Microglia Contribution to the Regulation of the Retinal and Choroidal Vasculature in Age-Related Macular Degeneration
Abstract
1. Introduction
2. Development and Structure of Retinal and Choroidal Vasculature
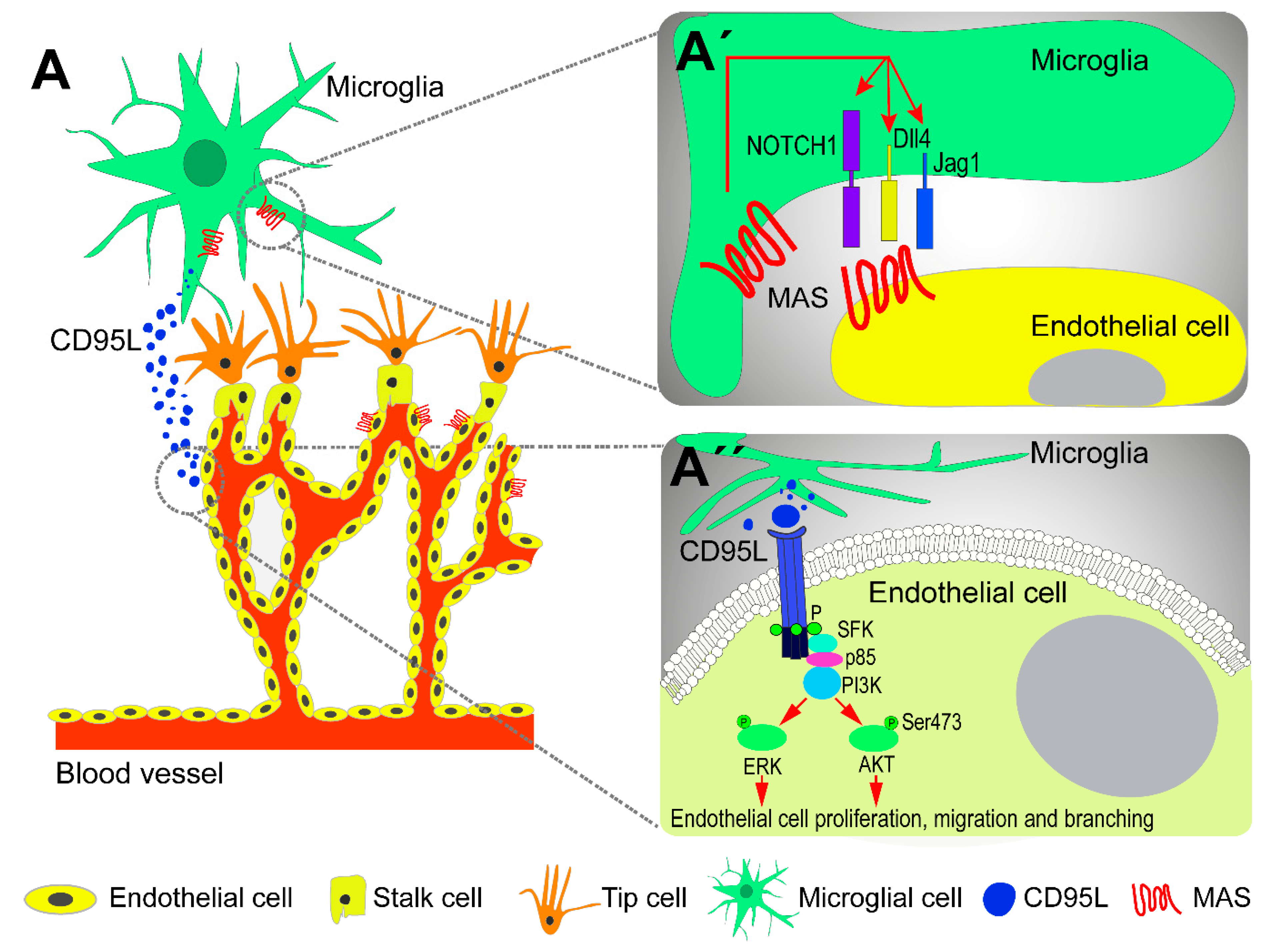
3. The Role of Microglia in Retinal Vascular Development
4. Changes in Retinal and Choroidal Vascular Structure and Function in Age-Related Macular Degeneration (AMD)
5. The Contribution of Microglia to Retinal and Choroidal Neovascularization in AMD
6. Modulation of Microglial Cells as a Potential Treatment for Neovascular AMD
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Quinn, P.M.J.; Wijnholds, J. Retinogenesis of the Human Fetal Retina: An Apical Polarity Perspective. Genes 2019, 10, 987. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Teotia, P.; Erickson, H.; Xia, X. Recapitulating developmental mechanisms for retinal regeneration. Prog. Retin. Eye Res. 2019, 100824. [Google Scholar] [CrossRef] [PubMed]
- Heavner, W.; Pevny, L. Eye development and retinogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008391. [Google Scholar] [CrossRef] [PubMed]
- Alliot, F.; Godin, I.; Pessac, B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res. Dev. Brain Res. 1999, 117, 145–152. [Google Scholar] [CrossRef]
- Santos, A.M.; Calvente, R.; Tassi, M.; Carrasco, M.-C.; Martín-Oliva, D.; Marín-Teva, J.L.; Navascués, J.; Cuadros, M.A. Embryonic and postnatal development of microglial cells in the mouse retina. J. Comp. Neurol. 2008, 506, 224–239. [Google Scholar] [CrossRef]
- Marín-Teva, J.L.; Almendros, A.; Calvente, R.; Cuadros, M.A.; Navascués, J. Tangential migration of ameboid microglia in the developing quail retina: Mechanism of migration and migratory behavior. Glia 1998, 22, 31–52. [Google Scholar] [CrossRef]
- Li, F.; Jiang, D.; Samuel, M.A. Microglia in the developing retina. Neural Dev. 2019, 14, 12. [Google Scholar] [CrossRef]
- Madeira, M.H.; Boia, R.; Santos, P.F.; Ambrósio, A.F.; Santiago, A.R. Contribution of microglia-mediated neuroinflammation to retinal degenerative diseases. Mediat. Inflamm. 2015, 2015, 673090. [Google Scholar] [CrossRef]
- Curcio, C.A.; Sloan, K.R.; Kalina, R.E.; Hendrickson, A.E. Human photoreceptor topography. J. Comp. Neurol. 1990, 292, 497–523. [Google Scholar] [CrossRef]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef]
- Khanani, A.M.; Skelly, A.; Bezlyak, V.; Griner, R.; Torres, L.R.; Sagkriotis, A. SIERRA-AMD: A retrospective, real-world evidence study of patients with neovascular age-related macular degeneration in the USA. Ophthalmol. Retin. 2020, 4, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Ting, D.S.W.; Thakku, S.G.; Wong, T.-Y.; Cheng, C.-Y.; Wong, E.; Mathur, R.; Wong, D.; Yeo, I.; Gemmy Cheung, C.M. Detailed characterization of choroidal morphologic and vascular features in age-related macular degeneration ad polypoidal choroidal vasculopathy. Retina 2017, 37, 2269–2280. [Google Scholar] [CrossRef] [PubMed]
- Heng, L.Z.; Comyn, O.; Peto, T.; Tadros, C.; Ng, E.; Sivaprasad, S.; Hykin, P.G. Diabetic retinopathy: Pathogenesis, clinical grading, management and future developments. Diabet. Med. 2013, 30, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Nonobe, N.; Kaneko, H.; Ito, Y.; Takayama, K.; Kataoka, K.; Tsunekawa, T.; Matsuura, T.; Suzumura, A.; Shimizu, H.; Terasaki, H. Optical coherence tomography angiography of the foveal avascular zone in children with a history of treatment requiring retinopathy of prematurity. Retina 2019, 39, 111–117. [Google Scholar] [CrossRef]
- Arrigo, A.; Romano, F.; Albertini, G.; Aragona, E.; Bandello, F.; Battaglia Parodi, M. Vascular patterns in retinitis pigmentosa on swept-source optical coherence tomography angiography. J. Clin. Med. 2019, 8, 1425. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Waldstein, S.M. A paradigm shift in imaging biomarkers in neovascular age-related macular degeneration. Prog. Retin. Eye Res. 2016, 50, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Perrott-Reynolds, R.; Cann, R.; Cronbach, N.; Neo, Y.N.; Ho, V.; McNally, O.; Madi, H.A.; Cochran, C.; Chakravarthy, U. The diagnostic accuracy of OCT angiography in naive and treated neovascular age-related macular degeneration: A review. Eye 2019, 33, 274–282. [Google Scholar] [CrossRef]
- Chiquita, S.; Rodrigues-Neves, A.C.; Baptista, F.I.; Carecho, R.; Moreira, P.I.; Castelo-Branco, M.; Ambrósio, A.F. The retina as a window or mirror of the brain changes detected in Alzheimer’s disease: Critical aspects to unravel. Mol. Neurobiol. 2019, 56, 5416–5435. [Google Scholar] [CrossRef]
- Zhao, L.; Zabel, M.K.; Wang, X.; Ma, W.; Shah, P.; Fariss, R.N.; Qian, H.; Parkhurst, C.N.; Gan, W.-B.; Wong, W.T. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol. Med. 2015, 7, 1179–1197. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, L.; Fontainhas, A.M.; Fariss, R.N.; Wong, W.T. Microglia in the mouse retina alter the structure and function of retinal pigmented epithelial cells: A potential cellular interaction relevant to AMD. PLoS ONE 2009, 4, e7945. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, L.; Zhang, J.; Fariss, R.N.; Ma, W.; Kretschmer, F.; Wang, M.; Qian, H.H.; Badea, T.C.; Diamond, J.S.; et al. Requirement for microglia for the aintenance of synaptic function and integrity in the mature retina. J. Neurosci. 2016, 36, 2827–2842. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Silverman, S.M.; Zhao, L.; Villasmil, R.; Campos, M.M.; Amaral, J.; Wong, W.T. Absence of TGFβ signaling in retinal microglia induces retinal degeneration and exacerbates choroidal neovascularization. Elife 2019, 8, e42049. [Google Scholar] [CrossRef] [PubMed]
- Lutty, G.A.; McLeod, D.S. Development of the hyaloid, choroidal and retinal vasculatures in the fetal human eye. Prog. Retin. Eye Res. 2018, 62, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Lutty, G.A.; Hasegawa, T.; Baba, T.; Grebe, R.; Bhutto, I.; McLeod, D.S. Development of the human choriocapillaris. Eye 2010, 24, 408–415. [Google Scholar] [CrossRef]
- Hayreh, S.S. The blood supply of the optic nerve head and the evaluation of it-myth and reality. Prog. Retin. Eye Res. 2001, 20, 563–593. [Google Scholar] [CrossRef]
- Yeung, S.C.; You, Y.; Howe, K.L.; Yan, P. Choroidal thickness in patients with cardiovascular disease: A review. Surv. Ophthalmol. 2020, 65, 473–486. [Google Scholar] [CrossRef]
- Nickla, D.L.; Wallman, J. The multifunctional choroid. Prog. Retin. Eye Res. 2010, 29, 144–168. [Google Scholar] [CrossRef]
- Sugiyama, K.; Cioffi, G.A.; Bacon, D.R.; Van Buskirk, E.M. Optic nerve and peripapillary choroidal microvasculature in the primate. J. Glaucoma 1994, 3, 45–54. [Google Scholar] [CrossRef]
- Chirco, K.R.; Sohn, E.H.; Stone, E.M.; Tucker, B.A.; Mullins, R.F. Structural and molecular changes in the aging choroid: Implications for age-related macular degeneration. Eye 2017, 31, 10–25. [Google Scholar] [CrossRef]
- Sun, Y.; Smith, L.E.H. Retinal Vasculature in Development and Diseases. Annu. Rev. Vis. Sci. 2018, 4, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Daufenbach, D.R.; Ruttum, M.S.; Pulido, J.S.; Keech, R.V. Chorioretinal colobomas in a pediatric population. Ophthalmology 1998, 105, 1455–1458. [Google Scholar] [CrossRef]
- McLeod, D.S.; Grebe, R.; Bhutto, I.; Merges, C.; Baba, T.; Lutty, G.A. Relationship between RPE and choriocapillaris in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4982–4991. [Google Scholar] [CrossRef]
- Alvarez, Y.; Cederlund, M.L.; Cottell, D.C.; Bill, B.R.; Ekker, S.C.; Torres-Vazquez, J.; Weinstein, B.M.; Hyde, D.R.; Vihtelic, T.S.; Kennedy, B.N. Genetic determinants of hyaloid and retinal vasculature in zebrafish. BMC Dev. Biol. 2007, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Fruttiger, M. Development of the retinal vasculature. Angiogenesis 2007, 10, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Korte, N.; Nortley, R.; Sethi, H.; Tang, Y.; Attwell, D. Targeting pericytes for therapeutic approaches to neurological disorders. Acta Neuropathol. 2018, 136, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Dorrell, M.I.; Aguilar, E.; Friedlander, M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3500–3510. [Google Scholar]
- Pau, H. Hypothesis on the pathogenesis of retinopathy of prematurity-it is not VEGF alone but anatomical structures that are crucial. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 1–3. [Google Scholar] [CrossRef][Green Version]
- Selvam, S.; Kumar, T.; Fruttiger, M. Retinal vasculature development in health and disease. Prog. Retin. Eye Res. 2018, 63, 1–19. [Google Scholar] [CrossRef]
- Provis, J.M. Development of the primate retinal vasculature. Prog. Retin. Eye Res. 2001, 20, 799–821. [Google Scholar] [CrossRef]
- Gariano, R.F.; Gardner, T.W. Retinal angiogenesis in development and disease. Nature 2005, 438, 960–966. [Google Scholar] [CrossRef]
- Chan-Ling, T.; McLeod, D.S.; Hughes, S.; Baxter, L.; Chu, Y.; Hasegawa, T.; Lutty, G.A. Astrocyte-endothelial cell relationships during human retinal vascular development. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2020–2032. [Google Scholar] [CrossRef] [PubMed]
- Flower, R.W.; McLeod, D.S.; Lutty, G.A.; Goldberg, B.; Wajer, S.D. Postnatal retinal vascular development of the puppy. Invest. Ophthalmol. Vis. Sci. 1985, 26, 957–968. [Google Scholar] [PubMed]
- Hughes, S.; Yang, H.; Chan-Ling, T. Vascularization of the human fetal retina: Roles of vasculogenesis and angiogenesis. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1217–1228. [Google Scholar]
- Dorey, C.K.; Aouididi, S.; Reynaud, X.; Dvorak, H.F.; Brown, L.F. Correlation of vascular permeability factor/vascular endothelial growth factor with extraretinal neovascularization in the rat. Arch. Ophthalmol. 1996, 114, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Powner, M.B.; Gandhi, P.; Clarkin, C.; Gutmann, D.H.; Johnson, R.S.; Ferrara, N.; Fruttiger, M. Astrocyte-derived vascular endothelial growth factor stabilizes vessels in the developing retinal vasculature. PLoS ONE 2010, 5, e11863. [Google Scholar] [CrossRef]
- Dorrell, M.I.; Friedlander, M. Mechanisms of endothelial cell guidance and vascular patterning in the developing mouse retina. Prog. Retin. Eye Res. 2006, 25, 277–295. [Google Scholar] [CrossRef]
- Chappell, J.C.; Darden, J.; Payne, L.B.; Fink, K.; Bautch, V.L. Blood vessel patterning on retinal astrocytes requires endothelial Flt-1 (VEGFR-1). J. Dev. Biol. 2019, 7, 18. [Google Scholar] [CrossRef]
- Yamamoto, H.; Rundqvist, H.; Branco, C.; Johnson, R.S. Autocrine VEGF isoforms differentially regulate endothelial cell behavior. Front. Cell Dev. Biol. 2016, 4, 99. [Google Scholar] [CrossRef]
- Stalmans, I.; Ng, Y.-S.; Rohan, R.; Fruttiger, M.; Bouché, A.; Yuce, A.; Fujisawa, H.; Hermans, B.; Shani, M.; Jansen, S.; et al. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J. Clin. Investig. 2002, 109, 327–336. [Google Scholar] [CrossRef]
- Ishida, S.; Usui, T.; Yamashiro, K.; Kaji, Y.; Amano, S.; Ogura, Y.; Hida, T.; Oguchi, Y.; Ambati, J.; Miller, J.W.; et al. VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J. Exp. Med. 2003, 198, 483–489. [Google Scholar] [CrossRef]
- Ozaki, H.; Seo, M.-S.; Ozaki, K.; Yamada, H.; Yamada, E.; Okamoto, N.; Hofmann, F.; Wood, J.M.; Campochiaro, P.A. Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am. J. Pathol. 2000, 156, 697–707. [Google Scholar] [CrossRef]
- Sandercoe, T.M.; Geller, S.F.; Hendrickson, A.E.; Stone, J.; Provis, J.M. VEGF expression by ganglion cells in central retina before formation of the foveal depression in monkey retina: Evidence of developmental hypoxia. J. Comp. Neurol. 2003, 462, 42–54. [Google Scholar] [CrossRef]
- Provis, J.M.; Hendrickson, A.E. The foveal avascular region of developing human retina. Arch. Ophthalmol. 2008, 126, 507–511. [Google Scholar] [CrossRef]
- Kozulin, P.; Natoli, R.; O’Brien, K.M.B.; Madigan, M.C.; Provis, J.M. The cellular expression of antiangiogenic factors in fetal primate macula. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4298–4306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kozulin, P.; Natoli, R.; O’Brien, K.M.B.; Madigan, M.C.; Provis, J.M. Differential expression of anti-angiogenic factors and guidance genes in the developing macula. Mol. Vis. 2009, 15, 45–59. [Google Scholar] [PubMed]
- Penfold, P.L.; Provis, J.M.; Madigan, M.C.; van Driel, D.; Billson, F.A. Angiogenesis in normal human retinal development the involvement of astrocytes and macrophages. Graefe’s Arch. Clin. Exp. Ophthalmol. 1990, 228, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Usui, Y.; Westenskow, P.D.; Kurihara, T.; Aguilar, E.; Sakimoto, S.; Paris, L.P.; Wittgrove, C.; Feitelberg, D.; Friedlander, M.S.H.; Moreno, S.K.; et al. Neurovascular crosstalk between interneurons and capillaries is required for vision. J. Clin. Investig. 2015, 125, 2335–2346. [Google Scholar] [CrossRef]
- Hanisch, U.-K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef]
- Tay, T.L.; Mai, D.; Dautzenberg, J.; Fernández-Klett, F.; Lin, G.; Sagar, S.; Datta, M.; Drougard, A.; Stempfl, T.; Ardura-Fabregat, A.; et al. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat. Neurosci. 2017, 20, 793–803. [Google Scholar] [CrossRef]
- Checchin, D.; Sennlaub, F.; Levavasseur, E.; Leduc, M.; Chemtob, S. Potential Role of Microglia in Retinal Blood Vessel Formation. Investig. Opthalmology Vis. Sci. 2006, 47, 3595–3602. [Google Scholar] [CrossRef]
- Ashwell, K.W.S.; Holländer, H.; Streit, W.; Stone, J. The appearance and distribution of microglia in the developing retina of the rat. Vis. Neurosci. 1989, 2, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Rymo, S.F.; Gerhardt, H.; Sand, F.W.; Lang, R.; Uv, A.; Betsholtz, C. A two-way communication between microglial cells and angiogenic sprouts regulates angiogenesis in aortic ring cultures. PLoS ONE 2011, 6, e15846. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tisch, N.; Kegel, M.; Yerbes, R.; Hermann, R.; Hudalla, H.; Zuliani, C.; Gülcüler, G.S.; Zwadlo, K.; von Engelhardt, J.; et al. CNS macrophages control neurovascular development via CD95L. Cell Rep. 2017, 19, 1378–1393. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Xu, W.Q.; Ye, M.X.; Zhang, Y.; Wang, H.Y.; Zhang, J.; Li, Y.; Wang, Y.S. Up-regulated basigin-2 in microglia induced by hypoxia promotes retinal angiogenesis. J. Cell. Mol. Med. 2017, 21, 3467–3480. [Google Scholar] [CrossRef] [PubMed]
- Foulquier, S.; Caolo, V.; Swennen, G.; Milanova, I.; Reinhold, S.; Recarti, C.; Alenina, N.; Bader, M.; Steckelings, U.M.; Vanmierlo, T.; et al. The role of receptor MAS in microglia-driven retinal vascular development. Angiogenesis 2019, 22, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Outtz, H.H.; Tattersall, I.W.; Kofler, N.M.; Steinbach, N.; Kitajewski, J. Notch1 controls macrophage recruitment and Notch signaling is activated at sites of endothelial cell anastomosis during retinal angiogenesis in mice. Blood 2011, 118, 3436–3439. [Google Scholar] [CrossRef] [PubMed]
- Haupt, F.; Krishnasamy, K.; Napp, L.C.; Augustynik, M.; Limbourg, A.; Gamrekelashvili, J.; Bauersachs, J.; Haller, H.; Limbourg, F.P. Retinal myeloid cells regulate tip cell selection and vascular branching morphogenesis via Notch ligand Delta-like 1. Sci. Rep. 2019, 9, 9798. [Google Scholar] [CrossRef]
- Hong, H.K.; Lee, H.J.; Ko, J.H.; Park, J.H.; Park, J.Y.; Choi, C.W.; Yoon, C.H.; Ahn, S.J.; Park, K.H.; Woo, S.J.; et al. Neonatal systemic inflammation in rats alters retinal vessel development and simulates pathologic features of retinopathy of prematurity. J. Neuroinflamm. 2014, 11, 87. [Google Scholar] [CrossRef]
- Sun, J.; Hopkins, B.D.; Tsujikawa, K.; Perruzzi, C.; Adini, I.; Swerlick, R.; Bornstein, P.; Lawler, J.; Benjamin, L.E. Thrombospondin-1 modulates VEGF-A-mediated Akt signaling and capillary survival in the developing retina. Am. J. Physiol. Circ. Physiol. 2009, 296, 1344–1351. [Google Scholar] [CrossRef]
- Tremblay, S.; Miloudi, K.; Chaychi, S.; Favret, S.; Binet, F.; Polosa, A.; Lachapelle, P.; Chemtob, S.; Sapieha, P. Systemic inflammation perturbs developmental retinal angiogenesis and neuroretinal function. Invest. Ophthalmol. Vis. Sci. 2013, 54, 8125–8139. [Google Scholar] [CrossRef]
- Mammadzada, P.; Corredoira, P.M.; André, H. The role of hypoxia-inducible factors in neovascular age-related macular degeneration: A gene therapy perspective. Cell. Mol. Life Sci. 2020, 77, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health. 2014, 2, 106–116. [Google Scholar] [CrossRef]
- Gehrs, K.M.; Anderson, D.H.; Johnson, L.V.; Hageman, G.S. Age-related macular degeneration-emerging pathogenetic and therapeutic concepts. Ann. Med. 2006, 38, 450–471. [Google Scholar] [CrossRef] [PubMed]
- Algvere, P.V.; Kvanta, A.; Seregard, S. Drusen maculopathy: A risk factor for visual deterioration. Acta Ophthalmol. 2016, 94, 427–433. [Google Scholar] [CrossRef]
- Wang, J.J.; Mitchell, P.; Rochtchina, E.; Tan, A.G.; Wong, T.Y.; Klein, R. Retinal vessel wall signs and the 5 year incidence of age related maculopathy: The Blue Mountains Eye Study. Br. J. Ophthalmol. 2004, 88, 104–109. [Google Scholar] [CrossRef]
- Remsch, H.; Spraul, C.W.; Lang, G.K.; Lang, G.E. Changes od retinal capillary blood flow in age-related maculopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2000, 238, 960–964. [Google Scholar] [CrossRef]
- Anderson, D.H.; Mullins, R.F.; Hageman, G.S.; Johnson, L.V. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 2002, 134, 411–431. [Google Scholar] [CrossRef]
- Hageman, G.S.; Luthert, P.J.; Victor Chong, N.H.; Johnson, L.V.; Anderson, D.H.; Mullins, R.F. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 2001, 20, 705–732. [Google Scholar] [CrossRef]
- Johnson, L.V.; Leitner, W.P.; Staples, M.K.; Anderson, D.H. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp. Eye Res. 2001, 73, 887–896. [Google Scholar] [CrossRef]
- Anderson, D.H.; Radeke, M.J.; Gallo, N.B.; Chapin, E.A.; Johnson, P.T.; Curletti, C.R.; Hancox, L.S.; Hu, J.; Ebright, J.N.; Malek, G.; et al. The pivotal role of the complement system in aging and age-related macular degeneration: Hypothesis re-visited. Prog. Retin. Eye Res. 2010, 29, 95–112. [Google Scholar] [CrossRef]
- Newman, A.M.; Gallo, N.B.; Hancox, L.S.; Miller, N.J.; Radeke, C.M.; Maloney, M.A.; Cooper, J.B.; Hageman, G.S.; Anderson, D.H.; Johnson, L.V.; et al. Systems-level analysis of age-related macular degeneration reveals global biomarkers and phenotype-specific functional networks. Genome Med. 2012, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Mullins, R.F.; Aptsiauri, N.; Hageman, G.S. Structure and composition of drusen associated with glomerulonephritis: Implications for the role of complement activation in drusen biogenesis. Eye 2001, 15, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, I.; Lutty, G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Aspects Med. 2012, 33, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, S.; Spee, C.; Barron, E.; Ryan, S.J.; Kannan, R.; Hinton, D.R. A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells. Nat. Protoc. 2009, 4, 662–673. [Google Scholar] [CrossRef] [PubMed]
- van der Schaft, T.L.; Mooy, C.M.; de Bruijn, W.C.; de Jong, P.T. Early stages of age-related macular degeneration: An immunofluorescence and electron microscopy study. Br. J. Ophthalmol. 1993, 77, 657–661. [Google Scholar] [CrossRef]
- Mullins, R.F.; Russell, S.R.; Anderson, D.H.; Hageman, G.S. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000, 14, 835–846. [Google Scholar] [CrossRef]
- Johnson, L.V.; Ozaki, S.; Staples, M.K.; Erickson, P.A.; Anderson, D.H. A potential role for immune complex pathogenesis in drusen formation. Exp. Eye Res. 2000, 70, 441–449. [Google Scholar] [CrossRef]
- Crabb, J.W.; Miyagi, M.; Gu, X.; Shadrach, K.; West, K.A.; Sakaguchi, H.; Kamei, M.; Hasan, A.; Yan, L.; Rayborn, M.E.; et al. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 14682–14687. [Google Scholar] [CrossRef]
- Wang, L.; Clark, M.E.; Crossman, D.K.; Kojima, K.; Messinger, J.D.; Mobley, J.A.; Curcio, C.A. Abundant lipid and protein components of drusen. PLoS ONE 2010, 5, e10329. [Google Scholar] [CrossRef]
- Martin, D.F.; Maguire, M.G.; Ying, G.S.; Grunwald, J.E.; Fine, S.L.; Jaffe, G.J. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011, 364, 1897–1908. [Google Scholar]
- Klein, R.; Klein, B.E.K.; Knudtson, M.D.; Meuer, S.M.; Swift, M.; Gangnon, R.E. Fifteen-year cumulative incidence of age-related macular degeneration: The Beaver Dam Eye Study. Ophthalmology 2007, 114, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.D.; Mieler, W.F.; Miller, J.W. Age-related macular degeneration. N. Engl. J. Med. 2008, 358, 2606–2617. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, S.A.; Spaide, R.F.; Curcio, C.A.; Malek, G.; Imamura, Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology 2010, 117, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, M.; Malek, G.; Messinger, J.D.; Clark, M.E.; Wang, L.; Curcio, C.A. Sub-retinal drusenoid deposits in human retina: Organization and composition. Exp. Eye Res. 2008, 87, 402–408. [Google Scholar] [CrossRef]
- Cohen, S.Y.; Dubois, L.; Tadayoni, R.; Delahaye-Mazza, C.; Debibie, C.; Quentel, G. Prevalence of reticular pseudodrusen in age-related macular degeneration with newly diagnosed choroidal neovascularisation. Br. J. Ophthalmol. 2007, 91, 354–359. [Google Scholar] [CrossRef]
- Hamel, C.P.; Meunier, I.; Arndt, C.; Ben Salah, S.; Lopez, S.; Bazalgette, C.; Bazalgette, C.; Zanlonghi, X.; Arnaud, B.; Defoort-Dellhemmes, S.; et al. Extensive macular atrophy with pseudodrusen-like appearance: A new clinical entity. Am. J. Ophthalmol. 2009, 147, 609–620. [Google Scholar] [CrossRef]
- Spaide, R.F.; Klancnik, J.M.; Cooney, M.J. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015, 133, 45–50. [Google Scholar] [CrossRef]
- Toto, L.; Borrelli, E.; Di Antonio, L.; Carpineto, P.; Mastropasqua, R. Retinal vascular plexuses’ changes in dry age-related macular degeneration, evaluated by means of optical coherence tomography angiography. Retina 2016, 36, 1566–1572. [Google Scholar] [CrossRef]
- Trinh, M.; Kalloniatis, M.; Nivison-Smith, L. Vascular changes in intermediate age-related macular degeneration quantified using optical coherence tomography angiography. Transl. Vis. Sci. Technol. 2019, 8, 20. [Google Scholar] [CrossRef]
- Lee, E.K.; Yu, H.G. Ganglion cell-inner plexiform layer and peripapillary retinal nerve fiber layer thicknesses in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2015, 56, 3976–3983. [Google Scholar] [CrossRef]
- Borrelli, E.; Abdelfattah, N.S.; Uji, A.; Nittala, M.G.; Boyer, D.S.; Sadda, S.R. Postreceptor neuronal loss in intermediate age-related macular degeneration. Am. J. Ophthalmol. 2017, 181, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.; Dutra-Medeiros, M.; Páris, L. Ganglion cell complex in early and intermediate age-related macular degeneration: Evidence by SD-OCT manual segmentation. Ophthalmologica 2017, 238, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Muftuoglu, I.K.; Ramkumar, H.L.; Bartsch, D.-U.; Meshi, A.; Gaber, R.; Freeman, W.R. Quantitative analysis of the inner retinal layer thicknesses in age-related macular degeneration using corrected optical coherence tomography segmentation. Retina 2018, 38, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Savastano, M.C.; Minnella, A.M.; Tamburrino, A.; Giovinco, G.; Ventre, S.; Falsini, B. Differential vulnerability of retinal layers to early age-related macular degeneration: Evidence by SD-OCT segmentation analysis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Yenice, E.; Şengün, A.; Soyugelen Demirok, G.; Turaçlı, E. Ganglion cell complex thickness in nonexudative age-related macular degeneration. Eye 2015, 29, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Zucchiatti, I.; Parodi, M.B.; Pierro, L.; Cicinelli, M.V.; Gagliardi, M.; Castellino, N.; Bandello, F. Macular ganglion cell complex and retinal nerve fiber layer comparison in different stages of age-related macular degeneration. Am. J. Ophthalmol. 2015, 160, 602–607. [Google Scholar] [CrossRef]
- Lamin, A.; Oakley, J.D.; Dubis, A.M.; Russakoff, D.B.; Sivaprasad, S. Changes in volume of various retinal layers over time in early and intermediate age-related macular degeneration. Eye 2019, 33, 428–434. [Google Scholar] [CrossRef]
- Pauleikhoff, D.; Spital, G.; Radermacher, M.; Brumm, G.A.; Lommatzsch, A.; Bird, A.C. A fluorescein and indocyanine green angiographic study of choriocapillaris in age-related macular disease. Arch. Ophthalmol. 1999, 117, 1353–1358. [Google Scholar] [CrossRef]
- Capon, M.R.C.; Polkinghorne, P.J.; Fitzke, F.W.; Bird, A.C. Sorsby’s pseudoinfiammatory macula dystrophy—Sorsby’s fundus dystrophies. Eye 1988, 2, 114–122. [Google Scholar] [CrossRef]
- Polkinghorne, P.J.; Capon, M.R.C.; Berninger, T.; Lyness, A.L.; Sehmi, K.; Bird, A.C. Sorsby’s Fundus Dystrophy: A Clinical Study. Ophthalmology 1989, 96, 1763–1768. [Google Scholar] [CrossRef]
- Margolis, R.; Spaide, R.F. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am. J. Ophthalmol. 2009, 147, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, J.E.; Hariprasad, S.M.; DuPont, J.; Maguire, M.G.; Fine, S.L.; Brucker, A.J.; Maguire, A.M.; Ho, A.C. Foveolar choroidal blood flow in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 1998, 39, 385–390. [Google Scholar] [PubMed]
- Lee, J.Y.; Lee, D.H.; Lee, J.Y.; Yoon, Y.H. Correlation between subfoveal choroidal thickness and the severity or progression of nonexudative age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7812–7818. [Google Scholar] [CrossRef] [PubMed]
- Mullins, R.F.; Johnson, M.N.; Faidley, E.A.; Skeie, J.M.; Huang, J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Oll, M.; Yzer, S.; Chang, S.; Barile, G.R.; Merriam, J.C.; Tsang, S.H.; Bearelly, S. Reticular pseudodrusen in early age-related macular degeneration are associated with choroidal thinning. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7075–7081. [Google Scholar] [CrossRef] [PubMed]
- Lutty, G.A.; McLeod, D.S.; Bhutto, I.A.; Edwards, M.M.; Seddon, J.M. Choriocapillaris dropout in early age-related macular degeneration. Exp. Eye Res. 2020, 192, 107939. [Google Scholar] [CrossRef]
- Usman, M.; Iqbal, K.; Ali, M.H.; Nafees, K. Features and diagnostic accuracy of optical coherence tomography angiography in neovascular age-related macular degeneration. Cureus 2019, 11, e6485. [Google Scholar] [CrossRef]
- Provis, J.M.; Penfold, P.L.; Edwards, A.J.; van Driel, D. Human retinal microglia: Expression of immune markers and relationship to the glia limitans. Glia 1995, 14, 243–256. [Google Scholar] [CrossRef]
- Karlstetter, M.; Scholz, R.; Rutar, M.; Wong, W.T.; Provis, J.M.; Langmann, T. Retinal microglia: Just bystander or target for therapy? Prog. Retin. Eye Res. 2015, 45, 30–57. [Google Scholar] [CrossRef]
- Penfold, P.L.; Provis, J.M.; Liew, S.C. Human retinal microglia express phenotypic characteristics in common with dendritic antigen-presenting cells. J. Neuroimmunol. 1993, 45, 183–191. [Google Scholar] [CrossRef]
- Karlstetter, M.; Ebert, S.; Langmann, T. Microglia in the healthy and degenerating retina: Insights from novel mouse models. Immunobiology 2010, 215, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Yeo, N.J.Y.; Chan, E.J.J.; Cheung, C. Choroidal neovascularization: Mechanisms of endothelial dysfunction. Front. Pharmacol. 2019, 10, 1363. [Google Scholar] [CrossRef] [PubMed]
- Vielma, A.H.; Retamal, M.A.; Schmachtenberg, O. Nitric oxide signaling in the retina: What have we learned in two decades? Brain Res. 2012, 1430, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Cantó, A.; Olivar, T.; Romero, F.J.; Miranda, M. Nitrosative stress in retinal pathologies: Review. Antioxidants 2019, 8, 543. [Google Scholar]
- Schmetterer, L. Role of Nitric Oxide in the Control of Ocular Blood Flow. Prog. Retin. Eye Res. 2001, 20, 823–847. [Google Scholar] [CrossRef]
- Krilis, M.; Qi, M.; Madigan, M.C.; Wong, J.W.H.; Abdelatti, M.; Guymer, R.H.; Whitelock, J.; McCluskey, P.; Zhang, P.; Qi, J.; et al. Nitration of tyrosines in complement factor H domains alters its immunological activity and mediates a pathogenic role in age related macular degeneration. Oncotarget 2017, 8, 49016–49032. [Google Scholar] [CrossRef]
- Masuda, T.; Shimazawa, M.; Hara, H. Retinal diseases associated with oxidative stress and the effects of a free radical scavenger (Edaravone). Oxid. Med. Cell. Longev. 2017, 2017, 9208489. [Google Scholar] [CrossRef]
- Opatrilova, R.; Kubatka, P.; Caprnda, M.; Büsselberg, D.; Krasnik, V.; Vesely, P.; Saxena, S.; Ruia, S.; Mozos, I.; Rodrigo, L.; et al. Nitric oxide in the pathophysiology of retinopathy: Evidences from preclinical and clinical researches. Acta Ophthalmol. 2018, 96, 222–231. [Google Scholar] [CrossRef]
- Bhutto, I.A.; Baba, T.; Merges, C.; McLeod, D.S.; Lutty, G.A. Low nitric oxide synthases (NOSs) in eyes with age-related macular degeneration (AMD). Exp. Eye Res. 2010, 90, 155–167. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, K.; Zhang, K.; Zhou, F.; Zhu, L. Induction of oxidative and nitrosative stresses in human retinal pigment epithelial cells by all-trans-retinal. Exp. Cell Res. 2016, 348, 87–94. [Google Scholar] [CrossRef]
- Nag, T.C.; Kathpalia, P.; Gorla, S.; Wadhwa, S. Localization of nitro-tyrosine immunoreactivity in human retina. Ann. Anat. 2019, 223, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Mazzitello, K.I.; Arizmendi, C.M.; Family, F.; Grossniklaus, H.E. Formation and growth of lipofuscin in the retinal pigment epithelium cells. Phys. Rev. E. Stat. Nonlin. Soft Matter Phys. 2009, 80, 051908. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M.; Marshall, J. Effects of increasing numbers of phagocytic inclusions on human retinal pigment epithelial cells in culture: A model for aging. Br. J. Ophthalmol. 1986, 70, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Feeney-Burns, L. Lipofuscin of human retinal pigment epithelium. Am. J. Ophthalmol. 1980, 90, 783–787. [Google Scholar] [CrossRef]
- Wing, G.L.; Blanchard, G.C.; Weiter, J.J. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 1978, 17, 601–607. [Google Scholar]
- Lakkaraju, A.; Finnemann, S.C.; Rodriguez-Boulan, E. The lipofuscin fluorophore A2E perturbs cholesterol metabolism in retinal pigment epithelial cells. Proc. Natl. Acad. Sci. USA 2007, 104, 11026–11031. [Google Scholar] [CrossRef]
- Rattner, A.; Nathans, J. Macular degeneration: Recent advances and therapeutic opportunities. Nat. Rev. Neurosci. 2006, 7, 860–872. [Google Scholar] [CrossRef]
- Thornalley, P.J. Cell activation by glycated proteins. AGE receptors, receptor recognition factors and functional classification of AGEs. Cell. Mol. Biol. 1998, 44, 1013–1023. [Google Scholar]
- Lin, T.; Walker, G.B.; Kurji, K.; Fang, E.; Law, G.; Prasad, S.S.; Kojic, L.; Cao, S.; White, V.; Cui, J.Z.; et al. Parainflammation associated with advanced glycation endproduct stimulation of RPE in vitro: Implications for age-related degenerative diseases of the eye. Cytokine 2013, 62, 369–381. [Google Scholar] [CrossRef]
- Ma, W.; Song, E.L.; Guo, J.; Qu, W.; Hudson, B.I.; Schmidt, A.M.; Barile, G.R. RAGE ligand upregulation of VEGF secretion in ARPE-19 cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1355–1361. [Google Scholar] [CrossRef]
- Yamada, Y.; Ishibashi, K.; Ishibashi, K.; Bhutto, I.A.; Tian, J.; Lutty, G.A.; Handa, J.T. The expression of advanced glycation endproduct receptors in rpe cells associated with basal deposits in human maculas. Exp. Eye Res. 2006, 82, 840–848. [Google Scholar] [CrossRef]
- Howes, K.A.; Liu, Y.; Dunaief, J.L.; Milam, A.; Frederick, J.M.; Marks, A.; Baehr, W. Receptor for advanced glycation end products and age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3713–3720. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Schiekofer, S.; Schwaninger, M.; Andrassy, M.; Humpert, P.M.; Chen, J.; Hong, M.; Luther, T.; Henle, T.; Klöting, I.; et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes 2001, 50, 2792–2808. [Google Scholar] [CrossRef]
- Gasparotto, J.; Girardi, C.S.; Somensi, N.; Ribeiro, C.T.; Moreira, J.C.F.; Michels, M.; Sonai, B.; Rocha, M.; Steckert, A.V.; Barichello, T.; et al. Receptor for advanced glycation end products mediates sepsis-triggered amyloid-β accumulation, Tau phosphorylation, and cognitive impairment. J. Biol. Chem. 2018, 293, 226–244. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, M.T.; Thorburn, D.R.; Penfold, S.A.; Laskowski, A.; Harcourt, B.E.; Sourris, K.C.; Tan, A.L.Y.; Fukami, K.; Thallas-Bonke, V.; Nawroth, P.P.; et al. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J. Am. Soc. Nephrol. 2009, 20, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Kalea, A.Z.; Schmidt, A.M.; Hudson, B.I. RAGE: A novel biological and genetic marker for vascular disease. Clin. Sci. 2009, 116, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Méndez, J.D.; Méndez-Valenzuela, V.; Aguilar-Hernández, M.M. Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell. Signal. 2013, 25, 2185–2197. [Google Scholar] [CrossRef]
- Chen, M.; Glenn, J.V.; Dasari, S.; McVicar, C.; Ward, M.; Colhoun, L.; Quinn, M.; Bierhaus, A.; Xu, H.; Stitt, A.W. RAGE regulates immune cell infiltration and angiogenesis in choroidal neovascularization. PLoS ONE 2014, 9, e89548. [Google Scholar] [CrossRef]
- Nagineni, C.N.; Samuel, W.; Nagineni, S.; Pardhasaradhi, K.; Wiggert, B.; Detrick, B.; Hooks, J.J. Transforming growth factor-β induces expression of vascular endothelial growth factor in human retinal pigment epithelial cells: Involvement of mitogen-activated protein kinases. J. Cell. Physiol. 2003, 197, 453–462. [Google Scholar] [CrossRef]
- Balser, C.; Wolf, A.; Herb, M.; Langmann, T. Co-inhibition of PGF and VEGF blocks their expression in mononuclear phagocytes and limits neovascularization and leakage in the murine retina. J. Neuroinflamm. 2019, 16, 26. [Google Scholar] [CrossRef]
- Olson, J.L.; Courtney, R.J.; Rouhani, B.; Mandava, N.; Dinarello, C.A. Intravitreal anakinra inhibits choroidal neovascular membrane growth in a rat model. Ocul. Immunol. Inflamm. 2009, 17, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Marneros, A.G. NLRP3 inflammasome blockade inhibits VEGF-A-induced age-related macular degeneration. Cell Rep. 2013, 4, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Izumi-Nagai, K.; Nagai, N.; Ozawa, Y.; Mihara, M.; Ohsugi, Y.; Kurihara, T.; Koto, T.; Satofuka, S.; Inoue, M.; Tsubota, K.; et al. Interleukin-6 receptor-mediated activation of signal transducer and activator of transcription-3 (STAT3) promotes choroidal neovascularization. Am. J. Pathol. 2007, 170, 2149–2158. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Carrasco, E.; García-Ramírez, M.; Hernández, C. Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr. Diabetes Rev. 2006, 2, 71–98. [Google Scholar] [CrossRef]
- Nunes, I.; Munger, J.; Harpel, J.G.; Nagano, Y.; Shapiro, R.; Gleizes, P.E.; Rifkin, D.B. Structure and activation of the large latent transforming growth factor-Beta complex. J. Am. Optom. Assoc. 1998, 69, 643–648. [Google Scholar]
- Loukovaara, S.; Robciuc, A.; Holopainen, J.M.; Lehti, K.; Pessi, T.; Liinamaa, J.; Kukkonen, K.T.; Jauhiainen, M.; Koli, K.; Keski-Oja, J.; et al. Ang-2 upregulation correlates with increased levels of MMP-9, VEGF, EPO and TGFβ1 in diabetic eyes undergoing vitrectomy. Acta Ophthalmol. 2013, 91, 531–539. [Google Scholar] [CrossRef]
- Wang, X.; Ma, W.; Han, S.; Meng, Z.; Zhao, L.; Yin, Y.; Wang, Y.; Li, J. TGF-β participates choroid neovascularization through Smad2/3-VEGF/TNF-α signaling in mice with Laser-induced wet age-related macular degeneration. Sci. Rep. 2017, 7, 9672. [Google Scholar] [CrossRef]
- Ambati, J.; Anand, A.; Fernandez, S.; Sakurai, E.; Lynn, B.C.; Kuziel, W.A.; Rollins, B.J.; Ambati, B.K. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat. Med. 2003, 9, 1390–1397. [Google Scholar] [CrossRef]
- Tsutsumi, C.; Sonoda, K.-H.; Egashira, K.; Qiao, H.; Hisatomi, T.; Nakao, S.; Ishibashi, M.; Charo, I.F.; Sakamoto, T.; Murata, T.; et al. The critical role of ocular-infiltrating macrophages in the development of choroidal neovascularization. J. Leukoc. Biol. 2003, 74, 25–32. [Google Scholar] [CrossRef]
- Tuo, J.; Smith, B.C.; Bojanoeski, C.M.; Meleth, A.D.; Gery, I.; Csaky, K.G.; Chew, E.Y.; Chan, C.C. The involvement of sequence variation and expression of CX3CR1 in the pathogenesis of age-related macular degeneration. FASEB J. 2004, 18, 1297–1299. [Google Scholar] [CrossRef]
- Combadière, C.; Feumi, C.; Raoul, W.; Keller, N.; Rodéro, M.; Pézard, A.; Lavalette, S.; Houssier, M.; Jonet, L.; Picard, E.; et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J. Clin. Investig. 2007, 117, 2920–2928. [Google Scholar] [CrossRef] [PubMed]
- Umeda, S.; Suzuki, M.T.; Okamoto, H.; Ono, F.; Mizota, A.; Terao, K.; Yoshikawa, Y.; Tanaka, Y.; Iwata, T. Molecular composition of drusen and possible involvement of anti-retinal autoimmunity in two different forms of macular degeneration in cynomolgus monkey (Macaca fascicularis). FASEB J. 2005, 19, 1683–1685. [Google Scholar] [CrossRef] [PubMed]
- Natoli, R.; Fernando, N.; Jiao, H.; Racic, T.; Madigan, M.; Barnett, N.L.; Chu-Tan, J.A.; Valter, K.; Provis, J.; Rutar, M. Retinal macrophages synthesize C3 and activate complement in AMD and in models of focal retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2977–2990. [Google Scholar] [CrossRef] [PubMed]
- Cherepanoff, S.; McMenamin, P.; Gillies, M.C.; Kettle, E.; Sarks, S.H. Bruch’s membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br. J. Ophthalmol. 2010, 94, 918–925. [Google Scholar] [CrossRef]
- Sahu, A.; Lambris, J.D. Structure and biology of complement protein C3, a connecting link between innate and acquired immunity. Immunol. Rev. 2001, 180, 35–48. [Google Scholar] [CrossRef]
- Lennikov, A.; Saddala, M.S.; Mukwaya, A.; Tang, S.; Huang, H. Autoimmune-mediated retinopathy in CXCR5-deficient mice as the result of age-related macular degeneration associated proteins accumulation. Front. Immunol. 2019, 10, 1903. [Google Scholar] [CrossRef]
- Lashkari, K.; Teague, G.; Chen, H.; Lin, Y.-Q.; Kumar, S.; McLaughlin, M.M.; López, F.J. A monoclonal antibody targeting amyloid β (Aβ) restores complement factor I bioactivity: Potential implications in age-related macular degeneration and Alzheimer’s disease. PLoS ONE 2018, 13, e0195751. [Google Scholar] [CrossRef]
- Iannaccone, A.; Giorgianni, F.; New, D.D.; Hollingsworth, T.J.; Umfress, A.; Alhatem, A.H.; Neeli, I.; Lenchik, N.I.; Jennings, B.J.; Calzada, J.I.; et al. Circulating autoantibodies in age-related macular degeneration recognize human macular tissue antigens implicated in autophagy, immunomodulation, and protection from oxidative stress and apoptosis. PLoS ONE 2015, 10, e0145323. [Google Scholar] [CrossRef]
- Van Noort, J.M.; Bsibsi, M.; Gerritsen, W.H.; Van Der Valk, P.; Bajramovic, J.J.; Steinman, L.; Amor, S. αB-crystallin is a target for adaptive immune responses and a trigger of innate responses in preactive multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 2010, 69, 694–703. [Google Scholar] [CrossRef]
- Ambati, J.; Atkinson, J.P.; Gelfand, B.D. Immunology of age-related macular degeneration. Nat. Rev. Immunol. 2013, 13, 438–451. [Google Scholar] [CrossRef]
- Fritsche, L.G.; Fariss, R.N.; Stambolian, D.; Abecasis, G.R.; Curcio, C.A.; Swaroop, A. Age-related macular degeneration: Genetics and biology coming together. Annu. Rev. Genomics Hum. Genet. 2014, 15, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Tao, Y.; Neumaier, M.; Findeisen, P. Cytokine concentration in aqueous humour of eyes with exudative age-related macular degeneration. Acta Ophthalmol. 2012, 90, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, M.; Raisler, B.J.; Sakurai, E.; Sarma, J.V.; Barnum, S.R.; Lambris, J.D.; Chen, Y.; Zhang, K.; Ambati, B.K.; Baffi, J.Z.; et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Natl. Acad. Sci. 2006, 103, 2328–2333. [Google Scholar] [CrossRef] [PubMed]
- Vinores, S.A.; Xiao, W.H.; Aslam, S.; Shen, J.; Oshima, Y.; Nambu, H.; Liu, H.; Carmeliet, P.; Campochiaro, P.A. Implication of the hypoxia response element of the Vegf promoter in mouse models of retinal and choroidal neovascularization, but not retinal vascular development. J. Cell. Physiol. 2006, 206, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, P.; Kokona, D.; Ebneter, A.; Zinkernagel, M.S. Effect of inhibition of colony-stimulating factor 1 receptor on choroidal neovascularization in mice. Am. J. Pathol. 2020, 190, 412–425. [Google Scholar] [CrossRef]
- Dainichi, T.; Matsumoto, R.; Mostafa, A.; Kabashima, K. Immune Control by TRAF6-Mediated Pathways of Epithelial Cells in the EIME (Epithelial Immune Microenvironment). Front. Immunol. 2019, 10, 1107. [Google Scholar] [CrossRef]
- Ding, D.; Zhu, M.; Liu, X.; Jiang, L.; Xu, J.; Chen, L.; Liang, J.; Li, L.; Zhou, T.; Wang, Y.; et al. Inhibition of TRAF6 alleviates choroidal neovascularization in vivo. Biochem. Biophys. Res. Commun. 2018, 503, 2742–2748. [Google Scholar] [CrossRef]
- Karlstetter, M.; Kopatz, J.; Aslanidis, A.; Shahraz, A.; Caramoy, A.; Linnartz-Gerlach, B.; Lin, Y.; Lückoff, A.; Fauser, S.; Düker, K.; et al. Polysialic acid blocks mononuclear phagocyte reactivity, inhibits complement activation, and protects from vascular damage in the retina. EMBO Mol. Med. 2017, 9, 154–166. [Google Scholar] [CrossRef]
- Lavine, J.A.; Farnoodian, M.; Wang, S.; Darjatmoko, S.R.; Wright, L.S.; Gamm, D.M.; Ip, M.S.; Sorenson, C.M.; Sheibani, N. β2—Adrenergic receptor antagonism attenuates CNV through inhibition of VEGF and IL-6 expression. Investig. Opthalmology Vis. Sci. 2017, 58, 299–308. [Google Scholar] [CrossRef]
- Lückoff, A.; Caramoy, A.; Scholz, R.; Prinz, M.; Kalinke, U.; Langmann, T. Interferon-beta signaling in retinal mononuclear phagocytes attenuates pathological neovascularization. EMBO Mol. Med. 2016, 8, 670–678. [Google Scholar] [CrossRef]
- Wu, W.K.; Georgiadis, A.; Copland, D.A.; Liyanage, S.; Luhmann, U.F.O.; Robbie, S.J.; Liu, J.; Wu, J.; Bainbridge, J.W.; Bates, D.O.; et al. IL-4 Regulates Specific Arg-1+ Macrophage sFlt-1-Mediated Inhibition of Angiogenesis. Am. J. Pathol. 2015, 185, 2324–2335. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, G.J.; Ciulla, T.A.; Ciardella, A.P.; Devin, F.; Dugel, P.U.; Eandi, C.M.; Masonson, H.; Monés, J.; Pearlman, J.A.; Quaranta-El Maftouhi, M.; et al. Dual antagonism of PDGF and VEGF in neovascular age-related macular degeneration: A phase IIb, multicenter, randomized controlled trial. Ophthalmology 2017, 124, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.I.; Niec, M.; Wong, V. One year results of a phase 1 study of the safety and tolerability of combination therapy using sustained release intravitreal triamcinolone acetonide and ranibizumab for subfoveal neovascular AMD. Br. J. Ophthalmol. 2015, 99, 618–623. [Google Scholar] [CrossRef]
- Chaudhary, V.; Barbosa, J.; Lam, W.-C.; Mak, M.; Mavrikakis, E.; Mohaghegh P, S.M. Ozurdex in age-related macular degeneration as adjunct to ranibizumab (The OARA Study). Can. J. Ophthalmol. 2016, 51, 302–305. [Google Scholar] [CrossRef] [PubMed]
- MacLachlan, T.K.; Lukason, M.; Collins, M.; Munger, R.; Isenberger, E.; Rogers, C.; Malatos, S.; Dufresne, E.; Morris, J.; Calcedo, R.; et al. Preclinical safety evaluation of AAV2-sFLT01 a gene therapy for age-related macular degeneration. Mol. Ther. 2011, 19, 326–334. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Lauer, A.K.; Sohn, E.H.; Mir, T.A.; Naylor, S.; Anderton, M.C.; Kelleher, M.; Harrop, R.; Ellis, S.; Mitrophanous, K.A. Lentiviral vector gene transfer of Endostatin/Angiostatin for macular degeneration (GEM) study. Hum. Gene Ther. 2017, 28, 99–111. [Google Scholar] [CrossRef]
- Cashman, S.M.; Ramo, K.; Kumar-Singh, R. A non membrane-targeted human soluble CD59 attenuates choroidal neovascularization in a model of age-related macular degeneration. PLoS ONE 2011, 6, e19078. [Google Scholar] [CrossRef]
- Rasmussen, H.; Chu, K.W.; Campochiaro, P.; Gehlbach, P.L.; Haller, J.A.; Handa, J.T.; Nguyen, Q.D.; Sung, J.U. Clinical protocol. An open-label, phase I, single administration, dose-escalation study of ADGVPEDF.11D (ADPEDF) in neovascular age-related macular degeneration (AMD). Hum. Gene Ther. 2001, 12, 2029–2032. [Google Scholar]
- Kaiser, P.K.; Symons, R.C.A.; Shah, S.M.; Quinlan, E.J.; Tabandeh, H.; Do, D.V.; Reisen, G.; Lockridge, J.A.; Short, B.; Guerciolini, R.; et al. RNAi-based treatment for neovascular age-related macular degeneration by Sirna-027. Am. J. Ophthalmol. 2010, 150, 33–39. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, C.H.; Fernandes, R.; Santiago, A.R.; Ambrósio, A.F. Microglia Contribution to the Regulation of the Retinal and Choroidal Vasculature in Age-Related Macular Degeneration. Cells 2020, 9, 1217. https://doi.org/10.3390/cells9051217
Alves CH, Fernandes R, Santiago AR, Ambrósio AF. Microglia Contribution to the Regulation of the Retinal and Choroidal Vasculature in Age-Related Macular Degeneration. Cells. 2020; 9(5):1217. https://doi.org/10.3390/cells9051217
Chicago/Turabian StyleAlves, C. Henrique, Rosa Fernandes, Ana Raquel Santiago, and António Francisco Ambrósio. 2020. "Microglia Contribution to the Regulation of the Retinal and Choroidal Vasculature in Age-Related Macular Degeneration" Cells 9, no. 5: 1217. https://doi.org/10.3390/cells9051217
APA StyleAlves, C. H., Fernandes, R., Santiago, A. R., & Ambrósio, A. F. (2020). Microglia Contribution to the Regulation of the Retinal and Choroidal Vasculature in Age-Related Macular Degeneration. Cells, 9(5), 1217. https://doi.org/10.3390/cells9051217







