Platelet-Rich Plasma Modulates Gap Junction Functionality and Connexin 43 and 26 Expression During TGF-β1–Induced Fibroblast to Myofibroblast Transition: Clues for Counteracting Fibrosis
Abstract
1. Introduction
1.1. Fibrotic Response in Skeletal Muscle
1.2. PRP as an Anti-fibrotic Agent
1.3. Gap Junction Intercellular Communication (GJIC)
2. Materials and Methods
2.1. Platelet-Rich Plasma (PRP) Preparation
2.2. Cell Culture and Treatments
2.3. Electrophysiological Records
2.4. Silencing of Cx43 Expression by Short Interfering RNA
2.5. Reverse Transcription - Polymerase Chain Reaction (RT-PCR)
2.6. Confocal Laser Scanning Microscopy
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
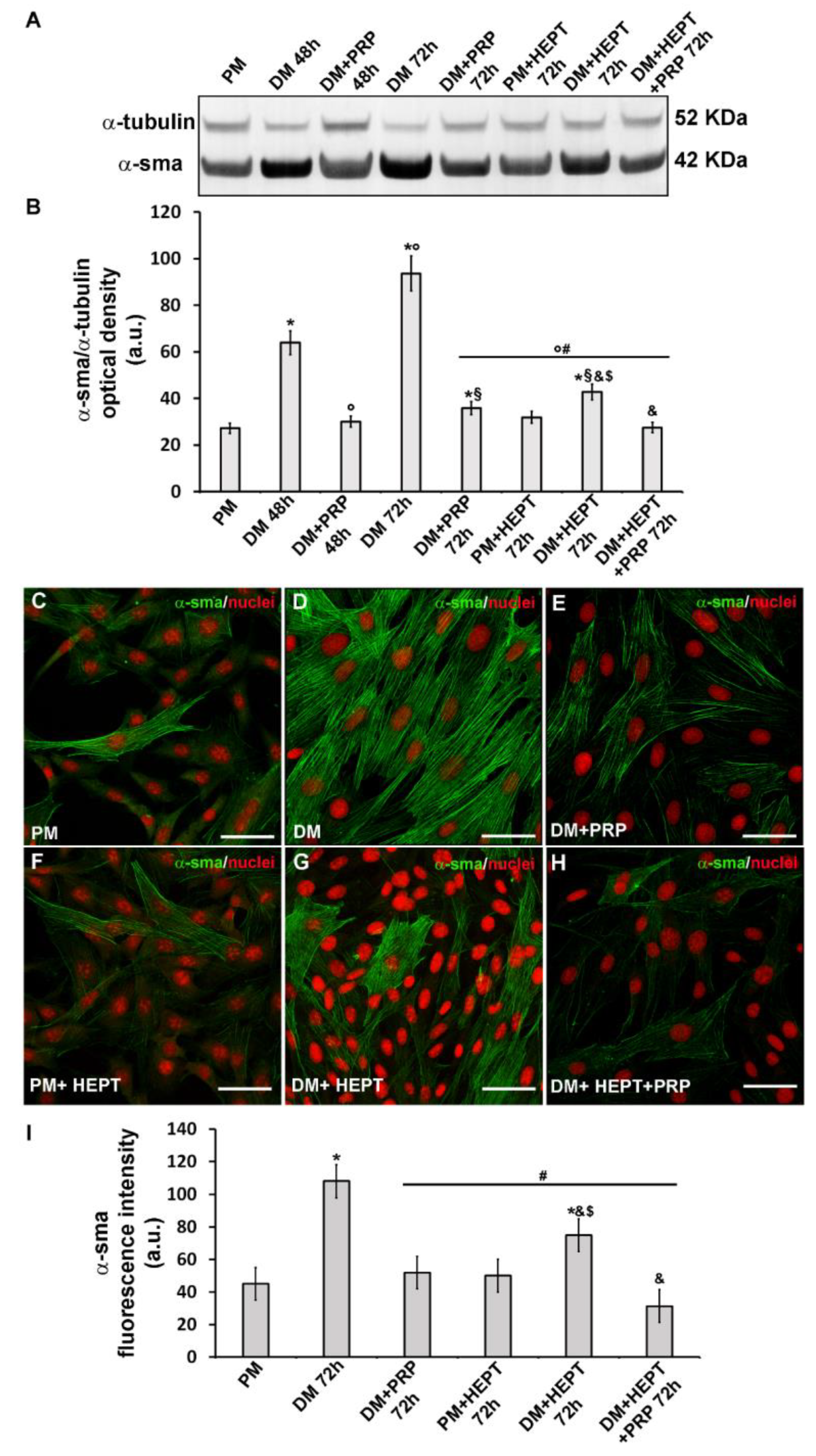
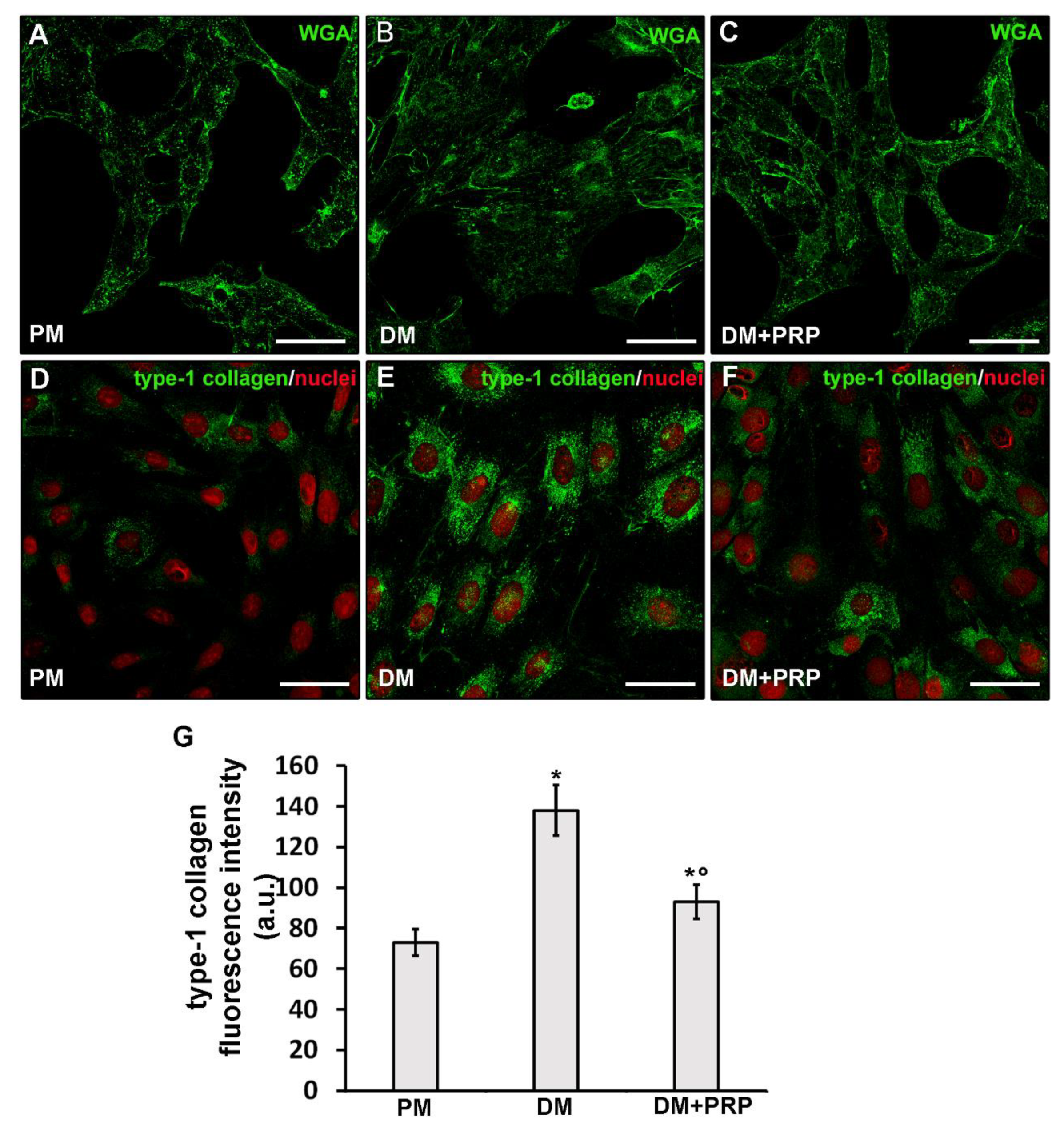
3.1. PRP Prevented TGF-β1- Induced Fibroblast to Myofibroblast Transition
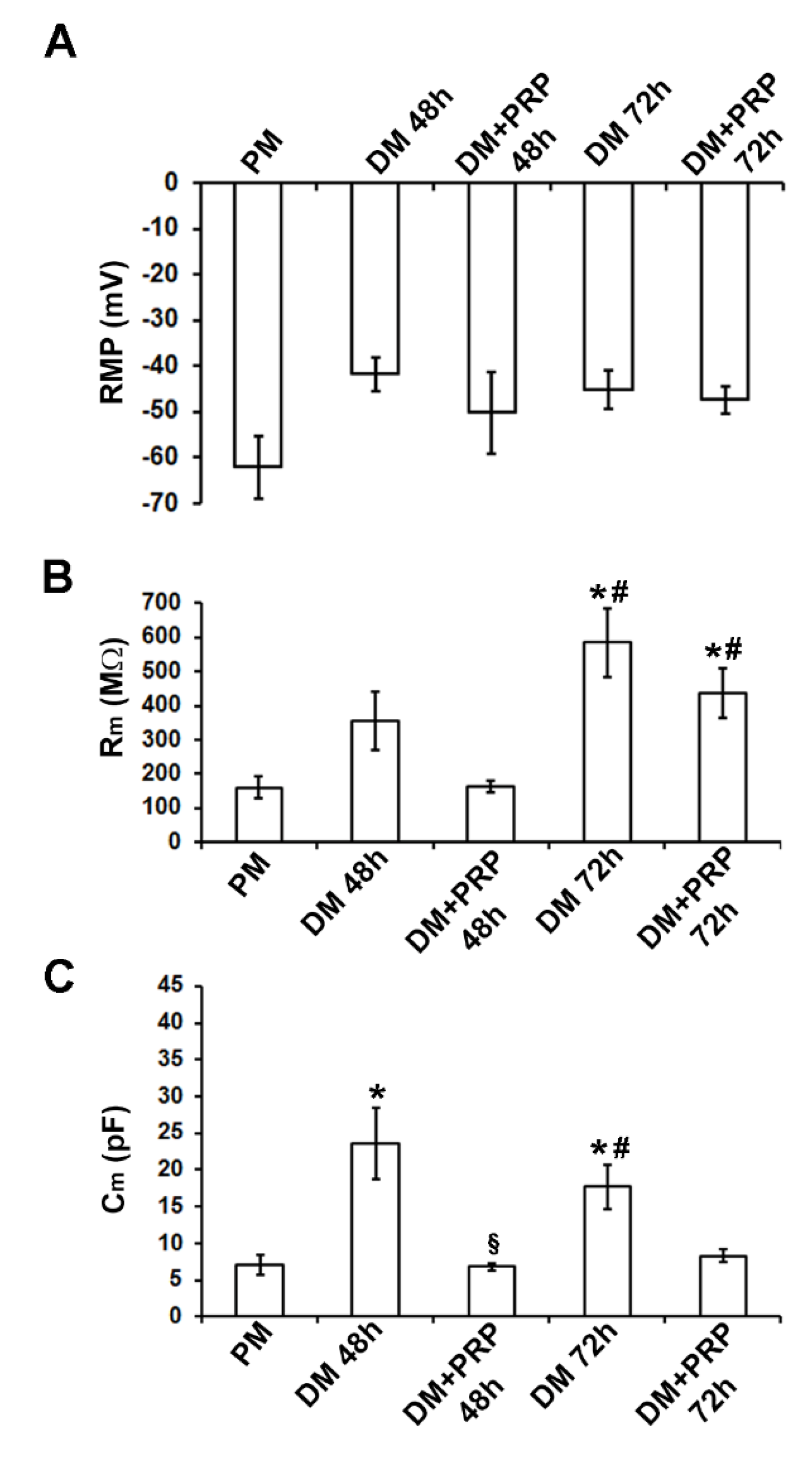
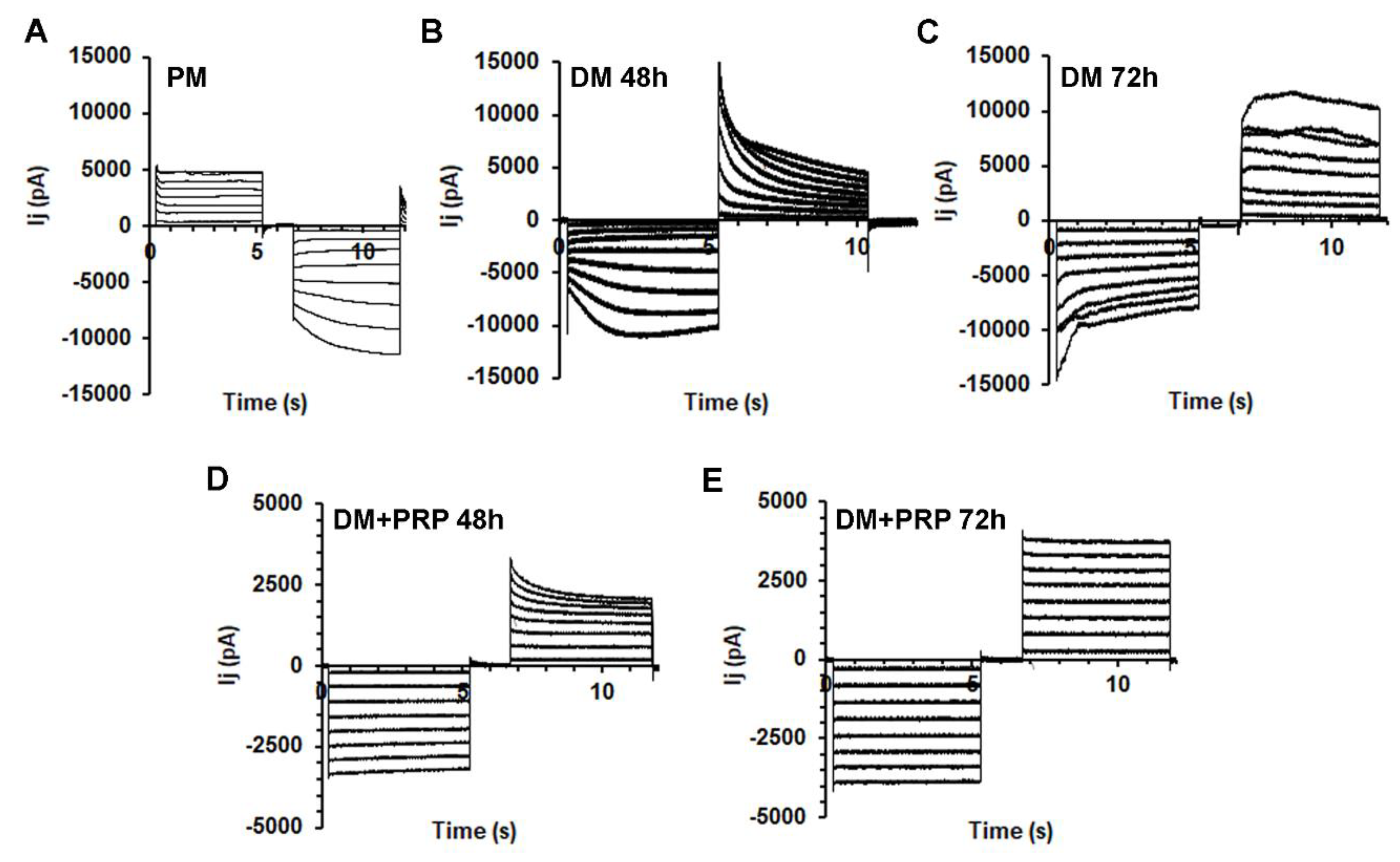
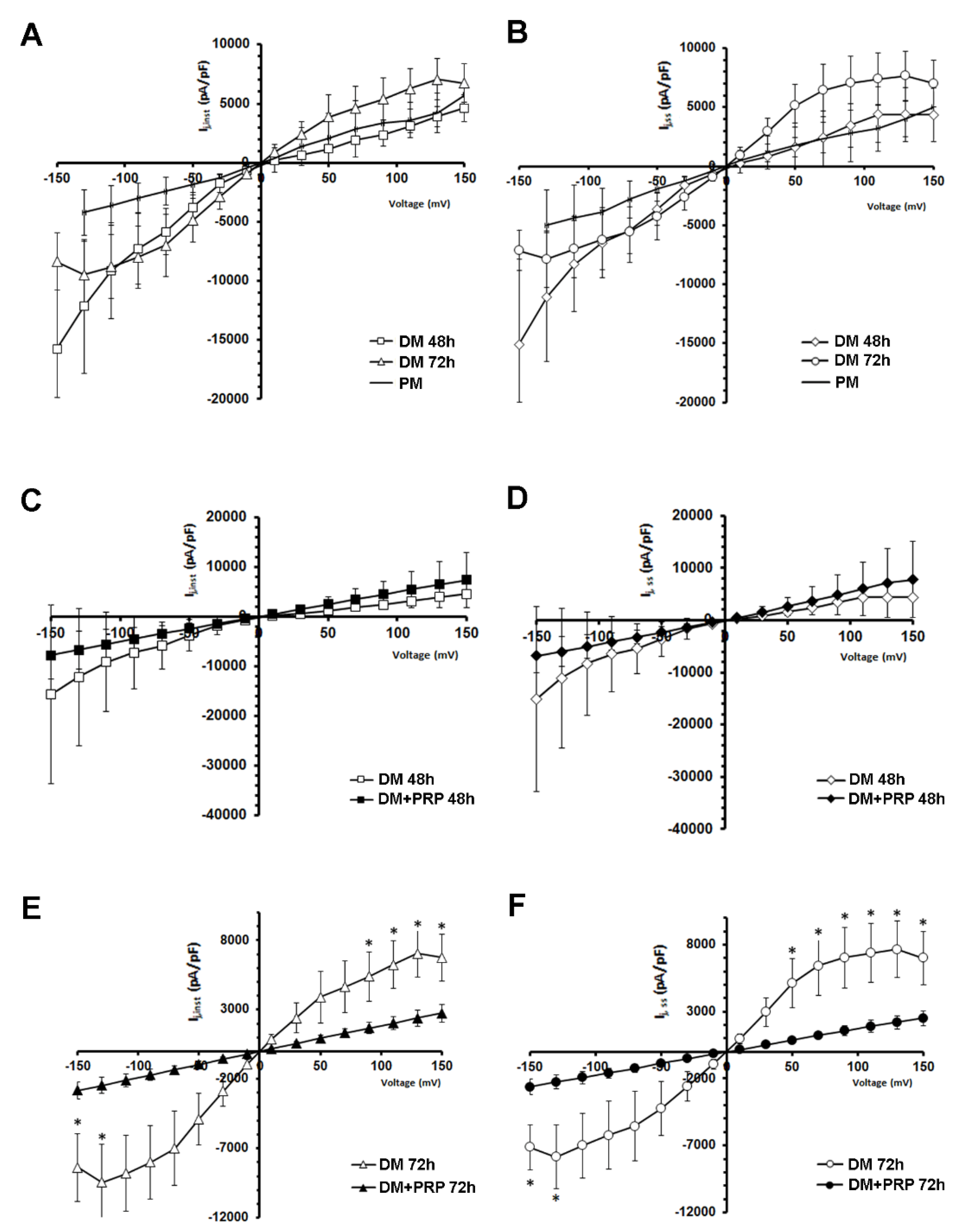
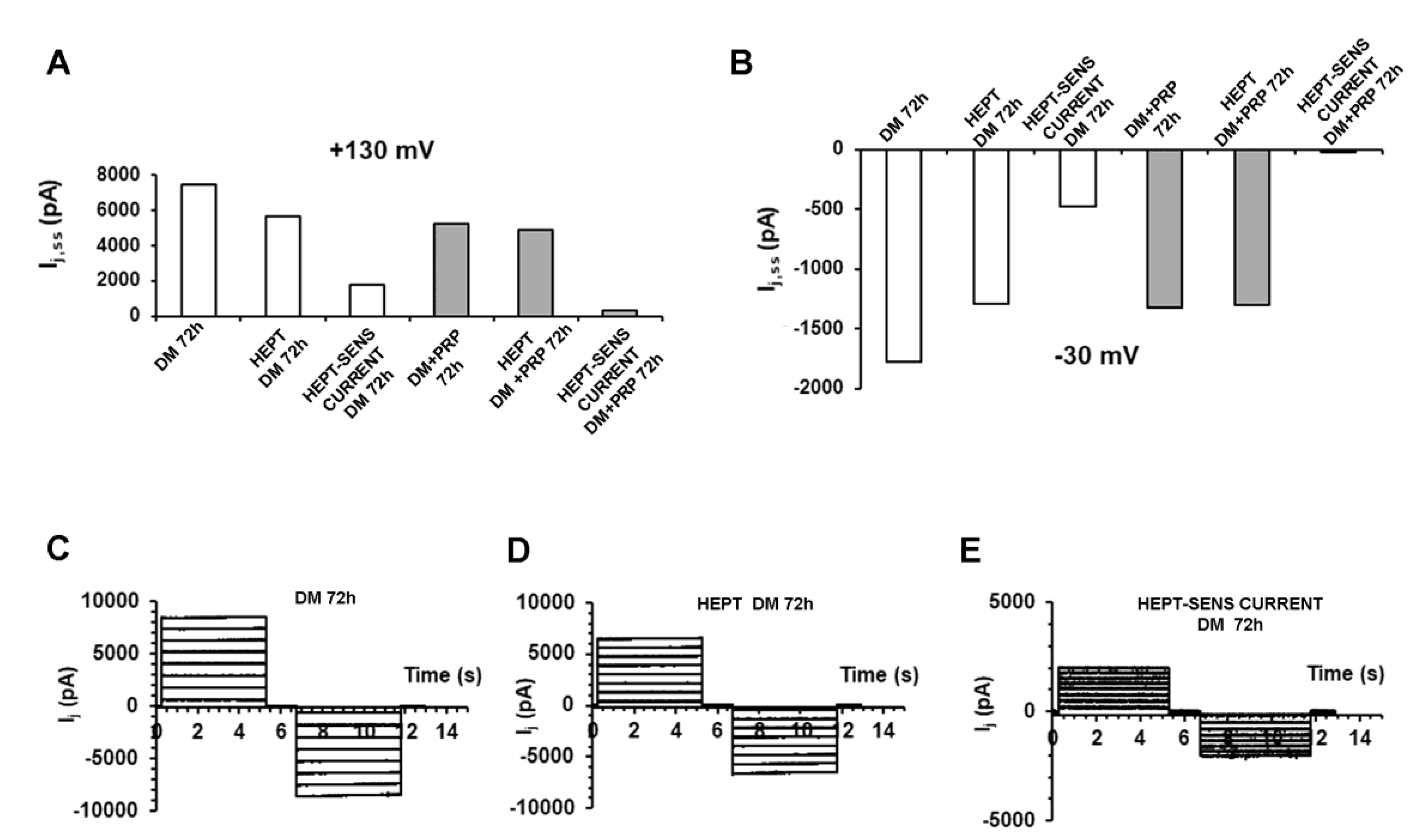
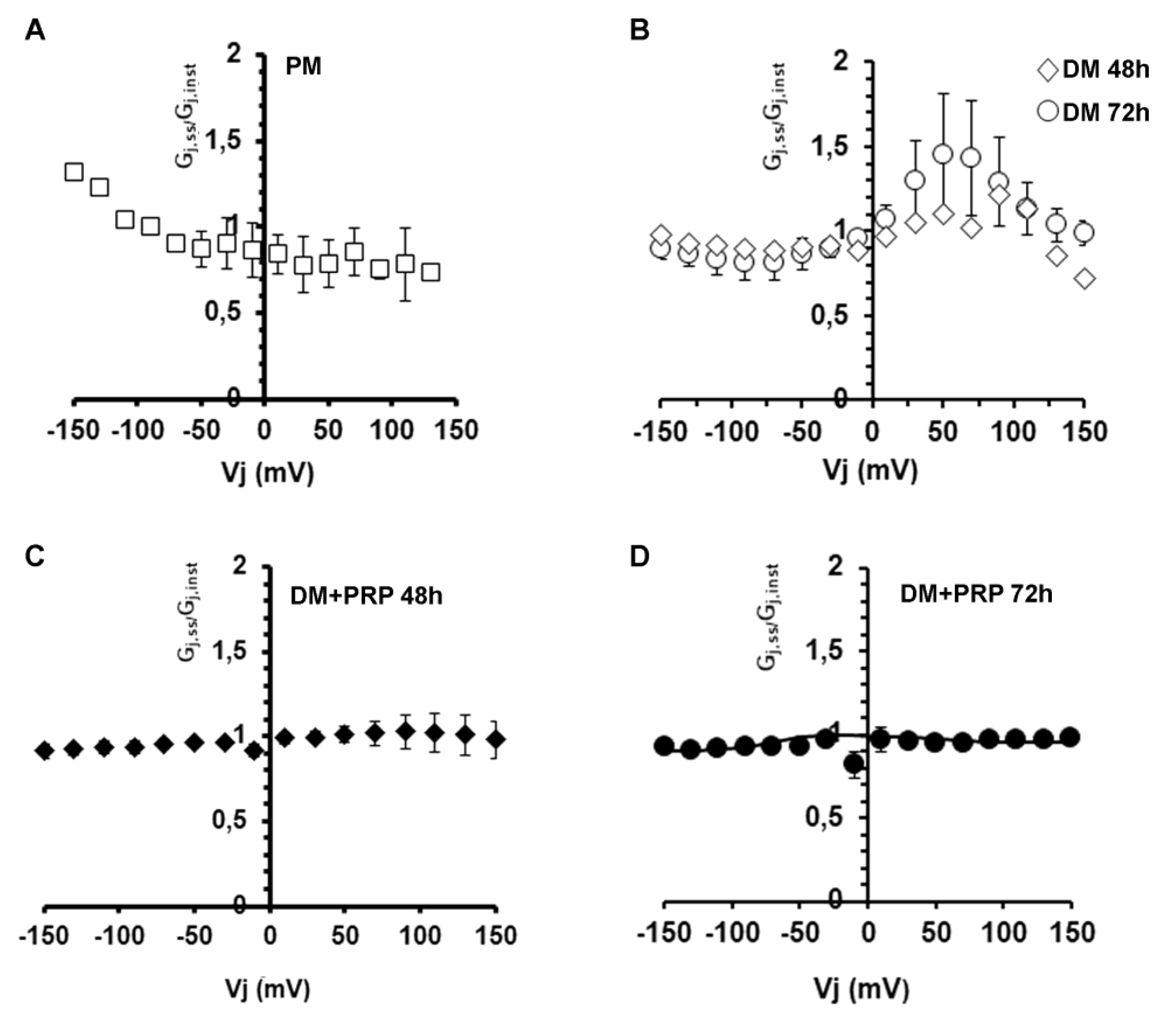
3.2. PRP Modifies Transjunctional Currents (Ij) and Gap Junctional Conductance (Gj) in Myofibroblast Pairs
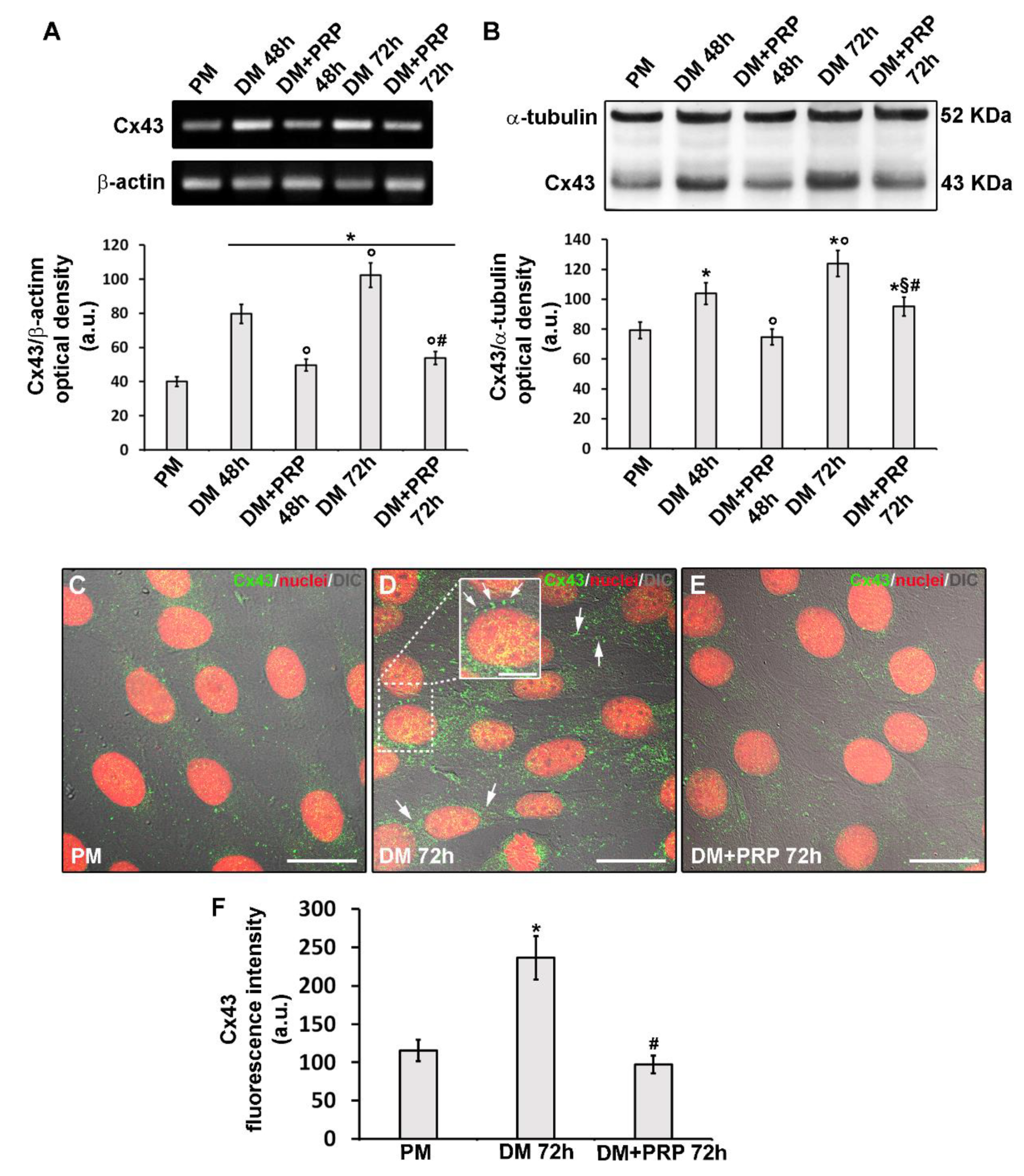
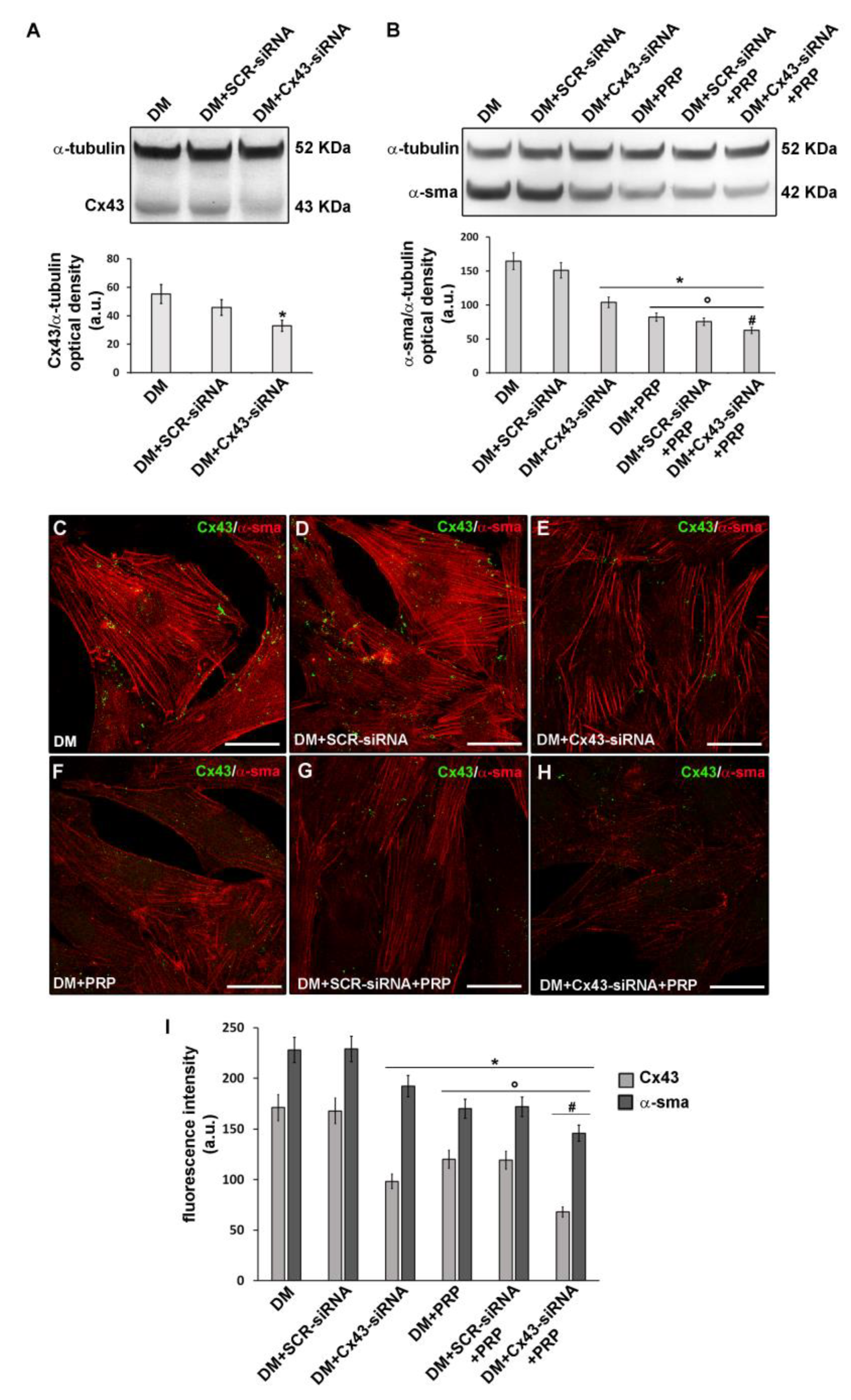
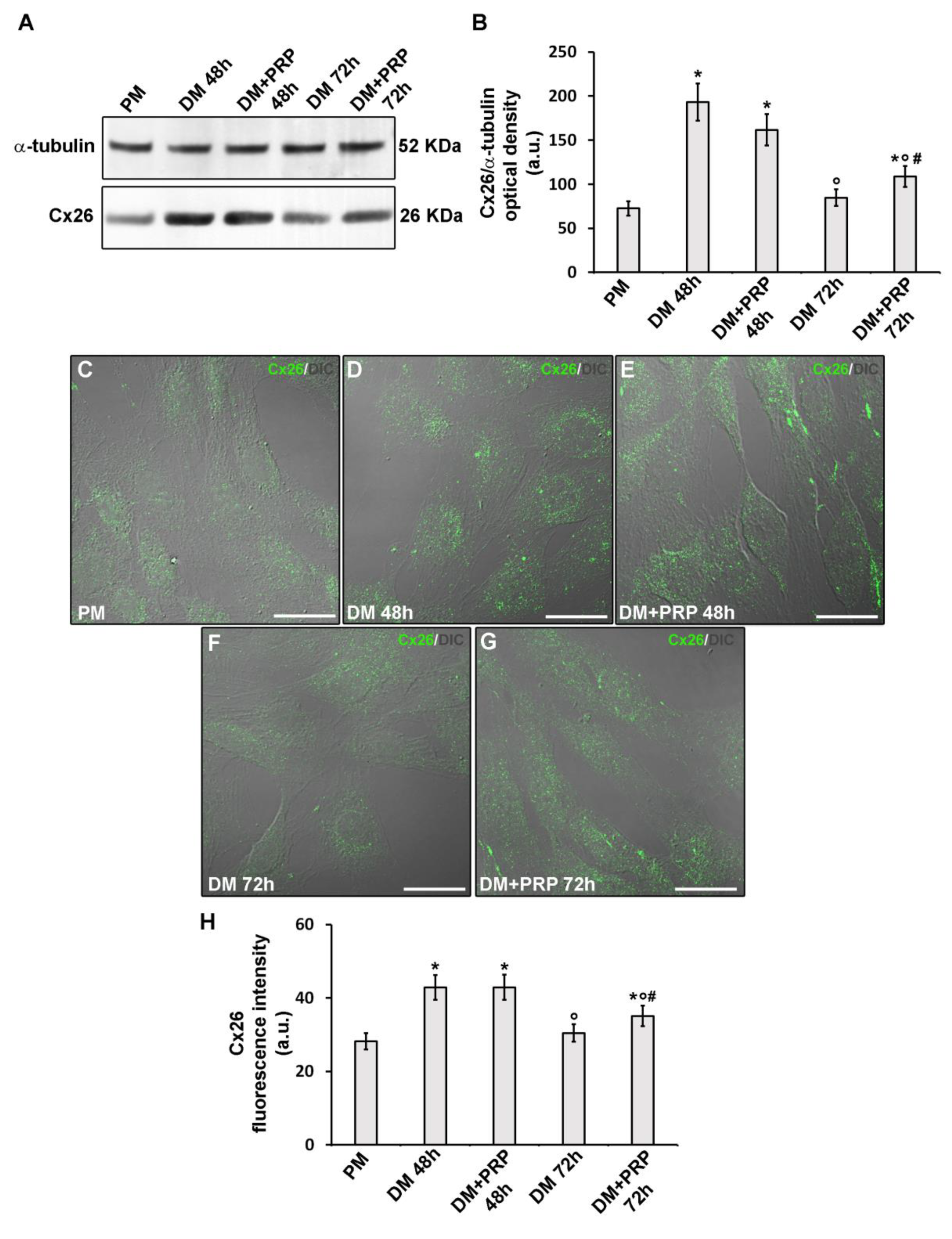
3.3. PRP Reduces Cx43 Expression and Increases Cx26 Expression in Differentiated Myofibroblasts
4. Discussion
4.1. PRP Counteracts Myofibroblast Generation
4.2. Role of GJIC and Cx43 in Myofibroblast Generation
4.3. GJs, Cx43, and Cx26 as Molecular Candidate Targets of the Potential Anti-fibrotic Action of PRP
4.4. Factors Released by PRP Possibly Modulating Cx Expression and GJ Functionality During Myofibroblast Generation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef]
- Doyle, M.J.; Zhou, S.; Tanaka, K.K.; Pisconti, A.; Farina, N.H.; Sorrentino, B.P.; Olwin, B.B. Abcg2 labels multiple cell types in skeletal muscle and participates in muscle regeneration. J. Cell. Biol. 2011, 195, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Judson, R.N.; Zhang, R.H.; Rossi, F.M. Tissue-resident mesenchymal stem/progenitor cells in skeletal muscle: Collaborators or saboteurs? FEBS J. 2013, 280, 4100–4108. [Google Scholar] [CrossRef]
- Meng, J.; Chun, S.; Asfahani, R.; Lochmüller, H.; Muntoni, F.; Morgan, J. Human skeletal muscle-derived CD133(+) cells form functional satellite cells after intramuscular transplantation in immunodeficient host mice. Mol. Ther. 2014, 22, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, B.M.; Sica, G.; Musarò, A. Stem Cells and Tissue Niche: Two Faces of the Same Coin of Muscle Regeneration. Eur. J. Transl. Myol. 2016, 26, 6125. [Google Scholar] [CrossRef] [PubMed]
- Tonlorenzi, R.; Rossi, G.; Messina, G. Isolation and Characterization of Vessel-Associated Stem/Progenitor Cells from Skeletal Muscle. Methods Mol. Biol. 2017, 1556, 149–177. [Google Scholar] [CrossRef] [PubMed]
- Dumont, N.A.; Wang, Y.X.; Rudnicki, M.A. Intrinsic and extrinsic mechanisms regulating satellite cell function. Development 2015, 142, 1572–1581. [Google Scholar] [CrossRef]
- Forcina, L.; Miano, C.; Pelosi, L.; Musarò, A. An Overview about the Biology of Skeletal Muscle Satellite Cells. Curr. Genomics 2019, 20, 24–37. [Google Scholar] [CrossRef]
- Sacco, A.; Puri, P.L. Regulation of Muscle Satellite Cell Function in Tissue Homeostasis and Aging. Cell Stem Cell. 2015, 16, 585–587. [Google Scholar] [CrossRef]
- Mann, C.J.; Perdiguero, E.; Kharraz, Y.; Aguilar, S.; Serrano, A.L.; Muñoz-Cánoves, P. Aberrant repair and fibrosis development in skeletal muscle. Skelet. Muscle 2011, 1, 21. [Google Scholar] [CrossRef]
- Farup, J.; Madaro, L.; Puri, P.L.; Mikkelsen, U.R. Interactions between muscle stem cells, mesenchymal-derived cells and immune cells in muscle homeostasis, regeneration and disease. Cell Death Dis. 2015, 6, e1830. [Google Scholar] [CrossRef] [PubMed]
- Ceafalan, L.C.; Fertig, T.E.; Popescu, A.C.; Popescu, B.O.; Hinescu, M.E.; Gherghiceanu, M. Skeletal muscle regeneration involves macrophage-myoblast bonding. Cell Adhes. Migr. 2018, 12, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Malecova, B.; Gatto, S.; Etxaniz, U.; Passafaro, M.; Cortez, A.; Nicoletti, C.; Giordani, L.; Torcinaro, A.; De Bardi, M.; Bicciato, S.; et al. Dynamics of cellular states of fibro-adipogenic progenitors during myogenesis and muscular dystrophy. Nat. Commun. 2018, 9, 3670. [Google Scholar] [CrossRef] [PubMed]
- Forcina, L.; Miano, C.; Scicchitano, B.M.; Musarò, A. Signals from the Niche: Insights into the Role of IGF-1 and IL-6 in Modulating Skeletal Muscle Fibrosis. Cells 2019, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Manetti, M.; Tani, A.; Rosa, I.; Chellini, F.; Squecco, R.; Idrizaj, E.; Zecchi-Orlandini, S.; Ibba-Manneschi, L.; Sassoli, C. Morphological evidence for telocytes as stromal cells supporting satellite cell activation in eccentric contraction-induced skeletal muscle injury. Sci. Rep. 2019, 9, 14515. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; McCulloch, C.A.; Coelho, N.M. Mechanical regulation of myofibroblast phenoconversion and collagen contraction. Exp. Cell Res. 2019, 379, 119–128. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Mol. Aspects Med. 2019, 65, 2–15. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell. Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Koivumäki, J.T.; Clark, R.B.; Belke, D.; Kondo, C.; Fedak, P.W.; Maleckar, M.M.; Giles, W.R. Na(+) current expression in human atrial myofibroblasts: Identity and functional roles. Front. Physiol. 2014, 5, 275. [Google Scholar] [CrossRef][Green Version]
- Zhan, H.; Zhang, J.; Jiao, A.; Wang, Q. Stretch-activated current in human atrial myocytes and Na+ current and mechano-gated channels’ current in myofibroblasts alter myocyte mechanical behavior: A computational study. Biomed. Eng. Online 2019, 18, 104. [Google Scholar] [CrossRef]
- Chilton, L.; Ohya, S.; Freed, D.; George, E.; Drobic, V.; Shibukawa, Y.; Maccannell, K.A.; Imaizumi, Y.; Clark, R.B.; Dixon, I.M.; et al. K+ currents regulate the resting membrane potential, proliferation, and contractile responses in ventricular fibroblasts and myofibroblasts. Am. J. Physiol. Heart. Circ. Physiol. 2005, 288, 2931–2939. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Zarzoso, M.; Ponce-Balbuena, D.; Guerrero-Serna, G.; Hou, L.; Musa, H.; Jalife, J. TGF-β1, released by myofibroblasts, differentially regulates transcription and function of sodium and potassium channels in adult rat ventricular myocytes. PLoS ONE 2013, 8, e55391. [Google Scholar] [CrossRef] [PubMed]
- Squecco, R.; Sassoli, C.; Garella, R.; Chellini, F.; Idrizaj, E.; Nistri, S.; Formigli, L.; Bani, D.; Francini, F. Inhibitory effects of relaxin on cardiac fibroblast-to-myofibroblast transition: An electrophysiological study. Exp. Physiol. 2015, 100, 652–666. [Google Scholar] [CrossRef] [PubMed]
- Sassoli, C.; Chellini, F.; Squecco, R.; Tani, A.; Idrizaj, E.; Nosi, D.; Giannelli, M.; Zecchi-Orlandini, S. Low intensity 635 nm diode laser irradiation inhibits fibroblast-myofibroblast transition reducing TRPC1 channel expression/activity: New perspectives for tissue fibrosis treatment. Lasers Surg. Med. 2016, 48, 318–332. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care (New Rochelle). 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.I.; Lau, L.F. Resolution of organ fibrosis. J. Clin. Investig. 2018, 128, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.C.; Thannickal, V.J. Mechanisms for the Resolution of Organ Fibrosis. Physiology (Bethesda) 2019, 34, 43–55. [Google Scholar] [CrossRef]
- Bochaton-Piallat, M.L.; Gabbiani, G.; Hinz, B. The myofibroblast in wound healing and fibrosis: Answered and unanswered questions. F1000Research 2016, 5, F1000 Faculty Rev-752. [Google Scholar] [CrossRef]
- Rosenbloom, J.; Macarak, E.; Piera-Velazquez, S.; Jimenez, S.A. Human Fibrotic Diseases: Current Challenges in Fibrosis Research. Methods Mol. Biol. 2017, 1627, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Chellini, F.; Tani, A.; Zecchi-Orlandini, S.; Sassoli, C. Influence of Platelet-Rich and Platelet-Poor Plasma on Endogenous Mechanisms of Skeletal Muscle Repair/Regeneration. Int. J. Mol. Sci. 2019, 20, 683. [Google Scholar] [CrossRef]
- Vu, T.D.; Pal, S.N.; Ti, L.K.; Martinez, E.C.; Rufaihah, A.J.; Ling, L.H.; Lee, C.N.; Richards, A.M.; Kofidis, T. An autologous platelet-rich plasma hydrogel compound restores left ventricular structure, function and ameliorates adverse remodeling in a minimally invasive large animal myocardial restoration model: A translational approach: Vu and Pal “Myocardial Repair: PRP, Hydrogel and Supplements”. Biomaterials 2015, 45, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.Y.; Myoung, S.M.; Choe, J.M.; Kim, T.; Cheon, Y.P.; Kim, Y.M.; Park, H. Effects of autologous platelet-rich plasma on regeneration of damaged endometrium in female rats. Yonsei Med. J. 2017, 58, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, A.; Khozani, T.T.; Mafi, A.; Namavar, M.R.; Dehghani, F. Effects of platelet-rich plasma on kidney regeneration in gentamicin-induced nephrotoxicity. J. Korean Med. Sci. 2017, 32, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Martin, J.I.; Maffulli, N. Advances with platelet rich plasma therapies for tendon regeneration. Expert. Opin. Biol. Ther. 2018, 18, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Avila, R.M.; Merayo-Lloves, J.; Riestra, A.C.; Berisa, S.; Lisa, C.; Sánchez, J.A.; Muruzabal, F.; Orive, G.; Anitua, E. Plasma rich in growth factors membrane as coadjuvant treatment in the surgery of ocular surface disorders. Medicine (Baltimore) 2018, 97, e0242. [Google Scholar] [CrossRef] [PubMed]
- Sayadi, L.R.; Obagi, Z.; Banyard, D.A.; Ziegler, M.E.; Prussak, J.; Tomlinson, L.; Evans, G.R.D.; Widgerow, A.D. Platelet-rich plasma, adipose tissue, and scar modulation. Aesthet. Surg. J. 2018, 38, 1351–1362. [Google Scholar] [CrossRef]
- Shoeib, H.M.; Keshk, W.A.; Foda, A.M.; Abo El Noeman, S.E. A study on the regenerative effect of platelet-rich plasma on experimentally induced hepatic damage in albino rats. Can. J. Physiol. Pharmacol. 2018, 96, 630–636. [Google Scholar] [CrossRef]
- Tavukcu, H.H.; Aytaç, Ö.; Atuğ, F.; Alev, B.; Çevik, Ö.; Bülbül, N.; Yarat, A.; Çetinel, S.; Sener, G.; Kulaksızoğlu, H. Protective effect of platelet-rich plasma on urethral injury model of male rats. Neurourol. Urodyn. 2018, 37, 1286–1293. [Google Scholar] [CrossRef]
- Terada, S.; Ota, S.; Kobayashi, M.; Kobayashi, T.; Mifune, Y.; Takayama, K.; Witt, M.; Vadalà, G.; Oyster, N.; Otsuka, T.; et al. Use of an anti-fibrotic agent improves the effect of platelet-rich plasma on muscle healing after injury. J. Bone Jt. Surg. Am. 2013, 95, 980–988. [Google Scholar] [CrossRef]
- Cunha, R.C.; Francisco, J.C.; Cardoso, M.A.; Matos, L.F.; Lino, D.; Simeoni, R.B.; Pereira, G.; Irioda, A.C.; Simeoni, P.R.; Guarita-Souza, L.C.; et al. Effect of platelet-rich plasma therapy associated with exercise training in musculoskeletal healing in rats. Transpl. Proc. 2014, 46, 1879–1881. [Google Scholar] [CrossRef]
- Anitua, E.; Pelacho, B.; Prado, R.; Aguirre, J.J.; Sánchez, M.; Padilla, S.; Aranguren, X.L.; Abizanda, G.; Collantes, M.; Hernandez, M.; et al. Infiltration of plasma rich in growth factors enhances in vivo angiogenesis and improves reperfusion and tissue remodeling after severe hind limb ischemia. J. Control. Release 2015, 202, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Cianforlini, M.; Mattioli-Belmonte, M.; Manzotti, S.; Chiurazzi, E.; Piani, M.; Orlando, F.; Provinciali, M.; Gigante, A. Effect of platelet rich plasma concentration on skeletal muscle regeneration: An experimental study. J. Biol. Regul. Homeost. Agents 2015, 29, 47–55. [Google Scholar] [PubMed]
- Denapoli, P.M.; Stilhano, R.S.; Ingham, S.J.; Han, S.W.; Abdalla, R.J. Platelet-Rich Plasma in a Murine Model: Leukocytes, Growth Factors, Flt-1, and Muscle Healing. Am. J. Sports Med. 2016, 44, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hicks, J.J.; Wang, L.; Oyster, N.; Philippon, M.J.; Hurwitz, S.; Hogan, M.V.; Huard, J. Customized platelet-rich plasma with transforming growth factor β1 neutralization antibody to reduce fibrosis in skeletal muscle. Biomaterials 2016, 87, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zanon, G.; Combi, F.; Combi, A.; Perticarini, L.; Sammarchi, L.; Benazzo, F. Platelet-rich plasma in the treatment of acute hamstring injuries in professional football players. Joints 2016, 4, 17–23. [Google Scholar] [CrossRef]
- Contreras-Muñoz, P.; Torrella, J.R.; Serres, X.; Rizo-Roca, D.; De la Varga, M.; Viscor, G.; Martínez-Ibáñez, V.; Peiró, J.L.; Järvinen, T.A.H.; Rodas, G.; et al. Postinjury Exercise and Platelet-Rich Plasma Therapies Improve Skeletal Muscle Healing in Rats But Are Not Synergistic When Combined. Am. J. Sports Med. 2017, 45, 2131–2141. [Google Scholar] [CrossRef]
- Anitua, E.; Troya, M.; Orive, G. Plasma rich in growth factors promote gingival tissue regeneration by stimulating fibroblast proliferation and migration and by blocking transforming growth factor-β1-induced myodifferentiation. J. Periodontol. 2012, 83, 1028–1037. [Google Scholar] [CrossRef]
- Chellini, F.; Tani, A.; Vallone, L.; Nosi, D.; Pavan, P.; Bambi, F.; Zecchi Orlandini, S.; Sassoli, C. Platelet-Rich Plasma Prevents In Vitro Transforming Growth Factor-β1- Induced Fibroblast to Myofibroblast Transition: Involvement of Vascular Endothelial Growth Factor (VEGF)-A/VEGF Receptor- 1-Mediated Signaling. Cells 2018, 7, 142. [Google Scholar] [CrossRef]
- Willebrords, J.; Crespo Yanguas, S.; Maes, M.; Decrock, E.; Wang, N.; Leybaert, L.; da Silva, T.C.; Veloso Alves Pereira, I.; Jaeschke, H.; Cogliati, B.; et al. Structure, Regulation and Function of Gap Junctions in Liver. Cell. Commun. Adhes. 2015, 22, 29–37. [Google Scholar] [CrossRef]
- Valiunas, V.; Gemel, J.; Brink, P.R.; Beyer, E.C. Gap junction channels formed by coexpressed connexin40 and connexin43. Am. J. Physiol. Heart. Circ. Physiol. 2001, 281, H1675–H1689. [Google Scholar] [CrossRef]
- Gaudesius, G.; Miragoli, M.; Thomas, S.P.; Rohr, S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ. Res. 2003, 93, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Chilton, L.; Giles, W.R.; Smith, G.L. Evidence of intercellular coupling between co-cultured adult rabbit ventricular myocytes and myofibroblasts. J. Physiol. 2007, 583, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Zlochiver, S.; Muñoz, V.; Vikstrom, K.L.; Taffet, S.M.; Berenfeld, O.; Jalife, J. Electrotonic myofibroblast-to-myocyte coupling increases propensity to reentrant arrhythmias in two-dimensional cardiac monolayers. Biophys. J. 2008, 95, 4469–4480. [Google Scholar] [CrossRef]
- Kakkar, R.; Lee, R.T. Intramyocardial fibroblast myocyte communication. Circ. Res. 2010, 106, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Sassoli, C.; Chellini, F.; Pini, A.; Tani, A.; Nistri, S.; Nosi, D.; Zecchi-Orlandini, S.; Bani, D.; Formigli, L. Relaxin prevents cardiac fibroblast-myofibroblast transition via notch-1-mediated inhibition of TGF-β/Smad3 signaling. PLoS ONE 2013, 8, e63896. [Google Scholar] [CrossRef] [PubMed]
- Sassoli, C.; Nosi, D.; Tani, A.; Chellini, F.; Mazzanti, B.; Quercioli, F.; Zecchi-Orlandini, S.; Formigli, L. Defining the role of mesenchymal stromal cells on the regulation of matrix metalloproteinases in skeletal muscle cells. Exp. Cell Res. 2014, 323, 297–313. [Google Scholar] [CrossRef]
- Chellini, F.; Tani, A.; Vallone, L.; Nosi, D.; Pavan, P.; Bambi, F.; Zecchi-Orlandini, S.; Sassoli, C. Platelet-Rich Plasma and Bone Marrow-Derived Mesenchymal Stromal Cells Prevent TGF-β1-Induced Myofibroblast Generation but Are Not Synergistic when Combined: Morphological in vitro Analysis. Cells Tissues Organs 2018, 206, 283–295. [Google Scholar] [CrossRef]
- Sassoli, C.; Vallone, L.; Tani, A.; Chellini, F.; Nosi, D.; Zecchi-Orlandini, S. Combined use of bone marrow-derived mesenchymal stromal cells (BM-MSCs) and platelet rich plasma (PRP) stimulates proliferation and differentiation of myoblasts in vitro: New therapeutic perspectives for skeletal muscle repair/regeneration. Cell Tissue Res. 2018, 372, 549–570. [Google Scholar] [CrossRef]
- Squecco, R.; Sassoli, C.; Nuti, F.; Martinesi, M.; Chellini, F.; Nosi, D.; Zecchi-Orlandini, S.; Francini, F.; Formigli, L.; Meacci, E. Sphingosine 1-phosphate induces myoblast differentiation through Cx43 protein expression: A role for a gap junction-dependent and -independent function. Mol. Biol. Cell. 2006, 17, 4896–4910. [Google Scholar] [CrossRef]
- Formigli, L.; Sassoli, C.; Squecco, R.; Bini, F.; Martinesi, M.; Chellini, F.; Luciani, G.; Sbrana, F.; Zecchi-Orlandini, S.; Francini, F.; et al. Regulation of transient receptor potential canonical channel 1 (TRPC1) by sphingosine 1-phosphate in C2C12 myoblasts and its relevance for a role of mechanotransduction in skeletal muscle differentiation. J. Cell. Sci. 2009, 122, 1322–1333. [Google Scholar] [CrossRef]
- Pierucci, F.; Frati, A.; Squecco, R.; Lenci, E.; Vicenti, C.; Slavik, J.; Francini, F.; Machala, M. Meacci, E. Non-dioxin-like organic toxicant PCB153 modulates sphingolipid metabolism in liver progenitor cells: Its role in Cx43-formed gap junction impairment. Arch. Toxicol. 2017, 91, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Sassoli, C.; Formigli, L.; Bini, F.; Tani, A.; Squecco, R.; Battistini, C.; Zecchi-Orlandini, S.; Francini, F.; Meacci, E. Effects of S1P on skeletal muscle repair/regeneration during eccentric contraction. J. Cell. Mol. Med. 2011, 15, 2498–2511. [Google Scholar] [CrossRef] [PubMed]
- Pappone, P.A.; Lee, S.C. Purinergic receptor stimulation increases membrane trafficking in brown adipocytes. J. Gen. Physiol. 1996, 108, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Formigli, L.; Meacci, E.; Sassoli, C.; Chellini, F.; Giannini, R.; Quercioli, F.; Tiribilli, B.; Squecco, R.; Bruni, P.; Francini, F.; et al. Sphingosine 1-phosphate induces cytoskeletal reorganization in C2C12 myoblasts: Physiological relevance for stress fibres in the modulation of ion current through stretch-activated channels. J. Cell. Sci. 2005, 118, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Meacci, E.; Bini, F.; Sassoli, C.; Martinesi, M.; Squecco, R.; Chellini, F.; Zecchi-Orlandini, S.; Francini, F.; Formigli, L. Functional interaction between TRPC1 channel and connexin-43 protein: A novel pathway underlying S1P action on skeletal myogenesis. Cell. Mol. Life Sci. 2010, 67, 4269–4285. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Abate, M. Platelet-rich plasma in the treatment of skeletal muscle injuries. Expert. Opin. Biol. 2015, 15, 987–999. [Google Scholar] [CrossRef]
- Cole, B.J.; Seroyer, S.T.; Filardo, G.; Bajaj, S.; Fortier, L.A. Platelet-rich plasma: Where are we now and where are we going? Sports Health 2010, 2, 203–210. [Google Scholar] [CrossRef]
- Delos, D.; Leineweber, M.J.; Chaudhury, S.; Alzoobaee, S.; Gao, Y.; Rodeo, S.A. The effect of platelet-rich plasma on muscle contusion healing in a rat model. Am. J. Sports Med. 2014, 42, 2067–2074. [Google Scholar] [CrossRef]
- Dimauro, I.; Grasso, L.; Fittipaldi, S.; Fantini, C.; Mercatelli, N.; Racca, S.; Geuna, S.; Di Gianfrancesco, A.; Caporossi, D.; Pigozzi, F.; et al. Platelet-rich plasma and skeletal muscle healing: A molecular analysis of the early phases of the regeneration process in an experimental animal model. PLoS ONE 2014, 9, e102993. [Google Scholar] [CrossRef]
- Reurink, G.; Goudswaard, G.J.; Moen, M.H.; Weir, A.; Verhaar, J.A.; Bierma-Zeinstra, S.M.; Maas, M.; Tol, J.L. Dutch HIT-study Investigators. Rationale, secondary outcome scores and 1-year follow-up of a randomised trial of platelet-rich plasma injections in acute hamstring muscle injury: The Dutch Hamstring Injection Therapy study. Br. J. Sports Med. 2015, 49, 1206–1212. [Google Scholar] [CrossRef]
- Kelc, R.; Vogrin, M. Concerns about fibrosis development after scaffolded PRP therapy of muscle injuries: Commentary on an article by Sanchez et al.: Muscle repair: Platelet-rich plasma derivates as a bridge from spontaneity to intervention. Injury 2015, 46, 428. [Google Scholar] [CrossRef]
- Guillodo, Y.; Madouas, G.; Simon, T.; Le Dauphin, H.; Saraux, A. Platelet-rich plasma (PRP) treatment of sports-related severe acute hamstring injuries. Muscles Ligaments Tendons J. 2016, 5, 284–288. [Google Scholar] [CrossRef]
- Grassi, A.; Napoli, F.; Romandini, I.; Samuelsson, K.; Zaffagnini, S.; Candrian, C.; Filardo, G. Is Platelet-Rich Plasma (PRP) Effective in the Treatment of Acute Muscle Injuries? A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 971–989. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.A.; Molina Rómoli, A.R.; Bertona Altieri, B.A.; Burgos Flor, J.A.; Scordo, W.E.; Elizondo, C.M. Does platelet-rich plasma decrease time to return to sports in acute muscle tear? A randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 3319–3325. [Google Scholar] [CrossRef] [PubMed]
- Tonogai, I.; Hayashi, F.; Iwame, T.; Takasago, T.; Matsuura, T.; Sairyo, K. Platelet-rich plasma does not reduce skeletal muscle fibrosis after distraction osteogenesis. J Exp Orthop. 2018, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; de la Fuente, M.; Muruzabal, F.; Riestra, A.; Merayo-Lloves, J.; Orive, G. Plasma rich in growth factors (PRGF) eye drops stimulates scarless regeneration compared to autologous serum in the ocular surface stromal fibroblasts. Exp. Eye Res. 2015, 135, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Van der Bijl, I.; Vlig, M.; Middelkoop, E.; de Korte, D. Allogeneic platelet-rich plasma (PRP) is superior to platelets or plasma alone in stimulating fibroblast proliferation and migration, angiogenesis, and chemotaxis as relevant processes for wound healing. Transfusion 2019, 59, 3492–3500. [Google Scholar] [CrossRef]
- Verhoekx, J.S.N.; Verjee, L.S.; Izadi, D.; Chan, J.K.K.; Nicolaidou, V.; Davidson, D.; Midwood, K.S.; Nanchahal, J. Isometric contraction of Dupuytren’s myofibroblasts is inhibited by blocking intercellular junctions. J. Investig. Dermatol. 2013, 133, 2664–2671. [Google Scholar] [CrossRef]
- Tarzemany, R.; Jiang, G.; Jiang, J.X.; Larjava, H.; Häkkinen, L. Connexin 43 Hemichannels Regulate the Expression of Wound Healing-Associated Genes in Human Gingival Fibroblasts. Sci. Rep. 2017, 7, 14157. [Google Scholar] [CrossRef]
- Au, S.R.; Au, K.; Saggers, G.C.; Karne, N.; Ehrlich, H.P. Rat mast cells communicate with fibroblasts via gap junction intercellular communications. J. Cell. Biochem. 2007, 100, 1170–1177. [Google Scholar] [CrossRef]
- Pistorio, A.L.; Ehrlich, H.P. Modulatory effects of connexin-43 expression on gap junction intercellular communications with mast cells and fibroblasts. J. Cell. Biochem. 2011, 112, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Foley, T.T.; Ehrlich, H.P. Through gap junction communications, co-cultured mast cells and fibroblasts generate fibroblast activities allied with hypertrophic scarring. Plast. Reconstr. Surg. 2013, 131, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Santos-Miranda, A.; Noureldin, M.; Bai, D. Effects of temperature on transjunctional voltage-dependent gating kinetics in Cx45 and Cx40 gap junction channels. J. Mol. Cell. Cardiol. 2019, 127, 185–193. [Google Scholar] [CrossRef]
- Lastwika, K.J.; Dunn, C.A.; Solan, J.L.; Lampe, P.D. Phosphorylation of connexin 43 at MAPK, PKC or CK1 sites each distinctly alter the kinetics of epidermal wound repair. J. Cell. Sci. 2019, 132, jcs234633. [Google Scholar] [CrossRef]
- Asazuma-Nakamura, Y.; Dai, P.; Harada, Y.; Jiang, Y.; Hamaoka, K.; Takamatsu, T. Cx43 contributes to TGF-beta signaling to regulate differentiation of cardiac fibroblasts into myofibroblasts. Exp. Cell. Res. 2009, 315, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Paw, M.; Borek, I.; Wnuk, D.; Ryszawy, D.; Piwowarczyk, K.; Kmiotek, K.; Wójcik-Pszczoła, K.A.; Pierzchalska, M.; Madeja, Z.; Sanak, M.; et al. Connexin43 Controls the Myofibroblastic Differentiation of Bronchial Fibroblasts from Patients with Asthma. Am. J. Respir. Cell. Mol. Biol. 2017, 57, 100–110. [Google Scholar] [CrossRef]
- Coutinho, P.; Qiu, C.; Frank, S.; Tamber, K.; Becker, D. Dynamic changes in connexin expression correlate with key events in the wound healing process. Cell. Biol. Int. 2003, 27, 525–541. [Google Scholar] [CrossRef]
- Kretz, M.; Euwens, C.; Hombach, S.; Eckardt, D.; Teubner, B.; Traub, O.; Willecke, K.; Ott, T. Altered connexin expression and wound healing in the epidermis of connexin-deficient mice. J. Cell. Sci. 2003, 116, 3443–3452. [Google Scholar] [CrossRef]
- Qiu, C.; Coutinho, P.; Frank, S.; Franke, S.; Law, L.Y.; Martin, P.; Green, C.R.; Becker, D.L. Targeting connexin43 expression accelerates the rate of wound repair. Curr. Biol. 2003, 13, 1697–1703. [Google Scholar] [CrossRef]
- Nakano, Y.; Oyamada, M.; Dai, P.; Nakagami, T.; Kinoshita, S.; Takamatsu, T. Connexin43 knockdown accelerates wound healing but inhibits mesenchymal transition after corneal endothelial injury in vivo. Investig. Ophthalmol. Vis. Sci. 2008, 49, 93–104. [Google Scholar] [CrossRef]
- Montgomery, J.; Ghatnekar, G.S.; Grek, C.L.; Moyer, K.E.; Gourdie, R.G. Connexin 43-Based Therapeutics for Dermal Wound Healing. Int. J. Mol. Sci. 2018, 19, 1778. [Google Scholar] [CrossRef]
- Cogliati, B.; Mennecier, G.; Willebrords, J.; Da Silva, T.C.; Maes, M.; Pereira, I.V.A.; Crespo-Yanguas, S.; Hernandez-Blazquez, F.J.; Dagli, M.L.Z.; Vinken, M. Connexins, Pannexins, and Their Channels in Fibroproliferative Diseases. J. Membr. Biol. 2016, 249, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.W.; Lampe, P.D. Therapeutic strategies targeting connexins. Nat. Rev. Drug. Discov. 2018, 17, 905–921. [Google Scholar] [CrossRef] [PubMed]
- Kameritsch, P.; Pogoda, K.; Pohl, U. Channel-independent influence of connexin 43 on cell migration. Biochim. Biophys. Acta. 2012, 1818, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Riteau, N.; Gasse, P.; Fauconnier, L.; Gombault, A.; Couegnat, M.; Fick, L.; Kanellopoulos, J.; Quesniaux, V.F.; Marchand-Adam, S.; Crestani, B.; et al. Extracellular ATP Is a Danger Signal Activating P2X7 Receptor in Lung Inflammation and Fibrosis. Am. J. Respir. Crit. Care Med. 2010, 182, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Soleymani, S.; Madakshire, R.; Insel, P.A. ATP released from cardiac fibroblasts via connexin hemichannels activates profibrotic P2Y2 receptor. FASEB 2012, 26, 2580–2591. [Google Scholar] [CrossRef]
- Ferrari, D.; Gambari, R.; Idzko, M.; Müller, T.; Albanesi, C.; Pastore, S.; La Manna, G.; Robson, S.C.; Cronstein, B. Purinergic signaling in scarring. FASEB J. 2016, 30, 3–12. [Google Scholar] [CrossRef]
- Hills, C.; Price, G.W.; Wall, M.J.; Kaufmann, T.J.; Chi-Wai Tang, S.; Yiu, W.H.; Squires, P.E. Transforming Growth Factor Beta 1 Drives a Switch in Connexin Mediated Cell-to-Cell Communication in Tubular Cells of the Diabetic Kidney. Cell. Physiol. Biochem. 2018, 45, 2369–2388. [Google Scholar] [CrossRef]
- Iyer, R.K.; Odedra, D.; Chiu, L.L.; Vunjak-Novakovic, G.; Radisic, M. Vascular endothelial growth factor secretion by nonmyocytes modulates Connexin-43 levels in cardiac organoids. Tissue Eng. Part. A. 2012, 18, 1771–1783. [Google Scholar] [CrossRef]
- Wuestefeld, R.; Chen, J.; Meller, K.; Brand-Saberi, B.; Theiss, C. Impact of vegf on astrocytes: Analysis of gap junctional intercellular communication, proliferation, and motility. Glia 2012, 60, 936–947. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; Xu, C.; Deng, M.; Song, M.; Yu, X.; Xu, S.; Zhao, X. VEGF promotes endothelial progenitor cell differentiation and vascular repair through connexin 43. Stem. Cell. Res. Ther. 2017, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Boeldt, D.S.; Grummer, M.A.; Yi, F.; Magness, R.R.; Bird, I.M. Phosphorylation of Ser-279/282 and Tyr-265 positions on Cx43 as possible mediators of VEGF-165 inhibition of pregnancy-adapted Ca2+ burst function in ovine uterine artery endothelial cells. Mol. Cell. Endocrinol. 2015, 412, 73–84. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nimlamool, W.; Andrews, R.M.; Falk, M.M. Connexin43 phosphorylation by PKC and MAPK signals VEGF-mediated gap junction internalization. Mol. Biol. Cell. 2015, 26, 2755–2768. [Google Scholar] [CrossRef]
- Solan, J.L.; Lampe, P.D. Spatio-temporal regulation of connexin43 phosphorylation and gap junction dynamics. Biochim. Biophys. Acta Biomembr. 2018, 1860, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Boyle, D.L.; Takemoto, D.J. IGF-I-induced phosphorylation of connexin 43 by PKCgamma: Regulation of gap junctions in rabbit lens epithelial cells. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; An, N.; Ouyang, X. Quantification of growth factors in different platelet concentrates. Platelets 2017, 28, 774–778. [Google Scholar] [CrossRef]
- Dong, Y.; Lakhia, R.; Thomas, S.S.; Dong, Y.; Wang, X.H.; Silva, K.A.; Zhang, L. Interactions between p-Akt and Smad3 in injured muscles initiate myogenesis or fibrogenesis. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E367–E375. [Google Scholar] [CrossRef]
- Andrade, D.; Oliveira, G.; Menezes, L.; Nascimento, A.L.; Carvalho, S.; Stumbo, A.C.; Thole, A.; Garcia-Souza, É.; Moura, A.; Carvalho, L.; et al. Insulin-like growth factor-1 short-period therapy improves cardiomyopathy stimulating cardiac progenitor cells survival in obese mice. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 151–161. [Google Scholar] [CrossRef]
- Sassoli, C.; Pierucci, F.; Zecchi-Orlandini, S.; Meacci, E. Sphingosine 1-Phosphate (S1P)/ S1P Receptor Signaling and Mechanotransduction: Implications for Intrinsic Tissue Repair/Regeneration. Int. J. Mol. Sci. 2019, 20, 5545. [Google Scholar] [CrossRef]
- Lynch, M.D.; Watt, F.M. Fibroblast heterogeneity: Implications for human disease. J. Clin. Investig. 2018, 128, 26–35. [Google Scholar] [CrossRef]
- Foote, A.G.; Wang, Z.; Kendziorski, C.; Thibeault, S.L. Tissue specific human fibroblast differential expression based on RNA sequencing analysis. BMC Genomics 2019, 20, 308. [Google Scholar] [CrossRef] [PubMed]
- LeBleu, V.S.; Neilson, E.G. Origin and functional heterogeneity of fibroblasts. FASEB J. 2020, 34, 3519–3536. [Google Scholar] [CrossRef] [PubMed]
| PM | DM 48 h | DM + PRP 48 h | DM 72 h | DM + PRP 72 h | |
|---|---|---|---|---|---|
| RMP (mV) | −62.0 ± 6.8 (n = 6) | −41.7 ± 3.75 (n = 5) | −50.2 ± 8.9 (n = 7) | −45.16 ± 4.06 (n = 7) | −47.37 ± 3.1 (n = 6) |
| Rm (MῺ) | 159.2 ± 31.0 (n = 6) | 353.8 ± 85.9 (n = 5) | 164.7 ± 17.3 (n = 14) | 585.3 ± 100.9 *,# (n=11) | 436.2 ± 71.3 *,# (n=15) |
| Cm (pF) | 7.05 ± 1.2 (n = 6) | 23.5 ± 4.7 * (n = 9) | 6.82 ± 0.5 § (n = 9) | 17.6 ± 2.9 *,# (n = 8) | 8.3 ± 0.9 (n = 14) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Squecco, R.; Chellini, F.; Idrizaj, E.; Tani, A.; Garella, R.; Pancani, S.; Pavan, P.; Bambi, F.; Zecchi-Orlandini, S.; Sassoli, C. Platelet-Rich Plasma Modulates Gap Junction Functionality and Connexin 43 and 26 Expression During TGF-β1–Induced Fibroblast to Myofibroblast Transition: Clues for Counteracting Fibrosis. Cells 2020, 9, 1199. https://doi.org/10.3390/cells9051199
Squecco R, Chellini F, Idrizaj E, Tani A, Garella R, Pancani S, Pavan P, Bambi F, Zecchi-Orlandini S, Sassoli C. Platelet-Rich Plasma Modulates Gap Junction Functionality and Connexin 43 and 26 Expression During TGF-β1–Induced Fibroblast to Myofibroblast Transition: Clues for Counteracting Fibrosis. Cells. 2020; 9(5):1199. https://doi.org/10.3390/cells9051199
Chicago/Turabian StyleSquecco, Roberta, Flaminia Chellini, Eglantina Idrizaj, Alessia Tani, Rachele Garella, Sofia Pancani, Paola Pavan, Franco Bambi, Sandra Zecchi-Orlandini, and Chiara Sassoli. 2020. "Platelet-Rich Plasma Modulates Gap Junction Functionality and Connexin 43 and 26 Expression During TGF-β1–Induced Fibroblast to Myofibroblast Transition: Clues for Counteracting Fibrosis" Cells 9, no. 5: 1199. https://doi.org/10.3390/cells9051199
APA StyleSquecco, R., Chellini, F., Idrizaj, E., Tani, A., Garella, R., Pancani, S., Pavan, P., Bambi, F., Zecchi-Orlandini, S., & Sassoli, C. (2020). Platelet-Rich Plasma Modulates Gap Junction Functionality and Connexin 43 and 26 Expression During TGF-β1–Induced Fibroblast to Myofibroblast Transition: Clues for Counteracting Fibrosis. Cells, 9(5), 1199. https://doi.org/10.3390/cells9051199






