.; Mancuso, R.; Chen, W.T.; Woodbury, M.E.; Srivastava, G.; et al. The Major Risk Factors for Alzheimer’s Disease: Age, Sex, and Genes Modulate the Microglia Response to Abeta Plaques. Cell Rep. 2019, 27, 1293–1306. [Google Scholar] [CrossRef]
- Gouras, G.K. Aging, Metabolism, Synaptic Activity, and Abeta in Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 185. [Google Scholar] [CrossRef]
- Maciel-Baron, L.A.; Moreno-Blas, D.; Morales-Rosales, S.L.; Gonzalez-Puertos, V.Y.; Lopez-Diazguerrero, N.E.; Torres, C.; Castro-Obregon, S.; Konigsberg, M. Cellular Senescence, Neurological Function, and Redox State. Antioxid. Redox Signal. 2018, 28, 1704–1723. [Google Scholar] [CrossRef]
- Fouad, G.I. Stem cells as a promising therapeutic approach for Alzheimer’s disease: A review. Bull. Natl. Res. Cent. 2019, 43, 52. [Google Scholar] [CrossRef]
- Ahmed Nel, M.; Murakami, M.; Hirose, Y.; Nakashima, M. Therapeutic Potential of Dental Pulp Stem Cell Secretome for Alzheimer’s Disease Treatment: An In Vitro Study. Stem. Cells Int. 2016, 2016, 8102478. [Google Scholar] [CrossRef]
- Griffith, C.M.; Eid, T.; Rose, G.M.; Patrylo, P.R. Evidence for altered insulin receptor signaling in Alzheimer’s disease. Neuropharmacology 2018, 136, 202–215. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Zebrowska, E.; Chabowski, A. Insulin Resistance and Oxidative Stress in the Brain: What’s New? Int. J. Mol. Sci. 2019, 20, 874. [Google Scholar] [CrossRef]
- Bedse, G.; Di Domenico, F.; Serviddio, G.; Cassano, T. Aberrant insulin signaling in Alzheimer’s disease: Current knowledge. Front. Neurosci. 2015, 9, 204. [Google Scholar] [CrossRef]
- Li, H.H.; Lin, S.L.; Huang, C.N.; Lu, F.J.; Chiu, P.Y.; Huang, W.N.; Lai, T.J.; Lin, C.L. miR-302 Attenuates Amyloid-beta-Induced Neurotoxicity through Activation of Akt Signaling. J. Alzheimer’s Dis. 2016, 50, 1083–1098. [Google Scholar] [CrossRef]
- Kuijk, E.W.; van Mil, A.; Brinkhof, B.; Penning, L.C.; Colenbrander, B.; Roelen, B.A. PTEN and TRP53 independently suppress Nanog expression in spermatogonial stem cells. Stem. Cells Dev. 2010, 19, 979–988. [Google Scholar] [CrossRef]
- Han, J.; Mistriotis, P.; Lei, P.; Wang, D.; Liu, S.; Andreadis, S.T. Nanog reverses the effects of organismal aging on mesenchymal stem cell proliferation and myogenic differentiation potential. Stem Cells 2012, 30, 2746–2759. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Chang, C.C.; Chang, T.S.; Li, H.H.; Hung, H.C.; Liu, G.Y.; Lin, C.L. Abeta stimulates microglial activation through antizyme-dependent downregulation of ornithine decarboxylase. J. Cell. Physiol. 2019, 234, 9733–9745. [Google Scholar] [CrossRef]
- Lin, C.L.; Cheng, Y.S.; Li, H.H.; Chiu, P.Y.; Chang, Y.T.; Ho, Y.J.; Lai, T.J. Amyloid-beta suppresses AMP-activated protein kinase (AMPK) signaling and contributes to alpha-synuclein-induced cytotoxicity. Exp. Neurol. 2016, 275 Pt 1, 84–98. [Google Scholar] [CrossRef]
- Huang, C.K.; Chang, Y.T.; Amstislavskaya, T.G.; Tikhonova, M.A.; Lin, C.L.; Hung, C.S.; Lai, T.J.; Ho, Y.J. Synergistic effects of ceftriaxone and erythropoietin on neuronal and behavioral deficits in an MPTP-induced animal model of Parkinson’s disease dementia. Behav. Brain. Res. 2015, 294, 198–207. [Google Scholar] [CrossRef]
- Toral-Rios, D.; Pichardo-Rojas, P.S.; Alonso-Vanegas, M.; Campos-Pena, V. GSK3beta and Tau Protein in Alzheimer’s Disease and Epilepsy. Front. Cell. Neurosci. 2020, 14, 19. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Kazantsev, A.G. The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J. 2018, 285, 3576–3590. [Google Scholar] [CrossRef]
- Chakravorty, A.; Jetto, C.T.; Manjithaya, R. Dysfunctional Mitochondria and Mitophagy as Drivers of Alzheimer’s Disease Pathogenesis. Front. Aging Neurosci. 2019, 11, 311. [Google Scholar] [CrossRef]
- Martinez-Cue, C.; Rueda, N. Cellular Senescence in Neurodegenerative Diseases. Front. Cell. Neurosci. 2020, 14, 16. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Pardo, J.; Uriarte, M.; Console, G.M.; Reggiani, P.C.; Outeiro, T.F.; Morel, G.R.; Goya, R.G. Insulin-like growth factor-I gene therapy increases hippocampal neurogenesis, astrocyte branching and improves spatial memory in female aging rats. Eur. J. Neurosci. 2016, 44, 2120–2128. [Google Scholar] [CrossRef]
- Lin, C.L.; Huang, C.N. The neuroprotective effects of the anti-diabetic drug linagliptin against Abeta-induced neurotoxicity. Neural Regen. Res. 2016, 11, 236–237. [Google Scholar] [CrossRef]
- Lee, S.H.; Zabolotny, J.M.; Huang, H.; Lee, H.; Kim, Y.B. Insulin in the nervous system and the mind: Functions in metabolism, memory, and mood. Mol. Metab. 2016, 5, 589–601. [Google Scholar] [CrossRef]
- Duarte, A.I.; Moreira, P.I.; Oliveira, C.R. Insulin in central nervous system: More than just a peripheral hormone. J. Aging Res. 2012, 2012, 384017. [Google Scholar] [CrossRef]
- Spinelli, M.; Fusco, S.; Grassi, C. Brain Insulin Resistance and Hippocampal Plasticity: Mechanisms and Biomarkers of Cognitive Decline. Front. Neurosci. 2019, 13, 788. [Google Scholar] [CrossRef]
- Rad, S.K.; Arya, A.; Karimian, H.; Madhavan, P.; Rizwan, F.; Koshy, S.; Prabhu, G. Mechanism involved in insulin resistance via accumulation of beta-amyloid and neurofibrillary tangles: Link between type 2 diabetes and Alzheimer’s disease. Drug Des. Dev. 2018, 12, 3999–4021. [Google Scholar] [CrossRef]
- Wu, C.C.; Lien, C.C.; Hou, W.H.; Chiang, P.M.; Tsai, K.J. Gain of BDNF Function in Engrafted Neural Stem Cells Promotes the Therapeutic Potential for Alzheimer’s Disease. Sci. Rep. 2016, 6, 27358. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Mohammadi, R.; Noruzi, S.; Mohamadi, Y.; Azizian, M.; Mousavy, S.M.; Ghasemi, F.; Hesari, A.; Sahebkar, A.; Salarinia, R.; et al. Stem cell- and gene-based therapies as potential candidates in Alzheimer’s therapy. J. Cell Biochem. 2018, 119, 8723–8736. [Google Scholar] [CrossRef]
- Apple, D.M.; Solano-Fonseca, R.; Kokovay, E. Neurogenesis in the aging brain. Biochem. Pharm. 2017, 141, 77–85. [Google Scholar] [CrossRef]
- Teng, C.F.; Jeng, L.B.; Shyu, W.C. Role of Insulin-like Growth Factor 1 Receptor Signaling in Stem Cell Stemness and Therapeutic Efficacy. Cell Transpl. 2018, 27, 1313–1319. [Google Scholar] [CrossRef]
- Sarubbo, F.; Moranta, D.; Pani, G. Dietary polyphenols and neurogenesis: Molecular interactions and implication for brain ageing and cognition. Neurosci. Biobehav. Rev. 2018, 90, 456–470. [Google Scholar] [CrossRef]
- Ray, S.; Corenblum, M.J.; Anandhan, A.; Reed, A.; Ortiz, F.O.; Zhang, D.D.; Barnes, C.A.; Madhavan, L. A Role for Nrf2 Expression in Defining the Aging of Hippocampal Neural Stem Cells. Cell Transpl. 2018, 27, 589–606. [Google Scholar] [CrossRef]
- Murphy, K.E.; Park, J.J. Can Co-Activation of Nrf2 and Neurotrophic Signaling Pathway Slow Alzheimer’s Disease? Int. J. Mol. Sci. 2017, 18, 1168. [Google Scholar] [CrossRef]
- Brookhouser, N.; Tekel, S.J.; Standage-Beier, K.; Nguyen, T.; Schwarz, G.; Wang, X.; Brafman, D.A. BIG-TREE: Base-Edited Isogenic hPSC Line Generation Using a Transient Reporter for Editing Enrichment. Stem Cell Rep. 2020, 14, 184–191. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-C.; Li, H.-H.; Tsou, S.-H.; Hung, H.-C.; Liu, G.-Y.; Korolenko, T.A.; Lai, T.-J.; Ho, Y.-J.; Lin, C.-L. The Pluripotency Factor Nanog Protects against Neuronal Amyloid β-Induced Toxicity and Oxidative Stress through Insulin Sensitivity Restoration. Cells 2020, 9, 1339. https://doi.org/10.3390/cells9061339
Chang C-C, Li H-H, Tsou S-H, Hung H-C, Liu G-Y, Korolenko TA, Lai T-J, Ho Y-J, Lin C-L. The Pluripotency Factor Nanog Protects against Neuronal Amyloid β-Induced Toxicity and Oxidative Stress through Insulin Sensitivity Restoration. Cells. 2020; 9(6):1339. https://doi.org/10.3390/cells9061339
Chicago/Turabian StyleChang, Ching-Chi, Hsin-Hua Li, Sing-Hua Tsou, Hui-Chih Hung, Guang-Yaw Liu, Tatiana A. Korolenko, Te-Jen Lai, Ying-Jui Ho, and Chih-Li Lin. 2020. "The Pluripotency Factor Nanog Protects against Neuronal Amyloid β-Induced Toxicity and Oxidative Stress through Insulin Sensitivity Restoration" Cells 9, no. 6: 1339. https://doi.org/10.3390/cells9061339
APA StyleChang, C.-C., Li, H.-H., Tsou, S.-H., Hung, H.-C., Liu, G.-Y., Korolenko, T. A., Lai, T.-J., Ho, Y.-J., & Lin, C.-L. (2020). The Pluripotency Factor Nanog Protects against Neuronal Amyloid β-Induced Toxicity and Oxidative Stress through Insulin Sensitivity Restoration. Cells, 9(6), 1339. https://doi.org/10.3390/cells9061339