HLA-DPB1 Reactive T Cell Receptors for Adoptive Immunotherapy in Allogeneic Stem Cell Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Primary Material
2.2. TCR Sequences and Cloning
2.3. Flow Cytometry and Antibodies
2.4. Retroviral Transduction and RNA Electroporation of T Cells
2.5. In Vitro Assays of TCR Modified T Cells
2.6. AML Xenograft Mouse Model
2.7. Statistical Analysis
3. Results
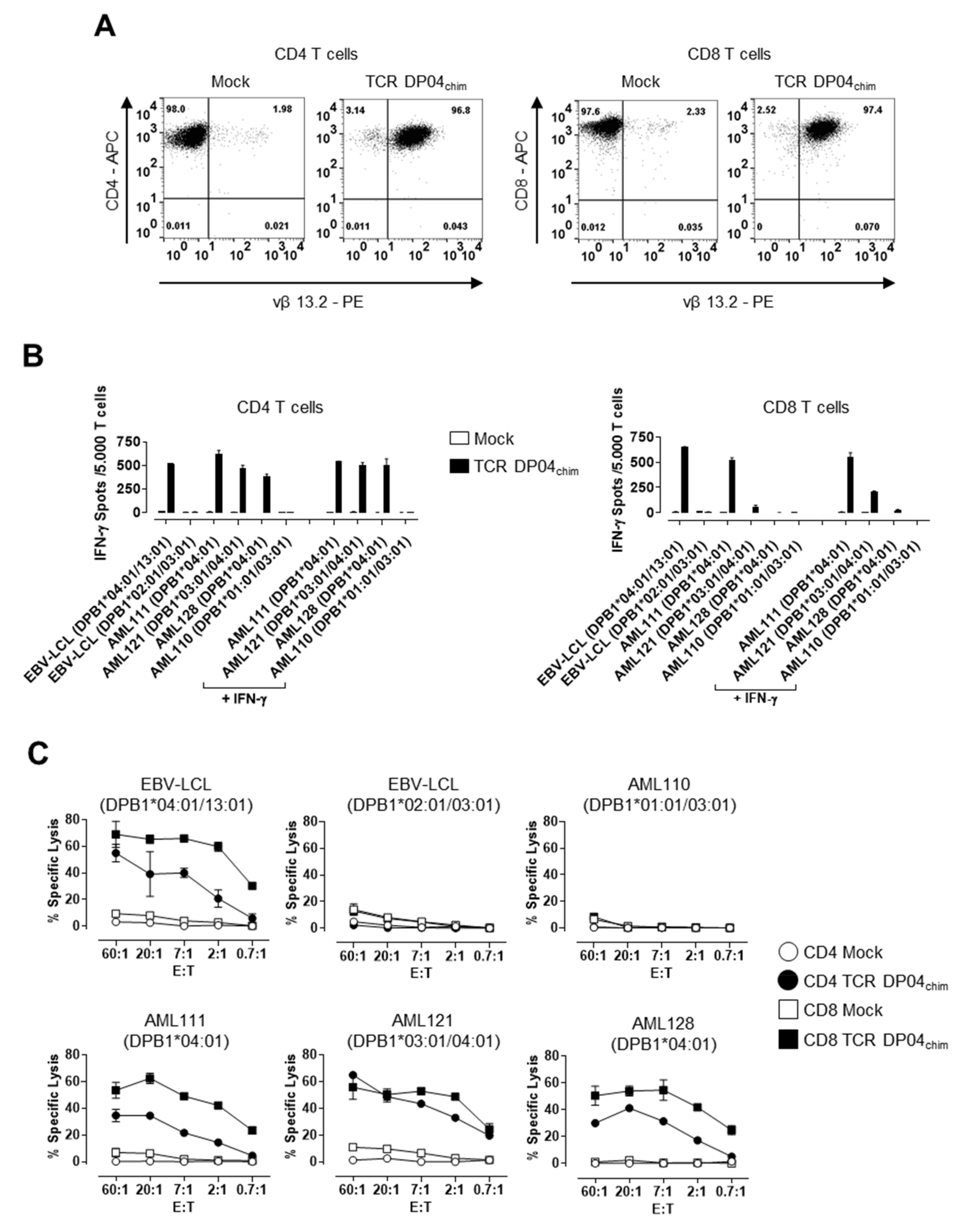
3.1. TCR DP04 Triggers Specific Recognition and Lysis of AML Blasts by CD4 and CD8 T Cells
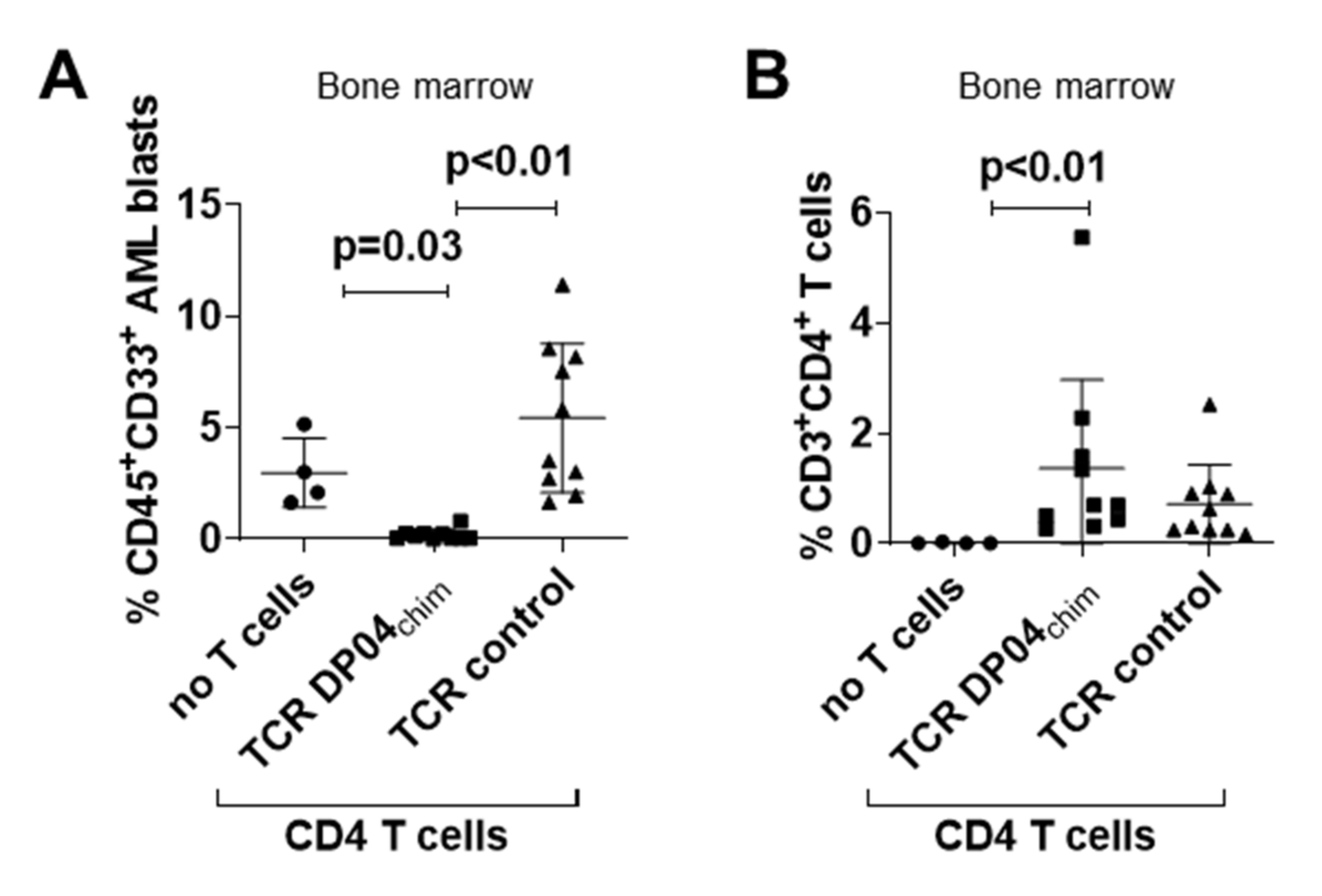
3.2. TCR DP04chim Modified CD4 T Cells Effectively Eliminate Human AML Blasts in NSG Mice
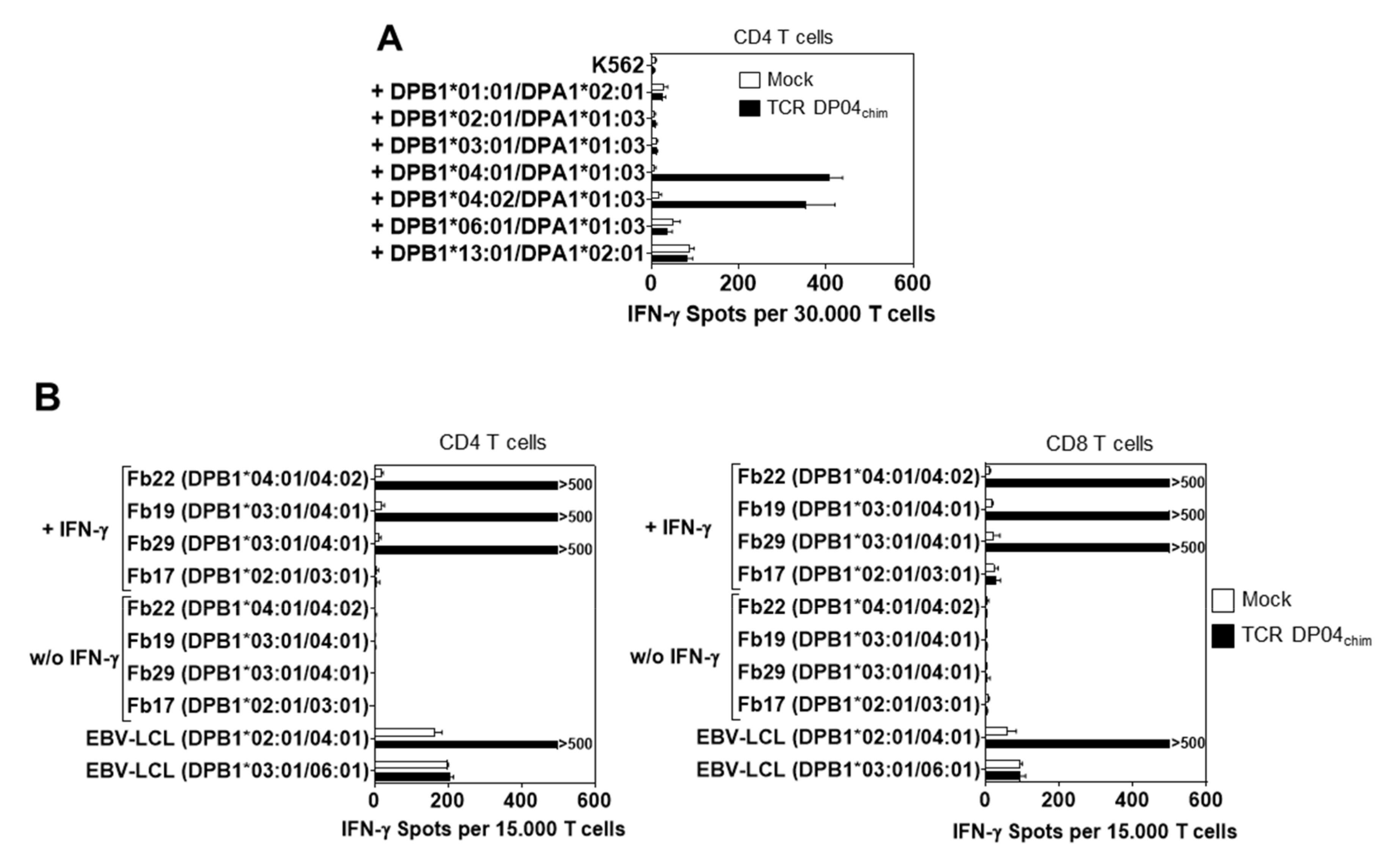
3.3. Reactivity of TCR DP04chim is Restricted to HLA-DPB1*04:01 Positive Cells, but not to Hematopoietic Cells
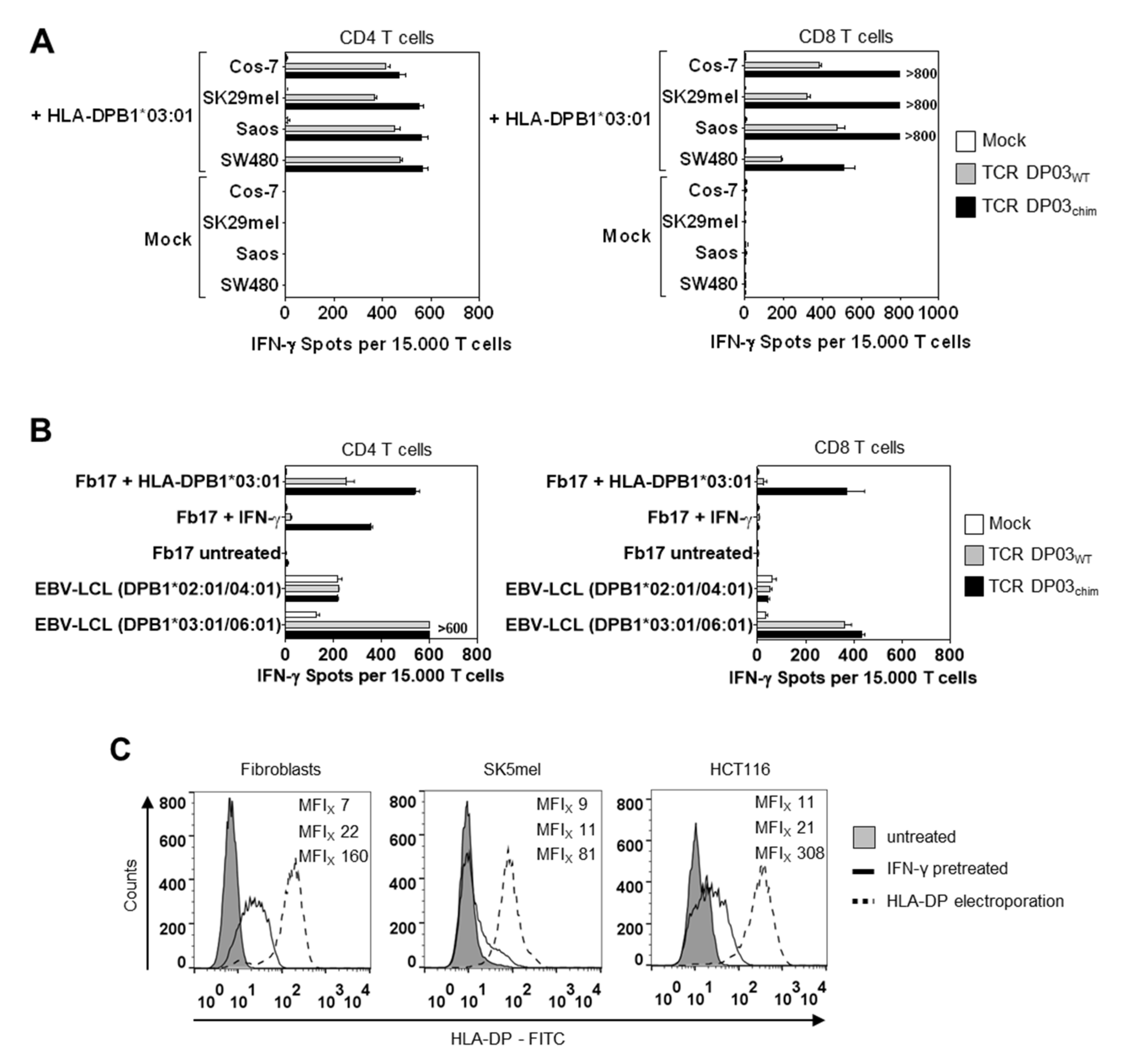
3.4. HLA-DPB1*03:01 Specific TCR Recognizes AML Blasts, but not Fibroblasts under Physiological Conditions
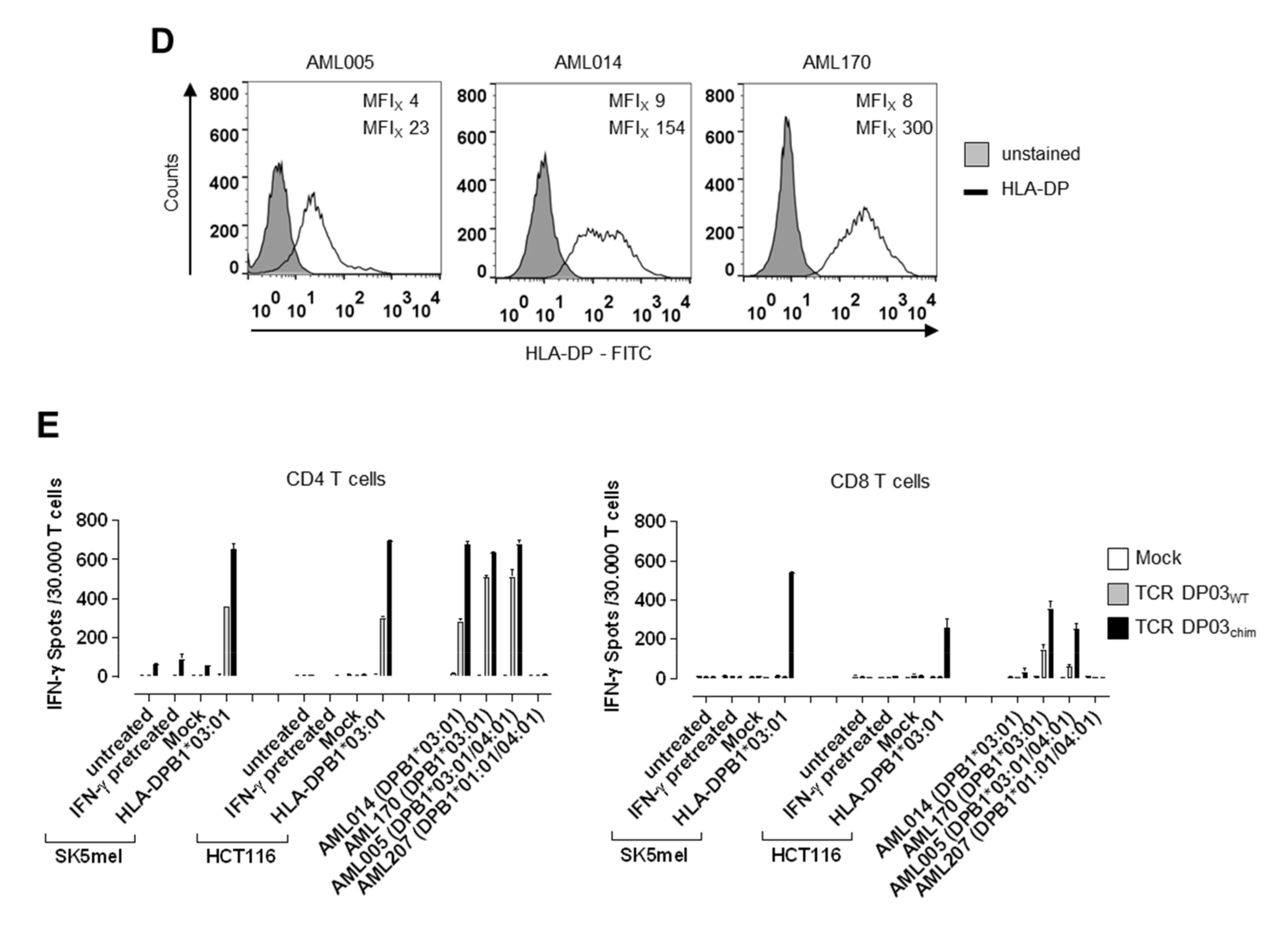
3.5. Expression Level of HLA-DPB1 Determines Recognition of Target Cells by TCR DP03
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Van Bergen, C.A.; van Luxemburg-Heijs, S.A.; de Wreede, L.C.; Eefting, M.; von dem Borne, P.A.; van Balen, P.; Heemskerk, M.H.; Mulder, A.; Claas, F.H.; Navarrete, M.A.; et al. Selective graft-versus-leukemia depends on magnitude and diversity of the alloreactive T cell response. J. Clin. Invest. 2017, 127, 517–529. [Google Scholar] [CrossRef]
- Loke, J.; Malladi, R.; Moss, P.; Craddock, C. The role of allogeneic stem cell transplantation in the management of acute myeloid leukaemia: A triumph of hope and experience. Br. J. Haematol 2019. [Google Scholar] [CrossRef]
- Chapuis, A.G.; Egan, D.N.; Bar, M.; Schmitt, T.M.; McAfee, M.S.; Paulson, K.G.; Voillet, V.; Gottardo, R.; Ragnarsson, G.B.; Bleakley, M.; et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat. Med. 2019, 25, 1064–1072. [Google Scholar] [CrossRef]
- Majzner, R.G.; Mackall, C.L. Clinical lessons learned from the first leg of the CAR T cell journey. Nat. Med. 2019, 25, 1341–1355. [Google Scholar] [CrossRef]
- Petersdorf, E.W.; Gooley, T.; Malkki, M.; Anasetti, C.; Martin, P.; Woolfrey, A.; Smith, A.; Mickelson, E.; Hansen, J.A. The biological significance of HLA-DP gene variation in haematopoietic cell transplantation. Br. J. Haematol. 2001, 112, 988–994. [Google Scholar] [CrossRef]
- Petersdorf, E.W.; Malkki, M.; O’HUigin, C.; Carrington, M.; Gooley, T.; Haagenson, M.D.; Horowitz, M.M.; Spellman, S.R.; Wang, T.; Stevenson, P. High HLA-DP Expression and Graft-versus-Host Disease. N. Engl. J. Med. 2015, 373, 599–609. [Google Scholar] [CrossRef]
- Fleischhauer, K.; Locatelli, F.; Zecca, M.; Orofino, M.G.; Giardini, C.; De Stefano, P.; Pession, A.; Iannone, A.M.; Carcassi, C.; Zino, E.; et al. Graft rejection after unrelated donor hematopoietic stem cell transplantation for thalassemia is associated with nonpermissive HLA-DPB1 disparity in host-versus-graft direction. Blood 2006, 107, 2984–2992. [Google Scholar] [CrossRef]
- Fleischhauer, K.; Shaw, B.E.; Gooley, T.; Malkki, M.; Bardy, P.; Bignon, J.D.; Dubois, V.; Horowitz, M.M.; Madrigal, J.A.; Morishima, Y.; et al. Effect of T-cell-epitope matching at HLA-DPB1 in recipients of unrelated-donor haemopoietic-cell transplantation: A retrospective study. Lancet. Oncol. 2012, 13, 366–374. [Google Scholar] [CrossRef]
- Fleischhauer, K.; Shaw, B.E. HLA-DP in unrelated hematopoietic cell transplantation revisited: Challenges and opportunities. Blood 2017, 130, 1089–1096. [Google Scholar] [CrossRef]
- Zino, E.; Frumento, G.; Marktel, S.; Sormani, M.P.; Ficara, F.; Di Terlizzi, S.; Parodi, A.M.; Sergeant, R.; Martinetti, M.; Bontadini, A.; et al. A T-cell epitope encoded by a subset of HLA-DPB1 alleles determines nonpermissive mismatches for hematologic stem cell transplantation. Blood 2004, 103, 1417–1424. [Google Scholar] [CrossRef]
- Christopher, M.J.; Petti, A.A.; Rettig, M.P.; Miller, C.A.; Chendamarai, E.; Duncavage, E.J.; Klco, J.M.; Helton, N.M.; O’Laughlin, M.; Fronick, C.C.; et al. Immune Escape of Relapsed AML Cells after Allogeneic Transplantation. N. Engl. J. Med. 2018, 379, 2330–2341. [Google Scholar] [CrossRef]
- Toffalori, C.; Zito, L.; Gambacorta, V.; Riba, M.; Oliveira, G.; Bucci, G.; Barcella, M.; Spinelli, O.; Greco, R.; Crucitti, L.; et al. Immune signature drives leukemia escape and relapse after hematopoietic cell transplantation. Nat. Med. 2019, 25, 603–611. [Google Scholar] [CrossRef]
- Herr, W.; Eichinger, Y.; Beshay, J.; Bloetz, A.; Vatter, S.; Mirbeth, C.; Distler, E.; Hartwig, U.F.; Thomas, S. HLA-DPB1 mismatch alleles represent powerful leukemia rejection antigens in CD4 T-cell immunotherapy after allogeneic stem-cell transplantation. Leukemia 2017, 31, 434–445. [Google Scholar] [CrossRef]
- Vatter, S.; Schmid, M.; Gebhard, C.; Mirbeth, C.; Klobuch, S.; Rehli, M.; Herr, W.; Thomas, S. In-vitro blockade of the CD4 receptor co-signal in antigen-specific T-cell stimulation cultures induces the outgrowth of potent CD4 independent T-cell effectors. J. Immunol. Methods 2018, 454, 80–85. [Google Scholar] [CrossRef]
- Nonn, M.; Herr, W.; Khan, S.; Todorova, M.; Link, I.; Thies, J.; Distler, E.; Kaltwasser, M.; Hoffmann, J.; Huber, C.; et al. Selective depletion of alloreactive T lymphocytes using patient-derived nonhematopoietic stimulator cells in allograft engineering. Transplantation 2008, 86, 1427–1435. [Google Scholar] [CrossRef]
- Cohen, C.J.; Zhao, Y.; Zheng, Z.; Rosenberg, S.A.; Morgan, R.A. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006, 66, 8878–8886. [Google Scholar] [CrossRef]
- Thomas, S.; Klobuch, S.; Podlech, J.; Plachter, B.; Hoffmann, P.; Renzaho, A.; Theobald, M.; Reddehase, M.J.; Herr, W.; Lemmermann, N.A. Evaluating Human T-Cell Therapy of Cytomegalovirus Organ Disease in HLA-Transgenic Mice. PLOS Pathog. 2015, 11, e1005049. [Google Scholar] [CrossRef]
- Thomas, S.; Klobuch, S.; Besold, K.; Plachter, B.; Dorrie, J.; Schaft, N.; Theobald, M.; Herr, W. Strong and sustained effector function of memory- versus naive-derived T cells upon T-cell receptor RNA transfer: Implications for cellular therapy. Eur. J. Immunol. 2012, 42, 3442–3453. [Google Scholar] [CrossRef]
- Dorrschuck, A.; Schmidt, A.; Schnurer, E.; Gluckmann, M.; Albrecht, C.; Wolfel, C.; Lennerz, V.; Lifke, A.; Di Natale, C.; Ranieri, E.; et al. CD8+ cytotoxic T lymphocytes isolated from allogeneic healthy donors recognize HLA class Ia/Ib-associated renal carcinoma antigens with ubiquitous or restricted tissue expression. Blood 2004, 104, 2591–2599. [Google Scholar] [CrossRef][Green Version]
- Giudicelli, V.; Chaume, D.; Lefranc, M.P. IMGT/V-QUEST, an integrated software program for immunoglobulin and T cell receptor V-J and V-D-J rearrangement analysis. Nucleic. Acids. Res. 2004, 32, W435–W440. [Google Scholar] [CrossRef]
- Matsushita, M.; Yamazaki, R.; Ikeda, H.; Mori, T.; Sumimoto, H.; Fujita, T.; Okamoto, S.; Ikeda, Y.; Kawakami, Y. Possible involvement of allogeneic antigens recognised by donor-derived CD4 cytotoxic T cells in selective GVL effects after stem cell transplantation of patients with haematological malignancy. Br. J. Haematol. 2006, 132, 56–65. [Google Scholar] [CrossRef]
- Stevanovic, S.; Griffioen, M.; Nijmeijer, B.A.; van Schie, M.L.; Stumpf, A.N.; Rutten, C.E.; Willemze, R.; Falkenburg, J.H. Human allo-reactive CD4+ T cells as strong mediators of anti-tumor immunity in NOD/scid mice engrafted with human acute lymphoblastic leukemia. Leukemia 2012, 26, 312–322. [Google Scholar] [CrossRef][Green Version]
- Markley, J.C.; Sadelain, M. IL-7 and IL-21 are superior to IL-2 and IL-15 in promoting human T cell-mediated rejection of systemic lymphoma in immunodeficient mice. Blood 2010, 115, 3508–3519. [Google Scholar] [CrossRef]
- Britten, C.M.; Meyer, R.G.; Kreer, T.; Drexler, I.; Wolfel, T.; Herr, W. The use of HLA-A*0201-transfected K562 as standard antigen-presenting cells for CD8(+) T lymphocytes in IFN-gamma ELISPOT assays. J. Immunol. Methods 2002, 259, 95–110. [Google Scholar] [CrossRef]
- Hollenbach, J.A.; Madbouly, A.; Gragert, L.; Vierra-Green, C.; Flesch, S.; Spellman, S.; Begovich, A.; Noreen, H.; Trachtenberg, E.; Williams, T.; et al. A combined DPA1~DPB1 amino acid epitope is the primary unit of selection on the HLA-DP heterodimer. Immunogenetics 2012, 64, 559–569. [Google Scholar] [CrossRef]
- Stevanovic, S.; van Bergen, C.A.; van Luxemburg-Heijs, S.A.; van der Zouwen, B.; Jordanova, E.S.; Kruisselbrink, A.B.; van de Meent, M.; Harskamp, J.C.; Claas, F.H.; Marijt, E.W.; et al. HLA class II upregulation during viral infection leads to HLA-DP-directed graft-versus-host disease after CD4+ donor lymphocyte infusion. Blood 2013, 122, 1963–1973. [Google Scholar] [CrossRef]
- Reith, W.; LeibundGut-Landmann, S.; Waldburger, J.M. Regulation of MHC class II gene expression by the class II transactivator. Nat. Rev. Immunol. 2005, 5, 793–806. [Google Scholar] [CrossRef]
- Rutten, C.E.; van Luxemburg-Heijs, S.A.; van der Meijden, E.D.; Griffioen, M.; Oudshoorn, M.; Willemze, R.; Falkenburg, J.H. HLA-DPB1 mismatching results in the generation of a full repertoire of HLA-DPB1-specific CD4+ T cell responses showing immunogenicity of all HLA-DPB1 alleles. Biol. Blood Marrow. Transplant. 2010, 16, 1282–1292. [Google Scholar] [CrossRef]
- Rutten, C.E.; van Luxemburg-Heijs, S.A.; van der Meijden, E.D.; Griffioen, M.; Oudshoorn, M.; Willemze, R.; Falkenburg, J.H. Both permissive and nonpermissive HLA-DPB1 mismatches can induce polyclonal HLA-DPB1 specific immune responses in vivo and in vitro. Blood 2010, 115, 151–153. [Google Scholar] [CrossRef][Green Version]
- Rutten, C.E.; van Luxemburg-Heijs, S.A.; Halkes, C.J.; van Bergen, C.A.; Marijt, E.W.; Oudshoorn, M.; Griffioen, M.; Falkenburg, J.H. Patient HLA-DP-specific CD4+ T cells from HLA-DPB1-mismatched donor lymphocyte infusion can induce graft-versus-leukemia reactivity in the presence or absence of graft-versus-host disease. Biol. Blood Marrow Transplant. 2013, 19, 40–48. [Google Scholar] [CrossRef]
- Van Balen, P.; van Bergen, C.A.M.; van Luxemburg-Heijs, S.A.P.; de Klerk, W.; van Egmond, E.H.M.; Veld, S.A.J.; Halkes, C.J.M.; Zwaginga, J.J.; Griffioen, M.; Jedema, I.; et al. CD4 Donor Lymphocyte Infusion Can Cause Conversion of Chimerism Without GVHD by Inducing Immune Responses Targeting Minor Histocompatibility Antigens in HLA Class II. Front. Immunol. 2018, 9, 3016. [Google Scholar] [CrossRef] [PubMed]
- Falkenburg, W.J.; Melenhorst, J.J.; van de Meent, M.; Kester, M.G.; Hombrink, P.; Heemskerk, M.H.; Hagedoorn, R.S.; Gostick, E.; Price, D.A.; Falkenburg, J.H.; et al. Allogeneic HLA-A*02-restricted WT1-specific T cells from mismatched donors are highly reactive but show off-target promiscuity. J. Immunol. 2011, 187, 2824–2833. [Google Scholar] [CrossRef]
- Bassani-Sternberg, M.; Coukos, G. Mass spectrometry-based antigen discovery for cancer immunotherapy. Curr. Opin. Immunol. 2016, 41, 9–17. [Google Scholar] [CrossRef]
- Caron, E.; Kowalewski, D.J.; Chiek Koh, C.; Sturm, T.; Schuster, H.; Aebersold, R. Analysis of Major Histocompatibility Complex (MHC) Immunopeptidomes Using Mass Spectrometry. Mol. Cell Proteomics. 2015, 14, 3105–3117. [Google Scholar] [CrossRef]
- Rudolph, M.G.; Stanfield, R.L.; Wilson, I.A. How TCRs bind MHCs, peptides, and coreceptors. Annu. Rev. Immunol. 2006, 24, 419–466. [Google Scholar] [CrossRef]
- Laghmouchi, A.; Hoogstraten, C.; van Balen, P.; Falkenburg, J.H.F.; Jedema, I. The allogeneic HLA-DP-restricted T-cell repertoire provoked by allogeneic dendritic cells contains T cells that show restricted recognition of hematopoietic cells including primary malignant cells. Haematologica 2019, 104, 197–206. [Google Scholar] [CrossRef]
- Wang, X.; Chang, W.C.; Wong, C.W.; Colcher, D.; Sherman, M.; Ostberg, J.R.; Forman, S.J.; Riddell, S.R.; Jensen, M.C. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 2011, 118, 1255–1263. [Google Scholar] [CrossRef]
- Wu, C.Y.; Roybal, K.T.; Puchner, E.M.; Onuffer, J.; Lim, W.A. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science 2015, 350, aab4077. [Google Scholar] [CrossRef]
- Roybal, K.T.; Williams, J.Z.; Morsut, L.; Rupp, L.J.; Kolinko, I.; Choe, J.H.; Walker, W.J.; McNally, K.A.; Lim, W.A. Engineering T Cells with Customized Therapeutic Response Programs Using Synthetic Notch Receptors. Cell 2016, 167, 419–432. [Google Scholar] [CrossRef]
- Di Stasi, A.; Tey, S.K.; Dotti, G.; Fujita, Y.; Kennedy-Nasser, A.; Martinez, C.; Straathof, K.; Liu, E.; Durett, A.G.; Grilley, B.; et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011, 365, 1673–1683. [Google Scholar] [CrossRef]
- Spear, T.T.; Evavold, B.D.; Baker, B.M.; Nishimura, M.I. Understanding TCR affinity, antigen specificity, and cross-reactivity to improve TCR gene-modified T cells for cancer immunotherapy. Cancer Immunol. Immunother. 2019, 68, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Kuball, J.; Dossett, M.L.; Wolfl, M.; Ho, W.Y.; Voss, R.H.; Fowler, C.; Greenberg, P.D. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood 2007, 109, 2331–2338. [Google Scholar] [CrossRef] [PubMed]
- Linette, G.P.; Stadtmauer, E.A.; Maus, M.V.; Rapoport, A.P.; Levine, B.L.; Emery, L.; Litzky, L.; Bagg, A.; Carreno, B.M.; Cimino, P.J.; et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013, 122, 863–871. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klobuch, S.; Hammon, K.; Vatter-Leising, S.; Neidlinger, E.; Zwerger, M.; Wandel, A.; Neuber, L.M.; Heilmeier, B.; Fichtner, R.; Mirbeth, C.; et al. HLA-DPB1 Reactive T Cell Receptors for Adoptive Immunotherapy in Allogeneic Stem Cell Transplantation. Cells 2020, 9, 1264. https://doi.org/10.3390/cells9051264
Klobuch S, Hammon K, Vatter-Leising S, Neidlinger E, Zwerger M, Wandel A, Neuber LM, Heilmeier B, Fichtner R, Mirbeth C, et al. HLA-DPB1 Reactive T Cell Receptors for Adoptive Immunotherapy in Allogeneic Stem Cell Transplantation. Cells. 2020; 9(5):1264. https://doi.org/10.3390/cells9051264
Chicago/Turabian StyleKlobuch, Sebastian, Kathrin Hammon, Sarah Vatter-Leising, Elisabeth Neidlinger, Michael Zwerger, Annika Wandel, Laura Maria Neuber, Bernhard Heilmeier, Regina Fichtner, Carina Mirbeth, and et al. 2020. "HLA-DPB1 Reactive T Cell Receptors for Adoptive Immunotherapy in Allogeneic Stem Cell Transplantation" Cells 9, no. 5: 1264. https://doi.org/10.3390/cells9051264
APA StyleKlobuch, S., Hammon, K., Vatter-Leising, S., Neidlinger, E., Zwerger, M., Wandel, A., Neuber, L. M., Heilmeier, B., Fichtner, R., Mirbeth, C., Herr, W., & Thomas, S. (2020). HLA-DPB1 Reactive T Cell Receptors for Adoptive Immunotherapy in Allogeneic Stem Cell Transplantation. Cells, 9(5), 1264. https://doi.org/10.3390/cells9051264




