Molecular Chaperones in Cancer Stem Cells: Determinants of Stemness and Potential Targets for Antitumor Therapy
Abstract
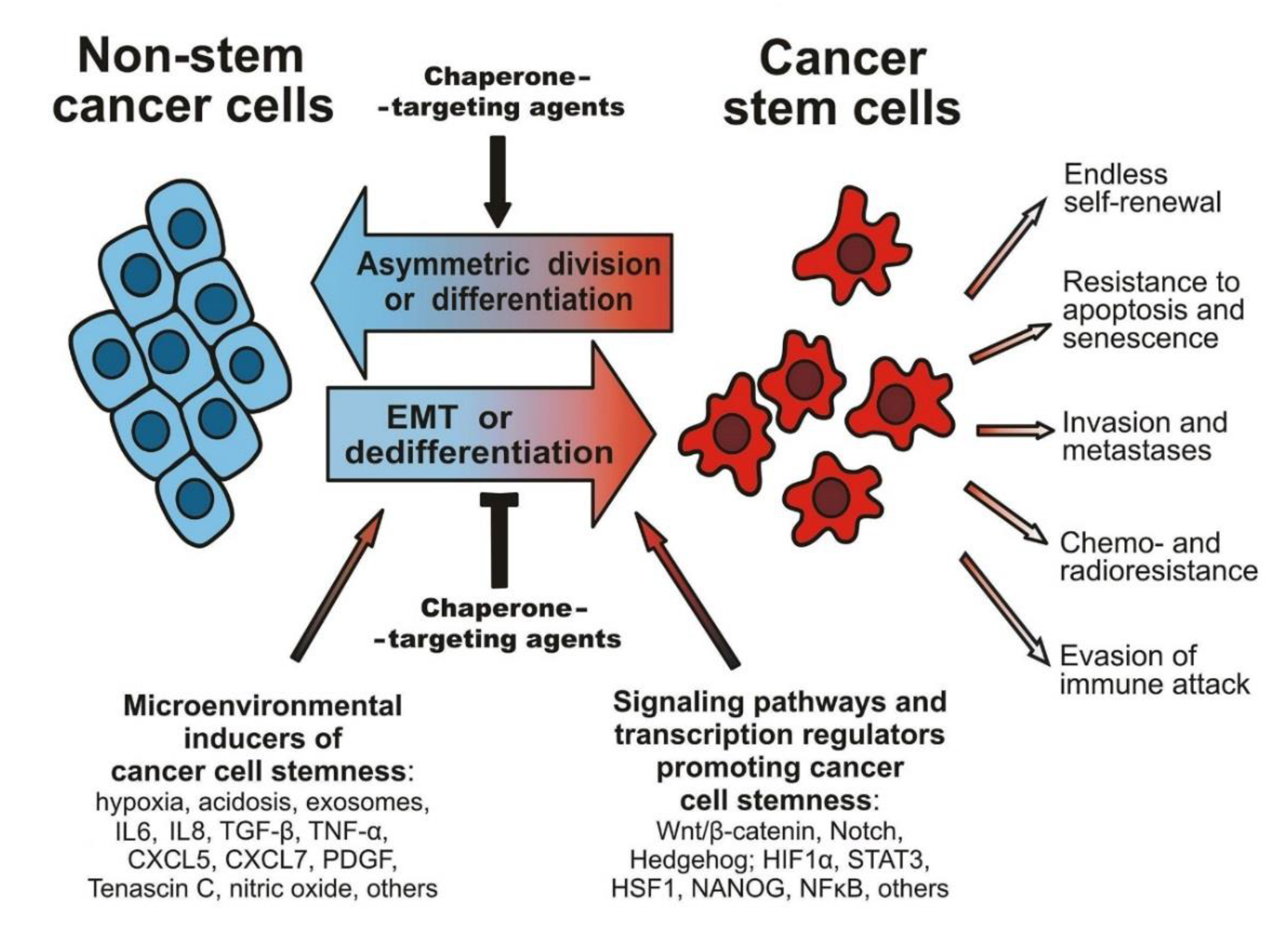
1. Overviewing Introduction: Cancer-Associated Stemness, Its Molecular Basis, and a Role in the Pathogenesis
1.1. Tumorigenicity of CSCs and Maintaining the Cancer Stemness
1.2. EMT and Other Factors Affecting the CSC Phenotype
1.3. Resistance of CSCs to Therapeutics, Immune Attack, and Stressful Conditions
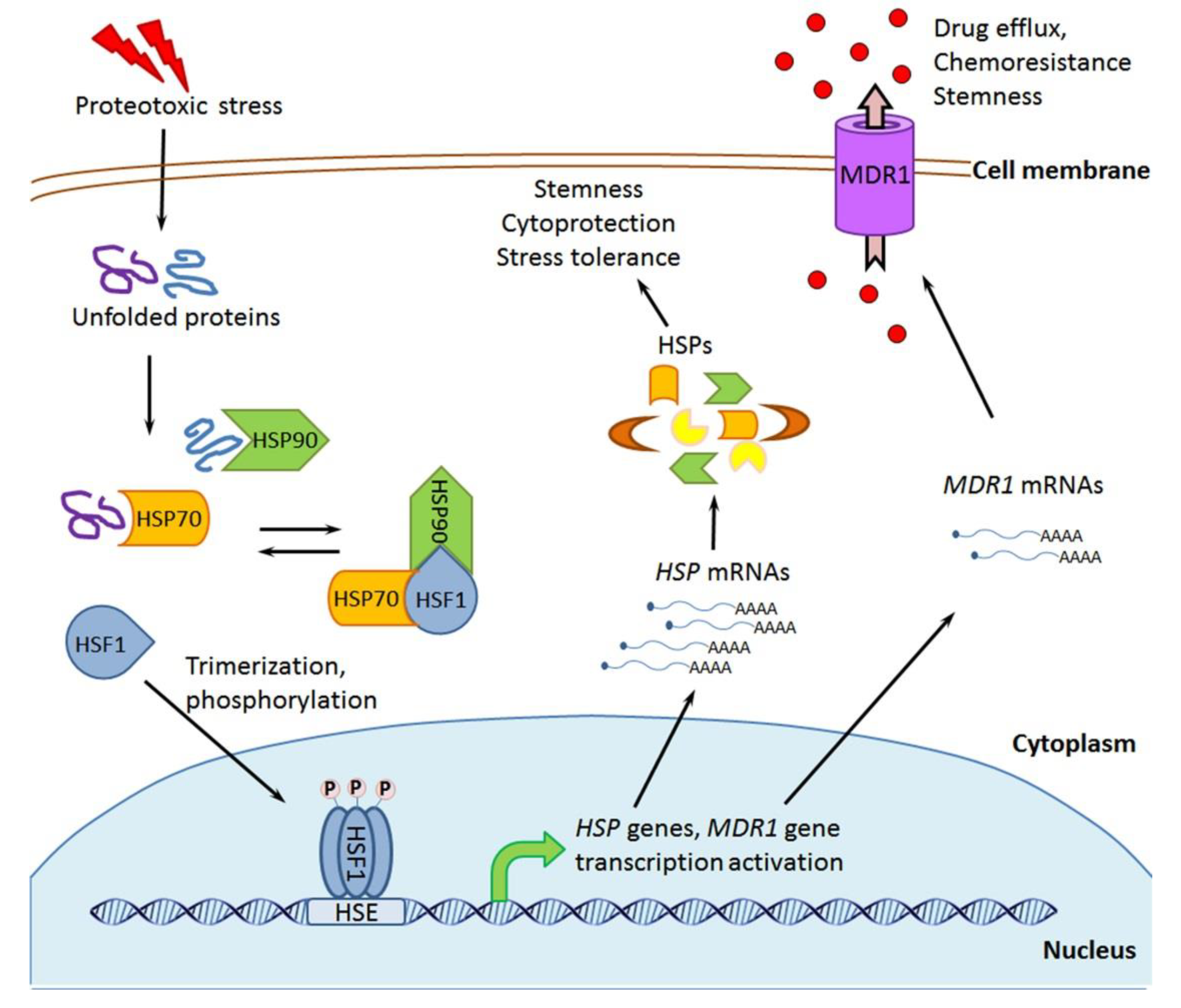
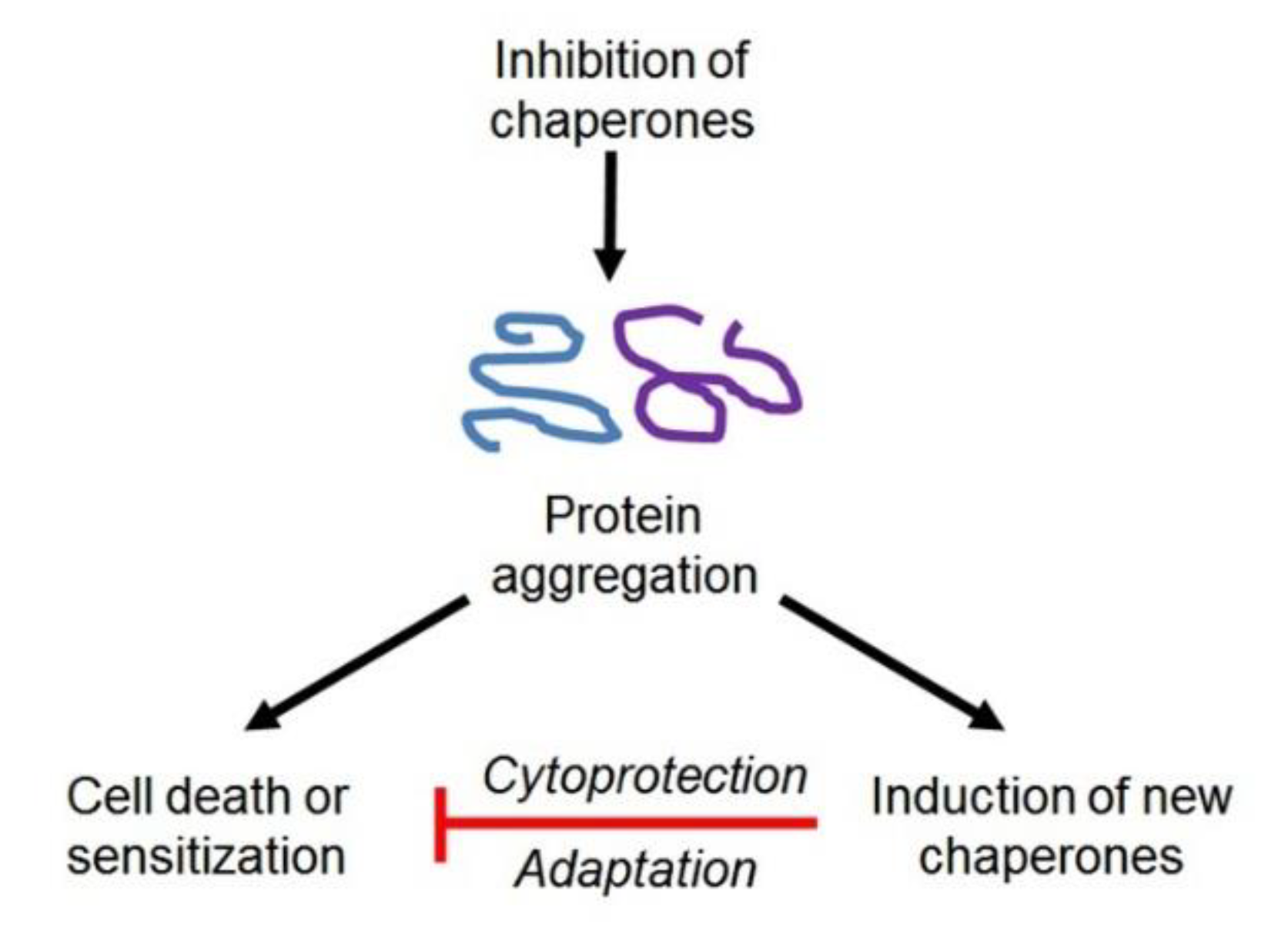
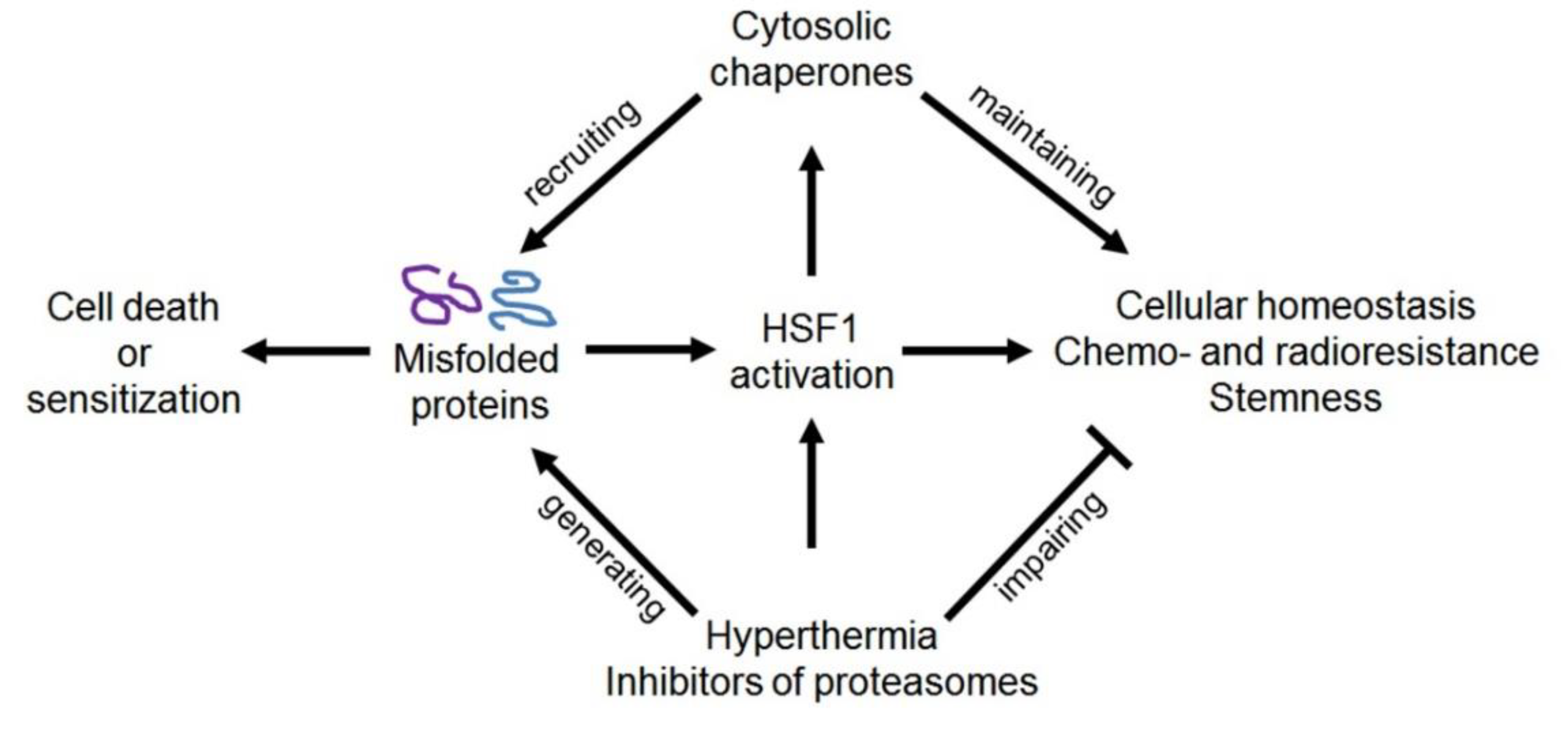
2. Molecular Chaperones: Localization, Activities, and Implication in Cellular Stress Responses
3. Contribution of HSPs, HSF1, and Immunophilins to the Regulation of the CSC Phenotype, and Approaches to Overcoming Chaperone- or HSF1-Conferred Cancer Stemness
3.1. HSP90
3.1.1. Intracellular HSP90 and Some of Its Partners in Chaperoning Client Proteins
Targeting Intracellular HSP90
3.1.2. Extracellular HSP90
Targeting Extracellular HSP90
3.2. HSP70
3.2.1. Intracellular HSP70
Targeting Intracellular HSP70
3.2.2. Extracellular and Cell Surface-Bound HSP70
3.3. HSP40
3.4. HSP27
Targeting HSP27
3.5. HSF1 and HSF1-Activating Exposure
Targeting HSF1
3.6. Immunophilins and Immunophilin-Like Peptidyl-Prolyl Isomerases
Targeting Peptidyl-Prolyl Isomerases and Use of FKBPL-Derived Peptides
4. Contribution of GRPs, TRAP1, Protein Disulfide Isomerases, and Calreticulin to the Regulation of the CSC Phenotype, and also Approaches to Overcoming Their Cancer Stemness-Promoting Activities
4.1. GRP94
4.2. GRP78
4.2.1. Intracellular GRP78
Targeting Intracellular GRP78
4.2.2. Extracellular (Plasma Membrane-Bound or Secreted) GRP78
Targeting Extracellular GRP78
4.3. GRP75
4.4. TRAP1 (Tumor Necrosis Factor Receptor-Associated Protein 1)
4.5. Protein Disulfide Isomerases
4.6. Calreticulin
5. Conclusion and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Lathia, J.D.; Liu, H. Overview of Cancer Stem Cells and Stemness for Community Oncologists. Target. Oncol. 2017, 12, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Vila, M.; Takahashi, R.-U.; Usuba, W.; Kohama, I.; Ochiya, T. Drug Resistance Driven by Cancer Stem Cells and Their Niche. Int. J. Mol. Sci. 2017, 18, 2574. [Google Scholar] [CrossRef]
- Ayob, A.Z.; Ramasamy, T.S. Cancer stem cells as key drivers of tumour progression. J. Biomed. Sci. 2018, 25, 20. [Google Scholar] [CrossRef] [PubMed]
- Prager, B.C.; Xie, Q.; Bao, S.; Rich, J.N. Cancer Stem Cells: The Architects of the Tumor Ecosystem. Cell Stem Cell 2019, 24, 41–53. [Google Scholar] [CrossRef]
- Blanpain, C.; Fuchs, E. Plasticity of epithelial stem cells in tissue regeneration. Science 2014, 344, 1242281. [Google Scholar] [CrossRef]
- Klauzinska, M.; Castro, N.P.; Rangel, M.C.; Spike, B.T.; Gray, P.C.; Bertolette, D.; Cuttitta, F.; Salomon, D.S. The multifaceted role of the embryonic gene Cripto-1 in cancer, stem cells and epithelial-mesenchymal transition. Semin. Cancer Biol. 2014, 29, 51–58. [Google Scholar] [CrossRef]
- Taniguchi, H.; Imai, K. PRDM14, a Zinc Finger Protein, Regulates Cancer Stemness. Methods Mol Biol. 2018, 1867, 3–13. [Google Scholar]
- Khandekar, D.; Amara, S.; Tiriveedhi, V. Immunogenicity of Tumor Initiating Stem Cells: Potential Applications in Novel Anticancer Therapy. Front. Oncol. 2019, 9, 315. [Google Scholar] [CrossRef]
- Abbaszadegan, M.R.; Bagheri, V.; Razavi, M.S.; Momtazi-Borojeni, A.A.; Sahebkar, A.; Gholamin, M. Isolation, identification, and characterization of cancer stem cells: A review. J. Cell. Physiol. 2017, 232, 2008–2018. [Google Scholar] [CrossRef]
- Hannen, R.; Bartsch, J.W. Essential roles of telomerase reverse transcriptase hTERT in cancer stemness and metastasis. FEBS Lett. 2018, 592, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Nazio, F.; Bordi, M.; Cianfanelli, V.; Locatelli, F.; Cecconi, F. Autophagy and cancer stem cells: Molecular mechanisms and therapeutic applications. Cell Death Differ. 2019, 26, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.-S.; Tseng, H.-Y.; Chen, Y.-A.; Shen, P.-C.; Al Haq, A.T.; Chen, L.-M.; Tung, Y.-C.; Hsu, H.-L. MCT-1/miR-34a/IL-6/IL-6R signaling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Mol. Cancer 2019, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Chai, B.; Guo, Y.; Cui, X.; Liu, J.; Suo, Y.; Dou, Z.; Li, N. MiR-223-3p promotes the proliferation, invasion and migration of colon cancer cells by negative regulating PRDM1. Am. J. Transl. Res. 2019, 11, 4516–4523. [Google Scholar]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Santibanez, J.; Obradović, H.; Kukolj, T.; Krstić, J. Transforming growth factor-β, matrix metalloproteinases, and urokinase-type plasminogen activator interaction in the cancer epithelial to mesenchymal transition. Dev. Dyn. 2017, 247, 382–395. [Google Scholar] [CrossRef]
- Jolly, M.K.; Celià-Terrassa, T. Dynamics of Phenotypic Heterogeneity Associated with EMT and Stemness during Cancer Progression. J. Clin. Med. 2019, 8, 1542. [Google Scholar] [CrossRef]
- Yao, D.; Dai, C.; Peng, S. Mechanism of the Mesenchymal-Epithelial Transition and Its Relationship with Metastatic Tumor Formation. Mol. Cancer Res. 2011, 9, 1608–1620. [Google Scholar] [CrossRef]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef]
- Ramteke, A.; Ting, H.; Agarwal, C.; Mateen, S.; Somasagara, R.; Hussain, A.; Graner, M.; Frederick, B.; Agarwal, R.; Deep, G. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol. Carcinog. 2013, 54, 554–565. [Google Scholar] [CrossRef]
- Matchuk, O.N.; Orlova, N.V.; Zamulaeva, I.A. Changes in the relative number of SP cells of melanoma line B16 after radiation exposure in vivo. Radiats. Biol. Radioecol. 2016, 56, 487–493. [Google Scholar]
- Kim, R.-K.; Kaushik, N.; Suh, Y.; Yoo, K.-C.; Cui, Y.-H.; Kim, M.-J.; Lee, H.-J.; Kim, I.-G.; Lee, S.-J. Radiation driven epithelial-mesenchymal transition is mediated by Notch signaling in breast cancer. Oncotarget 2016, 7, 53430–53442. [Google Scholar] [CrossRef] [PubMed]
- Zang, C.; Liu, X.; Li, B.; He, Y.; Jing, S.; He, Y.; Wu, W.; Zhang, B.; Ma, S.; Dai, W.; et al. IL-6/STAT3/TWIST inhibition reverses ionizing radiation-induced EMT and radioresistance in esophageal squamous carcinoma. Oncotarget 2017, 8, 11228–11238. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, P.; Morgado-Díaz, J.A. The Role of EphA4 Signaling in Radiation-Induced EMT-Like Phenotype in Colorectal Cancer Cells. J. Cell. Biochem. 2016, 118, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Wang, W.; Zhu, Y.; Chen, Y.; Tian, B. Gemcitabine treatment induces endoplasmic reticular (ER) stress and subsequently upregulates urokinase plasminogen activator (uPA) to block mitochondrial-dependent apoptosis in Panc-1 cancer stem-like cells (CSCs). PLoS ONE 2017, 12, e0184110. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.H.; Kim, B.W.; Song, K.-H.; Cho, H.; Lee, Y.-H.; Kim, J.H.; Chung, J.-Y.; Kim, J.-H.; Hewitt, S.M.; Seong, S.-Y.; et al. Nanog signaling in cancer promotes stem-like phenotype and immune evasion. J. Clin. Investig. 2012, 122, 4077–4093. [Google Scholar] [CrossRef] [PubMed]
- Song, K.-H.; Oh, S.J.; Kim, S.; Cho, H.; Lee, H.-J.; Song, J.S.; Chung, J.-Y.; Cho, E.; Lee, J.; Jeon, S.; et al. HSP90A inhibition promotes anti-tumor immunity by reversing multi-modal resistance and stem-like property of immune-refractory tumors. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Pasello, M.; Giudice, A.M.; Scotlandi, K. The ABC subfamily A transporters: Multifaceted players with incipient potentialities in cancer. Semin. Cancer Biol. 2020, 60, 57–71. [Google Scholar] [CrossRef]
- Vilaboa, N.E.; Galán, A.; Troyano, A.; de Blas, E.; Aller, P. Regulation of multidrug resistance 1 (MDR1)/P-glycoprotein gene expression and activity by heat-shock transcription factor 1 (HSF1). J. Biol. Chem. 2000, 275, 24970–24976. [Google Scholar] [CrossRef]
- Stacy, A.E.; Jansson, P.; Richardson, D.R. Molecular Pharmacology of ABCG2 and Its Role in Chemoresistance. Mol. Pharmacol. 2013, 84, 655–669. [Google Scholar] [CrossRef]
- Dahan, P.; Gala, J.M.; Delmas, C.; Monferran, S.; Malric, L.; Zentkowski, D.; Lubrano, V.; Toulas, C.; Moyal, E.C.-J.; Lemarié, A. Ionizing radiations sustain glioblastoma cell dedifferentiation to a stem-like phenotype through survivin: Possible involvement in radioresistance. Cell Death Dis. 2014, 5, e1543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, X.; Bian, L.; Wang, Y.; Liu, H. CD44+/CD24+-Expressing Cervical Cancer Cells and Radioresistant Cervical Cancer Cells Exhibit Cancer Stem Cell Characteristics. Gynecol. Obstet. Investig. 2018, 84, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhou, C.; Hu, J.; Xiong, L.; Cong, Z.; Shen, Y. DKK1 maintained cancer stem-like properties of esophageal carcinoma cells via ALDH1A1/SOX2 axis. Int. J. Clin. Exp. Pathol. 2017, 10, 9489–9495. [Google Scholar] [PubMed]
- Flavahan, W.A.; Wu, Q.; Hitomi, M.; Rahim, N.; Kim, Y.; Sloan, A.E.; Weil, R.J.; Nakano, I.; Sarkaria, J.N.; Stringer, B.W.; et al. Brain tumor initiating cells adapt to restricted nutrition through preferential glucose uptake. Nat. Neurosci. 2013, 16, 1373–1382. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Han, X.; Tkach, D.; Huang, S.-G.; Zhang, D. AMPK promotes the survival of colorectal cancer stem cells. Anim. Model. Exp. Med. 2018, 1, 134–142. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Chien, M.-H.; Liu, H.-Y.; Chang, Y.-C.; Chen, C.-K.; Lee, W.-J.; Kuo, T.-C.; Hsiao, M.; Hua, K.; Cheng, T.-Y. Nuclear translocation of PKM2/AMPK complex sustains cancer stem cell populations under glucose restriction stress. Cancer Lett. 2018, 421, 28–40. [Google Scholar] [CrossRef]
- Lee, H.-J.; Li, C.-F.; Ruan, D.; He, J.; Montal, E.D.; Lorenz, S.; Girnun, G.D.; Chan, C.-H. Non-proteolytic ubiquitination of Hexokinase 2 by HectH9 controls tumor metabolism and cancer stem cell expansion. Nat. Commun. 2019, 10, 2625. [Google Scholar] [CrossRef]
- Dahiya, V.; Buchner, J. Functional principles and regulation of molecular chaperones. DNA Repair. 2019, 114, 1–60. [Google Scholar]
- Wu, J.; Liu, T.; Rios, Z.; Mei, Q.; Lin, X.; Cao, S. Heat Shock Proteins and Cancer. Trends Pharmacol. Sci. 2017, 38, 226–256. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.; E Cheetham, M.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2008, 14, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Im, C.-N. Past, present, and emerging roles of mitochondrial heat shock protein TRAP1 in the metabolism and regulation of cancer stem cells. Cell Stress Chaperones 2016, 21, 553–562. [Google Scholar] [CrossRef]
- Lee, A. Glucose-regulated proteins in cancer: Molecular mechanisms and therapeutic potential. Nat. Rev. Cancer 2014, 14, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-C.; Weng, W.-C.; Lee, H. Functional Roles of Calreticulin in Cancer Biology. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, K.; Verma, A.; Bhatt, A.N.; Shrivastava, A.; Manda, K.; Raj, H.G.; Prasad, A.; Len, C.; Parmar, V.S.; Dwarakanath, B.S. Emerging Roles of Calreticulin in Cancer: Implications for Therapy. Curr. Protein Pept. Sci. 2018, 19, 344–357. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, K.; Braakman, I. Protein quality control at the endoplasmic reticulum. Essays Biochem. 2016, 60, 227–235. [Google Scholar]
- Lee, E.; Lee, D.H. Emerging roles of protein disulfide isomerase in cancer. BMB Rep. 2017, 50, 401–410. [Google Scholar] [CrossRef]
- Harikishore, A.; Yoon, H. Immunophilins: Structures, Mechanisms and Ligands. Curr. Mol. Pharmacol. 2015, 9, 37–47. [Google Scholar] [CrossRef]
- Kabakov, A.E.; Kudryavtsev, V.A.; Gabai, V. Hsp90 inhibitors as promising agents for radiotherapy. J. Mol. Med. 2009, 88, 241–247. [Google Scholar] [CrossRef]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; You, Q.; Xu, X.-L. Heat Shock Protein 90 Inhibitors: An Update on Achievements, Challenges, and Future Directions. J. Med. Chem. 2019, 63, 1798–1822. [Google Scholar] [CrossRef] [PubMed]
- Acunzo, J.; Katsogiannou, M.; Rocchi, P. Small heat shock proteins HSP27 (HspB1), αB-crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. Int. J. Biochem. Cell Biol. 2012, 44, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Dokladny, K.; Myers, O.B.; Moseley, P.L. Heat shock response and autophagy—cooperation and control. Autophagy 2015, 11, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.; Jäger, R.; Mosser, D.D.; Samali, A. Regulation of apoptosis by heat shock proteins. IUBMB Life 2014, 66, 327–338. [Google Scholar] [CrossRef]
- Naidu, S.D.; Dinkova-Kostova, A.T. Regulation of the mammalian heat shock factor 1. FEBS J. 2017, 284, 1606–1627. [Google Scholar] [CrossRef]
- Calderwood, S.K. Heat shock proteins and cancer: Intracellular chaperones or extracellular signalling ligands? Philos. Trans. R. Soc. B: Biol. Sci. 2017, 373, 20160524. [Google Scholar] [CrossRef]
- Kabakov, A.E.; Kudryavtsev, V.A. Heat Shock Proteins as Molecular Targets for Anticancer Therapy: Approaches, Agents, and Trends. In Heat shock proteins. Classifications, functions, and applications; Usmani, S., Ed.; Nova Science Publishers: New York, NY, USA, 2013; pp. 25–56. [Google Scholar]
- Shevtsov, M.A.; Multhoff, G.; Mikhaylova, E.; Shibata, A.; Guzhova, I.V.; Margulis, B. Combination of Anti-Cancer Drugs with Molecular Chaperone Inhibitors. Int. J. Mol. Sci. 2019, 20, 5284. [Google Scholar] [CrossRef]
- Olotu, F.; Adeniji, E.; Agoni, C.; Bjij, I.; Khan, S.; ElRashedy, A.; Soliman, M.E.S.; Bjiji, I. An update on the discovery and development of selective heat shock protein inhibitors as anti-cancer therapy. Expert Opin. Drug Discov. 2018, 13, 903–918. [Google Scholar] [CrossRef]
- Shende, P.; Bhandarkar, S.; Prabhakar, B. Heat Shock Proteins and their Protective Roles in Stem Cell Biology. Stem Cell Rev. Rep. 2019, 15, 637–651. [Google Scholar] [CrossRef]
- Lettini, G.; Lepore, S.; Crispo, F.; Sisinni, L.; Esposito, F.; Landriscina, M. Heat shock proteins in cancer stem cell maintenance: A potential therapeutic target? Histol. Histopathol. 2019, 35, 18153. [Google Scholar]
- Mahalingam, D.; Swords, R.; Carew, J.S.; Nawrocki, S.T.; Bhalla, K.; Giles, F.J. Targeting HSP90 for cancer therapy. Br. J. Cancer 2009, 100, 1523–1529. [Google Scholar] [CrossRef]
- Lee, C.-H.; Hong, H.-M.; Chang, Y.-Y.; Chang, W.-W. Inhibition of heat shock protein (Hsp) 27 potentiates the suppressive effect of Hsp90 inhibitors in targeting breast cancer stem-like cells. Biochimie 2012, 94, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Bhatia, A.; Dong, H.; Jayaprakash, P.; Guo, J.; Sahu, D.; Hou, Y.; Tsen, F.; Tong, C.; O’Brien, K.; et al. Evolutionarily conserved dual lysine motif determines the non-chaperone function of secreted Hsp90alpha in tumour progression. Oncogene 2016, 36, 2160–2171. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Long, T.-E.; Park, W.; Landry, J.C.; Taliaferro-Smith, L.; Farris, A.B.; Diaz, R.; El-Rayes, B.F. Heat shock protein 90 promotes epithelial to mesenchymal transition, invasion, and migration in colorectal cancer. Mol. Carcinog. 2014, 54, 1147–1158. [Google Scholar] [CrossRef]
- Kao, C.-Y.; Yang, P.-M.; Wu, M.-H.; Huang, C.-C.; Lee, Y.-C.; Lee, K.-H. Heat shock protein 90 is involved in the regulation of HMGA2-driven growth and epithelial-to-mesenchymal transition of colorectal cancer cells. Peer. J. 2016, 4, e1683. [Google Scholar] [CrossRef] [PubMed]
- Deskin, B.; Lasky, J.; Zhuang, Y.; Shan, B. Requirement of HDAC6 for activation of Notch1 by TGF-β1. Sci. Rep. 2016, 6, 31086. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, S.; Seike, M.; Miyanaga, A.; Chiba, M.; Zou, F.; Takahashi, A.; Ishikawa, A.; Kunugi, S.; Noro, R.; Kubota, K.; et al. Overcoming drug-tolerant cancer cell subpopulations showing AXL activation and epithelial–mesenchymal transition is critical in conquering ALK-positive lung cancer. Oncotarget 2018, 9, 27242–27255. [Google Scholar] [CrossRef]
- Meng, J.; Chen, S.; Lei, Y.-Y.; Han, J.-X.; Zhong, W.-L.; Wang, X.-R.; Liu, Y.-R.; Gao, W.-F.; Zhang, Q.; Tan, Q.; et al. Hsp90β promotes aggressive vasculogenic mimicry via epithelial–mesenchymal transition in hepatocellular carcinoma. Oncogene 2018, 38, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Deng, G.; Ai, M.; Xu, Z.; Mou, T.; Yu, J.; Liu, H.; Wang, S.; Li, G. Hsp90ab1 stabilizes LRP5 to promote epithelial–mesenchymal transition via activating of AKT and Wnt/β-catenin signaling pathways in gastric cancer progression. Oncogene 2018, 38, 1489–1507. [Google Scholar] [CrossRef]
- Chong, K.Y.; Kang, M.; Garofalo, F.; Ueno, D.; Liang, H.; Cady, S.; Madarikan, O.; Pitruzzello, N.; Tsai, C.-H.; Hartwich, T.M.; et al. Inhibition of Heat Shock Protein 90 suppresses TWIST1 Transcription. Mol. Pharmacol. 2019, 96, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, C.; Kovatch, K.; Sim, M.; Wang, G.; Prince, M.; Carey, T.; Davis, R.; Blagg, B.; Cohen, M.S. Novel C-Terminal Heat Shock Protein 90 Inhibitors (KU711 and Ku757) Are Effective in Targeting Head and Neck Squamous Cell Carcinoma Cancer Stem Cells. Neoplasia 2017, 19, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Newman, B.; Liu, Y.; Lee, H.-F.; Sun, D.; Wang, Y. HSP90 inhibitor 17-AAG selectively eradicates lymphoma stem cells. Cancer Res. 2012, 72, 4551–4561. [Google Scholar] [CrossRef] [PubMed]
- White, P.T.; Subramanian, C.; Zhu, Q.; Zhang, H.; Zhao, H.; Gallagher, R.; Timmermann, B.N.; Blagg, B.S.J.; Cohen, M.S. Novel HSP90 inhibitors effectively target functions of thyroid cancer stem cell preventing migration and invasion. Surgery 2016, 159, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.-S.; Jung, J.; Lim, I.; Lee, J.-Y.; Park, M.-J. Emodin Suppresses Maintenance of Stemness by Augmenting Proteosomal Degradation of Epidermal Growth Factor Receptor/Epidermal Growth Factor Receptor Variant III in Glioma Stem Cells. Stem Cells Dev. 2014, 24, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Filatova, A.; Seidel, S.; Bogurcu, N.; Gräf, S.; Garvalov, B.K.; Acker, T. Acidosis acts through HSP90 in a PHD/VHL-independent manner to promote HIF function and stem cell maintenance in glioma. Cancer Res. 2016, 76, 5845–5856. [Google Scholar] [CrossRef] [PubMed]
- Canella, A.; Welker, A.; Yoo, J.Y.; Xu, J.; Abas, F.S.; Kesanakurti, D.; Nagarajan, P.; Beattie, C.E.; Sulman, E.P.; Liu, J.L.; et al. Efficacy of Onalespib, a Long-Acting Second-Generation HSP90 Inhibitor, as a Single Agent and in Combination with Temozolomide against Malignant Gliomas. Clin. Cancer Res. 2017, 23, 6215–6226. [Google Scholar] [CrossRef]
- Tawfeeq, A.T.; Mahmood, N.A.-A.; Abd-Alghni, Z.S. Starvation contributes to elevated levels of heat shock proteins and cancer stem cell markers in an esophageal cancer cell line. Biomed. Res. 2018, 29, 3815–3823. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Chang, W.-W.; Chen, Y.-Y.; Tsai, Y.-H.; Chou, Y.-H.; Tseng, H.-C.; Chen, H.-L.; Wu, C.-C.; Chang-Chien, J.; Lee, H.-T.; et al. Hsp90α Mediates BMI1 Expression in Breast Cancer Stem/Progenitor Cells through Facilitating Nuclear Translocation of c-Myc and EZH2. Int. J. Mol. Sci. 2017, 18, 1986. [Google Scholar] [CrossRef]
- Moriya, C.; Taniguchi, H.; Nagatoishi, S.; Igarashi, H.; Tsumoto, K.; Imai, K. PRDM14 directly interacts with heat shock proteins HSP90α and glucose-regulated protein 78. Cancer Sci. 2017, 109, 373–383. [Google Scholar] [CrossRef]
- Cho, T.-M.; Kim, J.Y.; Kim, Y.-J.; Sung, D.; Oh, E.; Jang, S.; Farrand, L.; Hoang, V.-H.; Nguyen, C.-T.; Ann, J.; et al. C-terminal HSP90 inhibitor L80 elicits anti-metastatic effects in triple-negative breast cancer via STAT3 inhibition. Cancer Lett. 2019, 447, 141–153. [Google Scholar] [CrossRef]
- Liu, K.; Xu, S.-H.; Chen, Z.; Zeng, Q.-X.; Li, Z.-J.; Chen, Z.-M. TRPM7 overexpression enhances the cancer stem cell-like and metastatic phenotypes of lung cancer through modulation of the Hsp90α/uPA/MMP2 signaling pathway. BMC Cancer 2018, 18, 1167. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, S.; Chen, T.; Shu, X.; Mo, X.; Chang, G.; Chen, J.-J.; Li, C.; Luo, H.; Lee, J.-D. Verteporfin blocks Clusterin which is required for survival of gastric cancer stem cell by modulating HSP90 function. Int. J. Biol. Sci. 2019, 15, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Machida, H.; Nakajima, S.; Shikano, N.; Nishio, J.; Okada, S.; Asayama, M.; Shirai, M.; Kubota, N. Heat shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin potentiates the radiation response of tumor cells grown as monolayer cultures and spheroids by inducing apoptosis. Cancer Sci. 2005, 96, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.S.; Baek, J.-H.; Yim, J.-H.; Um, H.-D.; Park, J.K.; Song, J.-Y.; Park, I.-C.; Kim, J.-S.; Lee, S.-J.; Lee, C.-W.; et al. Radiotherapy diagnostic biomarkers in radioresistant human H460 lung cancer stem-like cells. Cancer Biol. Ther. 2016, 17, 208–218. [Google Scholar] [CrossRef]
- Giordano, C.; Chemi, F.; Panza, S.; Barone, I.; Bonofiglio, D.; Lanzino, M.; Cordella, A.; Campana, A.; Hashim, A.; Rizza, P.; et al. Leptin as a mediator of tumor-stromal interactions promotes breast cancer stem cell activity. Oncotarget 2015, 7, 1262–1275. [Google Scholar] [CrossRef]
- Guzman, M.L.; Yang, N.; Sharma, K.K.; Balys, M.; Corbett, C.A.; Jordan, C.T.; Becker, M.; Steidl, U.; Abdel-Wahab, O.; Levine, R.L.; et al. Selective activity of the histone deacetylase inhibitor AR-42 against leukemia stem cells: A novel potential strategy in acute myelogenous leukemia. Mol. Cancer Ther. 2014, 13, 1979–1990. [Google Scholar] [CrossRef]
- Hiyoshi, H.; Goto, N.; Tsuchiya, M.; Iida, K.; Nakajima, Y.; Hirata, N.; Kanda, Y.; Nagasawa, K.; Yanagisawa, J. 2-(4-Hydroxy-3-methoxyphenyl)-benzothiazole suppresses tumor progression and metastatic potential of breast cancer cells by inducing ubiquitin ligase CHIP. Sci. Rep. 2014, 4, 7095. [Google Scholar] [CrossRef]
- Cho, Y.; Kang, H.G.; Kim, S.-J.; Lee, S.; Jee, S.; Ahn, S.G.; Kang, M.J.; Song, J.S.; Chung, J.-Y.; Yi, E.C.; et al. Post-translational modification of OCT4 in breast cancer tumorigenesis. Cell Death Differ. 2018, 25, 1781–1795. [Google Scholar] [CrossRef]
- Cano, L.Q.; Lavery, D.N.; Sin, S.; Spanjaard, E.; Brooke, G.; Tilman, J.D.; Abroaf, A.; Gaughan, L.; Robson, C.N.; Heer, R.; et al. The co-chaperone p23 promotes prostate cancer motility and metastasis. Mol. Oncol. 2014, 9, 295–308. [Google Scholar] [CrossRef]
- Iglesia, R.; Prado, M.; Cruz, L.; Martins, V.; Dos Santos, T.G.; Lopes, M. Engagement of cellular prion protein with the co-chaperone Hsp70/90 organizing protein regulates the proliferation of glioblastoma stem-like cells. Stem Cell Res. Ther. 2017, 8, 76. [Google Scholar] [CrossRef]
- Kim, H.-B.; Lee, S.-H.; Um, J.-H.; Kim, M.-J.; Hyun, S.-K.; Gong, E.-J.; Oh, W.K.; Kang, C.-D.; Kim, S.-H. Sensitization of Chemo-Resistant Human Chronic Myeloid Leukemia Stem-Like Cells to Hsp90 Inhibitor by SIRT1 Inhibition. Int. J. Biol. Sci. 2015, 11, 923–934. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, R.; Tang, Q.; Miao, F.; An, Y.; Li, M.; Han, Y.; Wang, X.; Wang, J.; Liu, P.; Chen, R. Inhibition of heat-shock protein 90 sensitizes liver cancer stem-like cells to magnetic hyperthermia and enhances anti-tumor effect on hepatocellular carcinoma-burdened nude mice. Int. J. Nanomed. 2015, 10, 7345–7358. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moon, H.-J.; Park, S.-Y.; Lee, S.-H.; Kang, C.-D.; Kim, S.-H. Nonsteroidal Anti-inflammatory Drugs Sensitize CD44-Overexpressing Cancer Cells to Hsp90 Inhibitor Through Autophagy Activation. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2019, 27, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.; Nguyen, H.T.; Min, H.-Y.; Hyun, S.Y.; Kwon, S.; Lee, Y.; Van Le, T.H.; Lee, J.; Park, J.H.; Lee, H.-Y. Panaxynol, a natural Hsp90 inhibitor, effectively targets both lung cancer stem and non-stem cells. Cancer Lett. 2018, 412, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Zaarur, N.; Kalin, T.V.; Wang, I.-C.; Ackerson, T.J.; Major, M.L.; Detrisac, C.J.; Kalinichenko, V.V.; Lyubimov, A.V.; Costa, R.H. Targeting Heat Shock Response to Sensitize Cancer Cells to Proteasome and Hsp90 Inhibitors. Cancer Res. 2006, 66, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Schilling, D.; Kühnel, A.; Konrad, S.; Tetzlaff, F.; Bayer, C.; Yaglom, J.; Multhoff, G. Sensitizing tumor cells to radiation by targeting the heat shock response. Cancer Lett. 2015, 360, 294–301. [Google Scholar] [CrossRef]
- Kudryavtsev, V.A.; Khokhlova, A.; Mosina, V.A.; Selivanova, E.I.; Kabakov, A.E. Induction of Hsp70 in tumor cells treated with inhibitors of the Hsp90 activity: A predictive marker and promising target for radiosensitization. PLoS ONE 2017, 12, e0173640. [Google Scholar] [CrossRef]
- Kudryavtsev, V.A.; Khokhlova, A.V.; Selivanova, E.I.; Mosina, V.A.; Makarova, Y.M.; Kabakov, A.E. Enhanced Radiosensitization of Tumor Cells by Means of Combination of Inhibitors of Chaperone Activity and Chaperone Expression. Radiats. Biol. Radioecol. 2018, 58, 26–34. [Google Scholar]
- Booth, L.; Roberts, J.L.; Cash, D.; Tavallai, S.; Jean, S.; Fidanza, A.; Cruz-Luna, T.; Siembiba, P.; Cycon, K.A.; Cornelissen, C.N.; et al. GRP78/BiP/HSPA5/Dna K is a universal therapeutic target for human disease. J. Cell. Physiol. 2015, 230, 1661–1676. [Google Scholar] [CrossRef]
- Tharmarajah, L.; Samarakoon, S.; Ediriweera, M.K.; Piyathilaka, P.; Tennekoon, K.; Senathilake, K.S.; Rajagopalan, U.; Galhena, P.B.; Thabrew, I. In Vitro Anticancer Effect of Gedunin on Human Teratocarcinomal (NTERA-2) Cancer Stem-Like Cells. BioMed Res. Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Hance, M.W.; Dole, K.; Gopal, U.; Bohonowych, J.E.; Jezierska-Drutel, A.; Neumann, C.A.; Liu, H.; Garraway, I.P.; Isaacs, J.S. Secreted Hsp90 Is a Novel Regulator of the Epithelial to Mesenchymal Transition (EMT) in Prostate Cancer. J. Biol. Chem. 2012, 287, 37732–37744. [Google Scholar] [CrossRef] [PubMed]
- Nolan, K.D.; Franco, O.E.; Hance, M.W.; Hayward, S.W.; Isaacs, J.S. Tumor-secreted Hsp90 Subverts Polycomb Function to Drive Prostate Tumor Growth and Invasion. J. Biol. Chem. 2015, 290, 8271–8282. [Google Scholar] [CrossRef] [PubMed]
- Nolan, K.D.; Kaur, J.; Isaacs, J.S. Secreted heat shock protein 90 promotes prostate cancer stem cell heterogeneity. Oncotarget 2016, 8, 19323–19341. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Sogawa, C.; Okusha, Y.; Uchibe, K.; Iinuma, R.; Ono, K.; Nakano, K.; Murakami, J.; Itoh, M.; Arai, K.; et al. Organoids with cancer stem cell-like properties secrete exosomes and HSP90 in a 3D nanoenvironment. PLoS ONE 2018, 13, e0191109. [Google Scholar] [CrossRef]
- Stivarou, T.; Stellas, D.; Vartzi, G.; Thomaidou, D.; Patsavoudi, E. Targeting highly expressed extracellular HSP90 in breast cancer stem cells inhibits tumor growth in vitro and in vivo. Cancer Biol. Ther. 2016, 17, 799–812. [Google Scholar] [CrossRef]
- Liu, Y.; Suo, X.; Peng, H.; Yan, W.; Li, H.; Yang, X.; Li, Z.; Zhang, J.; Liu, D. Multifunctional Magnetic Nanoplatform Eliminates Cancer Stem Cells via Inhibiting the Secretion of Extracellular Heat Shock Protein 90. Adv. Heal. Mater. 2019, 8, e1900160. [Google Scholar] [CrossRef]
- Chen, W.-S.; Chen, C.-C.; Chen, L.-L.; Lee, C.-C.; Huang, T.-S. Secreted Heat Shock Protein 90α (HSP90α) Induces Nuclear Factor-κB-mediated TCF12 Protein Expression to Down-regulate E-cadherin and to Enhance Colorectal Cancer Cell Migration and Invasion. J. Biol. Chem. 2013, 288, 9001–9010. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, C.; Jiang, Y.; Zhang, S.; Feng, S.; Fu, Y.; Luo, Y. Metformin Inhibits Tumor Metastasis through Suppressing Hsp90α Secretion in an AMPKα1-PKCγ Dependent Manner. Cells 2020, 9, 144. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Scroggins, B.; Koga, F.; Lee, M.-J.; Trepel, J.; Felts, S.; Carreras, C.; Neckers, L. A small molecule cell-impermeant Hsp90 antagonist inhibits tumor cell motility and invasion. Oncogene 2007, 27, 2478–2487. [Google Scholar] [CrossRef]
- Crowe, L.; Hughes, P.F.; Alcorta, D.A.; Osada, T.; Smith, A.P.; Totzke, J.; Loiselle, D.R.; Lutz, I.D.; Gargesha, M.; Roy, D.; et al. A Fluorescent Hsp90 Probe Demonstrates the Unique Association between Extracellular Hsp90 and Malignancy in Vivo. ACS Chem. Biol. 2017, 12, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Radons, J. The human HSP70 family of chaperones: Where do we stand? Cell Stress Chaperones 2016, 21, 379–404. [Google Scholar] [CrossRef] [PubMed]
- Boudesco, C.; Cause, S.; Jego, G.; Garrido, C. Hsp70: A Cancer Target Inside and Outside the Cell. Methods Mol Biol. 2017, 1709, 371–396. [Google Scholar]
- Yu, D.; Shin, H.-S.; Choi, G.; Lee, Y.C. Proteomic analysis of CD44(+) and CD44(−) gastric cancer cells. Mol. Cell. Biochem. 2014, 396, 213–220. [Google Scholar] [CrossRef]
- Ronci, M.; Catanzaro, G.; Pieroni, L.; Po, A.; Besharat, Z.M.; Greco, V.; Mortera, S.L.; Screpanti, I.; Ferretti, E.; Urbani, A. Proteomic analysis of human sonic hedgehog (SHH) medulloblastoma stem-like cells. Mol. BioSyst. 2015, 11, 1603–1611. [Google Scholar] [CrossRef]
- Gong, J.; Weng, D.; Eguchi, T.; Murshid, A.; Sherman, M.Y.; Song, B.; Calderwood, S.K. Targeting the hsp70 gene delays mammary tumor initiation and inhibits tumor cell metastasis. Oncogene 2015, 34, 5460–5471. [Google Scholar] [CrossRef]
- Gupta, N.; Jagadish, N.; Surolia, A.; Suri, A. Heat shock protein 70-2 (HSP70-2) a novel cancer testis antigen that promotes growth of ovarian cancer. Am. J. Cancer Res. 2017, 7, 1252–1269. [Google Scholar]
- Kasioumi, P.; Vrazeli, P.; Vezyraki, P.; Zerikiotis, S.; Katsouras, C.; Damalas, A.; Angelidis, C. Hsp70 (HSP70A1A) downregulation enhances the metastatic ability of cancer cells. Int. J. Oncol. 2018, 54, 821–832. [Google Scholar] [CrossRef]
- Matsuda, Y.; Ishiwata, T.; Yoshimura, H.; Hagio, M.; Arai, T. Inhibition of nestin suppresses stem cell phenotype of glioblastomas through the alteration of post-translational modification of heat shock protein HSPA8/HSC71. Cancer Lett. 2015, 357, 602–611. [Google Scholar] [CrossRef]
- Matchuk, O.N.; Zamulaeva, I.A. High level of radiation-induced heat shock protein with a molecular weight of 27 and 70 kDa is the hallmark of radioresistant SP cells of MCF-7 breast cancer culture. Radiats. Biol. Radioecol. 2016, 56, 382–388. [Google Scholar]
- Fani, S.; Dehghan, F.; Karimian, H.; Lo, K.M.; Nigjeh, S.E.; Keong, Y.S.; Soori, R.; Chow, K.M.; Kamalidehghan, B.; Ali, H.M.; et al. Monobenzyltin Complex C1 Induces Apoptosis in MCF-7 Breast Cancer Cells through the Intrinsic Signaling Pathway and through the Targeting of MCF-7-Derived Breast Cancer Stem Cells via the Wnt/β-Catenin Signaling Pathway. PLoS ONE 2016, 11, e0160836. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, Y.-J.; Park, K.-S.; Heo, S.-H.; Nam, H.-S.; Cho, M.-K.; Lee, S.-H. Pifithrin-μ induces necroptosis through oxidative mitochondrial damage but accompanies epithelial-mesenchymal transition-like phenomenon in malignant mesothelioma cells under lactic acidosis. Arch. Pharm. Res. 2019, 42, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Y.; Liu, D.; Sun, H.; Su, N.; Yang, F.; Liu, J. Extracellular HSP70/HSP70-PCs Promote Epithelial-Mesenchymal Transition of Hepatocarcinoma Cells. PLoS ONE 2013, 8, e84759. [Google Scholar] [CrossRef] [PubMed]
- Nigro, A.; Mauro, L.; Giordano, F.; Panza, S.; Iannacone, R.; Liuzzi, G.M.; Aquila, S.; De Amicis, F.; Cellini, F.; Indiveri, C.; et al. Recombinant Arabidopsis HSP70 sustains cell survival and metastatic potential of breast cancer cells. Mol. Cancer Ther. 2016, 15, 1063–1073. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Breuninger, S.; Stangl, S.; Werner, C.; Sievert, W.; Lobinger, D.; Foulds, G.; Wagner, S.; Pickhard, A.; Piontek, G.; Kokowski, K.; et al. Membrane Hsp70—A Novel Target for the Isolation of Circulating Tumor Cells After Epithelial-to-Mesenchymal Transition. Front. Oncol. 2018, 8, 497. [Google Scholar] [CrossRef] [PubMed]
- Sterrenberg, J.; Blatch, G.L.; Edkins, A.L. Human DNAJ in cancer and stem cells. Cancer Lett. 2011, 312, 129–142. [Google Scholar] [CrossRef]
- Nishizawa, S.; Hirohashi, Y.; Torigoe, T.; Takahashi, A.; Tamura, Y.; Mori, T.; Kanaseki, T.; Kamiguchi, K.; Asanuma, H.; Morita, R.; et al. HSP DNAJB8 Controls Tumor-Initiating Ability in Renal Cancer Stem-like Cells. Cancer Res. 2012, 72, 2844–2854. [Google Scholar] [CrossRef]
- Morita, R.; Nishizawa, S.; Torigoe, T.; Takahashi, A.; Tamura, Y.; Tsukahara, T.; Kanaseki, T.; Sokolovskaya, A.; Kochin, V.; Kondo, T.; et al. Heat shock protein DNAJB8 is a novel target for immunotherapy of colon cancer-initiating cells. Cancer Sci. 2014, 105, 389–395. [Google Scholar] [CrossRef]
- Yamashita, M.; Hirohashi, Y.; Torigoe, T.; Kusumoto, H.; Murai, A.; Imagawa, T.; Sato, N. Dnajb8, a Member of the Heat Shock Protein 40 Family Has a Role in the Tumor Initiation and Resistance to Docetaxel but Is Dispensable for Stress Response. PLoS ONE 2016, 11, e0146501. [Google Scholar] [CrossRef]
- Kusumoto, H.; Hirohashi, Y.; Nishizawa, S.; Yamashita, M.; Yasuda, K.; Murai, A.; Takaya, A.; Mori, T.; Kubo, T.; Nakatsugawa, M.; et al. Cellular stress induces cancer stem-like cells through expression of DNAJB8 by activation of heat shock factor 1. Cancer Sci. 2018, 109, 741–750. [Google Scholar] [CrossRef]
- Kagiali, Z.C.U.; Şanal, E.; Karayel, Ö.; Polat, A.N.; Saatci, Ö.; Ersan, P.G.; Trappe, K.; Renard, B.Y.; Önder, T.; Tuncbag, N.; et al. Systems-level Analysis Reveals Multiple Modulators of Epithelial-mesenchymal Transition and Identifies DNAJB4 and CD81 as Novel Metastasis Inducers in Breast Cancer. Mol. Cell. Proteom. 2019, 18, 1756–1771. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Kitahara, M.; Nagata, K. Benzylidene lactam compound, KNK437, a novel inhibitor of acquisition of thermotolerance and heat shock protein induction in human colon carcinoma cells. Cancer Res. 2000, 60, 2942–2948. [Google Scholar] [PubMed]
- Yang, S.; Ren, X.; Liang, Y.; Yan, Y.; Zhou, Y.; Hu, J.; Wang, Z.; Song, F.; Wang, F.; Liao, W.; et al. KNK437 restricts the growth and metastasis of colorectal cancer via targeting DNAJA1/CDC45 axis. Oncogene 2019, 39, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.-P. Mammalian HspB1 (Hsp27) is a molecular sensor linked to the physiology and environment of the cell. Cell Stress Chaperones 2017, 22, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, J.; Sang, D.; Lan, Q. Phosphorylation of AKT induced by phosphorylated Hsp27 confers the apoptosis-resistance in t-AUCB-treated glioblastoma cells in vitro. J. Neuro-Oncology 2014, 121, 83–89. [Google Scholar] [CrossRef]
- Choi, S.-K.; Kam, H.; Kim, K.-Y.; Park, S.I.; Lee, Y.-S. Targeting Heat Shock Protein 27 in Cancer: A Druggable Target for Cancer Treatment? Cancers 2019, 11, 1195. [Google Scholar] [CrossRef]
- Mizutani, H.; Okano, T.; Minegishi, Y.; Matsuda, K.; Sudoh, J.; Kitamura, K.; Noro, R.; Soeno, C.; Yoshimura, A.; Seike, M.; et al. HSP27 modulates epithelial to mesenchymal transition of lung cancer cells in a Smad-independent manner. Oncol. Lett. 2010, 1, 1011–1016. [Google Scholar] [CrossRef]
- Wei, L.; Liu, T.-T.; Wang, H.-H.; Hong, H.-M.; Yu, A.L.; Feng, H.-P.; Chang, W.-W. Hsp27 participates in the maintenance of breast cancer stem cells through regulation of epithelial-mesenchymal transition and nuclear factor-κB. Breast Cancer Res. 2011, 13, R101. [Google Scholar] [CrossRef]
- Nagata, Y.; Kudo, M.; Nagai, T.; Watanabe, T.; Kawasaki, M.; Asakuma, Y.; Hagiwara, S.; Nishida, N.; Matsui, S.; Kashida, H.; et al. Heat Shock Protein 27 Expression is Inversely Correlated with Atrophic Gastritis and Intraepithelial Neoplasia. Dig. Dis. Sci. 2012, 58, 381–388. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Qian, Y.; Dai, X.; Yang, L.; Chen, J.; Guo, S.; Hisamitsu, T. Research on the efficacy of Celastrus Orbiculatus in suppressing TGF-β1-induced epithelial-mesenchymal transition by inhibiting HSP27 and TNF-α-induced NF-κ B/Snail signaling pathway in human gastric adenocarcinoma. BMC Complement. Altern. Med. 2014, 14, 433. [Google Scholar] [CrossRef]
- Shiota, M.; Bishop, J.; Nip, K.M.; Zardan, A.; Takeuchi, A.; Cordonnier, T.; Beraldi, E.; Bazov, J.; Fazli, L.; Chi, K.; et al. Hsp27 Regulates Epithelial Mesenchymal Transition, Metastasis, and Circulating Tumor Cells in Prostate Cancer. Cancer Res. 2013, 73, 3109–3119. [Google Scholar] [CrossRef] [PubMed]
- Cordonnier, T.; Bishop, J.; Shiota, M.; Nip, K.M.; Thaper, D.; Vahid, S.; Heroux, D.; Gleave, M.; Zoubeidi, A. Hsp27 regulates EGF/β-catenin mediated epithelial to mesenchymal transition in prostate cancer. Int. J. Cancer 2014, 136, E496–E507. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qian, J.; Li, X.; Chen, W.; Xu, A.; Zhao, K.; Hua, Y.; Huang, Z.; Zhang, J.; Liang, C.; et al. Long noncoding RNA BX357664 regulates cell proliferation and epithelial-to-mesenchymal transition via inhibition of TGF-β1/p38/HSP27 signaling in renal cell carcinoma. Oncotarget 2016, 7, 81410–81422. [Google Scholar] [PubMed]
- Chen, W.; Ren, X.; Wu, J.; Gao, X.; Cen, X.; Wang, S.; Sheng, S.; Chen, Q.; Tang, Y.-J.; Liang, X.; et al. HSP27 associates with epithelial–mesenchymal transition, stemness and radioresistance of salivary adenoid cystic carcinoma. J. Cell. Mol. Med. 2018, 22, 2283–2298. [Google Scholar] [CrossRef]
- Lin, S.-P.; Lee, Y.-T.; Wang, J.-Y.; Miller, S.A.; Chiou, S.-H.; Hung, M.-C.; Hung, S.-C. Survival of Cancer Stem Cells under Hypoxia and Serum Depletion via Decrease in PP2A Activity and Activation of p38-MAPKAPK2-Hsp27. PLoS ONE 2012, 7, e49605. [Google Scholar] [CrossRef]
- Chen, S.-F.; Nieh, S.; Jao, S.-W.; Liu, C.-L.; Wu, C.-H.; Chang, Y.-C.; Yang, C.-Y.; Lin, Y.-S. Quercetin Suppresses Drug-Resistant Spheres via the p38 MAPK–Hsp27 Apoptotic Pathway in Oral Cancer Cells. PLoS ONE 2012, 7, e49275. [Google Scholar] [CrossRef]
- Liu, C.-L.; Chen, S.-F.; Wu, M.-Z.; Jao, S.-W.; Lin, Y.-S.; Yang, C.-Y.; Lee, T.-Y.; Wen, L.-W.; Lan, G.-L.; Nieh, S. The molecular and clinical verification of therapeutic resistance via the p38 MAPK–Hsp27 axis in lung cancer. Oncotarget 2016, 7, 14279–14290. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, J.; Wang, G.; Zhou, C.; Wang, P.; Zhao, S.; Zhao, S.; Huang, S.; Su, W.; Jiang, P.; et al. Inactivation of p38 MAPK contributes to stem cell-like properties of non-small cell lung cancer. Oncotarget 2017, 8, 26702–26717. [Google Scholar] [CrossRef]
- Li, G.; Zhao, F.; Cui, Y. Proteomics using mammospheres as a model system to identify proteins deregulated in breast cancer stem cells. Curr. Mol. Med. 2013, 13, 459–463. [Google Scholar]
- Rajesh, Y.; Banerjee, A.; Pal, I.; Biswas, A.; Das, S.; Dey, K.K.; Kapoor, N.; Ghosh, A.K.; Mitra, P.; Mandal, M. Delineation of crosstalk between HSP27 and MMP-2/MMP-9: A synergistic therapeutic avenue for glioblastoma management. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1196–1209. [Google Scholar] [CrossRef]
- Lee, C.-H.; Wu, Y.; Hsieh, H.-C.; Yu, Y.; Yu, A.L.; Chang, W.-W. Epidermal growth factor/heat shock protein 27 pathway regulates vasculogenic mimicry activity of breast cancer stem/progenitor cells. Biochimie 2014, 104, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-S.; Lin, J.-H.; Huang, W.-C.; Hsu, T.-W.; Su, K.; Chiou, S.-H.; Tsai, Y.-T.; Hung, S. Chemoresistance of lung cancer stemlike cells depends on activation of Hsp27. Cancer 2010, 117, 1516–1528. [Google Scholar] [CrossRef]
- Lin, S.-P.; Lee, Y.-T.; Yang, S.-H.; Miller, S.A.; Chiou, S.-H.; Hung, M.-C.; Hung, S. Colon cancer stem cells resist antiangiogenesis therapy-induced apoptosis. Cancer Lett. 2013, 328, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Chou, K.; Hsu, J.; Lin, J.; Hsu, T.; Yen, D.H.; Hung, S.; Hsu, H. High metabolic rate and stem cell characteristics of esophageal cancer stem-like cells depend on the Hsp27–AKT–HK2 pathway. Int. J. Cancer 2019, 145, 2144–2156. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.-T.; Wang, B.-Y.; Chi, W.-Y.; Chang-Chien, J.; Yang, J.-J.; Lee, H.-T.; Tzeng, Y.-M.; Chang, W.-W. Ovatodiolide Inhibits Breast Cancer Stem/Progenitor Cells through SMURF2-Mediated Downregulation of Hsp27. Toxins 2016, 8, 127. [Google Scholar] [CrossRef]
- Peng, C.-Y.; Fong, P.-C.; Yu, C.-C.; Tsai, W.-C.; Tzeng, Y.-M.; Chang, W.-W. Methyl Antcinate A suppresses the Population of Cancer Stem-Like Cells in MCF7 Human Breast Cancer Cell Line. Molecules 2013, 18, 2539–2548. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Wu, X.; Zhou, X.; Wolfram, J.; Shen, J.; Zhang, D.; Mai, J.; Xia, X.; Holder, A.M.; Ferrari, M.; et al. Chemotherapy Sensitizes Therapy-Resistant Cells to Mild Hyperthermia by Suppressing Heat Shock Protein 27 Expression in Triple-Negative Breast Cancer. Clin. Cancer Res. 2018, 24, 4900–4912. [Google Scholar] [CrossRef]
- Dai, C. The heat-shock, or HSF1-mediated proteotoxic stress, response in cancer: From proteomic stability to oncogenesis. Philos. Trans. R. Soc. B: Biol. Sci. 2017, 373, 20160525. [Google Scholar] [CrossRef]
- Dong, B.; Jaeger, A.M.; Thiele, D.J. Inhibiting Heat Shock Factor 1 in Cancer: A Unique Therapeutic Opportunity. Trends Pharmacol. Sci. 2019, 40, 986–1005. [Google Scholar] [CrossRef]
- Xi, C.; Hu, Y.; Buckhaults, P.; Moskophidis, D.; Mivechi, N.F. Heat Shock Factor Hsf1 Cooperates with ErbB2 (Her2/Neu) Protein to Promote Mammary Tumorigenesis and Metastasis. J. Biol. Chem. 2012, 287, 35646–35657. [Google Scholar] [CrossRef]
- Powell, C.D.; Paullin, T.R.; Aoisa, C.; Menzie, C.J.; Ubaldini, A.; Westerheide, S. The Heat Shock Transcription Factor HSF1 Induces Ovarian Cancer Epithelial-Mesenchymal Transition in a 3D Spheroid Growth Model. PLoS ONE 2016, 11, e0168389. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Sun, L.; Qin, X.; Liu, T.; Zhang, S.; Liu, Y.; Li, S.; Guo, K. HSF1 promotes the inhibition of EMT-associated migration by low glucose via directly regulating Snail1 expression in HCC cells. Discov. Med. 2016, 22, 87–96. [Google Scholar] [PubMed]
- Chen, K.; Qian, W.; Li, J.; Jiang, Z.; Cheng, L.; Yan, B.; Cao, J.; Sun, L.; Zhou, C.; Lei, M.; et al. Loss of AMPK activation promotes the invasion and metastasis of pancreatic cancer through an HSF1-dependent pathway. Mol. Oncol. 2017, 11, 1475–1492. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.-C.; Zhu, L.-F.; Pan, L.; Yu, M.; Shen, W.-L.; Li, B.; Zhang, W.; Liu, L.-K. Heat shock factor 1 in cancer-associated fibroblasts is a potential prognostic factor and drives progression of oral squamous cell carcinoma. Cancer Sci. 2019, 110, 1790–1803. [Google Scholar] [CrossRef] [PubMed]
- Kourtis, N.; Moubarak, R.S.; Aranda-Orgillés, B.; Lui, K.; Aydin, I.T.; Trimarchi, T.; Darvishian, F.; Salvaggio, C.; Zhong, J.; Bhatt, K.; et al. FBXW7 modulates cellular stress response and metastatic potential through HSF1 post-translational modification. Nat. Cell Biol. 2015, 17, 322–332. [Google Scholar] [CrossRef]
- Wang, B.; Lee, C.-W.; Witt, A.; Thakkar, A.; Ince, T. Heat shock factor 1 induces cancer stem cell phenotype in breast cancer cell lines. Breast Cancer Res. Treat. 2015, 153, 57–66. [Google Scholar] [CrossRef]
- Im, C.-N.; Yun, H.H.; Lee, J.-H. Heat Shock Factor 1 Depletion Sensitizes A172 Glioblastoma Cells to Temozolomide via Suppression of Cancer Stem Cell-Like Properties. Int. J. Mol. Sci. 2017, 18, 468. [Google Scholar] [CrossRef]
- Chou, S.-D.; Murshid, A.; Eguchi, T.; Gong, J.; Calderwood, S.K. HSF1 regulation of β-catenin in mammary cancer cells through control of HuR/elavL1 expression. Oncogene 2014, 34, 2178–2188. [Google Scholar] [CrossRef]
- Carpenter, R.; Sirkisoon, S.; Zhu, N.; Rimkus, T.; Harrison, A.; Anderson, A.; Paw, I.; Qasem, S.; Xing, F.; Liu, Y.; et al. Combined inhibition of AKT and HSF1 suppresses breast cancer stem cells and tumor growth. Oncotarget 2017, 8, 73947–73963. [Google Scholar] [CrossRef]
- Yasuda, K.; Hirohashi, Y.; Mariya, T.; Murai, A.; Tabuchi, Y.; Kuroda, T.; Kusumoto, H.; Takaya, A.; Yamamoto, E.; Kubo, T.; et al. Phosphorylation of HSF1 at serine 326 residue is related to the maintenance of gynecologic cancer stem cells through expression of HSP27. Oncotarget 2017, 8, 31540–31553. [Google Scholar] [CrossRef]
- Oei, A.L.; Vriend, L.E.M.; Krawczyk, P.M.; Horsman, M.; Franken, N.A.P.; Crezee, J. Targeting therapy-resistant cancer stem cells by hyperthermia. Int. J. Hyperth. 2017, 33, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.D.; Lee, D.; Cha-Molstad, H.; Kim, H.; Mun, S.R.; Ji, C.; Park, S.H.; Sung, K.S.; Choi, S.A.; Hwang, J.; et al. Glioma-derived cancer stem cells are hypersensitive to proteasomal inhibition. EMBO Rep. 2016, 18, 150–168. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, N.; Hirayoshi, K.; Kudo, H.; Takechi, H.; Aoike, A.; Kawai, K.; Nagata, K. Inhibition of the activation of heat shock factor in vivo and in vitro by flavonoids. Mol. Cell. Biol. 1992, 12, 3490–3498. [Google Scholar] [CrossRef] [PubMed]
- Zgajnar, N.R.; De Leo, S.A.; Lotufo, C.M.; Erlejman, A.G.; Piwien-Pilipuk, G.; Galigniana, M. Biological Actions of the Hsp90-binding Immunophilins FKBP51 and FKBP52. Biomolecules 2019, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Porter, G.A.; Beutner, G. Cyclophilin D, Somehow a Master Regulator of Mitochondrial Function. Biomolecules 2018, 8, 176. [Google Scholar] [CrossRef]
- Cheng, C.-W.; Tse, E. PIN1 in Cell Cycle Control and Cancer. Front. Pharmacol. 2018, 9, 1367. [Google Scholar] [CrossRef]
- McClements, L.; Yakkundi, A.; Papaspyropoulos, A.; Harrison, H.; Ablett, M.P.; Jithesh, P.V.; McKeen, H.D.; Bennett, R.; Donley, C.; Kissenpfennig, A.; et al. Targeting Treatment-Resistant Breast Cancer Stem Cells with FKBPL and Its Peptide Derivative, AD-01, via the CD44 Pathway. Clin. Cancer Res. 2013, 19, 3881–3893. [Google Scholar] [CrossRef]
- McClements, L.; Annett, S.; Yakkundi, A.; O’Rourke, M.; Valentine, A.; Moustafa, N.; Alqudah, A.; Simões, B.M.; Furlong, F.; Short, A.; et al. FKBPL and its peptide derivatives inhibit endocrine therapy resistant cancer stem cells and breast cancer metastasis by downregulating DLL4 and Notch4. BMC Cancer 2019, 19, 351. [Google Scholar] [CrossRef]
- Annett, S.; Moore, G.; Short, A.; Marshall, A.; McCrudden, C.; Yakkundi, A.; Das, S.; McCluggage, W.G.; Nelson, L.; Harley, I.; et al. FKBPL-based peptide, ALM201, targets angiogenesis and cancer stem cells in ovarian cancer. Br. J. Cancer 2019, 122, 361–371. [Google Scholar] [CrossRef]
- Posada, I.M.D.; Lectez, B.; Sharma, M.; Oetken-Lindholm, C.; Yetukuri, L.; Zhou, Y.; Aittokallio, T.; Abankwa, D. Rapalogs can promote cancer cell stemness in vitro in a Galectin-1 and H-ras-dependent manner. Oncotarget 2017, 8, 44550–44566. [Google Scholar] [CrossRef]
- Haskins, W.E.; Eedala, S.; Jadhav, Y.A.; Labhan, M.S.; Pericherla, V.C.; Perlman, E.J. Insights on neoplastic stem cells from gel-based proteomics of childhood germ cell tumors. Pediatr. Blood Cancer 2011, 58, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, S.; Khora, S.; Suresh, A. Cancer stem cell based molecular predictors of tumor recurrence in oral squamous cell carcinoma. Arch. Oral Biol. 2019, 99, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Lemma, S.; Avnet, S.; Salerno, M.; Chano, T.; Baldini, N. Identification and Validation of Housekeeping Genes for Gene Expression Analysis of Cancer Stem Cells. PLoS ONE 2016, 11, e0149481. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.R.; Choi, H.-K.; Bin Cho, K.; Kim, H.S.; Kang, K.-W. Involvement of Pin1 induction in epithelial-mesenchymal transition of tamoxifen-resistant breast cancer cells. Cancer Sci. 2009, 100, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.-L.; Gong, C.; Chen, C.-H.; Lee, D.Y.; Hu, H.; Huang, P.; Yao, Y.; Guo, W.; Reinhardt, F.; Wulf, G.; et al. Prolyl isomerase Pin1 acts downstream of miR200c to promote cancer stem-like cell traits in breast cancer. Cancer Res. 2014, 74, 3603–3616. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, Y.; Nishikiori, H.; Hirai, S.; Yamaguchi, M.; Yamada, G.; Watanabe, A.; Hasegawa, T.; Kojima, T.; Niki, T.; Takahashi, H. Prolyl isomerase Pin1 promotes survival in EGFR-mutant lung adenocarcinoma cells with an epithelial–mesenchymal transition phenotype. Lab. Investig. 2016, 96, 391–398. [Google Scholar] [CrossRef]
- Nakada, S.; Kuboki, S.; Nojima, H.; Yoshitomi, H.; Furukawa, K.; Takayashiki, T.; Takano, S.; Miyazaki, M.; Ohtsuka, M. Roles of Pin1 as a Key Molecule for EMT Induction by Activation of STAT3 and NF-κB in Human Gallbladder Cancer. Ann. Surg. Oncol. 2019, 26, 907–917. [Google Scholar] [CrossRef]
- Zhang, Z.-Z.; Yu, W.-X.; Zheng, M.; Liao, X.-H.; Wang, J.-C.; Yang, D.-Y.; Lu, W.-X.; Wang, L.; Zhang, S.; Liu, H.-K.; et al. Pin1 inhibition sensitizes chemotherapy in gastric cancer cells by targeting stem-cell like traits and multiple biomarkers. Mol. Cancer Ther. 2020, 19, 906–919. [Google Scholar] [CrossRef]
- Romano, S.; Staibano, S.; Greco, A.; Brunetti, A.; Nappo, G.; Ilardi, G.; Martinelli, R.; Sorrentino, A.; Di Pace, A.L.; Mascolo, M.; et al. FK506 binding protein 51 positively regulates melanoma stemness and metastatic potential. Cell Death Dis. 2013, 4, e578. [Google Scholar] [CrossRef]
- D’Angelillo, A.; Staibano, S.; Russo, M.; Romano, M.; Zambrano, N. Molecular Aspects of FKBP51 that Enable Melanoma Dissemination. Curr. Mol. Pharmacol. 2015, 9, 141–147. [Google Scholar] [CrossRef]
- Gao, Y.; Elamin, E.; Zhou, R.; Yan, H.; Liu, S.; Hu, S.; Dong, J.; Wei, M.; Sun, L.; Zhao, Y. FKBP51 promotes migration and invasion of papillary thyroid carcinoma through NF-κB-dependent epithelial-to-mesenchymal transition. Oncol. Lett. 2018, 16, 7020–7028. [Google Scholar] [CrossRef] [PubMed]
- Senturk, S.; Yao, Z.; Camiolo, M.; Stiles, B.; Rathod, T.; Walsh, A.M.; Nemajerova, A.; Lazzara, M.J.; Altorki, N.K.; Krainer, A.; et al. p53Ψ is a transcriptionally inactive p53 isoform able to reprogram cells toward a metastatic-like state. Proc. Natl. Acad. Sci. USA 2014, 111, E3287–E3296. [Google Scholar] [CrossRef] [PubMed]
- Rustighi, A.; Zannini, A.; Tiberi, L.; Sommaggio, R.; Piazza, S.; Sorrentino, G.; Nuzzo, S.; Tuscano, A.; Eterno, V.; Benvenuti, F.; et al. Prolyl-isomerase Pin1 controls normal and cancer stem cells of the breast. EMBO Mol. Med. 2013, 6, 99–119. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.-L.; Gong, C.; Chen, C.-H.; Hu, H.; Huang, P.; Zheng, M.; Yao, Y.; Wei, S.; Wulf, G.; Lieberman, J.; et al. The Rab2A GTPase promotes breast cancer stem cells and tumorigenesis via Erk signaling activation. Cell Rep. 2015, 11, 111–124. [Google Scholar] [CrossRef]
- Chen, L.; Xu, X.; Wen, X.; Xu, S.; Wang, L.; Lu, W.; Jiang, M.; Huang, J.; Yang, D.; Wang, J.; et al. Targeting PIN1 exerts potent antitumor activity in pancreatic ductal carcinoma via inhibiting tumor metastasis. Cancer Sci. 2019, 110, 2442–2455. [Google Scholar]
- Zhang, J.; Chen, M.; Zhu, Y.; Dai, X.; Dang, F.; Ren, J.; Ren, S.; Shulga, Y.V.; Beca, F.; Gan, W.; et al. SPOP Promotes Nanog Destruction to Suppress Stem Cell Traits and Prostate Cancer Progression. Dev. Cell 2019, 48, 329–344. [Google Scholar] [CrossRef]
- Wang, G.; Shen, J.; Sun, J.; Jiang, Z.; Fan, J.; Wang, H.; Yu, S.; Long, Y.; Liu, Y.; Bao, H.; et al. Cyclophilin A Maintains Glioma-Initiating Cell Stemness by Regulating Wnt/β-Catenin Signaling. Clin. Cancer Res. 2017, 23, 6640–6649. [Google Scholar] [CrossRef]
- Thacker, P.C.; Karunagaran, D. Curcumin and Emodin Down-Regulate TGF-β Signaling Pathway in Human Cervical Cancer Cells. PLoS ONE 2015, 10, e0120045. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Ding, J.; Nie, S.; Wang, L.; Zhang, L.; Ren, S. Celastrol strongly inhibits proliferation, migration and cancer stem cell properties through suppression of Pin1 in ovarian cancer cells. Eur. J. Pharmacol. 2019, 842, 146–156. [Google Scholar] [CrossRef]
- Campaner, E.; Rustighi, A.; Zannini, A.; Cristiani, A.; Piazza, S.; Ciani, Y.; Kalid, O.; Golan, G.; Baloglu, E.; Shacham, S.; et al. A covalent PIN1 inhibitor selectively targets cancer cells by a dual mechanism of action. Nat. Commun. 2017, 8, 15772. [Google Scholar] [CrossRef]
- Saw, P.E.; Zhang, A.; Nie, Y.; Zhang, L.; Xu, Y.; Xu, X. Tumor-Associated Fibronectin Targeted Liposomal Nanoplatform for Cyclophilin A siRNA Delivery and Targeted Malignant Glioblastoma Therapy. Front. Pharmacol. 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, X. Shrimp miRNA suppresses the stemness of human cancer stem cells via the PIN1 pathway. FASEB J. 2019, 33, 10767–10779. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.X.; Hong, F.; Zhang, Y.; Ansa-Addo, E.; Li, Z. GRP94/gp96 in Cancer. Adv. Cancer Res. 2016, 129, 165–190. [Google Scholar] [PubMed]
- Hong, F.; Liu, B.; Chiosis, G.; Gewirth, D.T.; Li, Z. α7 Helix Region of αI Domain Is Crucial for Integrin Binding to Endoplasmic Reticulum Chaperone gp96. J. Biol. Chem. 2013, 288, 18243–18248. [Google Scholar] [CrossRef]
- Bartkowiak, K.; Effenberger, K.E.; Harder, S.; Andreas, A.; Buck, F.; Peter-Katalinić, J.; Pantel, K.; Brandt, B. Discovery of a Novel Unfolded Protein Response Phenotype of Cancer Stem/Progenitor Cells from the Bone Marrow of Breast Cancer Patients. J. Proteome Res. 2010, 9, 3158–3168. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Zhao, M.; Matsuura, Y.; Laurent, S.; Yang, P.C.; Bernstein, D.; Ruiz-Lozano, P.; Serpooshan, V. Infection-resistant MRI-visible scaffolds for tissue engineering applications. BioImpacts 2016, 6, 111–115. [Google Scholar] [CrossRef]
- Li, Y.-C.; Chang, J.T.-C.; Chiu, C.; Lu, Y.-C.; Li, Y.-L.; Chiang, C.-H.; You, G.-R.; Lee, L.-Y.; Cheng, A.-J. Areca nut contributes to oral malignancy through facilitating the conversion of cancer stem cells. Mol. Carcinog. 2015, 55, 1012–1023. [Google Scholar] [CrossRef]
- Hu, T.; Xie, N.; Qin, C.; Wang, J.; You, Y. Glucose-regulated protein 94 is a novel glioma biomarker and promotes the aggressiveness of glioma via Wnt/β-catenin signaling pathway. Tumor Biol. 2015, 36, 9357–9364. [Google Scholar] [CrossRef]
- Khandelwal, A.; Crowley, V.; Blagg, B.S.J. Resorcinol-Based Grp94-Selective Inhibitors. ACS Med. Chem. Lett. 2017, 8, 1013–1018. [Google Scholar] [CrossRef]
- Gifford, J.B.; Hill, R. GRP78 Influences Chemoresistance and Prognosis in Cancer. Curr. Drug Targets 2018, 19, 701–708. [Google Scholar] [CrossRef]
- Miłoszewska, J.; Gos, M.; Przybyszewska, M.; Trembacz, H.; Koronkiewicz, M.; Janik, P. Mouse sarcoma L1 cell line holoclones have a stemness signature. Cell Prolif. 2010, 43, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-J.; Jan, C.-I.; Tsay, Y.-G.; Yu, Y.-H.; Huang, C.-Y.; Lin, S.-C.; Liu, C.J.; Chen, Y.-S.; Lo, J.-F.; Yu, C.-C. Elimination of head and neck cancer initiating cells through targeting glucose regulated protein 78 signaling. Mol. Cancer 2010, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-C.; Lee, L.-Y.; Li, Y.-C.; Chen, Y.-J.; Lu, Y.-C.; Li, Y.-L.; Wang, H.-M.; Chang, J.T.; Cheng, A.-J. Grp78 as a therapeutic target for refractory head–neck cancer with CD24−CD44+ stemness phenotype. Cancer Gene Ther. 2013, 20, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-J.; Chen, W.-Y.; Huang, C.-Y.; Liu, H.-H.; Wei, P.-L. Glucose-regulated protein 78 (GRP78) regulates colon cancer metastasis through EMT biomarkers and the NRF-2/HO-1 pathway. Tumor Biol. 2014, 36, 1859–1869. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-M.; Sun, L.-L.; Fang, R.-M.; Lin, L.-Z. YiQi ChuTan Recipe Inhibits Epithelial Mesenchymal Transition of A549 Cells under Hypoxia. Cell. Mol. Biol. 2016, 62, 10–15. [Google Scholar]
- Qiu, X.; Guan, X.; Liu, W.; Zhang, Y. DAL-1 attenuates epithelial to mesenchymal transition and metastasis by suppressing HSPA5 expression in non-small cell lung cancer. Oncol. Rep. 2017, 38, 3103–3113. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, Y.; Wei, X.; Chen, S.; Jia, N.; Zhou, Q.; Zhang, S.; Gui, S.; Wang, Y. Hyperbaric oxygen rescues lung cancer cells from chemical hypoxia-induced low differentiation and apoptosis resistance. Exp. Lung Res. 2018, 44, 417–423. [Google Scholar] [CrossRef]
- Sun, L.-L.; Chen, C.-M.; Zhang, J.; Wang, J.; Yang, C.-Z.; Lin, L.-Z. Glucose-Regulated Protein 78 Signaling Regulates Hypoxia-Induced Epithelial-Mesenchymal Transition in A549 Cells. Front. Oncol. 2019, 9, 137. [Google Scholar] [CrossRef]
- Cuevas, E.P.; Eraso, P.; Mazón, M.J.; Santos, V.; Moreno-Bueno, G.; Cano, A.; Portillo, F. LOXL2 drives epithelial-mesenchymal transition via activation of IRE1-XBP1 signalling pathway. Sci. Rep. 2017, 7, 44988. [Google Scholar] [CrossRef]
- Nayak, D.; Katoch, A.; Sharma, D.; Faheem, M.M.; Chakraborty, S.; Sahu, P.K.; Chikan, N.A.; Amin, H.; Gupta, A.P.; Gandhi, S.G.; et al. Indolylkojyl methane analogue IKM5 potentially inhibits invasion of breast cancer cells via attenuation of GRP78. Breast Cancer Res. Treat. 2019, 177, 307–323. [Google Scholar] [CrossRef]
- Feng, X.; Lv, W.; Wang, S.; He, Q. miR-495 enhances the efficacy of radiotherapy by targeting GRP78 to regulate EMT in nasopharyngeal carcinoma cells. Oncol. Rep. 2018, 40, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Cultrara, C.N.; Kozuch, S.D.; Ramasundaram, P.; Heller, C.J.; Shah, S.; Beck, A.E.; Sabatino, D.; Zilberberg, J. GRP78 modulates cell adhesion markers in prostate Cancer and multiple myeloma cell lines. BMC Cancer 2018, 18, 1263. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.P.; Dupre, T.; Siskind, L.J.; Beverly, L.J. Common cytotoxic chemotherapeutics induce epithelial-mesenchymal transition (EMT) downstream of ER stress. Oncotarget 2017, 8, 22625–22639. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.-J.; Chin-Sheng, H.; Kuo, L.-J.; Wei, P.-L.; Lu, H.-H.; Chen, H.-A.; Liu, T.-Z.; Liu, J.-J.; Liu, D.-Z.; Ho, Y.-S.; et al. Survivin-Mediated Cancer Cell Migration Through GRP78 and Epithelial-Mesenchymal Transition (EMT) Marker Expression in Mahlavu Cells. Ann. Surg. Oncol. 2011, 19, 336–343. [Google Scholar] [CrossRef]
- Wei, P.-L.; Kuo, L.-J.; Wang, W.; Lin, F.-Y.; Liu, H.-H.; Tseng, H.; Ho, Y.-S.; Huang, M.-T.; Wu, C.-H.; Chang, Y.-J. Silencing of Glucose-Regulated Protein 78 (GRP78) Enhances Cell Migration Through the Upregulation of Vimentin in Hepatocellular Carcinoma Cells. Ann. Surg. Oncol. 2011, 19, 572–579. [Google Scholar] [CrossRef]
- Xia, W.; Zhuang, J.; Wang, G.; Ni, J.; Wang, J.; Ye, Y. P4HB promotes HCC tumorigenesis through downregulation of GRP78 and subsequent upregulation of epithelial-to-mesenchymal transition. Oncotarget 2016, 8, 8512–8521. [Google Scholar] [CrossRef]
- Chang, H.-L.; Chen, H.-A.; Bamodu, O.A.; Lee, K.-F.; Tzeng, Y.-M.; Lee, W.-H.; Tsai, J.-T. Ovatodiolide suppresses yes-associated protein 1-modulated cancer stem cell phenotypes in highly malignant hepatocellular carcinoma and sensitizes cancer cells to chemotherapy in vitro. Toxicol. In Vitro 2018, 51, 74–82. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Fan, Y.; Li, H.; Li, Z.; Li, Y. Overexpressed GRP78 affects EMT and cell-matrix adhesion via autocrine TGF-β/Smad2/3 signaling. Int. J. Biochem. Cell Biol. 2015, 64, 202–211. [Google Scholar] [CrossRef]
- Evensen, N.A.; Kuscu, C.; Nguyen, H.-L.; Zarrabi, K.; Dufour, A.; Kadam, P.; Hu, Y.-J.; Pulkoski-Gross, A.; Bahou, W.F.; Zucker, S.; et al. Unraveling the role of KIAA1199, a novel endoplasmic reticulum protein, in cancer cell migration. J. Natl. Cancer Inst. 2013, 105, 1402–1416. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, X.; Yang, Y.P.; Li, B. GRP78 mediates radiation resistance of a stem cell-like subpopulation within the MCF-7 breast cancer cell line. Oncol. Rep. 2013, 30, 2119–2126. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Z.; Peng, C.; You, J.; Shen, J.; Han, S.; Chen, J. Dietary compound isoliquiritigenin targets GRP78 to chemosensitize breast cancer stem cells via β-catenin/ABCG2 signaling. Carcinogenesis 2014, 35, 2544–2554. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.-W.; Yu, C.-C.; Hsieh, P.-L.; Liao, Y.-W.; Lu, M.-Y.; Chu, P.-M. Targeting oral cancer stemness and chemoresistance by isoliquiritigenin-mediated GRP78 regulation. Oncotarget 2017, 8, 93912–93923. [Google Scholar] [CrossRef]
- Huynh, T.-T.; Lin, C.-M.; Lee, W.-H.; Wu, A.T.H.; Lin, Y.-K.; Lin, Y.-F.; Yeh, C.-T.; Wang, L.-S. Pterostilbene suppressed irradiation-resistant glioma stem cells by modulating GRP78/miR-205 axis. J. Nutr. Biochem. 2015, 26, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-W.; Chen, Y.-S.; Tsay, Y.-G.; Han, C.-L.; Chen, Y.-J.; Yang, C.-C.; Hung, K.-F.; Lin, C.-H.; Huang, T.-Y.; Kao, S.-Y.; et al. ROS-independent ER stress-mediated NRF2 activation promotes warburg effect to maintain stemness-associated properties of cancer-initiating cells. Cell Death Dis. 2018, 9, 194. [Google Scholar] [CrossRef]
- Dauer, P.; Sharma, N.S.; Gupta, V.K.; Durden, B.; Hadad, R.; Banerjee, S.; Dudeja, V.; Saluja, A.; Banerjee, S. ER stress sensor, glucose regulatory protein 78 (GRP78) regulates redox status in pancreatic cancer thereby maintaining “stemness”. Cell Death Dis. 2019, 10, 132. [Google Scholar] [CrossRef]
- Su, S.-F.; Chang, Y.-W.; Andreu-Vieyra, C.; Fang, J.Y.; Yang, Z.; Han, B.; Lee, A.; Liang, G. miR-30d, miR-181a and miR-199a-5p cooperatively suppress the endoplasmic reticulum chaperone and signaling regulator GRP78 in cancer. Oncogene 2012, 32, 4694–4701. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-M.; Lin, Y.-T.; Shun, C.-T.; Lin, S.-H.; Wei, T.-T.; Chuang, S.-H.; Wu, M.-S.; Chen, C.-C. Zebularine inhibits tumorigenesis and stemness of colorectal cancer via p53-dependent endoplasmic reticulum stress. Sci. Rep. 2013, 3, 3219. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, B.; Arumugam, P.; Ju, H.; Kulsi, G.; Samson, A.A.S.; Song, J.M. Novel ruthenium(II) triazine complex [Ru(bdpta)(tpy)]2+ co-targeting drug resistant GRP78 and subcellular organelles in cancer stem cells. Eur. J. Med. Chem. 2018, 156, 747–759. [Google Scholar] [CrossRef]
- Shen, J.; Xu, R.; Mai, J.; Kim, H.-C.; Guo, X.; Qin, G.; Yang, Y.; Wolfram, J.; Mu, C.; Xia, X.; et al. High Capacity Nanoporous Silicon Carrier for Systemic Delivery of Gene Silencing Therapeutics. ACS Nano 2013, 7, 9867–9880. [Google Scholar] [CrossRef]
- Samson, A.A.S.; Park, S.; Kim, S.-Y.; Min, D.-H.; Jeon, N.L.; Song, J.M. Liposomal co-delivery-based quantitative evaluation of chemosensitivity enhancement in breast cancer stem cells by knockdown of GRP78/CLU. J. Liposome Res. 2018, 29, 44–52. [Google Scholar] [CrossRef]
- Mo, L.; Bachelder, R.E.; Kennedy, M.; Chen, P.-H.; Chi, J.-T.; Berchuck, A.; Cianciolo, G.; Pizzo, S.V. Syngeneic murine ovarian cancer model reveals that ascites enriches for ovarian cancer stem-like cells expressing membrane GRP78. Mol. Cancer Ther. 2015, 14, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; Li, H.-D.; Zhao, S.; Zhao, L.; Song, H.-J.; Wang, G.; Guo, Q.-J.; Luan, Z.-D.; Su, R. The Cell Surface GRP78 Facilitates the Invasion of Hepatocellular Carcinoma Cells. BioMed Res. Int. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Stanciauskas, R.; Zhang, P.; Woo, D.; Wu, K.; Kelly, K.; Gill, P.S.; Yu, M.; Pinaud, F.; Lee, A. GRP78 regulates CD44v membrane homeostasis and cell spreading in tamoxifen-resistant breast cancer. Life Sci. Alliance 2019, 2, e201900377. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Chang, J.T.-C.; Chien, K.-Y.; Lee, Y.-S.; You, G.-R.; Cheng, A.-J. The Endogenous GRP78 Interactome in Human Head and Neck Cancers: A Deterministic Role of Cell Surface GRP78 in Cancer Stemness. Sci. Rep. 2018, 8, 536. [Google Scholar] [CrossRef] [PubMed]
- Bachelder, R.E. Chapter 6: Cell surface GRP78: A targetable marker of cancer stem-like cells. In Cell Surface GRP78, a New Paradigm in Signal Transduction Biology; Pizzo, S.V., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 99–109. [Google Scholar]
- Conner, C.; Lager, T.W.; Guldner, I.H.; Wu, M.-Z.; Hishida, Y.; Hishida, T.; Ruiz, S.; Yamasaki, A.E.; Gilson, R.C.; Belmonte, J.C.I.; et al. Cell surface GRP78 promotes stemness in normal and neoplastic cells. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Z.; Li, Z. GRP78 secreted by tumor cells stimulates differentiation of bone marrow mesenchymal stem cells to cancer-associated fibroblasts. Biochem. Biophys. Res. Commun. 2013, 440, 558–563. [Google Scholar] [CrossRef]
- La, X.; Zhang, L.; Li, H.; Li, Z.; Song, G.; Yang, P.; Yang, Y. Ajuba receptor mediates the internalization of tumor-secreted GRP78 into macrophages through different endocytosis pathways. Oncotarget 2018, 9, 15464–15479. [Google Scholar] [CrossRef][Green Version]
- Deocaris, C.C.; Lu, W.-J.; Kaul, S.; Wadhwa, R. Druggability of Mortalin for Cancer and Neuro-Degenerative Disorders. Curr. Pharm. Des. 2013, 19, 418–429. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.-B.; Jia, W.-D.; Xu, G.; Ma, J.-L.; Huang, M.; Deng, Y.-R.; Li, J.-S. Overexpression of Mortalin in hepatocellular carcinoma and its relationship with angiogenesis and epithelial to mesenchymal transition. Int. J. Oncol. 2013, 44, 247–255. [Google Scholar] [CrossRef]
- Na, Y.; Kaul, S.; Ryu, J.; Lee, J.-S.; Ahn, H.M.; Kalra, R.S.; Li, L.; Widodo, N.; Yun, C.-O.; Wadhwa, R. Stress chaperone mortalin contributes to epithelial-mesenchymal transition and cancer metastasis. Cancer Res. 2016, 76, 2754–2765. [Google Scholar] [CrossRef]
- Kang, Q.; Cai, J.-B.; Dong, R.-Z.; Liu, L.-X.; Zhang, C.; Zhang, P.-F.; Zou, H.; Xie, N.; Zhang, L.; Zhang, X.-Y.; et al. Mortalin promotes cell proliferation and epithelial mesenchymal transition of intrahepatic cholangiocarcinoma cells in vitro. J. Clin. Pathol. 2017, 70, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Jin, T.; Chen, L.; Zhang, X.; Zhu, G.; Wang, Q.; Lin, Z. Mortalin is a distinct bio-marker and prognostic factor in serous ovarian carcinoma. Gene 2019, 696, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, Y.; Cui, M.; Wang, X.; Lin, Z. Mortalin contributes to colorectal cancer by promoting proliferation and epithelial–mesenchymal transition. IUBMB Life 2019, 72, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.-O.; Bhargava, P.; Na, Y.; Lee, J.-S.; Ryu, J.; Kaul, S.; Wadhwa, R. Relevance of mortalin to cancer cell stemness and cancer therapy. Sci. Rep. 2017, 7, 42016. [Google Scholar] [CrossRef]
- Abdullah, A.; Sane, S.; Branick, K.A.; Freeling, J.L.; Wang, H.; Zhang, D.; Rezvani, K. A plant alkaloid, veratridine, potentiates cancer chemosensitivity by UBXN2A-dependent inhibition of an oncoprotein, mortalin-2. Oncotarget 2015, 6, 23561–23581. [Google Scholar] [CrossRef] [PubMed]
- Matassa, D.S.; Agliarulo, I.; Avolio, R.; Landriscina, M.; Esposito, F. TRAP1 Regulation of Cancer Metabolism: Dual Role as Oncogene or Tumor Suppressor. Genes 2018, 9, 195. [Google Scholar] [CrossRef]
- Lettini, G.; Sisinni, L.; Condelli, V.; Matassa, D.S.; Simeon, V.; Maddalena, F.; Gemei, M.; Lopes, E.; Vita, G.; Del Vecchio, L.; et al. TRAP1 regulates stemness through Wnt/β-catenin pathway in human colorectal carcinoma. Cell Death Differ. 2016, 23, 1792–1803. [Google Scholar] [CrossRef]
- Amoroso, M.R.; Matassa, D.S.; Agliarulo, I.; Avolio, R.; Lu, H.; Sisinni, L.; Lettini, G.; Gabra, H.; Landriscina, M.; Esposito, F. TRAP1 downregulation in human ovarian cancer enhances invasion and epithelial-mesenchymal transition. Cell Death Dis. 2016, 7, e2522. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Cho, K.; Dong, X.; Teng, L.; Han, D.; Liu, H.; Chen, X.; Chen, X.; Hou, X.; et al. Downregulation of TRAP1 sensitizes glioblastoma cells to temozolomide chemotherapy through regulating metabolic reprogramming. NeuroReport 2016, 27, 136–144. [Google Scholar] [CrossRef]
- Park, H.-K.; Hong, J.-H.; Oh, Y.T.; Kim, S.S.; Yin, J.; Lee, A.-J.; Chae, Y.C.; Kim, J.H.; Park, S.-H.; Park, C.-K.; et al. Interplay between TRAP1 and sirtuin-3 modulates mitochondrial respiration and oxidative stress to maintain stemness of glioma stem cells. Cancer Res. 2019, 79, 1369–1382. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Ishida, C.T.; Shang, E.; Shu, C.; Bianchetti, E.; Karpel-Massler, G.; Siegelin, M.D. Activation of LXR Receptors and Inhibition of TRAP1 Causes Synthetic Lethality in Solid Tumors. Cancers 2019, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Vo, V.T.; Choi, J.-W.; Phan, A.N.; Hua, T.N.; Kim, M.-K.; Kang, B.H.; Jung, S.-H.; Yong, S.J.; Jeong, Y. TRAP1 Inhibition Increases Glutamine Synthetase Activity in Glutamine Auxotrophic Non-small Cell Lung Cancer Cells. Anticancer. Res. 2018, 38, 2187–2193. [Google Scholar] [PubMed]
- Jia, M.; Guo, Y.; Zhu, D.; Zhang, N.; Li, L.; Jiang, J.; Dong, Y.; Xu, Q.; Zhang, X.; Wang, M.; et al. Pro-metastatic activity of AGR2 interrupts angiogenesis target bevacizumab efficiency via direct interaction with VEGFA and activation of NF-κB pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Delom, F.; Nazaraliyev, A.; Fessart, D. The role of protein disulphide isomerase AGR2 in the tumour niche. Biol. Cell 2018, 110, 271–282. [Google Scholar] [CrossRef]
- Yosudjai, J.; Inpad, C.; Chomwong, S.; Dana, P.; Sawanyawisuth, K.; Phimsen, S.; Wongkham, S.; Jirawatnotai, S.; Kaewkong, W. An aberrantly spliced isoform of anterior gradient-2, AGR2vH promotes migration and invasion of cholangiocarcinoma cell. Biomed. Pharmacother. 2018, 107, 109–116. [Google Scholar] [CrossRef]
- Jung, S.Y.; Yun, J.; Kim, S.J.; Kang, S.; Kim, D.Y.; Kim, Y.J.; Park, J.H.; Jang, W.B.; Ji, S.T.; Ha, J.S.; et al. Basic helix-loop-helix transcription factor Twist1 is a novel regulator of anterior gradient protein 2 homolog (AGR2) in breast cancer. Biochem. Biophys. Res. Commun. 2019, 516, 149–156. [Google Scholar] [CrossRef]
- Lamichane, B.D.; Jung, S.Y.; Yun, J.; Kang, S.; Kim, D.Y.; Lamichane, S.; Kim, Y.J.; Park, J.H.; Jang, W.B.; Ji, S.T.; et al. AGR2 is a target of canonical Wnt/β-catenin signaling and is important for stemness maintenance in colorectal cancer stem cells. Biochem. Biophys. Res. Commun. 2019, 515, 600–606. [Google Scholar] [CrossRef]
- Chi, J.; Zhang, H.; Hu, J.; Song, Y.; Li, J.; Wang, L.; Wang, Z. AGR3 promotes the stemness of colorectal cancer via modulating Wnt/β-catenin signalling. Cell. Signal. 2019, 65, 109419. [Google Scholar] [CrossRef]
- Obacz, J.; Sommerova, L.; Sicari, D.; Durech, M.; Avril, T.; Iuliano, F.; Pastorekova, S.; Hrstka, R.; Chevet, E.; Delom, F.; et al. Extracellular AGR3 regulates breast cancer cells migration via Src signaling. Oncol. Lett. 2019, 18, 4449–4456. [Google Scholar] [CrossRef]
- Yang, S.; Shergalis, A.; Lu, D.; Kyani, A.; Liu, Z.; Ljungman, M.; Neamati, N.; Lu, Z. Design, Synthesis, and Biological Evaluation of Novel Allosteric Protein Disulfide Isomerase Inhibitors. J. Med. Chem. 2019, 62, 3447–3474. [Google Scholar] [CrossRef]
- Liu, C.-C.; LeClair, P.; Pedari, F.; Vieira, H.; Monajemi, M.; Sly, L.; Reid, G.S.; Lim, C.J. Integrins and ERp57 Coordinate to Regulate Cell Surface Calreticulin in Immunogenic Cell Death. Front. Oncol. 2019, 9, 411. [Google Scholar] [CrossRef] [PubMed]
- Pampalakis, G.; Prosnikli, E.; Agalioti, T.; Vlahou, A.; Zoumpourlis, V.; Sotiropoulou, G. A Tumor-Protective Role for Human Kallikrein-Related Peptidase 6 in Breast Cancer Mediated by Inhibition of Epithelial-to-Mesenchymal Transition. Cancer Res. 2009, 69, 3779–3787. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Lee, W.-J.; Lai, D.-W.; Wu, S.-M.; Liu, C.-Y.; Tien, H.-R.; Chiu, C.-S.; Peng, Y.-C.; Jan, Y.-J.; Chao, T.-H.; et al. Honokiol confers immunogenicity by dictating calreticulin exposure, activating ER stress and inhibiting epithelial-to-mesenchymal transition. Mol. Oncol. 2015, 9, 834–849. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, X.; Ma, L.; Yi, Q.; Sun, W.; Tang, L. Calreticulin regulates TGF-β1-induced epithelial mesenchymal transition through modulating Smad signaling and calcium signaling. Int. J. Biochem. Cell Biol. 2017, 90, 103–113. [Google Scholar] [CrossRef]
- Sheng, W.; Chen, C.; Dong, M.; Wang, G.; Zhou, J.; Song, H.; Li, Y.; Zhang, J.; Ding, S. Calreticulin promotes EGF-induced EMT in pancreatic cancer cells via Integrin/EGFR-ERK/MAPK signaling pathway. Cell Death Dis. 2017, 8, e3147. [Google Scholar] [CrossRef] [PubMed]
- Matsukuma, S.; Yoshimura, K.; Ueno, T.; Oga, A.; Inoue, M.; Watanabe, Y.; Kuramasu, A.; Fuse, M.; Tsunedomi, R.; Nagaoka, S.; et al. Calreticulin is highly expressed in pancreatic cancer stem-like cells. Cancer Sci. 2016, 107, 1599–1609. [Google Scholar] [CrossRef]
- Mead, A.J.; Mullally, A. Myeloproliferative neoplasm stem cells. Blood 2017, 129, 1607–1616. [Google Scholar] [CrossRef]
- Han, A.; Li, C.; Zahed, T.; Wong, M.; Smith, I.; Hoedel, K.; Green, D.; Boiko, A.D. Calreticulin is a Critical Cell Survival Factor in Malignant Neoplasms. PLoS Biol. 2019, 17, e3000402. [Google Scholar] [CrossRef]
- Gameiro, S.R.; Malamas, A.S.; Bernstein, M.B.; Tsang, K.Y.; Vassantachart, A.; Sahoo, N.; Tailor, R.; Pidikiti, R.; Guha, C.P.; Hahn, S.M.; et al. Tumor Cells Surviving Exposure to Proton or Photon Radiation Share a Common Immunogenic Modulation Signature, Rendering Them More Sensitive to T Cell–Mediated Killing. Int. J. Radiat. Oncol. 2016, 95, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Q.; Guo, Q.M.; Xu, X.P.; Liang, J.C.; He, Y.Y.; An, S.H.; Su, F.; Li, C.Y.; Huang, C.X. Preparation of chaperone-antigen peptide vaccine derived from human gastric cancer stem cells and its immune function. Zhonghua zhong liu za zhi Chinese J. Oncol. 2017, 39, 109–114. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabakov, A.; Yakimova, A.; Matchuk, O. Molecular Chaperones in Cancer Stem Cells: Determinants of Stemness and Potential Targets for Antitumor Therapy. Cells 2020, 9, 892. https://doi.org/10.3390/cells9040892
Kabakov A, Yakimova A, Matchuk O. Molecular Chaperones in Cancer Stem Cells: Determinants of Stemness and Potential Targets for Antitumor Therapy. Cells. 2020; 9(4):892. https://doi.org/10.3390/cells9040892
Chicago/Turabian StyleKabakov, Alexander, Anna Yakimova, and Olga Matchuk. 2020. "Molecular Chaperones in Cancer Stem Cells: Determinants of Stemness and Potential Targets for Antitumor Therapy" Cells 9, no. 4: 892. https://doi.org/10.3390/cells9040892
APA StyleKabakov, A., Yakimova, A., & Matchuk, O. (2020). Molecular Chaperones in Cancer Stem Cells: Determinants of Stemness and Potential Targets for Antitumor Therapy. Cells, 9(4), 892. https://doi.org/10.3390/cells9040892




